ABSTRACT
Anion exchanger 2 (AE2) has a critical role in epithelial cells and is involved in the ionic homeostasis such as Cl− uptake and HCO3− secretion. However, little is known about the regulatory mechanism of AE2. The main goal of the present study was to investigate potential regulators, such as spinophilin (SPL), inositol-1,4,5-trisphosphate [IP3] receptors binding protein released with IP3 (IRBIT), STE20/SPS1-related proline/alanine-rich kinase (SPAK) kinase, and carbonic anhydrase XII (CA XII). We found that SPL binds to AE2 and markedly increased the Cl−/HCO3− exchange activity of AE2. Especially SPL 1–480 domain is required for enhancing AE2 activity. For other regulatory components that affect the fidelity of fluid and HCO3− secretion, IRBIT and SPAK had no effect on the activity of AE2 and no protein-protein interaction with AE2. It has been proposed that CA activity is closely associated with AE activity. In this study, we provide evidence that the basolateral membrane-associated CA isoform CA XII significantly increased the activity of AE2 and co-localized with AE2 to the plasma membrane. Collectively, SPL and CA XII enhanced the Cl−/HCO3− exchange activity of AE2. The modulating action of these regulatory proteins could serve as potential therapeutic targets for secretory diseases mediated by AE2.
KEYWORDS: anion exchanger 2, carbonic anhydrase XII, fluid secretion, IRBIT, SPAK, spinophilin
Introduction
Transmembrane bicarbonate (HCO3−) transport is critical for maintaining mammalian cell functions, such as the maintenance of intracellular pH and the ionic composition and homeostasis of fluid volume or concentration. HCO3− transportation is conducted by numerous kinds of ion transporters in epithelial cells. Fluid and HCO3− secretion requires HCO3− influx at the basolateral membrane (BLM) and subsequent HCO3− efflux at the luminal membrane (LM) of secretory epithelia such as salivary and pancreatic glands.1 Dysfunction of secretory processes, such as in cystic fibrosis, Sjögren's syndrome, and pancreatitis,2,3 leads to aberrant fluid and HCO3− secretion in secretory epithelia. HCO3− transport is directed by 2 major classes of Cl−/HCO3− exchangers: the solute carrier family member 4 (SLC4) and sulfate permease SLC26 superfamilies. Members of the SLC4A superfamily are crucial transporters involved in the maintenance of cellular HCO3− and Cl− concentrations.2,4,5 Of these, AE2 (SLC4A2) is widely expressed in most cells, including epithelial cells such as airway epithelia, proximal colon, and salivary glands cells.6-8 AE activity is regulated by many signals in epithelial cells. At the cellular level, muscarinic receptor stimulation enhances AE activity in salivary acinar cells.9 The global mechanism by which extracellular stimuli including activation of muscarinic receptors are converted to intracellular Ca2+ responses involves the activation of G protein-coupled receptors (GPCRs). The GPCRs recruit large complex proteins including the Gα subunit, Gßγ, PLCß, and regulators of G protein signaling (RGS) proteins.10 The RGS proteins recruit the scaffolding protein spinophilin (SPL) that mediates the inhibition of GPCR to reduce the intensity of Ca2+ signals triggered by GPCR activation.11 Although the regulatory role of SPL on GPCRs is well characterized,10,11 little is known about the possible role of SPL on Cl−/HCO3− exchange. Moreover, the signaling elements involved in AE activation are unclear. These uncertainties led to our hypothesis that SPL interacts with AE2.
Several reports have shown that regulatory components affect the fidelity of fluid and HCO3− secretion.1,12-14 Several regulators of the electrogenic Na+-HCO3− cotransporter 1 (NBCe1) were recently shown to be involved in regulating the transporter's activity. For example, an inositol-1,4,5-trisphosphate (IP3) receptor-binding protein released with IP3 (IRBIT) was associated with the auto-inhibitory domain of NBCe1-B, which contains 2 phosphorylation sites by STE20/SPS1-related proline/alanine-rich kinase (SPAK).1,12 Our previous study demonstrated that NBCe1-B activity is modulated via both IRBIT and SPAK at the auto-inhibitory domain (AID) of NBCe1-B. The auto-inhibitory domain consists of positively charged amino acids that make up the NBC regulatory module that facilitates IRBIT binding to activate and SPAK phosphorylation to inhibit the activity of NBCe1-B.13 Generally, activated GPCR by stimulation leads to the release of IRBIT from IP3 receptors. The released IRBIT as a regulatory factor binds ion transporters such as NBCe1-B and then triggers or accelerates fluid secretion.15 Interestingly, an analysis of the amino acid sequences reveals extensive homology between the IRBIT binding module and the intracellular N-terminal domain of AE2. However, little is known about the mechanism by which IRBIT regulates AE2 activity. Here, we test the hypothesis that a putative regulatory module of IRBIT may modulate AE2 activity.
For another potential regulatory factor, carbonic anhydrases (CAs) determine local HCO3− concentrations by regulating the bidirectional catalytic reaction of carbon dioxide and water to HCO3− and hydrogen ions.16 The local HCO3− concentration affects the activity of transporters involved in HCO3− secretion. It has been reported that Cl−/HCO3− exchange activity is modulated by the CA inhibitor acetazolamide.9 Moreover, physical interaction between CA and AE is necessary for maximal AE activity, suggesting that AE2 activity is dependent on CA activity17 and the physical interaction at the similar position of the BLM should be considered. Here, we evaluated that the BLM-associated CA XII may provide fuel to activate AE2.
AE activity is regulated by various signals, including the activation of GPCRs and adenylyl cyclases by the coordinated function of cAMP and intracellular Ca2+. However, how these signaling elements modulate AE2 activity and which regulatory mechanism is involved in the modulation of AE2 activity remains unknown. Consequently, we have focused on AE2 in this study. This is the first report demonstrating a regulatory role of signaling elements such as SPL, IRBIT, SPAK, and CA XII in the modulation of AE2 activity.
Material and methods
Reagents, plasmids, and solutions
The hemagglutinin (HA)-tagged AE2 construct and cDNA were transferred to an expression vector (p3xFLAG-CMV-7.1, Clontech, Mountain View, CA) encoding IRBIT and SPAK, pEGFP encoding SPL, EGFP-C1, and pEGFP encoding electrogenic Na+-HCO3− cotransporter 1-B (NBCe1-B) that were provided by Dr. Shmuel Muallem (National Institutes of Health, Bethesda, MD). CA XII cDNA in a pCMV6-AC-Myc-DDK vector was purchased from Origene Technologies (Rockville, MD). The cDNA encoding CA XII was excised from the original vectors and transferred to pCMV6-AC-mKate vectors (Origene Technologies). The reagents 2′,7′-bis-(carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF)-AM and Pluronic F-127 (20% in DMSO) were purchased from TEFlabs (Austin, TX). HA antibodies and spinophilin (SPL) antibodies were purchased from Cell Signaling Technology Inc. (Beverly, MA). GFP (green fluorescent protein), Myc, and mKate antibodies were purchased from Invitrogen (Carlsbad, CA). Flag and β-actin antibodies were obtained from Sigma (St. Louis, MO). CA XII antibody was purchased from Proteintech Inc. (Chicago, IL). All other chemicals not mentioned here were purchased from Sigma. The standard bath solution (Solution A) contained (mM) 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES, and 10 D-glucose and was adjusted to pH 7.4 with NaOH. The HCO3−-buffered solution was prepared by replacing 25 mM Na+ with 25 mM Na+-HCO3− and reducing HEPES to 2.5 mM. The solution containing HCO3− and Cl−-free was prepared by replacing NaCl with Na+-gluconate and NaHCO3. HCO3−-buffered solutions were gassed with 5% CO2 and 95% O2. The osmolarity of all solutions was adjusted to 310 mOsm with the major salt.
Cell culture and transfection
HeLa cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum (FBS) with 100 U/mL penicillin and 100 μg/mL streptomycin and incubated at 37°C in a humidified incubator composed of 5% CO2 and 95% air. Cells were washed with phosphate buffered saline (PBS) and treated with trypsin/ trypsin-ethylenediaminetetraacetic acid (EDTA) for 2 min at 37°C when the density reached 80% confluence. Dispersed cells were then transferred to new culture dishes or dishes including glass coverslips for use. Plasmid DNA transfection by Lipofectamine 200 was followed by manufacturer's protocol (Invitrogen). Each plasmid DNA was diluted in 250 μl of Opti-Eagle's Minimum Essential Media (Opti-MEM™), and 4 μl Lipofectamine 2000 was diluted in 250 μl of the same medium and incubated for 5 min at room temperature. The diluted DNAs and Lipofectamine 2000 were mixed and after 25 min were added to the cell-cultured dish containing coverslip. After 4 hr, the medium was replaced with a fresh DMEM medium containing FBS and the cells were used 24 hr after the beginning of the transfection.
Intracellular pH (pHi) measurements
pHi was measured with BCECF at dual-excitation wavelengths of 495 nm and 440 nm. BCECF fluorescence was read at emission wavelengths above 530 nm. HeLa cells grown on coverslips were co-transfected with the indicated plasmids and GFP.
loaded in the chamber with BCECF in the presence of 0.05% Pluronic F-127 for 15 min incubation in Solution A at room temperature with 6 μM BCECF/AM. After stabilizing the fluorescence, cells were perfused with Solution A for at least 5 min before measuring pHi at 37°C. AE2 activity was measured by incubating the cells with CO2-saturated HCO3−-buffered media to acidify the cytosol. AE2 activity was initiated by perfusing the cells with Cl−-free HCO3−-buffered media containing 140 mM Na+. The emitted fluorescence was monitored with a CCD camera (Photometrics, Tucson, AZ) attached to an inverted microscope (Olympus, Japan) and analyzed with a MetaFluor system (Molecular Devices, PA). Fluorescence images were obtained at 1 sec intervals and region of interests were selected in GFP-positive cells. Fluorescence was subtracted from the raw background signals at each wavelength. AE2 activity was determined from the slopes the first derivatives of the first 30–45 sec of pHi increases and height of the derivatives between baseline and maximum of pHi increases in Cl−-free HCO3−-buffered media. Activity of AE2 with potent regulatory proteins is expressed as a percent fold change relative to that of AE2 only.
Intracellular Cl− (Cl−i) measurements
Intracellular Cl− was evaluated from N-(Ethoxycarbonylmethyl)-6-methoxyquinolinium bromide (MQAE) fluorescence. The HeLa cells on the coverslip were loaded with MQAE by 30 min of incubation at room temperature in bath solution containing 5 mM MQAE and were washed by perfusion with NaCl− based solution until stabilization of the signal. Fluorescence was recorded for at least 3 min to obtain the baseline and then switched perfusion solution with 0 mM Cl− (0Cl−). And then added back 126 mM Cl− (126Cl−) containing HCO3− solution. MQAE fluorescence was recorded at an excitation of 360 nm and light emitted at a wavelength higher than 530 nm was collected with a CCD camera (Photometrics) attached to an inverted microscope (Olympus). Images were analyzed with a MetaFluor system (Molecular Devices).
Co-immunoprecipitation (Co-IP) and surface biotinylation
For surface biotinylation experiments, cells were incubated with 0.5 mg/mL EZ-LINK Sulfo-NHS-LC-biotin (Thermo Scientific, Waltham, MA) for 30 min, incubated with 100 mM glycine for 10 min to quench the free biotin, and washed with PBS. All experiments were performed on ice. Proteins were extracted with ice-cold lysis buffer (1× PBS containing (mM) 50 NaF, 1 Na+ orthovanadate, 10 Na+ pyrophosphate, 1% Triton X-100, and a protease inhibitor cocktail at pH 7.4) by passing cell lysates through a 26-gauge needle 10–12 times after sonication. Extracts were incubated with HA, SPL, and GFP antibodies at 4°C for overnight and then incubated with Protein G Sepharose beads (Invitrogen) for 4 hr at 0°C for Co-IP. The beads were collected and washed 3 times with a lysis buffer. Immunoprecipitated proteins were recovered and denatured by heating in SDS sample buffer at 37°C for 30 min. The heated samples were subjected to SDS-PAGE and subsequent western blot analysis. For the western blot analysis, 20 μg denatured proteins for the input and eluted proteins for the IP were electrophoresed in SDS-PAGE gels and proteins were visualized with appropriate antibodies by an enhanced luminescent solution (Thermo Scientific).
Confocal imaging
Cells expressing AE2 or CA XII were grown on glass coverslips and fixed/permeabilized by incubating with cold methanol for 10 min. After fixation, coverslips were soaked in cold 100 mM glycine for 10 min and washed 3 times with cold PBS. Nonspecific sites were blocked with 5% goat serum. Cells were stained overnight with primary antibodies, then stained for 1 hr at room temperature with fluorescent-tagged secondary antibodies, and washed 3 times with PBS. Coverslips were mounted on glass slides with Fluoromount-G™ (Electron Microscopy Sciences, Hatfield, PA) and analyzed using a LSM 700 Zeiss confocal microscope (Germany).
Statistical analyses
Data from the indicated number of experiments were expressed as mean ± SEM. Statistical significance was determined by analysis of variance in each experiment. A value of *P < 0.01 was considered statistically significant.
Results
AE2 activity is regulated by SPL
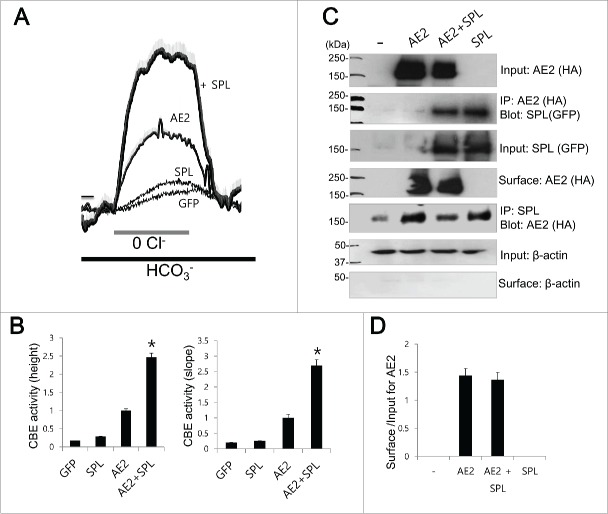
To elucidate the regulatory role of SPL, the Cl−/HCO3− exchange activity of AE2 was evaluated by measuring changes in pHi induced by acute Cl− removal and subsequent addition of Cl−. Removal of Cl− in the media induced intracellular alkalinization in AE2-expressing cells. The slope and height of the change in the pHi effect were measured in the Cl−-free HCO3−-buffered solution. Figure 1A shows that SPL increases the Cl−/HCO3− exchange activity of AE2. SPL markedly increased the activity of AE2 by 64.4% for pHi height and 68.7% for pHi slope (Fig. 1B, n = 26, 19, 114, and 97 cells for GFP, SPL, AE2, and AE2+SPL, respectively). The Co-IP data reveal that SPL interacts with AE2 (Fig. 1C), however, SPL also shows non-specific binding. To verify the non-specific binding of SPL, we performed bidirectional Co-IP with SPL antibody to immunoprecipatate endogenous SPL (Fig. 1C). The bidirectional Co-IP similarly showed non-specific binding. As determined by surface biotinylation assay, SPL did not affect surface expression of AE2 (Fig. 1C and 1D)
Figure 1.
AE2 activity is regulated by SPL (A and B) HeLa cells were transfected with indicated plasmids. Cells in Solution A were exposed to HCO3−-buffered solutions. Cl−/HCO3− exchanger (CBE) activity was assessed by slope of pHi in the absence of Cl− at the beginning of time course (30˜45 sec) and height to reach the maximum pHi point from the minimum point. The traces were the mean ± SEM of number of experiments under each condition. (*P < 0.01). (C) The Co-IP of AE2 with SPL. AE2 was tagged with HA and SPL was tagged with GFP. Anti-HA, anti-GFP and anti-SPL antibodies were used for the Co-IP and detection of proteins. β-Actin was used as a control. (D) Analysis of surface expression of AE2. The bar graphs show the mean ± SEM. The (-) represented cell lysates.
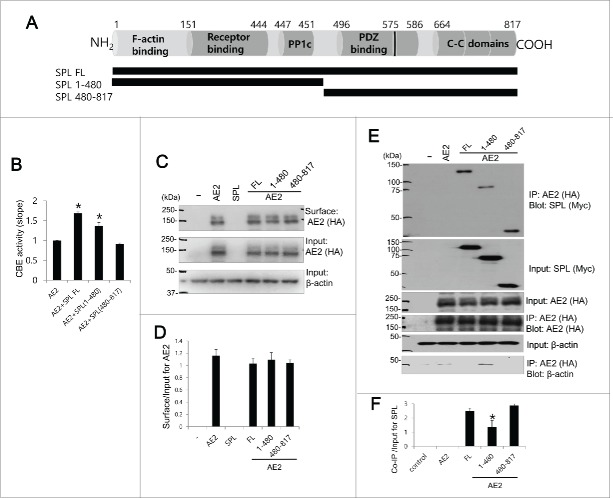
The essential SPL domain for functional interaction with AE2
We therefore asked which domain of SPL is involved in modulation of AE2 activity. Figure 2A provides a schematic representation of the main SPL structural domains of the full-length protein, which has the same predicted open reading frame in both rats and humans. SPL contains an F-actin binding, a receptor binding, a canonical PP1 binding (R-K-I-H-F motif), a PDZ domain, 3 coiled-coil (C-C) domains,18 and a predicted PP1 binding motif (R/K-V-X-F/I)19 in the PDZ domain. Having confirmed that AE2 and SPL interact, we next sought to determine the essential binding domains. Two SPL truncated mutants were constructed in which 1–480 and 480–817 domains had been included, respectively (Fig. 2A). The SPL 1–480 mutant lacked the PDZ domain and the 3 C—C domain, whereas the SPL 480–817 mutant retained the PDZ and C—C domains. We confirmed that full length (SPL FL) increased the Cl−/HCO3− exchange activity of AE2 (Fig. 2B). The SPL 1–480 mutant also increased the activity of AE2 by 37.0% for the pHi slope. However, the SPL 480–817 mutant did not activate AE2 (n = 6 experiments, n = 76, 45, 55, and 43 cells for AE2, AE2+SPL FL, AE2+SPL 1–480, and AE2+SPL 480–817, respectively). SPL and its truncated mutants did not affect the surface expression of AE2, as determined by the surface biotinylation assay (n = 3, Fig. 2C and 2D). In Figure 2E and 2F, the Co-IP results indicated that the SPL-interacting region could not be narrowed down to a primary amino acid sequence. Although SPL 1–480 mutant, deleted PDZ and C-C domain, revealed the reduced interaction with AE2 by about 55% (n = 3, p < 0.01), AE2 activity was enhanced by 1–480 mutant. Overall, the findings of Figure 2 suggest that SPL 1–480 domain requires the binding with AE2 and modulation of AE2 activity.
Figure 2.
The essential SPL domain for functional interaction with AE2 Schematic representation of SPL. SPL includes F-acting binding, receptor binding domains, PP1c domain including R-K-I-H-F motif, PDZ binding, another PP1 binding motif within PDZ binding domain (black bar at 575), and 3 coiled-coil (C—C) domains. Schematic diagram of SPL full length (FL), truncated mutants SPL 1–480 and SPL 480–817. HEK293T cells were transfected with AE2 only, or SPL FL, SPL 1–480, and SPL 480–817 with AE2. (B) CBE activity was assessed by the slope of pHi in the absence of Cl− at the beginning of time course (30∼45 sec). The bar graphs show the mean ± SEM. (*P < 0.01). (C and D) The surface expression of indicated plasmids and analysis of surface expression of AE2. AE2 was tagged with HA. Anti-HA antibody was used for the detection of biotinylated proteins. Input actin was used as a loading control. (E) AE2 was tagged with HA and SPL and mutants were tagged with Myc. Anti-HA and anti-Myc antibodies were used for the Co-IP and detection of proteins. Input β-actin was used as a loading control. (F) Analysis of Co-IP of SPL and mutants. The bar graphs show the mean ± SEM. (*P < 0.01). The (-) represented cell lysates.
IRBIT neither activates nor binds to AE2
Analysis of the amino acid sequences revealed shared sequence identity between the IRBIT binding module of NBCe1-B and the intracellular N-terminal domain of AE2 (Fig. 3A). To determine the role of IRBIT on the activity of AE2, we measured the slope and height of pHi change. Figure 3B and 3C show that IRBIT does not activate AE2 (n = 32, 27, 251 and 112 cells for GFP, IRBIT, AE2, and AE2 + IRBIT, respectively). The Co-IP of IRBIT with NBCe1-B was used as a positive control because IRBIT has been shown to bind to NBCe1-B,20 but IRBIT did not co-immunoprecipitate with AE2 in this experiment (Fig. 3D). Since IRBIT neither activates nor binds to AE2, our results suggest that IRBIT does not regulate AE2 activity.
Figure 3.
IRBIT neither activates nor binds to AE2 (A) Sequence alignment between (1–79) N-terminal domain of NBCe1-B as a putative IRBIT binding module and N- terminal cytoplasmic domain of AE2. (B and C) HeLa cells were transfected with GFP, IRBIT, AE2, or AE2 + IRBIT. CBE activity was assessed by the slope of pHi in the absence of Cl− at the beginning of time course (30 ∼45 sec) and height to reach the maximum pHi point from the minimum point. The bars show the mean ± SEM. (D) The Co-IP of IRBIT with AE2. NBCe1-B was tagged with GFP and IRBIT was tagged with flag. Anti-GFP, anti-HA, and anti-flag antibodies were used for the Co-IP. Input actin was used as a loading control. The (-) represented cell lysates.
SPAK neither activates nor binds to AE2
Our previous study suggested that SPAK has convergence of regulatory modalities with IRBIT, which is antagonized by IRBIT.13 We speculated whether AE2 activity is regulated by SPAK and found that SPAK does not modulate the activity of AE2 (n = 42, 44, 126, and 94 cells for GFP, SPAK, AE2, and AE2 + SPAK, Figure 4A and 4B). The Co-IP of SPAK with NBCe1-B was used as a positive control because SPAK binds to NBCe1-B1, but we found that SPAK does not co-immunoprecipitate with AE2 (Fig. 4C). These results suggest that AE2 is not regulated by SPAK.
Figure 4.
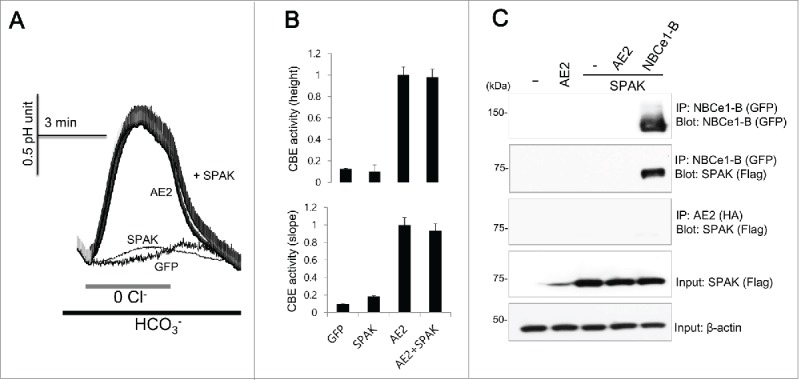
SPAK neither activates nor binds to AE2 (A and B) HeLa cells were transfected with AE2 only or AE2 + SPAK. CBE activity was assessed by the slope of pHi in the absence of Cl− at the beginning of time course (30 ˜45 sec) and height to reach the maximum pHi point from the minimum point. The bars show the mean ± SEM. (D) The Co-IP of SPAK with AE2. NBCe1-B was tagged with GFP and SPAK was tagged with flag. Anti-GFP, anti-HA, and anti-flag antibodies were used for the Co-IP. Input β-actin was used as a loading control. The (-) represented cell lysates.
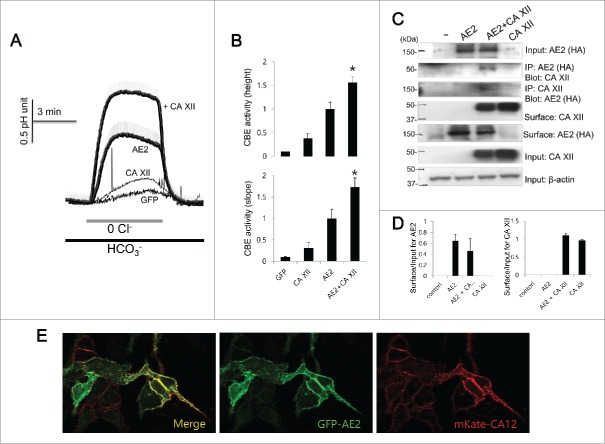
CA XII activates AE2 and shows analogous cellular distribution
To elucidate the role of CA XII on the activity of AE2, the intracellular pH measurements and the Co-IP and biotinylation assay were assessed (Fig. 5). CA XII significantly increased the activity of AE2 (Fig. 5A and 5B) by increasing the pHi height and slope by about 64.7% and 73.2%, respectively compared to those of AE2 only (n = 22, 19, 34, and 61 cells for GFP, CA XII, AE2, and AE2 + CA XII, respectively). The Co-IP and bidirectional Co-IP experiments showed that AE2 interacts with CA XII and the biotinylation assay revealed that CA XII did not appreciably affect surface expressions of AE2 (Fig. 5C and 5D). Co-expression experiments for imaging similarly show that AE2 and CA XII co-localize to the plasma membrane (Fig. 5E), suggesting that AE2 and CA XII interact at the plasma membrane and CA XII can be considered a potential regulatory factor of AE2.
Figure 5.
CA XII activates AE2 and shows analogous cellular distribution (A and B) HeLa cells were transfected with AE2 only or AE2 + CA XII. CBE activity was assessed by the slope of pHi in the absence of Cl− at the beginning of time course (30 ∼45 sec) and height to reach the maximum pHi point from the minimum point. The bars show the mean ± SEM. (*P < 0.01). (C) The Co-IP and surface expression of CA XII with AE2. Anti-HA and anti-CA XII antibodies were used for the Co-IP. Input β-actin was used as a loading control. Co-IP of actin was used as a loading control. (D) Analysis of Co-IP and surface expression of CA XII. The bar graphs show the mean ± SEM. (E) Images of HeLa cells transfected with the indicated plasmids showing the localization of AE2 (GFP, green) and CA XII (mKate, red). The (-) represented cell lysates.
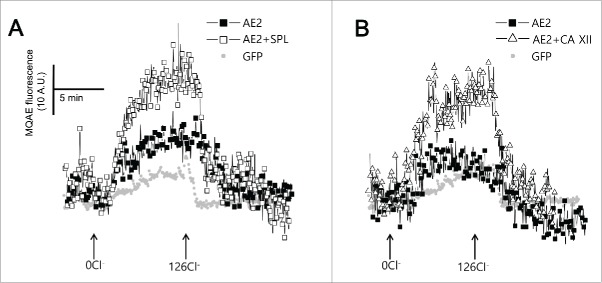
SPL and CA XII activate AE2 to transport Cl−
AE2 activity was measured by following intracellular Cl− transport with the Cl−-sensitive dye MQAE. The exchange of intracellular Cl− with extracellular HCO3− by AE2 results in de-quenching of MQAE. SPL (open square) and CA XII (open triangle) enhanced intracellular Cl− transport by AE2 (Fig. 6A and 6B).
Figure 6.
SPL and CA XII activate AE2 to transport Cl−. (A and B) Changes of intracellular Cl− concentration. HeLa cells were transfected with GFP (closed gray circle), AE2 (closed square), AE2 + SPL (open square), and AE2+ CA XII (open triangle). The traces were measured with arbitrary MQAE intensity at every 3 sec.
Discussion
Anion exchangers are fundamental to the function of various secretory organ systems such as fluid and HCO3− secretion. Nevertheless, precise regulatory mechanism of AE2 is generally lacking. In the present study, we have provided the demonstration that new candidates for the regulatory factors of AE2 activity, revealed SPL and CA XII bind to AE2 and enhance its Cl−/HCO3− exchange activity.
SPL is a widely distributed multi-domain scaffold protein that binds several GPCRs, including α1-adrenoceptors, all α2-adrenoceptors, and TRPC5/6 channels,21-24 suggesting that SPL is involved in multiple intracellular pathways. The discovery of partner proteins of SPL and its modulatory functions has persisted mostly in the field of neurobiology because SPL was characterized in rat brains.25 SPL regulates protein phosphorylation in neurons and alters the localization of the SPL-PP1 complex, functional activities that may have essential consequences for synaptic plasticity.26 Our present study provides a new scope of the regulatory role of SPL on the Cl−/HCO3− exchange protein. The AE2-binding site of SPL that is responsible for AE2 activation was found to be located in the 1–480 amino acids domain of SPL. At present, it is not clear how the PDZ and C-C domain of SPL are involved in non-specific binding of AE2. Although we are unaware of where the binding site for SPL is in AE2, the SPL 480–817 mutant almost abolished the ability of SPL to modulate AE2 (Fig. 2). Most of the interaction between SPL and AE2 occurs at the 1–480 domain of SPL that includes a receptor binding domain and a PP1 binding domain. Interestingly, SPL is known to be enriched in dendritic spines.25 However, the ability of SPL to regulate the Cl−/HCO3− exchange activity, a critical function of ion transporters for fluid secretion, is a novel role for secretory organs such as salivary glands and pancreas.
In this study, we propose that the SPL plays a crucial role in Cl−/HCO3− exchange activity. The importance of SPL in the regulation of AE2 activity in vivo remains to be tested by using an SPL knockdown system. Although, we found that SPL protein is expressed in submandibular glands (unpublished data not shown), however, our results do not provide sufficient evidence that SPL directly contributes to the secretory process. Muscarinic receptor-mediated signaling is attenuated by SPL, which can recruit RGS2 and 4 that reduce the half-life of activated G proteins.11,27 In addition, this muscarinic receptor stimulation involved in the activation of AE.28 Thus, the SPL machinery induced by receptor stimulation may play a role in secretory processes. SPL also has additional binding modules such as those for PP1 and F-actin that can recruit other binding partners and can assemble the complex to the cytoskeleton. Therefore, further studies are required to elucidate the underlying mechanisms linking to SPL to Cl−/HCO3− exchange proteins to explain the regulatory role of the fluid secretion for high fidelity.
The current study reveals that the BLM-associated CA XII is a potent activator of AE2. Although the functions of AE2 as a Cl−/HCO3− exchanger on the regulation of pHi and cell volume homeostasis are well addressed, AE is also involved in the survival of tumor cells.29 Tumor progression and metastasis are fueled by hypoxia and acidosis.30 CA XII is likely involved in modulating a variety of physiological processes such as pHi regulation that are affected by over-expressed AE2 related to tumor growth and survival advantages. Thus, the extracellular environment becomes acidic and hypoxic. However, the role of AE2 and CA XII on tumor cell survival and growth remains unknown.
Collectively, our findings highlight the role of several regulatory factors in the modulation of a Cl−/HCO3− exchanger 2. The modulating role of regulatory proteins, such as SPL and CA XII, could serve as a means to amplify the secretory response or prevent the impairment of secretory processes mediated by AE2. Moreover, the abilities of AE2 and CA XII to regulate cellular microenvironments might provide the basis for new therapeutic targets against secretory dysfunction such as hyposecretion.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The DNA constructs were kindly gifted by Dr. Shmuel Muallem in National Institutes of Health/National Institute of Dental and Craniofacial Research, Bethesda, MD, USA.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2014R1A1A3049477).
References
- [1].Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. IRBIT coordinates epithelial fluid and HCO3- secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest 2009; 119(1):193-202; PMID:19033647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev 2012; 92(1):39-74; PMID:22298651; http://dx.doi.org/ 10.1152/physrev.00011.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, et al.. Dynamic regulation of CFTR bicarbonate permeability by [cl-]i and its role in pancreatic bicarbonate secretion. Gastroenterology 2010; 139(2):620-31; PMID:20398666; http://dx.doi.org/ 10.1053/j.gastro.2010.04.004 [DOI] [PubMed] [Google Scholar]
- [4].Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev 2013; 93(2):803-959; PMID:23589833; http://dx.doi.org/ 10.1152/physrev.00023.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shcheynikov N, Son A, Hong JH, Yamazaki O, Ohana E, Kurtz I, Shin DM, Muallem S. Intracellular cl- as a signaling ion that potently regulates na+/HCO3- transporters. Proc Natl Acad Sci U S A 2015; 112(3):E329-37; PMID:25561556; http://dx.doi.org/ 10.1073/pnas.1415673112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 2005; 67:445-69; PMID:15709965; http://dx.doi.org/ 10.1146/annurev.physiol.67.041703.084745 [DOI] [PubMed] [Google Scholar]
- [7].Huang J, Shan J, Kim D, Liao J, Evagelidis A, Alper SL, Hanrahan JW. Basolateral chloride loading by the anion exchanger type 2: Role in fluid secretion by the human airway epithelial cell line calu-3. J Physiol 2012; 590(Pt 21):5299-316; PMID:22802585; http://dx.doi.org/ 10.1113/jphysiol.2012.236919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gawenis LR, Bradford EM, Alper SL, Prasad V, Shull GE. AE2 cl-/HCO3- exchanger is required for normal cAMP-stimulated anion secretion in murine proximal colon. Am J Physiol Gastrointest Liver Physiol 2010; 298(4):G493-503; PMID:20110461; http://dx.doi.org/ 10.1152/ajpgi.00178.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nguyen HV, Stuart-Tilley A, Alper SL, Melvin JE. Cl(-)/HCO(3)(-) exchange is acetazolamide sensitive and activated by a muscarinic receptor-induced [ca(2+)](i) increase in salivary acinar cells. Am J Physiol Gastrointest Liver Physiol 2004; 286(2):G312-20; PMID:12958022; http://dx.doi.org/ 10.1152/ajpgi.00158.2003 [DOI] [PubMed] [Google Scholar]
- [10].Wang X, Zeng W, Kim MS, Allen PB, Greengard P, Muallem S. Spinophilin/neurabin reciprocally regulate signaling intensity by G protein-coupled receptors. EMBO J 2007; 26(11):2768-76; PMID:17464283; http://dx.doi.org/ 10.1038/sj.emboj.7601701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang X, Zeng W, Soyombo AA, Tang W, Ross EM, Barnes AP, Milgram SL, Penninger JM, Allen PB, Greengard P, et al.. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat Cell Biol 2005; 7(4):405-11; PMID:15793568; http://dx.doi.org/ 10.1038/ncb1237 [DOI] [PubMed] [Google Scholar]
- [12].Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest 2011; 121(3):956-65; PMID:21317537; http://dx.doi.org/ 10.1172/JCI43475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hong JH, Yang D, Shcheynikov N, Ohana E, Shin DM, Muallem S. Convergence of IRBIT, phosphatidylinositol (4,5) bisphosphate, and WNK/SPAK kinases in regulation of the na+-HCO3- cotransporters family. Proc Natl Acad Sci U S A 2013; 110(10):4105-10; PMID:23431199; http://dx.doi.org/ 10.1073/pnas.1221410110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shcheynikov N, Son A, Hong JH, Yamazaki O, Ohana E, Kurtz I, Shin DM, Muallem S. Intracellular cl- as a signaling ion that potently regulates na+/HCO3- transporters. Proc Natl Acad Sci U S A 2015; 112(3):E329-37; PMID:25561556; http://dx.doi.org/ 10.1073/pnas.1415673112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park S, Shcheynikov N, Hong JH, Zheng C, Suh SH, Kawaai K, Ando H, Mizutani A, Abe T, Kiyonari H, et al.. Irbit mediates synergy between ca(2+) and cAMP signaling pathways during epithelial transport in mice. Gastroenterology 2013; 145(1):232-41; PMID:23542070; http://dx.doi.org/ 10.1053/j.gastro.2013.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McKenna R, Frost SC. Overview of the carbonic anhydrase family. Subcell Biochem 2014; 75:3-5; PMID:24146371; http://dx.doi.org/ 10.1007/978-94-007-7359-2_1 [DOI] [PubMed] [Google Scholar]
- [17].Sterling D, Reithmeier RA, Casey JR. A transport metabolon. functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem 2001; 276(51):47886-94; PMID:11606574 [DOI] [PubMed] [Google Scholar]
- [18].Sarrouilhe D, di Tommaso A, Metaye T, Ladeveze V. Spinophilin: From partners to functions. Biochimie 2006; 88(9):1099-113; PMID:16737766; http://dx.doi.org/ 10.1016/j.biochi.2006.04.010 [DOI] [PubMed] [Google Scholar]
- [19].Piechotta K, Garbarini N, England R, Delpire E. Characterization of the interaction of the stress kinase SPAK with the na+-K+-2Cl- cotransporter in the nervous system: Evidence for a scaffolding role of the kinase. J Biol Chem 2003; 278(52):52848-56; PMID:14563843; http://dx.doi.org/ 10.1074/jbc.M309436200 [DOI] [PubMed] [Google Scholar]
- [20].Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type na+/HCO3- cotransporter 1 (pNBC1). Proc Natl Acad Sci U S A 2006; 103(25):9542-7; PMID:16769890; http://dx.doi.org/ 10.1073/pnas.0602250103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xu J, Chen Y, Lu R, Cottingham C, Jiao K, Wang Q. Protein kinase A phosphorylation of spinophilin modulates its interaction with the alpha 2A-adrenergic receptor (AR) and alters temporal properties of alpha 2AAR internalization. J Biol Chem 2008; 283(21):14516-23; PMID:18367453; http://dx.doi.org/ 10.1074/jbc.M710340200 [DOI] [PubMed] [Google Scholar]
- [22].Richman JG, Brady AE, Wang Q, Hensel JL, Colbran RJ, Limbird LE. Agonist-regulated interaction between alpha2-adrenergic receptors and spinophilin. J Biol Chem 2001; 276(18):15003-8; PMID:11154706; http://dx.doi.org/ 10.1074/jbc.M011679200 [DOI] [PubMed] [Google Scholar]
- [23].Smith FD, Oxford GS, Milgram SL. Association of the D2 dopamine receptor third cytoplasmic loop with spinophilin, a protein phosphatase-1-interacting protein. J Biol Chem 1999; 274(28):19894-900; PMID:10391935; http://dx.doi.org/ 10.1074/jbc.274.28.19894 [DOI] [PubMed] [Google Scholar]
- [24].Goel M, Sinkins W, Keightley A, Kinter M, Schilling WP. Proteomic analysis of TRPC5- and TRPC6-binding partners reveals interaction with the plasmalemmal na(+)/K(+)-ATPase. Pflugers Arch 2005; 451(1):87-98; PMID:16025302; http://dx.doi.org/ 10.1007/s00424-005-1454-y [DOI] [PubMed] [Google Scholar]
- [25].Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci U S A 1997; 94(18):9956-61; PMID:9275233; http://dx.doi.org/ 10.1073/pnas.94.18.9956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hsieh-Wilson LC, Benfenati F, Snyder GL, Allen PB, Nairn AC, Greengard P. Phosphorylation of spinophilin modulates its interaction with actin filaments. J Biol Chem 2003; 278(2):1186-94; PMID:12417592; http://dx.doi.org/ 10.1074/jbc.M205754200 [DOI] [PubMed] [Google Scholar]
- [27].Ruiz de Azua I, Nakajima K, Rossi M, Cui Y, Jou W, Gavrilova O, Wess J. Spinophilin as a novel regulator of M3 muscarinic receptor-mediated insulin release in vitro and in vivo. FASEB J 2012; 26(10):4275-86; PMID:22730439; http://dx.doi.org/ 10.1096/fj.12-204644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nguyen HV, Stuart-Tilley A, Alper SL, Melvin JE. Cl(-)/HCO(3)(-) exchange is acetazolamide sensitive and activated by a muscarinic receptor-induced [ca(2+)](i) increase in salivary acinar cells. Am J Physiol Gastrointest Liver Physiol 2004; 286(2):G312-20; PMID:12958022; http://dx.doi.org/ 10.1152/ajpgi.00158.2003 [DOI] [PubMed] [Google Scholar]
- [29].Hwang JM, Kao SH, Hsieh YH, Li KL, Wang PH, Hsu LS, Liu JY. Reduction of anion exchanger 2 expression induces apoptosis of human hepatocellular carcinoma cells. Mol Cell Biochem 2009; 327(1-2):135-44; PMID:19224338; http://dx.doi.org/ 10.1007/s11010-009-0051-3 [DOI] [PubMed] [Google Scholar]
- [30].Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouysségur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res 2009; 69(1):358-68; PMID:19118021; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2470 [DOI] [PubMed] [Google Scholar]