ABSTRACT
Our previous work reported that KCa3.1 (IKCa) channels are expressed in CA1 hippocampal pyramidal cells and contribute to the slow afterhyperpolarization that regulates spike accommodation in these cells. The current report presents data from single cell RT-PCR that further reveals mRNA in CA1 cells that corresponds to the sequence of an IKCa channel from transmembrane segments 5 through 6 including the pore region, revealing the established binding sites for 4 different IKCa channel blockers. A comparison of methods to internally apply the IKCa channel blocker TRAM-34 shows that including the drug in an electrode from the onset of an experiment is unviable given the speed of drug action upon gaining access for whole-cell recordings. Together the data firmly establish IKCa channel expression in CA1 neurons and clarify methodological requirements to obtain a block of IKCa channel activity through internal application of TRAM-34.
KEYWORDS: CA1 pyramidal, IKCa, sAHP, slow AHP, TRAM-34
Introduction
It is well recognized that potassium channels exert powerful control over the excitability of central neurons, including the output of cortical cells. Nowhere is this more evident than for a potassium-mediated slow afterhyperpolarizing potential (sAHP), yet the molecular basis for this response has eluded identification for decades.1 Strong evidence has been reported for a contribution to the sAHP of CA1 hippocampal pyramidal cells by the intermediate conductance calcium-activated potassium channel (IKCa, KCa3.1, KCNN4).2-3 Data in support of IKCa channel expression in pyramidal cells include GFP expression tied to the promoter for IKCa channels, in situ hybridization, and immunolabeling.2-3 Direct recordings from CA1 pyramidal cells reveal a current with the unique pharmacological characteristics of IKCa channels in wild type animals that is absent in KCa3.1−/− mice, and intermediate conductance channels with an activity profile that recapitulates the IsAHP.3 Other work has considered contributions by SK (KCNN) channels and Kv7.2/7.3 (KCNQ2/3) channels and the Na-K pump to the sAHP.4-9 Here we provide further evidence for IKCa expression with respect to single cell RT-PCR data as an extension of our previous work,3 and clarify the requirements to obtain a block of IKCa channels by internal application of the drug TRAM-34.
Results
Expression
Initial studies using in situ hybridization failed to detect a signal for KCNN4 in brain tissue.10-12 However, KCNN4 mRNA has since been identified in several brain regions including the CA1 hippocampus.13 Our study by King et al.3 used a suite of tests to assess the pharmacology of currents and single channels contributing to the IsAHP (voltage clamp) or sAHP (current clamp) in the presence of 100 nM apamin and 10 μM XE-991 to block SK and Kv7 channels, respectively. While we had previously detected IKCa mRNA through single cell RT-PCR in cerebellar Purkinje cells,14 similar tests conducted in CA1 cells were not included in King et al.3 We thus present data on single cell RT-PCR in cells of the CA1 pyramidal cell layer as a further test for the expression of the IKCa channel in hippocampus. Here we used whole-cell patch recordings of cells in the CA1 region and recorded current-evoked firing patterns to assist in identifying cell types. We recorded one firing pattern expected for pyramidal cells with a relatively low frequency and evidence of spike accommodation, and another expected for inhibitory cells with a fast rate of firing that lacked spike accommodation (Fig. 1A). Cytoplasmic contents were then extracted into the electrode by negative pressure for 10 min and the electrode removed, with the integrity of cytoplasmic contents ensured by forming an outside-patch configuration to create a membrane seal on the electrode tip. Electrode contents were then placed in a sterile centrifuge tube and immediately frozen on dry ice for subsequent PCR analysis using primers surrounding the pore region of IKCa channels.
Figure 1.
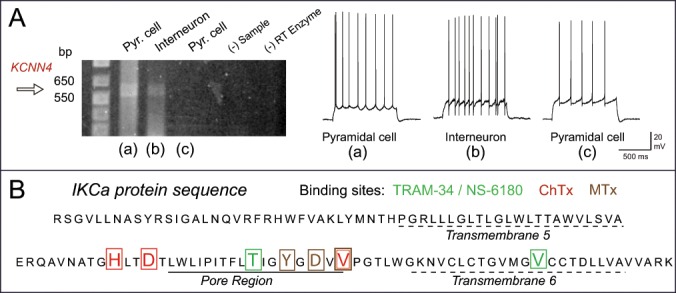
Single cell RT-PCR for KCNN4 mRNA isolated PCR surrounding the IKCa pore region. (A) The predicted band for KCNN4 DNA is identified in both a pyramidal cell (a) and presumed inhibitory interneuron (b) as judged by firing patterns, but is not evident in a third pyramidal cell sample (c). Control lanes lacking sample (RNA) or reverse transcriptase (RT) enzyme are negative. Primers and single cell RT-PCR procedures were as previously published.14 Shown are current-evoked recordings of spike output from each cell prior to extracting cytoplasm by negative pressure for 10 min reveals characteristic firing patterns of CA1 pyramidal cells or inhibitory interneurons. (B) The protein sequence of IKCa translated from the single cell RT-PCR DNA product using the web-base translation tool ExPASy. The sequence surrounding the pore region contains the unique binding sites for IKCa-specific blockers TRAM-34 / NS-6180, ChTx, and MTx, but not apamin.
These tests identified a band between 550 and 650 bp consistent with IKCa DNA in some but not all CA1 pyramidal cells as well as presumed inhibitory interneurons (Fig. 1A). Finding this band in both pyramidal and interneuron samples is consistent with previous results, where the most intensely immunolabeled cells in CA1 were often displaced slightly above or below the pyramidal cell layer as expected for putative inhibitory cells. Variability in detecting IKCa mRNA in pyramidal cell single cell samples is also expected given that up to ∼20% of pyramidal cells are reported to lack an sAHP.15 We have also applied TRAM-34 to CA1 neurons exhibiting a firing pattern characteristic of presumed inhibitory cells and found a comparable increase in the firing rate as for pyramidal cells (data not shown). Most importantly, subsequent sequence analysis of the DNA from single cell RT-PCR identified the protein sequence surrounding the channel pore from before transmembrane segment 5 through 6 as corresponding to the IKCa channel.16 Moreover, the sequence contained the known binding sites for all of the established IKCa blockers TRAM-34/NS-6180, ChTx, and maurotoxin (MTx)16-19 but not for apamin20 (Fig. 1B). These data thus provide further strong support for the expression of IKCa channels in hippocampal neurons.
Applying TRAM-34
Intensive work has been applied to developing pharmacological tools to target IKCa channels over the closely related SK channel isoforms given the involvement of IKCa channels in several disease states.18,21-23 TRAM-34 is membrane permeable and selectively blocks IKCa channels at sites near the inner pore of the channel.17 As such, it can be applied in the bath (at a concentration ≤ 1 μM) to block the channel after crossing the cell membrane. In cerebellar Purkinje cells we found that even low doses of bath applied TRAM-34 rapidly blocked a parallel fiber-mediated form of slow AHP.14 TRAM-34 can also be successfully applied in the bath for CA1 hippocampal pyramidal cells, and most effectively for cases of on-cell, outside-out and perforated patch configurations.3 However, a significant potential drawback to bath application of a drug is the expected effect on other cells or presynaptic elements outside the cell of interest. While initial analysis suggests that TRAM-34 has little effect on stratum radiatum-evoked synaptic responses, this issue was circumvented in our previous study3 whenever possible by applying TRAM-34 through an exchange of internal electrode solution (ALA Systems). In this way we obtained a control recording unaffected in any way by the drug in the presence of normal internal electrode solution. We then found that TRAM-34 was highly effective at blocking IKCa current within only a few minutes of initiating the electrode solution exchange.
An alternative strategy would be to include TRAM-34 in the electrode solution from the beginning of the experiment, with the expectation that a control recording could be obtained soon after gaining whole-cell recording access and before the drug enters the cell. However, our control tests comparing these 2 forms of drug application indicate that this is not the case. The data in Figure 2 compares the results of applying TRAM-34 using each of these methods on the IsAHP evoked by a step command to +40 mV (200 ms) in the presence of 100 nM apamin and 10 μM XE-991 to block SK and Kv7 channels. When 1 μM TRAM-34 was included in the electrode solution we recorded an IsAHP of 10-25 pA (21.68 ± 6.15 pA at 5 min) that was stable in not changing significantly in amplitude over the course of 15 min of recording (22.76 ± 6.29 pA at 15 min, n = 5, p = 0.75) (Fig. 2A). Moreover, there was no change in IsAHP amplitude when the electrode solution was exchanged with the same solution that also contained 1 μM TRAM-34 (20.6 ± 4.34 pA, n = 5, p = 0.73). The latter step was included to provide an important control that confirmed no affect by the pressure infusion process on cell activity. By comparison, using an electrode containing normal internal solution without TRAM-34 we first recorded an IsAHP of 20-100 pA peak amplitude (47.12 ± 11.78 pA at 5 min, n = 5). We found that when using an internal solution with 0.5 mM EGTA and 0 calcium IsAHP amplitude was stable and not significantly different from control after 15 min of recording (52.30 ± 10.86 pA at 15 min, n = 5, p = 0.154) (Fig. 2B). However, the IsAHP was rapidly reduced when a solution containing 1 µM TRAM-34 was infused into the electrode, with a statistically significant block by 3 min after initiating the electrode infusion (27.7 ± 7.79 pA, n = 5, p = 0.003) (Fig. 2B). While the absolute rate of decline over 3 min could vary, in 3/5 cases there was a substantial block of IsAHP amplitude even by 1 min after initiating electrode infusion. This is shown in Fig. 2C in which the mean IsAHP amplitude is plotted for every min during infusion of TRAM-34 into the electrode (typically over a 3 min period). These results are particularly striking considering that the tip of the infusion tube in the electrode was positioned at a distance away from the electrode tip, requiring some degree of TRAM-34 diffusion to reach the end of the electrode to enter the cell. These results are thus important in raising an important cautionary note that including TRAM-34 within the recording electrode for whole-cell recordings is not viable as a method for applying this particular drug.
Figure 2.
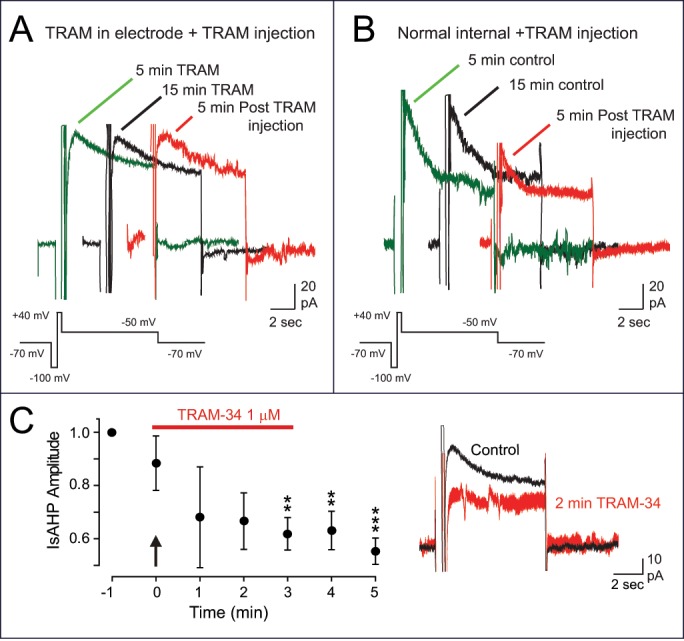
A comparison of methods for applying TRAM-34 to block the IsAHP in CA1 pyramidal cells. All recordings were performed in the presence of apamin and XE-991 at 32°C. (A) A whole-cell recording from a CA1 pyramidal cell using an electrode that includes 1 µM TRAM-34 shows a small IsAHP from the onset of recording that is stable 15 min later, and 5 min following infusion of 1 μM TRAM-34 by intra-electrode solution change. (B) A separate pyramidal cell recording in which the IsAHP first recorded with normal electrode solution (lacking TRAM-34) is stable in amplitude over 15 min time but is rapidly blocked upon exchange of internal solution containing 1 μM TRAM-34. (C) Plot of IsAHP amplitude beginning at the final recording of a stable response at 15 min with normal electrode solution (−1) (as in B), and at 1 min intervals during infusion of 1 μM TRAM-34 into the electrode (arrow, horizontal bar). A substantial block of IsAHP was often apparent by 1–2 min after initiating TRAM-34 infusion (n = 5), with the provided inset showing the block at 2 min for a representative cell.
Discussion
Multiple sources of ionic current have been proposed to contribute to generating the sAHP of CA1 pyramidal cells, including SK and Kv7 channels, and the Na-K pump. Here we extend our previous work3 and provide further evidence that CA1 pyramidal cells express IKCa channels that are sensitive to the selective blocker TRAM-34. This is in agreement with previous data in which a calcium-activated potassium current in pyramidal cells was reduced by 4 other established IKCa blockers that include Senicapoc, NS-6180, ChTx, and MTx.3 Indeed, the sequence of the mRNA extracted here from CA1 neurons contained the specific binding sites for each of these channel blockers. Together with previous data using in situ hybridization, immunocytochemistry, a knock-in animal expressing GFP in relation to the IKCa promoter, and recordings of a TRAM-34-sensitive potassium current that is activated by all the stimuli that evoke IsAHP, the evidence for IKCa expression in CA1 cells is overwhelming. Indeed, the characteristics of IKCa channels recorded here and in our recent study3 are entirely in line with the properties of the channel that underlies the sAHP of enteric/myenteric neurons and long established as corresponding to IKCa channels.16,24-26
The hallmark pharmacological signature for the IKCa channel is a block by the drug TRAM-34.17,27 This drug can be readily applied in the bath to block IKCa channels after entry into the cell and acting on sites near the channel pore (Fig. 1).14,17,27 Indeed, King et al. used bath application of TRAM-34 effectively during multiple forms of patch recordings, including on-cell, outside-out, and perforated patch configurations.3 However, to prevent any possible effects of TRAM-34 on synaptic transmission in the slice preparation we adopted the approach of applying the drug internally through an intra-electrode perfusion system for whole-cell recordings. The additional control tests provided here emphasize the need to employ this process of internal perfusion compared to the alternative strategy of just including TRAM-34 in the electrode from the onset of experiments. The results confirm that TRAM-34 is so effective at blocking IKCa channels that whole-cell current was substantially blocked within the timeframe required for initial equilibration of electrode and cell contents (≤2 min) that remained stable over a 15 min time period (Fig. 2C). This process may account by itself for a recently reported lack of TRAM-34 effects on the IsAHP in CA1 pyramidal cells, where all drug applications were conducted by including TRAM-34 in the electrode before gaining a whole-cell recording configuration.28 Unfortunately, as established here, this approach can not be used to assess the role of IKCa channels during whole-cell recordings.
The actual contribution of IKCa channels to the sAHP in CA1 cells in tissue not pre-treated by apamin and XE-991 to isolate IKCa channels remains to be determined. It is interesting to note that recent work indicates that the relative contributions of SK and Kv7 channels to the sAHP and spike accommodation is more complex than anticipated, with the role of SK channels becoming more evident if Kv7 channels are first blocked.4 More work will thus be needed to determine if a similar process applies to the contribution of IKCa channels to the sAHP.
Materials and methods
Animal care
Tests were conducted on male P16-25 Sprague-Dawley rats (Charles River) raised from timed-pregnant dams and maintained according to the guidelines of the Canadian Council of Animal Care.
Slice preparation
Details on slice preparation and recording conditions were as described in King et al. (2015). All drugs were obtained from Sigma unless otherwise specified. Briefly, animals were anaesthetized by isofluorane inhalation and transverse hippocampal slices cut by Vibratome in ice cold medium comprised of (in mM): 215 sucrose, 25 NaHCO3, 20 D-glucose, 2.5 KCl, 0.5 CaCl2, 1.25 NaH2PO4 and 3 MgCl2 bubbled with carbogen gas. Slices were incubated for 15 min (34°C) in medium comprised of (in mM): 125 NaCl, 3.25 KCl, 1.5 CaCl2, 1.5 MgCl2, 25 NaHCO3, and 25 D-glucose before storing at room temperature and subsequent recording in the same solution at 32-34 °C.
Electrophysiology
Recordings were carried out using Multiclamp 700B amplifiers with pClamp software and custom-made routines (Matlab). Control recordings were made >5 min after gaining whole-cell access to promote full exchange of the internal solution. Whole-cell recordings for current clamp used an electrolyte of (in mM): 130 K-gluconate, 0.1 EGTA, 10 HEPES, 7 NaCl, 0.3 MgCl2, pH 7.3 with KOH. Di-Tris-creatine phosphate (5), 2 Tris-ATP and 0.5 Na-GTP were added daily from frozen stock solutions. Whole-cell voltage clamp recordings were conducted using an electrolyte comprised of (in mM): 110 KMeSO4, 30 KCl, 0.5 EGTA, 2 MgCl2, 10 HEPES maintained at room temperature. The peak amplitude of IsAHP evoked by a step command was measured with respect to the stable current level following decay of the initial response.
TRAM-34 was made up as a stock solution in DMSO and stored at room temperature for dilution each day to include in electrode solutions at 1 μM (DMSO < 0.1%). The following drugs were used to block ion channels to isolate IKCa under voltage clamp (in mM): BK, Kv1.x (5 TEA), SK (0.0001 apamin), Kv7 (0.01 XE-991), Kv4.x (5 4-AP), sodium (0.001 TTX), HCN (external 2 CsCl,). Synaptic responses were blocked by (in mM): GABA-A (0.05 picrotoxin), NMDA (0.025 DL-AP5, Ascent Scientific), and AMPA/KA (0.01 DNQX, Tocris Scientific). Current clamp recordings were conducted in medium in which only synaptic transmission was blocked by DL-AP5, DNQX, and picrotoxin (as above).
RT-PCR
Single cell samples
Procedures were as described by Toledo-Rodriguez and Markram29 and used in Engbers et al.14 The cytoplasmic contents of CA1 neurons were extracted to conduct single cell RT-PCR using whole-cell recordings with an internal solution for current-clamp recordings that contained RNAse inhibitor (200 U/ml). After recording spike firing patterns the electrode was removed to form an outside-out patch configuration to prevent contamination of electrode contents and placed immediately into a sterile centrifuge tube immediately frozen on dry ice. The mRNA was reverse transcribed (RT) using an Omniscript reverse transcriptase (Qiagen) and oligo d(T) primers. Primers for KCa3.1 PCR were based on rat nucleotide sequences (accession numbers: NM_023021.1 for KCNN4). The cDNA samples were amplified using Taq DNA polymerase (Invitrogen) and primers: IK1F: 5′-ATG GGC GGG GAG CTG GTG ACT GGC CTG GGG-3′; IK2F: 5′-GGC CAT GCT GCT ACG TCT CTA CCT GGT GCC TCG-3′; IK3R: 5′-GCT GAT GCC TGC GAG CCG CTC GGG AGT CC-3′; and IK4R: 5′-CTA TGT GGC CTC CTG GAT GGG TTC TGG CGG CTG C-3′. A two-round PCR protocol was performed. The first was carried out after adding PCR buffer (Invitrogen), MgCl2 (2.5 mM), 2.5 U Taq Polymerase and corresponding primers (IK1F and IK4R) to the RT product. Forty-five cycles were performed (denaturation at 95°C, 1 min; annealing at 65°C, 2 min for the first 5 cycles, and at 52°C, 1 min, for the remaining cycles; extension at 72°C, 2 min; final elongation at 72°C, 10 min). An aliquot (5 μl) of the first round PCR product was used as template for the second PCR using the nested primers (IK2F and IK3R) and the same cycles and condition as above. KCa3.1 RT-PCR products were identified by agarose gel electrophoresis (1%, stained with ethidium bromide) and documented with the Alpha Innotech Gel Doc System. All KCa3.1 RT-PCR products were confirmed by sequence analysis in the University of Calgary Core DNA facility. The protein sequence was translated from the single cell RT-PCR DNA product using the web-based translation tool ExPASy (SIB Bioinformatics Resource Portal; http://web.expasy.org/translate/).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We gratefully acknowledge M. Kruskic and S. Hameed for expert technical assistance.
Funding
This work was supported by grants from the Canadian Institutes of Health Research (R.W.T., G.W.Z.). Trainee support was provided by Alberta Innovates - Health Solutions (AI-HS) Studentships (J.D.T.E., A.P.R.) and Postdoctoral Fellowship (G. S.), Canadian Institutes of Health Research - Canada Graduate Scholarship (J.D.T.E.), Eyes High Postdoctoral Scholarship (G.S), Killam Studentship (J.D.T.E.), Queen EII Scholarship (A.P.R.), and a Cumming School of Medicine Studentship (J.M.). R.W.T. and G.W.Z. are AI-HS Scientists and G.W.Z. holds a Canada Research Chair.
References
- [1].Andrade R, Foehring RC, Tzingounis AV. The calcium-activated slow AHP: cutting through the Gordian knot. Front Cell Neurosci 2012; 6:47; PMID:23112761; http://dx.doi.org/ 10.3389/fncel.2012.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Turner RW, Kruskic M, Teves M, Scheidl-Yee T, Hameed S, Zamponi GW. Neuronal expression of the intermediate conductance calcium-activated potassium channel KCa3.1 in the mammalian central nervous system. Eur J Physiol 2015; 467:311-28; http://dx.doi.org/ 10.1007/s00424-014-1523-1 [DOI] [PubMed] [Google Scholar]
- [3].King B, Rizwan AP, Asmara H, Heath NC, Engbers JD, Dykstra S, Bartoletti TM, Hameed S, Zamponi GW, Turner RW. IKCa channels are a critical determinant of the slow AHP in CA1 pyramidal neurons. Cell Rep 2015; 11:175-82; PMID:25865881; http://dx.doi.org/ 10.1016/j.celrep.2015.03.026 [DOI] [PubMed] [Google Scholar]
- [4].Chen S, Benninger F, Yaari Y. Role of Small Conductance Ca2+-Activated K+ Channels in Controlling CA1 Pyramidal Cell Excitability. J Neurosci 2014; 34:8219-30; PMID:24920626; http://dx.doi.org/ 10.1523/JNEUROSCI.0936-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature 1998; 395:900-5; PMID:9804423; http://dx.doi.org/ 10.1038/27674 [DOI] [PubMed] [Google Scholar]
- [6].Bowden SE, Fletcher S, Loane DJ, Marrion NV. Somatic colocalization of rat SK1 and D class (Ca(v)1.2) L-type calcium channels in rat CA1 hippocampal pyramidal neurons. J Neurosci 2001; 21:RC175; PMID:11588205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci 2004; 24:5301-6; PMID:15190101; http://dx.doi.org/ 10.1523/JNEUROSCI.0182-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gu N, Hu H, Vervaeke K, Storm JF. SK (KCa2) channels do not control somatic excitability in CA1 pyramidal neurons but can be activated by dendritic excitatory synapses and regulate their impact. J Neurophysiol 2008; 100:2589-604; PMID:18684909; http://dx.doi.org/ 10.1152/jn.90433.2008 [DOI] [PubMed] [Google Scholar]
- [9].Gulledge AT, Dasari S, Onoue K, Stephens EK, Hasse JM, Avesar D. A sodium-pump-mediated afterhyperpolarization in pyramidal neurons. J Neurosci 2013; 33:13025-41; PMID:23926257; http://dx.doi.org/ 10.1523/JNEUROSCI.0220-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Joiner WJ, Wang LY, Tang MD, Kaczmarek LK. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc Natl Acad Sci U S A 1997; 94:11013-8; PMID:9380751; http://dx.doi.org/ 10.1073/pnas.94.20.11013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci U S A 1997; 94:11651-6; PMID:9326665; http://dx.doi.org/ 10.1073/pnas.94.21.11651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Logsdon NJ, Kang J, Togo JA, Christian EP, Aiyar J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J Biol Chem 1997; 272:32723-6; PMID:9407042; http://dx.doi.org/ 10.1074/jbc.272.52.32723 [DOI] [PubMed] [Google Scholar]
- [13].Lein ES. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007; 445:168-76; PMID:17151600; http://dx.doi.org/ 10.1038/nature05453 [DOI] [PubMed] [Google Scholar]
- [14].Engbers JDT, Anderson D, Asmara H, Rehak R, Mehaffey WH, Hameed S, McKay BE, Kruskic M, Zamponi GW, Turner RW. Intermediate conductance calcium-activated potassium channels modulate summation of parallel fiber input in cerebellar Purkinje cells. Proc Natl Acad Sci U S A 2012; 109:2601-6; PMID:22308379; http://dx.doi.org/ 10.1073/pnas.1115024109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu WW, Chan CS, Disterhoft JF. Slow afterhyperpolarization governs the development of NMDA receptor-dependent afterdepolarization in CA1 pyramidal neurons during synaptic stimulation. J Neurophysiol 2004; 92:2346-56; PMID:15190096; http://dx.doi.org/ 10.1152/jn.00977.2003 [DOI] [PubMed] [Google Scholar]
- [16].Nguyen TV, Matsuyama H, Baell J, Hunne B, Fowler CJ, Smith JE, Nurgali K, Furness JB. Effects of compounds that influence IK (KCNN4) channels on afterhyperpolarizing potentials, and determination of IK channel sequence, in guinea pig enteric neurons. J Neurophysiol 2007; 97:2024-31; PMID:17229825; http://dx.doi.org/ 10.1152/jn.00935.2006 [DOI] [PubMed] [Google Scholar]
- [17].Wulff H, Gutman GA, Cahalan MD, Chandy KG. Delineation of the clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel, IKCa1. J Biol Chem 2001; 276:32040-5; PMID:11425865; http://dx.doi.org/ 10.1074/jbc.M105231200 [DOI] [PubMed] [Google Scholar]
- [18].Strobaek D, Brown DT, Jenkins DP, Chen YJ, Coleman N, Ando Y, Chiu P, Jørgensen S, Demnitz J, Wulff H, et al.. NS6180, a new K(Ca) 3.1 channel inhibitor prevents T-cell activation and inflammation in a rat model of inflammatory bowel disease. Br J Pharmacol 2013; 168:432-44; PMID:22891655; http://dx.doi.org/ 10.1111/j.1476-5381.2012.02143.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Visan V, Fajloun Z, Sabatier JM, Grissmer S. Mapping of maurotoxin binding sites on hKv1.2, hKv1.3, and hIKCa1 channels. Mol Pharmacol 2004; 66:1103-12; PMID:15286210; http://dx.doi.org/ 10.1124/mol.104.002774 [DOI] [PubMed] [Google Scholar]
- [20].Nolting A, Ferraro T, D'Hoedt D, Stocker M. An amino acid outside the pore region influences apamin sensitivity in small conductance Ca2+-activated K+ channels. J Biol Chem 2007; 282:3478-86; PMID:17142458; http://dx.doi.org/ 10.1074/jbc.M607213200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wulff H, Kohler R. Endothelial small-conductance and intermediate-conductance KCa channels: an update on their pharmacology and usefulness as cardiovascular targets. J Cardiovasc Pharmacol 2013; 61:102-12; PMID:23107876; http://dx.doi.org/ 10.1097/FJC.0b013e318279ba20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wulff H, Kolski-Andreaco A, Sankaranarayanan A, Sabatier JM, Shakkottai V. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr Med Chem 2007; 14:1437-57; PMID:17584055; http://dx.doi.org/ 10.2174/092986707780831186 [DOI] [PubMed] [Google Scholar]
- [23].Ataga KI, Smith WR, De Castro LM, Swerdlow P, Saunthararajah Y, Castro O, Vichinsky E, Kutlar A, Orringer EP, Rigdon GC, et al.. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood 2008; 111:3991-7; PMID:18192510; http://dx.doi.org/ 10.1182/blood-2007-08-110098 [DOI] [PubMed] [Google Scholar]
- [24].Vogalis F, Furness JB, Kunze WA. Afterhyperpolarization current in myenteric neurons of the guinea pig duodenum. J Neurophysiol 2001; 85:1941-51; PMID:11353011 [DOI] [PubMed] [Google Scholar]
- [25].Vogalis F, Harvey JR, Furness JB. TEA- and apamin-resistant K(Ca) channels in guinea-pig myenteric neurons: slow AHP channels. J Physiol 2002; 538:421-33; PMID:11790810; http://dx.doi.org/ 10.1113/jphysiol.2001.012952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vogalis F, Harvey JR, Furness JB. PKA-mediated inhibition of a novel K+ channel underlies the slow after-hyperpolarization in enteric AH neurons. J Physiol 2003; 548:801-14; PMID:12640013; http://dx.doi.org/ 10.1113/jphysiol.2002.037325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci U S A 2000; 97:8151-6; PMID:10884437; http://dx.doi.org/ 10.1073/pnas.97.14.8151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang K, Mateos-Aparicio P, Honigsperger C, Raghuram V, Wu WW, Ridder MC, Sah P, Maylie J, Storm JF, Adelman JP. IK1 channels do not contribute to the slow afterhyperpolarization in pyramidal neurons. Elife 2016; 5:e11206; PMID:26765773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Toledo-Rodriguez M, Markram H. Single-cell RT-PCR, a technique to decipher the electrical, anatomical, and genetic determinants of neuronal diversity. Methods Mol Biol 2007; 403:123-39; PMID:18827991; http://dx.doi.org/ 10.1007/978-1-59745-529-9_8 [DOI] [PubMed] [Google Scholar]


