abstract
Low-voltage-gated T-type calcium channels are expressed throughout the nervous system where they play an essential role in shaping neuronal excitability. Defects in T-type channel expression have been linked to various neuronal disorders including neuropathic pain and epilepsy. Currently, little is known about the cellular mechanisms controlling the expression and function of T-type channels. Asparagine-linked glycosylation has recently emerged as an essential signaling pathway by which the cellular environment can control expression of T-type channels. However, the role of N-glycans in the conducting function of T-type channels remains elusive. In the present study, we used human Cav3.2 glycosylation-deficient channels to assess the role of N-glycosylation on the gating of the channel. Patch-clamp recordings of gating currents revealed that N-glycans attached to hCav3.2 channels have a minimal effect on the functioning of the channel voltage-sensor. In contrast, N-glycosylation on specific asparagine residues may have an essential role in the conducting function of the channel by enhancing the channel permeability and / or the pore opening of the channel. Our data suggest that modulation of N-linked glycosylation of hCav3.2 channels may play an important physiological role, and could also support the alteration of T-type currents observed in disease states.
KEYWORDS: calcium channel, Cav3.2, gating, glycosylation, T-type channel
Introduction
Low-voltage-activated T-type calcium (Ca2+) channels consist of a pore-forming Cav3.x-subunit (Cav3.1, Cav3.2, and Cav3.3) embedded in the plasma membrane of most excitable cells including neurons.1 One of the key features of T-type channels arises from their low-threshold of activation that makes these channels perfectly suited to operate near the resting membrane potential of cells.2 Although T-type channels may require a preceding period of hyperpolarization that removes inactivation when cells are partially depolarized at rest, they are typically recruited by subthreshold membrane depolarizations, and generate a transient low-threshold Ca2+ current (so-called T-type current) as well as low-threshold Ca2+ spikes, which support high frequency bursts of action potentials essential to maintain various forms of neuronal rhythmogenesis.3-6 In addition, because of their unique gating properties, a small population of T-type channels remains open at rest, providing an incentive for Ca2+ to flow insight of the cell near typical neuronal resting membrane potentials (“window current”). Besides regulating neuronal excitability, T-type channels also contribute to low-threshold exocytosis by virtue of their functional coupling with the vesicular release machinery.7,8
Over the last few years, various pathways and mechanisms underlying the modulation of T-type channels have been identified.9-11 Besides numerous small molecules that acutely modulate channel activity,12 T-type channels are subject to regulation at the levels of transcription,13-15 alternative splicing,16-19 protein interaction,20-21 and post-translational modification including ubiquitination 22 and phosphorylation.23 In addition, asparagine (N)-linked glycosylation has emerged as an essential level of control of T-type channel expression. We have previously reported that N-glycosylation of human Cav3.2 channels (hCav3.2) is critical for the proper maturation, surface trafficking and stability of the channel.24 In addition, acute enzymatic removal of N-glycans attached at the surface of the channel altered recombinant and native T-type currents, suggesting that N-glycosylation can also modulate the activity of the channel while at the plasma membrane.24-27 However, the detailed mechanism by which glycosylation modulates T-type channel activity besides controlling surface expression of the channel remains elusive.
In the present study, we have specifically analyzed the role of N-linked glycosylation on the functioning of hCav3.2 T-type channels. Using recombinant glycosylation-deficient hCav3.2 channels, we compared charge movements and ionic currents measured from HEK-293 cell-expressing hCav3.2 channels and revealed that N-glycosylation on specific loci modulates channel conductance, by enhancing channel permeability and / or opening probability.
Results
Effect of N-glycosylation on the voltage-dependence of T-type currents
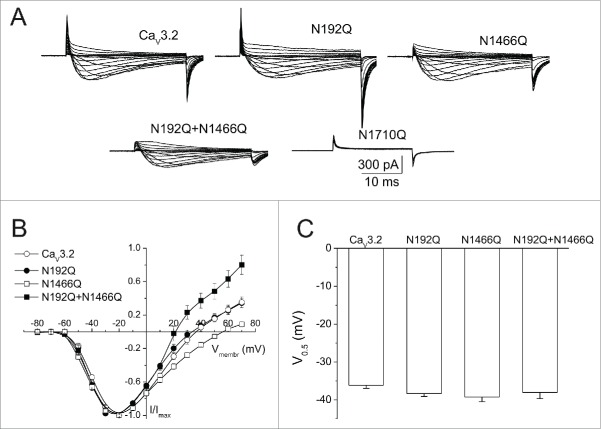
The human Cav3.2 channel (hCav3.2) presents 4 N-linked glycosylation loci, located in the extracellular loops of domains I, III and IV (Fig. 1). We and others have previously reported that acute enzymatic deglycosylation of hCav3.2 channels resulted in a significant decrease of the T-type current density.24,25 Because alteration of T-type currents may have resulted from an alteration of the negative surface potential, we assessed the voltage-dependence of T-type currents from cells expressing wild-type (WT) or glycosylation-deficient hCav3.2 channels where the potential N-glycosylation motifs (N-X-S/T) have been disrupted by replacing the asparagine residue (N) with glutamine (Q). Representative Ba2+ current traces in response to 30 ms depolarizing steps to values ranging between −80 mV and +70 mV, from a holding potential of −100 mV, are shown in Figure 2A for hCav3.2WT, N192Q, N1466Q, N192Q/N1466Q, and N1710Q channels. Figure 2B shows the corresponding mean normalized peak Ba2+ current density as a function of membrane voltage. The voltage-dependence of the T-type current activation was determined by fitting the values with a modified Boltzman equation (see Methods). The mean half-activation potential remained unaltered in cells expressing glycosylation-deficient hCav3.2 channels (−38.3 ± 0.8, n = 31 for N192Q; −39.2 ± 1.3 mV, n = 13 for N1466Q; and −38.0 ± 1.7 mV, n = 12 for N192Q/N1466Q) compared to cells expressing the wild-type channel (−36.1 ± 0.8 mV, n = 29) (Fig. 2C). In contrast, we observed a significant alteration of the reversal potential of Ba2+ currents in cells expressing glycosylation-deficient channels. For instance, the reversal potential was shifted by +9.3 mV (p < 0.01) in N1466Q-expressing cells (48.2 ± 2.9 mV, n = 13), and by −9.0 mV (p < 0.01) in N192Q/N1466Q-expressing cells (29.9 ± 2.1 mV, n = 12) as compared to cells expressing the wild-type hCav3.2 channel (38.9 ± 1.6 mV, n = 29). Consistent with our previous observation that preventing glycosylation at asparagine N1710 gives rise to a non-functional channel, we did not observe any measurable current from cells expressing the N1710Q mutant channel.
Figure 1.

Schematic representation of the human Cav3.2 T-type calcium channel showing the position of N-glycans. Glycosylation loci investigated in this study are labeled in bold.
Figure 2.
Effect of N-glycosylation on hCav3.2 ionic currents. (A) Representative Ba2+ current traces recorded in response to 30 ms depolarizing steps to values ranging between −80 mV and +70 mV, from a holding potential of −100 mV, for wild-type hCav3.2 and glycosylation-deficient N192Q, N1466Q, N192Q/N1466Q, and N1710Q channels. (B) Corresponding mean normalized current-voltage relationships for hCav3.2WT (open circles, n = 29), N192Q (filled circles, n = 31), N1466Q (open squares, n = 13), and N192Q/N1466Q channels (filled circles, n = 12). (C) Corresponding mean half-activation potential.
Effect of N-glycosylation on gating currents of hCav3.2 channels
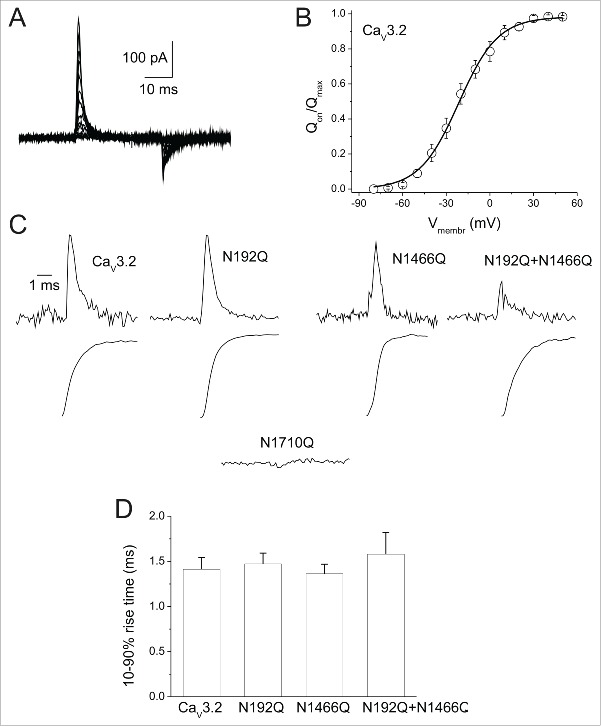
To determine whether N-glycosylation affects the functioning of the channel voltage-sensor, we analyzed charge movements that refer to the movement of the elements of the voltage-sensor following depolarization of the plasma membrane and produce gating currents (Q). Because glycosylation-deficient hCav3.2 channels usually are not sufficiently abundant at the plasma membrane to accurately assess charge movements over a large range of potentials, we measured the total charges (Qmax) at the reversal potential around +40 mV (Qrev), where we can consider Qrev to be equal to Qmax (Fig. 3A–B). Representative gating current traces recorded from cells expressing the wild-type hCav3.2 channel, or the various N-glycosylation mutants are shown in Figure 3C (top panels). No gating currents were detected in cells expressing N1710Q mutant channels (Fig. 3C; n = 54 cells from 5 independent transfections). Kinetics of charge movements were assessed by measuring the 10–90 % rise time of the gating current integral (Fig. 3C, bottom panels). The 10–90 % rise time of the gating current remained unaltered in cells expressing hCav3.2 channels (1.63 ± 0.20 ms, n = 24 for N192Q; 1.36 ± 0.11 ms, n = 12 for N1466Q; and 1.58 ± 0.24 ms, n = 12 for N192Q/N1466Q) compared to cells expressing the wild-type channel (1.41 ± 0.13 ms, n = 29) (Fig. 3D).
Figure 3.
Effect of N-glycosylation on hCav3.2 gating currents. (A) Representative ON-gating current traces recorded from hCav3.2WT-expressing cell in response to 30 ms depolarizing steps to values ranging between −80 mV and +70 mV, from a holding potential of −100 mV. (B) Corresponding mean normalized Qon-voltage relationship for hCav3.2WT channels. (C) Representative ON-gating current traces recorded from wild-type hCav3.2- and glycosylation-deficient N192Q-, N1466Q-, N192Q/N1466Q-, and N1710Q-expressing cells, at the reversal potential of each respective cell (top panels). Bottom panels show the corresponding time course of integral of gating currents. (D) Corresponding mean 10–90 % rise time of the ON-gating currents for wild-type hCav3.2 (n = 29) and glycosylation-deficient N192Q (n = 24), N1466Q (n = 12) and N192Q/N1466Q (n = 12) channels.
N-glycosylation controls the permeability of hCav3.2 channels
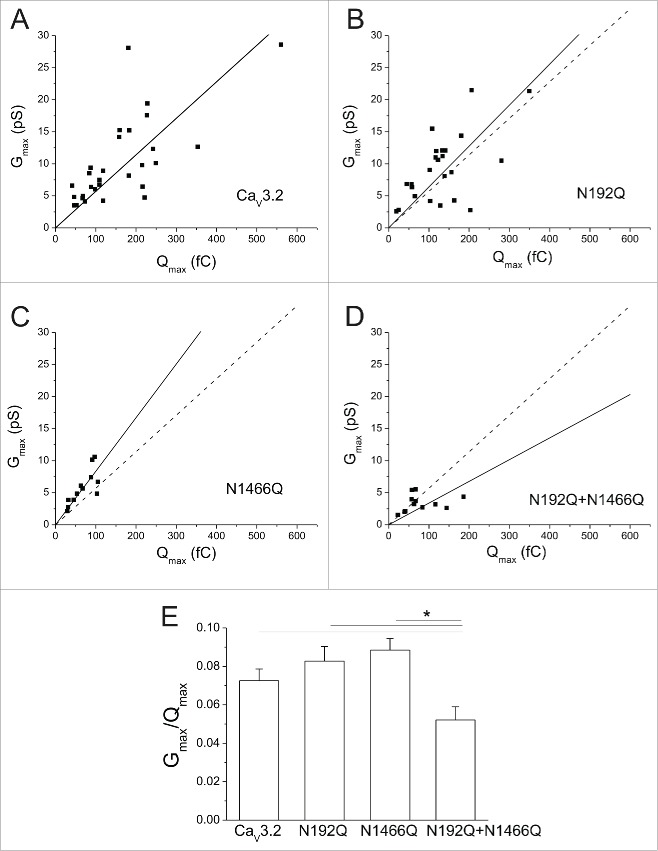
Activation of voltage-gated Ca2+ channels requires the initial mobilization of the channel voltage-sensor that generates charge movements (Q), followed by the opening of the pore evidenced by the occurrence of a Ca2+ conductance (G). Hence, comparing Q over G provide valuable insights into the functioning of the channel. Figure 4A shows the scatter plot of Gmax as a function of Qmax for wild type and glycosylation-deficient hCav3.2 channels, and fitted with the following linear regression:
with k being the steepness factor. Gmax was calculated from the Boltzmann-Ohm fit of the voltage-dependence of the peak Ca2+ current (see Fig. 2 and Methods). Whereas disrupting N-glycosylation at asparagines N192 and N1466 had no significant effect on the Qmax-Gmax dependency, the steepness factor was reduced by ≈1.7 fold (p < 0.05) in N192Q/N1466Q-expressing cells (0.034 ± 0.006, n = 12) as compared to cells expressing the wild-type hCav3.2 channel (0.057 ± 0.05, n = 29) (Fig. 4B). Qmax and Gmax can be described by the following equations (1) and (2), respectively:
| (1) |
| (2) |
with n being the number of functional channels, q the unitary charge per channel, g the unitary conductance, and Po the opening probability. Hence, dividing Gmax by Qmax as shown in equation (3) provides information on the single channel properties:
| (3) |
Figure 4.
N-glycosylation modulates the permeability of hCav3.2 channels. (A) Scatter plots of the maximal conductance (Gmax) as a function of the maximal ON-gating charge (Qmax) for wild-type hCav3.2 (n = 29) and glycosylation-deficient N192Q (n = 24), N1466Q (n = 12), and N192Q/N1466Q (n = 12) channels. Data were fitted with a linear regression (continuous line). The doted lines represent the fit of the wild-type channel. (B) Corresponding mean values for Gmax over Qmax.
Considering that the charge movements were found unaltered, this result is in support of a decreased channel conductance and / or opening probability of the glycosylation-deficient N192Q/N1466Q channel.
Discussion
In the present study, we provide evidence for an essential role of N-glycosylation in the control of human Cav3.2 T-type channel gating. Whereas glycosylation has minimal influence on the functioning of the channel voltage-sensor, N-glycans attached to the hCav3.2 subunit are essential to regulate the permeability of the channel.
Glycosylation has been shown to play an essential role in the expression and functioning of a wide variety of ion channels.28-30 To assess the role of N-glycosylation in T-type channel function, we used N-glycosylation-deficient hCav3.2 channels where glycosylation motifs have been disrupted by mutagenesis. It was previously proposed that sialic acid residues attached to the outermost ends of glycan chains may contribute to the negative surface potential, and contributing to the gating modulation of some voltage-gated Na+ and K+ channels by an electrostatic mechanism.31-35 In contrast, we showed that disruption of N-glycosylation had no influence on the mean half-activation potential of hCav3.2 currents. This result is consistent with previous reports showing that enzymatic deglycosylation of recombinant and native Cav3.2 channels with PNGase F or neuraminidase did not alter the voltage-dependence of T-type currents.24,25
In contrast, we showed that N-glycosylation plays an essential role in modulating the permeability of hCav3.2 channels. Whereas disruption of N-glycosylation at asparagine N192 and N1466 had no influence on single channel properties, simultaneous disruption of the two N-glycosylation sites resulted in a decrease of the single channel conductance and / or the opening probability. This result supports initial observations that acute deglycosylation of hCav3.2 with PNGase F, or desialylation with neuraminidase, attenuates the Ca2+ conductance of Cav3.2 channels.24,25 In addition, the alteration of the channel permeability is further supported by our observation that cells expressing glycosylation-deficient channels had altered reversal potential of the T-type current. As the potential for half-maximal current activation was unaffected, a shift of the reversal potential can be attributed to an altered outward current flowing through Cav3.2 glycosylation-deficient channels. However, this alteration was observed in non-physiological conditions with Cs+ as a main intracellular cation and with intracellular Cl− replaced by methansulfonate (see Methods). As one could expect, an outward current through T-type channels depends considerably on the type of intracellular cation.36 It is unlikely that the alteration of the reversal potential has any physiological relevance, but strongly supports the notion that N-glycans attached to the Cav3.2 subunit influences channel selectivity.
Asparagine N1466 is located in the third transmembrane domain of hCav3.2 in the pore-forming loop between segments S5 and S6 that project into the pore of the channel to form the selectivity filter. Thus, glycosylation of asparagine N1466 may provide a local negative environment that could affect the Ca2+ permeability. However, our observation that deglycosylation of asparagine N1466 is not sufficient to alter the channel conductance and requires the concomitant deglycosylation of asparagine N192 suggests the implication of other channel gating domains. In contrast, the asparagine N192 is located in the first domain of the channel in the short extracellular loop linking segments S3 and S4. It is thus conceivable that glycosylation of asparagine N192, by providing a negative charge in the vicinity of the S4 voltage-sensor may have an effect on the gating of the channel. Consistent with this idea, it was reported that the open state of the inwardly rectifying K+ channel Kir1.1 was destabilized in the absence of N-glycosylation.37 Although we have not observed any change in the ON kinetics of the gating currents of glycosylation-deficient channels, it is possible that the OFF kinetics may be affected. This aspect was not possible to investigate in our experimental conditions, as it would have required a full block of the ionic current. Nevertheless, our data clearly established N-glycosylation as an essential determinant of hCav3.2 channel gating.
Repeatedly, no gating current was measured in cells expressing N1710Q mutant channels. While previous experiments detected channel expression at the cell surface,24 lack of measurable charge movement suggests that either the channel is not properly inserted in the plasma membrane, or its voltage sensors are not functional.
Taken together, our data extend our understanding of the role of N-glycosylation in the functioning of T-type channels. Our observation that the conducting properties of hCav3.2 channels can be altered by the amount of occupied N-glycosylation sites in the protein may have important pathophysiological implications, as a defect in ion channel glycosylation has been reported in numerous disease states.38 Interestingly, a causal increased activity of T-type channels has been documented in various animal models of chronic pain11,39,40 including painful diabetic neuropathy.41 And consistent with the idea that the increased T-type channel activity may be caused by a defect in the glycosylation of the channel, in vivo desialylation by injection of neuraminidase in an animal model of diabetes restored normal T-type currents and pain behavior.25 In addition, an increased T-type channel activity has also been documented in animal model of epilepsy.42-45 Interestingly, a missense mutation (D1463N) in the CACNA1H gene encoding for Cav3.2 channels and located near to the asparagine N1466 was identified in patients with childhood absence epilepsy.46,47 Whether this mutation affects N-glycosylation of the channel remains to be determined.
Material and methods
Plasmid cDNA constructs
The human wild type and glycosylation-deficient hCav3.2 channels used in this study were previously described.24 Briefly, the wild-type hCav3.2 construct (hCav3.2WT) was used as a template for mutation of the consensus asparagine (N)-linked glycosylation sites by substituting the asparagine residues N192, N1466, and N1710 with glutamine (Q) residues. Glutamine was chosen because of its structural similarity with asparagine residues, differing only by one methyl group in the amino acid side chains, which is consequently expected to preserve the local charge distribution within the protein and the secondary structure of the channel. The N to Q substitution was introduced by site-directed mutagenesis using the QuikChange II™ Site-Directed Mutagenesis Kit (Agilent Technologies Inc.), and the final constructs were verified by sequencing of the full-length cDNAs.
Heterologous expression
Human embryonic kidney HEK-293 cells (DSMZ, Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, German Collection of Microorganisms and Cell Cultures) were grown in MEM with Earle's salts containing 10% fetal calf serum and 100 U/mL penicillin-streptomycin and maintained under standard conditions at 37°C in a humidified atmosphere containing 5% CO2 and 95 % air. Cells were harvested from their culture flasks by trypsinization, plated out onto 25 cm2 culture flasks (Sigma Aldrich), and transiently transfected using the calcium/phosphate method with the human hCav3.2 channel, along with a green fluorescent protein. For calcium/phosphate transfection we used Solution A (250 mM CaCl2) and Solution B (2x HEPES buffer containing in mM; NaCl, 250; HEPES, 40 Dextrose 12; Na2HPO4, 1.4; pH 7.05 with NaOH) to form a calcium phosphate precipitate that was directly layered onto the cells and 3 μg of plasmid cDNA.
Electrophysiology and data analysis
Patch-clamp recordings were performed 48–72 h after transfection at room temperature (22–24°C) using an EPC-10 patch clamp amplifier (HEKA Electronic, Lambrecht, Germany). The extracellular solution contained (in mM): CsCl, 95; HEPES, 10; glucose, 10; TEA-Cl, 40; BaCl2, 5; MgCl2, 1; pH 7.4 (CsOH). Background Cl− current in HEK-293 cells was minimized by use of cesium methansulfonate in the pipette solution. The composition of the intracellular solution was (in mM): CH3SO3Cs, 130; EGTA, 10; MgCl2, 5; TEA-Cl, 10; Na-ATP, 5; and HEPES, 10; pH 7.4 (CsOH). Patch pipettes were made out of borosilicate glass (Sutter Instrument, Novato, CA). When filled with the intracellular solution, the input resistance ranged between 1.6 and 2.0 MΩ. The capacitance of individual cells ranged between 10 and 40 pF. Series resistance reached values between 2.5 to 6 MΩ and was compensated by built-in circuits of the EPC 10 amplifier. Data were recorded with HEKA Patchmaster and analyzed offline using HEKA Fitmaster v2×73.1 and Origin 8.1 software. The holding potential (HP) in all experiments was −100 mV. Ionic currents were measured by 30-ms-long depolarizing pulses from the HP to membrane potentials between −80 mV and +70 mV applied with a frequency of 0.33 Hz. The linear components of leak current were subtracted off-line. The voltage-dependence of the peak Ca2+ current was fitted with the following modified Boltzman equation:
with I(V) being the peak current amplitude at the command potential V, Gmax the maximum conductance, Vrev the reversal potential, V0.5 the half activation potential, and k the steepness factor. ON-gating currents were measured by a series of 5 identical depolarizing pulse to the reversal potential of each respective cell. The linear components of leak current and capacitive transients were subtracted using the -P/8 procedure. The total QON charge was evaluated by integrating the area below averaged gating current traces at the beginning of the depolarizing pulse.
Statistical analysis
Data values are presented as mean ± S.E.M. for n recorded cells. Statistical significance was determined using ANOVA test. *p < 0.05, **p < 0.01, ***p < 0.001, and NS, statistically not different.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Dr. Gerald W. Zamponi (University of Calgary, Calgary, Canada) for kindly providing the Cav3.2 glycosylation-deficient channel constructs.
Funding
LL was supported by the VEGA (grant 2/0044/13). NW is supported by the Czech Science Foundation (grant 15-13556S), the Czech Ministry of Education Youth and Sports (grant 7AMB15FR015), and the Institute of Organic Chemistry and Biochemistry (IOCB). JL is supported by an IOCB fellowship.
References
- [1].Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 2005; 57:411-25; PMID:16382099; http://dx.doi.org/ 10.1124/pr.57.4.5 [DOI] [PubMed] [Google Scholar]
- [2].Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev 2003; 83:117-61; PMID:12506128; http://dx.doi.org/ 10.1152/physrev.00018.2002 [DOI] [PubMed] [Google Scholar]
- [3].Crunelli V, Cope DW, Hughes SW. Thalamic T-type Ca2+ channels and NREM sleep. Cell Calcium 2006; 40:175-90; PMID:16777223; http://dx.doi.org/ 10.1016/j.ceca.2006.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bal T, McCormick DA. Synchronized oscillations in the inferior olive are controlled by the hyperpolarization-activated cation current I(h). J Neurophysiol 1997; 77:3145-56; PMID:9212264 [DOI] [PubMed] [Google Scholar]
- [5].Beurrier C, Congar P, Bioulac B, Hammond C. Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. J Neurosci 1999; 19:599-609; PMID:9880580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J Physiol 2003; 551:927-43; PMID:12865506; http://dx.doi.org/ 10.1113/jphysiol.2003.046847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weiss N, Hameed S, Fernández-Fernández JM, Fablet K, Karmazinova M, Poillot C, Proft J, Chen L, Bidaud I, Monteil A, et al.. A Ca(v)3.2/syntaxin-1A signaling complex controls T-type channel activity and low-threshold exocytosis. J Biol Chem 2012; 287:2810-8; PMID:22130660; http://dx.doi.org/ 10.1074/jbc.M111.290882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Weiss N, Zamponi GW. Control of low-threshold exocytosis by T-type calcium channels. Biochim Biophys Acta 2013; 1828:1579-86; PMID:22885170; http://dx.doi.org/ 10.1016/j.bbamem.2012.07.031 [DOI] [PubMed] [Google Scholar]
- [9].Zhang Y, Jiang X, Snutch TP, Tao J. Modulation of low-voltage-activated T-type Ca2+ channels. Biochim Biophys Acta 2013; 1828:1550-9; PMID:22975282; http://dx.doi.org/ 10.1016/j.bbamem.2012.08.032 [DOI] [PubMed] [Google Scholar]
- [10].Peers C, Elies J, Gamper N. Novel ways to regulate T-type Ca2+ channels. Channels (Austin) 2015; 9:68-9; PMID:25715174; http://dx.doi.org/ 10.1080/19336950.2015.1017995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev 2015; 67:821-70; PMID:26362469; http://dx.doi.org/ 10.1124/pr.114.009654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].M'Dahoma S, Gadotti VM, Zhang FX, Park B, Nam JH, Onnis V, Balboni G, Lee JY, Zamponi GW. Effect of the T-type channel blocker KYS-05090S in mouse models of acute and neuropathic pain. Pflugers Arch 2016; 468:193-9; http://dx.doi.org/ 10.1007/s00424-015-1733-1. [DOI] [PubMed] [Google Scholar]
- [13].Yu H, Seo JB, Jung SR, Koh DS, Hille B. Noradrenaline upregulates T-type calcium channels in rat pinealocytes. J Physiol 2015; 593:887-904; PMID:25504572; http://dx.doi.org/ 10.1113/jphysiol.2014.284208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sellak H, Zhou C, Liu B, Chen H, Lincoln TM, Wu S. Transcriptional regulation of α1H T-type calcium channel under hypoxia. Am J Physiol Cell Physiol 2014; 307:C648-56; PMID:25099734; http://dx.doi.org/ 10.1152/ajpcell.00210.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].van Loo KM, Schaub C, Pernhorst K, Yaari Y, Beck H, Schoch S, Becker AJ. Transcriptional regulation of T-type calcium channel CaV3.2: bi-directionality by early growth response 1 (Egr1) and repressor element 1 (RE-1) protein-silencing transcription factor (REST). J Biol Chem 2012; 287:15489-501; PMID:22431737; http://dx.doi.org/ 10.1074/jbc.M111.310763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Murbartián J, Arias JM, Perez-Reyes E. Functional impact of alternative splicing of human T-type Cav3.3 calcium channels. J Neurophysiol 2004; 92:3399-407; PMID:15254077; http://dx.doi.org/ 10.1152/jn.00498.2004 [DOI] [PubMed] [Google Scholar]
- [17].Latour I, Louw DF, Beedle AM, Hamid J, Sutherland GR, Zamponi GW. Expression of T-type calcium channel splice variants in human glioma. Glia 2004; 48:112-9; PMID:15378657; http://dx.doi.org/ 10.1002/glia.20063 [DOI] [PubMed] [Google Scholar]
- [18].Powell KL, Cain SM, Ng C, Sirdesai S, David LS, Kyi M, Garcia E, Tyson JR, Reid CA, Bahlo M, et al.. A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy. J Neurosci 2009; 29:371-80; PMID:19144837; http://dx.doi.org/ 10.1523/JNEUROSCI.5295-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].David LS, Garcia E, Cain SM, Thau E, Tyson JR, Snutch TP. Splice-variant changes of the CaV3.2 T-type calcium channel mediate voltage-dependent facilitation and associate with cardiac hypertrophy and development. Channels (Austin) 2010; 4:375-89; PMID:20699644; http://dx.doi.org/ 10.4161/chan.4.5.12874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wolfe JT, Wang H, Howard J, Garrison JC, Barrett PQ. T-type calcium channel regulation by specific G-protein betagamma subunits. Nature 2003; 424:209-13; PMID:12853961; http://dx.doi.org/ 10.1038/nature01772 [DOI] [PubMed] [Google Scholar]
- [21].Aromolaran KA, Benzow KA, Cribbs LL, Koob MD, Piedras-Rentería ES. T-type current modulation by the actin-binding protein Kelch-like 1. Am J Physiol Cell Physiol 2010; 298:C1353-62; PMID:20147652; http://dx.doi.org/ 10.1152/ajpcell.00235.2009 [DOI] [PubMed] [Google Scholar]
- [22].García-Caballero A, Gadotti VM, Stemkowski P, Weiss N, Souza IA, Hodgkinson V, Bladen C, Chen L, Hamid J, Pizzoccaro A, et al.. The deubiquitinating enzyme USP5 modulates neuropathic and inflammatory pain by enhancing Cav3.2 channel activity. Neuron 2014; 83:1144-58; PMID:25189210; http://dx.doi.org/ 10.1016/j.neuron.2014.07.036 [DOI] [PubMed] [Google Scholar]
- [23].Blesneac I, Chemin J, Bidaud I, Huc-Brandt S, Vandermoere F, Lory P. Phosphorylation of the Cav3.2 T-type calcium channel directly regulates its gating properties. Proc Natl Acad Sci U S A 2015; 112:13705-10; PMID:26483470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Weiss N, Black SA, Bladen C, Chen L, Zamponi GW. Surface expression and function of Cav3.2 T-type calcium channels are controlled by asparagine-linked glycosylation. Pflugers Arch 2013; 465:1159-70; PMID:23503728; http://dx.doi.org/ 10.1007/s00424-013-1259-3 [DOI] [PubMed] [Google Scholar]
- [25].Orestes P, Osuru HP, McIntire WE, Jacus MO, Salajegheh R, Jagodic MM, Choe W, Lee J, Lee SS, Rose KE, et al.. Reversal of neuropathic pain in diabetes by targeting glycosylation of CaV3.2 T-type calcium channels. Diabetes 2013; 62:3828-38; PMID:23835327; http://dx.doi.org/ 10.2337/db13-0813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fermini B, Nathan RD. Removal of sialic acid alters both T- and L-type calcium currents in cardiac myocytes. Am J Physiol 1991; 260:H735-43; PMID:2000969 [DOI] [PubMed] [Google Scholar]
- [27].Yee HF, Weiss JN, Langer GA. Neuraminidase selectively enhances transient Ca2+ current in cardiac myocytes. Am J Physiol 1989; 256:C1267-72; PMID:2544097 [DOI] [PubMed] [Google Scholar]
- [28].Lazniewska J, Weiss N. The “sweet” side of ion channels. Rev Physiol Biochem Pharmacol 2014; 167:67-114; PMID:25239698 [DOI] [PubMed] [Google Scholar]
- [29].Ednie AR, Bennett ES. Modulation of voltage-gated ion channels by sialylation. Compr Physiol 2012; 2:1269-301; PMID:23798301 [DOI] [PubMed] [Google Scholar]
- [30].Penuela S, Lohman AW, Lai W, Gyenis L, Litchfield DW, Isakson BE, Laird DW. Diverse post-translational modifications of the pannexin family of channel-forming proteins. Channels (Austin) 2014; 8:124-30; PMID:24418849; http://dx.doi.org/ 10.4161/chan.27422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bennett E, Urcan MS, Tinkle SS, Koszowski AG, Levinson SR. Contribution of sialic acid to the voltage dependence of sodium channel gating. A possible electrostatic mechanism. J Gen Physiol 1997; 109:327-43; PMID:9089440; http://dx.doi.org/ 10.1085/jgp.109.3.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang Y, Hartmann HA, Satin J. Glycosylation influences voltage-dependent gating of cardiac and skeletal muscle sodium channels. J Membr Biol 1999; 171:195-207; PMID:10501828; http://dx.doi.org/ 10.1007/s002329900571 [DOI] [PubMed] [Google Scholar]
- [33].Watanabe I, Wang HG, Sutachan JJ, Zhu J, Recio-Pinto E, Thornhill WB. Glycosylation affects rat Kv1.1 potassium channel gating by a combined surface potential and cooperative subunit interaction mechanism. J Physiol 2003; 550:51-66; PMID:12879861; http://dx.doi.org/ 10.1113/jphysiol.2003.040337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Watanabe I, Zhu J, Sutachan JJ, Gottschalk A, Recio-Pinto E, Thornhill WB. The glycosylation state of Kv1.2 potassium channels affects trafficking, gating, and simulated action potentials. Brain Res 2007; 1144:1-18; PMID:17324383; http://dx.doi.org/ 10.1016/j.brainres.2007.01.092 [DOI] [PubMed] [Google Scholar]
- [35].Schwetz TA, Norring SA, Bennett ES. N-glycans modulate K(v)1.5 gating but have no effect on K(v)1.4 gating. Biochim Biophys Acta 2010; 1798:367-75; PMID:19961828; http://dx.doi.org/ 10.1016/j.bbamem.2009.11.018 [DOI] [PubMed] [Google Scholar]
- [36].Kurejová M, Pavlovicová M, Lacinová L. Monovalent currents through the T-type Cav3.1 channels and their block by Mg2+. Gen Physiol Biophys 2007; 26:234-9 [PubMed] [Google Scholar]
- [37].Schwalbe RA, Wang Z, Wible BA, Brown AM. Potassium channel structure and function as reported by a single glycosylation sequon. J Biol Chem 1995; 270:15336-40; PMID:7797521; http://dx.doi.org/ 10.1074/jbc.270.25.15336 [DOI] [PubMed] [Google Scholar]
- [38].Baycin-Hizal D, Gottschalk A, Jacobson E, Mai S, Wolozny D, Zhang H, Krag SS, Betenbaugh MJ. Physiologic and pathophysiologic consequences of altered sialylation and glycosylation on ion channel function. Biochem Biophys Res Commun 2014; 453:243-53; PMID:24971539; http://dx.doi.org/ 10.1016/j.bbrc.2014.06.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].François A, Laffray S, Pizzoccaro A, Eschalier A, Bourinet E. T-type calcium channels in chronic pain: mouse models and specific blockers. Pflugers Arch 2014; 466:707-17; PMID:24590509; http://dx.doi.org/ 10.1007/s00424-014-1484-4 [DOI] [PubMed] [Google Scholar]
- [40].Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW, Zamponi GW. Calcium-permeable ion channels in pain signaling. Physiol Rev 2014; 94:81-140; PMID:24382884; http://dx.doi.org/ 10.1152/physrev.00023.2013 [DOI] [PubMed] [Google Scholar]
- [41].Cao XH, Byun HS, Chen SR, Pan HL. Diabetic neuropathy enhances voltage-activated Ca2+ channel activity and its control by M4 muscarinic receptors in primary sensory neurons. J Neurochem 2011; 119:594-603; PMID:21883220; http://dx.doi.org/ 10.1111/j.1471-4159.2011.07456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tsakiridou E, Bertollini L, de Curtis M, Avanzini G, Pape HC. Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. J Neurosci 1995; 15:3110-7; PMID:7722649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang Y, Mori M, Burgess DL, Noebels JL. Mutations in high-voltage-activated calcium channel genes stimulate low-voltage-activated currents in mouse thalamic relay neurons. J Neurosci 2002; 22:6362-71; PMID:12151514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang Y, Vilaythong AP, Yoshor D, Noebels JL. Elevated thalamic low-voltage-activated currents precede the onset of absence epilepsy in the SNAP25-deficient mouse mutant coloboma. J Neurosci 2004; 24:5239-48; PMID:15175394; http://dx.doi.org/ 10.1523/JNEUROSCI.0992-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cain SM, Snutch TP. T-type calcium channels in burst-firing, network synchrony, and epilepsy. Biochim Biophys Acta 2013; 1828:1572-8; PMID:22885138; http://dx.doi.org/ 10.1016/j.bbamem.2012.07.028 [DOI] [PubMed] [Google Scholar]
- [46].Chen Y, Lu J, Pan H, Zhang Y, Wu H, Xu K, Liu X, Jiang Y, Bao X, Yao Z, et al.. Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann Neurol 2003; 54:239-43; PMID:12891677; http://dx.doi.org/ 10.1002/ana.10607 [DOI] [PubMed] [Google Scholar]
- [47].Khosravani H, Altier C, Simms B, Hamming KS, Snutch TP, Mezeyova J, McRory JE, Zamponi GW. Gating effects of mutations in the Cav3.2 T-type calcium channel associated with childhood absence epilepsy. J Biol Chem 2004; 279:9681-4; PMID:14729682; http://dx.doi.org/ 10.1074/jbc.C400006200 [DOI] [PubMed] [Google Scholar]