Many motile cells, including epithelial cells, keratinocytes, leukocytes and cancer cells, can sense extracellular weak electric fields (EFs), and migrate directionally, a phenomenon termed electrotaxis/galvanotaxis.1 Direct current EFs have been detected at wounds, tissue lesions and during development in many organisms, including human. The molecular mechanisms by which cell senses extracellular EFs, however, remain largely unknown.1
“Taxis” is the directional movement of a cell (or a free-moving organism) in response to environmental stimuli, and plays fundamental roles at both cellular and tissue levels.2 Many types of taxis have been identified, and some of them are well characterized.2 For example, directional migration of cells toward or away from a soluble chemical compound is known as chemotaxis, and many receptors that are necessary for sensing the chemical compound and transduce its signals to intracellular downstream pathways have been identified and well characterized.3 Previous research demonstrated that galvanotaxis shares some similar signaling pathways with chemotaxis.4 Like chemotaxis, galvanotaxis is thought to be a crucial cellular behavior for maintenance of homeostasis in our body, e.g. wound healing, angiogenesis, and development. In addition, electric stimulation could be a promising therapeutic method to treat non-healing chronic wounds such as diabetic foot ulceration, since keratinocyte migration can be controlled/enhanced by exogenously applied EFs.
To discover sensor molecules/sensing mechanisms for extracellular EFs in galvanotaxis, we first developed a more efficient method to determine galvanotaxis responses of cultured cells. We then knocked down individual genes in a human corneal epithelial cell line (hTCEpi cells) using a siRNA library of 381 ion channels, pumps, transporters and other putative channel encoding genes. Screening of the knockdown cells produced a comprehensive profile of galvanotaxis phenotypes. This analysis identified 35 gene knockdowns that showed significant effects on galvanotaxis. Knockdown of KCNJ15 and 8 others genes significantly decreased the directedness value (a quantification for how directionally cells move in an EF). Knockdown of CLCN1 or any of 8 other genes significantly increased the directedness. From the ion channels identified, we focused on KCNJ15 because its knockdown showed the most severe reduction of directionality without affecting migration speed, and revealed a unique sensing mechanism.5 KCNJ15 encodes inwardly rectifying K+ channel Kir4.2. We confirmed the knockdown efficiency by checking RNA and protein products of KCNJ15. Knockdown was also confirmed by membrane potential measurement; membrane potential of KCNJ15 knocked down cells (−39 mV) is less negative than that in control cells (−52 mV).
Knocking down of KCNJ15 in hTCEpi cells impaired galvanotaxis significantly, abolishing directionality without any effect on migration speed. Pharmacological inhibition of Kir4.2 by Ba2+ also strongly impaired galvanotaxis. In an EF, some types of cell migrate toward the anode, opposite to the direction of hTCEpi cell galvanotaxis. Importantly, knocking down of KCNJ15 also impaired anode-directed galvanotaxis of human keratinocyte-derived HaCaT and human breast cancer-derived MDA-MB-231 cells.
The inward rectification property of inwardly rectifying K+ (Kir) channels is mediated by positively charged intracellular small molecules called polyamines.6 Endogenous polyamines, spermine and spermidine in human and most mammals bind to negatively charged amino acid residues located in the pore region of the channel and block outward flux of K+. We next evaluated the effect of intracellular polyamines depletion on galvanotaxis. Depletion of polyamines significantly inhibited galvanotaxis, as in knock down and inhibitor experiments. We then replaced the negatively charged residue in the pore region (glutamic acid at position 157 of human Kir4.2) with asparagine, and expressed the mutant protein (E157N) in hTCEpi cells. Expression of E157N significantly inhibited galvanotaxis. We also tested if an EF affects distribution of Kir4.2 protein on the plasma membrane. However, distribution of Kir4.2 protein was not affected by an extracellular EF. Interestingly, application of an EF caused asymmetrical distribution of intracellular polyamines; polyamines were accumulated at the cathode-facing side of the cells. It has been reported that phosphatidylinositol-3, 4, 5-trisphosphate (PIP3) is recruited to the leading edge in cell undergoing directional migration, including galvanotaxis.4 Cathode-facing accumulation (accumulation at the leading edge of the cell) of PIP3 was not observed in KCNJ15 knocked down and Ba2+-treated hTCEpi cells. Furthermore, the expression of E157N mutant protein also impaired cathode-facing accumulation of PIP3 in hTCEpi cells. These observations suggest that KCNJ15/Kir4.2 and its interaction with polyamines are essential for EF sensing for galvanotaxis.
This study revealed that the concerted action of 2 molecules, potassium channel Kir4.2 and polyamines, are required for cells to sense an extracellular EF for galvanotaxis (Fig. 1). Importantly, this sensing mechanism appears to be important for both cathode- and anode- migrating galvanotaxis. These data suggest for the first time a 2-molecule sensing model in galvanotaxis. Caution should be noted here because this model is unlikely to be an exclusive one, because potassium transporter Trk1p, sodium channel ENaC, calcium channels and integrin have been shown to be required for EF sensing in yeast, and galvanotaxis of keratinocytes.7,8 Future studies should reveal a shared molecular basis of these different sensing mechanisms, and separation of sensing from signaling and motor machinery in directional migration. We expect an exciting and productive research field in the coming years in ion channel regulation of directional migration, which underlies many important biology and pathology processes.
Figure 1.
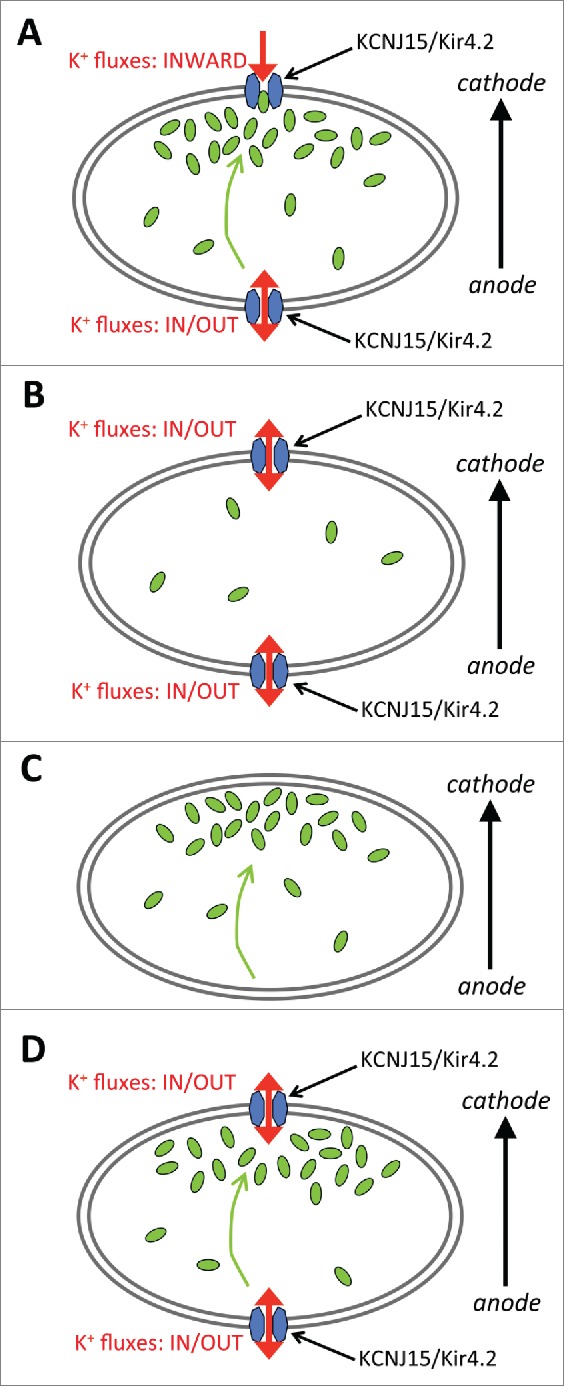
A hypothetical 2-molecule model for KCNJ15/Kir4.2 and intracellular polyamines in electric field sensing through biased inward rectification of potassium channels. (A) In an electric field, intracellular polyamines accumulate at the cathode-facing side and modulate the rectification property of KCNJ15/Kir4.2. The cathode would show an increased inward rectifying property, whereas the anode side would show a decreased inward rectifying property. This biased inward rectification of potassium channels to the cathode side would result in directional sensing. (B) In a polyamine-depleted cell, the rectification property of KCNJ15/Kir4.2 in the cell would be lost at both cathode and anode sides thus would decrease biased inward rectification in the cell, and result in loss of directional sensing. (C) In a KCNJ15 knocked down cell, as well as in pharmacological inhibition, biased inward rectification would also be significantly decreased, causing loss of directional sensing. (D) In polyamine-binding defective mutant E157N expressing cells, polyamines cannot bind to KCNJ15/Kir4.2, so rectification property of KCNJ15/Kir4.2 in the cell would be lost at both sides, thus preventing biased inward rectification of potassium channels in an electric field resulting in loss of directional sensing.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
English editing and proof reading by Dr. Brian Reid is gratefully acknowledged.
Funding
This work was supported by NIH EY019101 to M.Z. This study was supported in part by an Unrestricted Grant from Research to Prevent Blindness, Inc., and the UC Davis NEI core grant.
References
- [1].McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev 2005; 85(3):943-78; PMID:15987799; http://dx.doi.org/ 10.1152/physrev.00020.2004 [DOI] [PubMed] [Google Scholar]
- [2].Lara Rodriguez L, Schneider IC. Directed cell migration in multi-cue environments. Integrat Biol 2013; 5(11):1306-23; PMID:24089107; http://dx.doi.org/ 10.1039/c3ib40137e [DOI] [PubMed] [Google Scholar]
- [3].Swaney KF, Huang CH, Devreotes PN. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu Rev Biophys 2010; 39:265-89; PMID:20192768; http://dx.doi.org/ 10.1146/annurev.biophys.093008.131228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, et al.. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006; 442(7101):457-60; PMID:16871217; http://dx.doi.org/ 10.1038/nature04925 [DOI] [PubMed] [Google Scholar]
- [5].Nakajima K, Zhu K, Sun YH, Hegyi B, Zeng Q, Murphy CJ, Small JV, Chen-Izu Y, Izumiya Y, Penninger JM, Zhao M. KCNJ15/Kir4.2 couples with polyamines to sense weak extracellular electric fields in galvanotaxis. Nat Commun 2015; 6:8532; PMID:26449415; http://dx.doi.org/ 10.1038/ncomms9532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 2010; 90(1):291-366; PMID:20086079; http://dx.doi.org/ 10.1152/physrev.00021.2009 [DOI] [PubMed] [Google Scholar]
- [7].Yang HY, Charles RP, Hummler E, Baines DL, Isseroff RR. The epithelial sodium channel mediates the directionality of galvanotaxis in human keratinocytes. J Cell Sci 2013; 126(Pt 9):1942-51; PMID:23447677; http://dx.doi.org/ 10.1242/jcs.113225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Haupt A, Campetelli A, Bonazzi D, Piel M, Chang F, Minc N. Electrochemical regulation of budding yeast polarity. PLoS Biol 2014; 12(12):e1002029; PMID:25548923; http://dx.doi.org/ 10.1371/journal.pbio.1002029 [DOI] [PMC free article] [PubMed] [Google Scholar]


