Abstract
The links between muscle and bone have been recently examined because of the increasing number of patients with osteoporosis and sarcopenia. Myokines are skeletal muscle-derived humoral cytokines and growth factors, which exert physiological and pathological functions in various distant organs, including the regulation of glucose, energy and bone metabolism. Myostatin is a crucial myokine, the expression of which is mainly limited to muscle tissues. The inhibition of myostatin signaling increases bone remodeling, bone mass and muscle mass, and it may provide a target for the treatment of both sarcopenia and osteoporosis. As myostatin is involved in osteoclast formation and bone destruction in rheumatoid arthritis, myostatin may be a target myokine for the treatment of accelerated bone resorption and joint destruction in rheumatoid arthritis. Numerous other myokines, including transforming growth factor-β, follistatin, insulin-like growth factor-I, fibroblast growth factor-2, osteoglycin, FAM5C, irisin, interleukin (IL)-6, leukemia inhibitory factor, IL-7, IL-15, monocyte chemoattractant protein-1, ciliary neurotrophic factor, osteonectin and matrix metalloproteinase 2, also affect bone cells in various manners. However, the effects of myokines on bone metabolism are largely unknown. Further research is expected to clarify the interaction between muscle and bone, which may lead to greater diagnosis and the development of the treatment for muscle and bone disorders, such as osteoporosis and sarcopenia.
Introduction
Muscles exert motor function by their contraction under neuronal regulation. Moreover, muscles regulate glucose metabolism through glucose uptake as a target of insulin. It is well-known that adipose tissues influence systemic metabolic control and bone metabolism through adipocytokines, such as leptin, adiponectin and tumor necrosis factor-α. Recently, accumulating evidence suggests that bone tissues affect glucose and energy metabolism as an endocrine organ. Muscles affect various anatomically distant organs, by exhibiting biological effects on target organs in an endocrine manner, by secreting myokines.
Many researchers have recently examined muscle/bone relationships in skeletal tissues, as muscle and bone are anatomically adjacent to each other. The influences of muscle tissues on bone cells may be equally as important as those of bone on muscle cells. In this review, I will summarize recent findings concerning the effects of myokines on bone.
Interaction between Muscle and Bone
Osteoporosis and sarcopenia are age-related systemic and progressive disorders, which impair the activities of daily living and quality of life in elderly people. Sarcopenia is characterized as systemic and progressive decreases in skeletal muscle mass, strength and function.1 Numerous studies indicate that an increase in muscle mass is positively associated with an increase in bone mineral density (BMD), and a reduction in fracture risk.2 Our previous study suggested that lean body mass (muscle mass), measured by dual-energy x-ray bone absorptiometry, is positively associated with BMD in the lumbar spine and femoral neck of postmenopausal women.3 Moreover, several studies have suggested that muscle strength and function are correlated with BMD and fracture risk.2
Several factors simultaneously affect muscle and bone,2,4 including genes, nutrition, mechanical stress, inflammation and endocrine factors, such as vitamin D, growth hormone and androgens. In the pathological state, muscle wasting and osteoporosis are observed in patients with glucocorticoid excess and diabetes. In addition, calcium ions are critical for both muscle contraction and bone formation. Clinical evidence suggests that there are interactions between muscle and bone, which may have physiological and pathological roles in humans.
Systemic humoral factors produced from muscle or bone tissues affect each other. Bone-derived factors such as sclerostin and osteocalcin affect muscle cells as humoral factors.2,4 Weight-loss-induced increase of serum sclerostin levels is prevented by exercise in obese older adults, and changes in serum sclerostin levels were negatively correlated with muscle mass.5 Moreover, trabecular bone volume and muscle mass were increased and decreased in sclerostin-deficient mice, respectively.6 As canonical Wnt signaling regulates muscle regeneration and differentiation, sclerostin might affect skeletal muscles,4 although the direct effects of sclerostin remained unclear. Concerning the effects of muscle on bone, muscle-derived local factors are considered to positively affect osteoblastic bone formation, as muscle supports fracture healing and osteoblastic differentiation.2,4 Moreover, muscles influence bone through direct mechanical impact and an increase in vessel blood flow. In addition, numerous humoral factors, produced in muscle, affect distant bone tissues through the bloodstream.
Myokines
Intracellular organelles, such as the endoplasmic reticulum, are richly expressed in the cytoplasm of muscle cells, and muscle tissues produce a large amount of proteins, which may have a physiological role. Myokines are skeletal muscle-derived cytokines and growth factors. Myokines have various physiological roles, including the regulation of glucose metabolism, vascularization and bone metabolism.7 As the largest organ in the body, muscle is related to endocrine regulation of distant organs, such as bone, through the release of myokines into the bloodstream.
As shown in Figure 1, there are various kinds of muscle-derived humoral factors, including myostatin, transforming growth factor-β (TGF-β), bone morphogenetic proteins (BMPs), activin, follistatin, insulin-like growth factor (IGF)-I, fibroblast growth factor (FGF)-2, osteoglycin, FAM5C, irisin, interleukin (IL)-6, IL-7, IL-15, monocyte chemoattractant protein (MCP)-1, ciliary neurotrophic factor (CNTF), osteonectin and matrix metalloproteinase (MMP)-2. Here, I will describe the effects of these myokines on bone.
Figure 1.
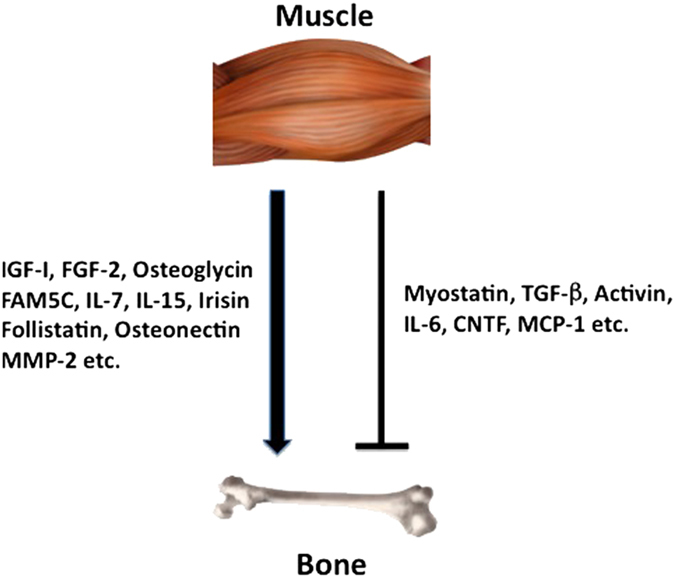
Various myokines affect bone. Numerous muscle-derived factors (myokines) positively or negatively affect bone metabolism, which may have physiological and pathological roles in the interactions between muscle and bone.
Myostatin
Myostatin, one of the TGF-β superfamily proteins, is a well-known myokine and potent suppressor of skeletal muscle mass and development, which is expressed mainly in skeletal muscle tissues. It binds to the activin receptor type IIB (ACVR2B), leading to the activation of activin-like kinase (ALK)4/5, and then phosphorylates Smad2 and Smad3, the TGF-β-specific Smads. Myostatin-deficient mice exhibit skeletal muscle hypertrophy,8 and myostatin-overexpressing mice show a decrease in muscle mass.9 There is also a negative correlation between exercise-induced muscle hypertrophy and myostatin expression.10 These findings indicate that myostatin is a negative regulator of skeletal muscle mass. On the other hand, myostatin deficiency led to improved gains in bone strength with exercise in mice.11 Moreover, immobilization, microgravity and inflammation enhance muscle and blood myostatin levels, which might be related to muscle wasting in these pathological states. With regard to the effects of myostatin on bone, osteogenic differentiation and in vitro calcification of bone marrow-derived mesenchymal stem cells are increased in myostatin-null mice,12 suggesting that myostatin negatively affects osteoblast differentiation. In contrast, myostatin enhances receptor activator of nuclear factor κB ligand (RANKL)-induced osteoclast formation through Smad2-dependent regulation of the nuclear factor of activated T cells (NFATc1).13 Indeed, myostatin enhanced RANKL-induced expression of osteoclast-related genes, including integrin αVβ3, DC-STAMP and calcitonin receptor in the same study. In addition, an in vitro study indicated that the paracrine and autocrine actions of myostatin are important for osteoclast differentiation. These findings suggest that myostatin exerts negative effects on bone mass through decreased bone formation and enhanced resorption.
Myostatin and its signaling might be a crucial target molecule for the treatment of both sarcopenia and osteoporosis. A clinical study found that myostatin gene polymorphism is associated with BMD,14 and genome-wide association studies suggested that the myostatin gene is related to both osteoporosis and sarcopenia.15 ACVR2B-Fc, a soluble myostatin decoy receptor, suppresses the binding of myostatin, some BMPs and activin to ACVR2B. Systemic administration of ACVR2B-Fc enhances hind limb skeletal muscle weight in osteogenesis imperfecta mice.16 In addition, ACVR2B-Fc enhances muscle mass and bone formation indices in postmenopausal women.17 These findings suggest that inhibiton of ACVR signaling might be effective for the treatment of both osteoporosis and sarcopenia. However, ACVR is a receptor for activin, which has numerous essential roles in various tissues. An inhibition of ACVR signaling might cause unexpected side effects in humans. Indeed, a recent study using ACVR2B-Fc in boys with muscular dystrophy was suspended because of the development of unexpected gum bleeds and nose bleeds.18 On the other hand, Arounleut et al.19 reported that myostatin propeptide-Fc, a myostatin inhibitor, did not affect BMD and bone strength in aged mice, although it increased muscle mass. These findings suggest that pharmacological inhibition using myostatin neutralization is not sufficient for the treatment of osteoporosis in mice.
The production of myostatin in tissues other than muscles has recently been reported. Myostatin is expressed during fracture repair and negatively regulates fracture callus size.20 Dankbar et al.13 revealed that myostatin is highly expressed in the synovial tissues of patients with rheumatoid arthritis (RA) and a mouse model of RA. Myostatin deficiency or inhibition with an antibody caused an improvement in arthritis, as well as increased grip strength and less bone destruction in a mouse model of RA.13 These findings suggest that myostatin is involved in osteoclast formation and bone destruction in RA, and that myostatin may be a target myokine for the treatment of RA-associated accelerated bone resorption and joint destruction (Figure 2). Future studies are likely to further elucidate the therapeutic potential of myostatin inhibition.
Figure 2.
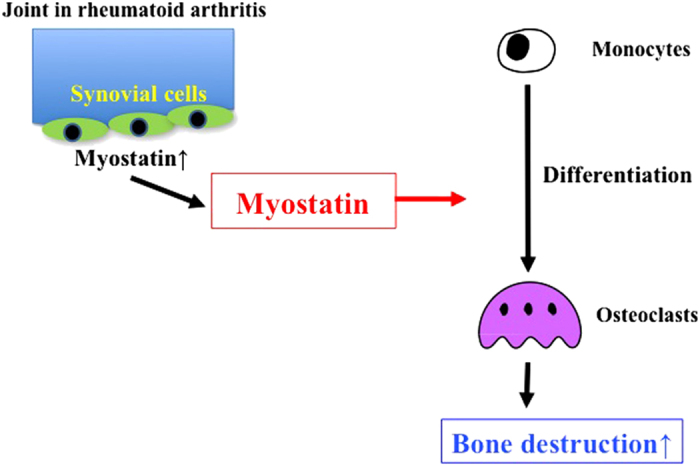
Role of myostatin in joint and bone destruction of rheumatoid arthritis. Exposure of synovial fibroblasts to inflammatory cytokines causes the upregulation of myostatin in patients with RA. Myostatin enhances RANKL-induced osteoclast differentiation in autocrine and paracrine manners. Myostatin stimulates osteoclast formation through increased Smad2-dependent nuclear translocation of NFATc1 and subsequent upregulation of osteoclast differentiation genes.13
The contributions of myostatin's direct action and its muscle mass-mediated secondary action on bone cannot be clearly discriminated at present, and both actions might be involved in its effects on bone.
TGF-β Superfamily Molecules
TGF-β superfamily molecules other than myostatin are produced from muscle tissues. BMPs and TGF-β are important regulators of bone and cartilage formation.21 These factors, released from muscle tissues, have physiological roles as antocrine, paracrine and endocrine factors. BMP-2 and BMP-4, especially, are crucial for the maintenance of osteogenesis. Loss of Smad1/5/8, BMP-specific Smads, induces severe chondrodysplasia and decreased bone formation. As BMPs are mainly produced from bone tissues, the significance of BMPs produced from muscle remains unknown.
Activin, a ligand for ACVR2B, which is a receptor of myostatin, is expressed in most tissues including muscle and bone. Activin has been shown to enhance osteoclast formation in vitro.22 Recent findings suggest that activin negatively affects BMP-induced osteoblastic differentiation, although an enhancement of osteoblast differentiation by activin has previously been reported.23 The impact of local activin may be more important than that of activin as a humoral factor in both muscle and bone.
Follistatin increases muscle mass and muscle regeneration after injury presumably by antagonizing activin and/or myostatin-induced phosphorylation of Smad2/3 and subsequent ACVR signaling.24 In addition, follistatin might modulate bone metabolism by affecting activin and myostatin signaling in bone cells. Low-magnitude mechanical stress positively affects both muscle and bone through a periosteum direction-dependent permeability-related mechanism.25 Muscle wasting and osteoporosis are serious problems in immobilization. These findings suggest that mechanical stress influences both muscle and bone.2,4 Muscle wasting and osteopenia is observed after long durations of space flight in astronauts.26,27 Our preliminary study suggested that follistatin may be a myokine produced from muscle in response to gravity change, and further study is currently in progress in our laboratory (Manuscript in preparation).
Irisin
Irisin is a novel myokine produced from skeletal muscle following exercise, and it is characterized by the induction of a browning response in white adipose tissues. Exercise enhances the expression of irisin in muscle tissues through a PGC-1α-dependent mechanism.28 Irisin is considered to be protective against insulin resistance, metabolic syndrome and cardiovascular disease in humans.29 These findings suggest that irisin may be a myokine, which is important for metabolic regulation (Figure 3).
Figure 3.
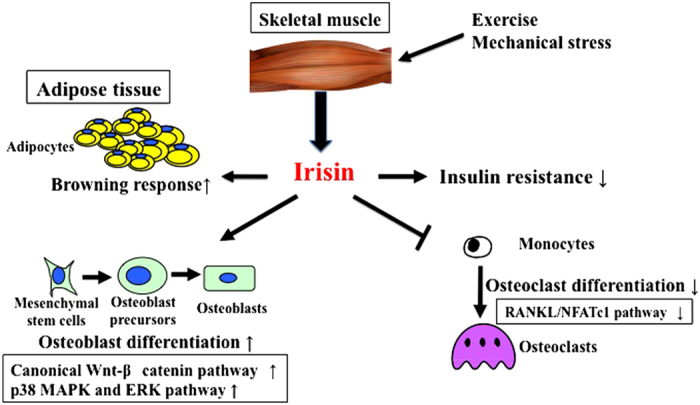
Role of irisin in the linkage of muscle to bone. Irisin is a myokine produced from skeletal muscle after exercise. It induces a browning response in white adipose tissues and protects from insulin resistance. In bone, irisin increases osteoblast differentiation through canonical Wnt-β-catenin, p38 MAPK and ERK pathways. On the other hand, irisin suppresses osteoclast differentiation through the suppression of RANKL/NFATc1 pathways.
In bone, irisin increases osteoblast differentiation through the Wnt-β-catenin pathway in MC3T3-E1 cells.30 Moreover, Qiao et al.31 revealed that irisin enhances proliferation, differentiation, alkaline phosphatase activity and mineralization in cultured osteoblasts through the activation of p38 mitogen-activated protein kinase and extracellular signal-regulated kinase. On the other hand, irisin suppresses osteoclast formation through the inhibition of RANKL/NFATc1 in RAW264.7 cells. In an in vivo study, recombinant irisin administration increased cortical, but not trabecular, bone mass and strength primarily through the stimulation of bone formation, but with a parallel reduction in osteoclast number in male mice.32 In the same study, irisin treatment upregulated the levels of osteogenic genes, including ATF4, Runx2, Osterix, low-density lipoprotein-related protein 5, β-catenin, alkaline phosphatase and type I collagen in bone marrow stromal cells (Figure 3). In humans, a previous study showed that serum irisin levels are correlated with the prevalence of osteoporotic fractures in postmenopausal women with osteopenia.33 Loffler et al.34 reported that acute strenuous exercise but not long-term elevations in physical activity increases serum irisin levels in children and adults. These findings suggest that irisin might be a useful marker for the assessment of muscle/bone disorders and metabolic disease. However, the importance of serum irisin in humans has recently been questioned. Serum irisin levels were measured by enzyme-linked immunoassays using antibodies with poor specificity. Moreover, the start codon of the human irisin-coding gene, membrane protein fibronetin type III domain containing protein 5 (FNDC5), is mutated, resulting in translation with very low efficiency to the protein.35 On the other hand, a study by Jedrychowski et al. used a quantitative mass spectrometry and unbiased assay for the detection of human irisin in plasma to show that human irisin is regularly translated from its noncanonical start codon.36 This study claimed the importance of human irisin, and found that its synthesis was increased by physical activity. Further studies are necessary to determine whether irisin would be a useful diagnostic marker and therapeutic target for clinical use.37
Osteoglycin and FAM5C
Fibrodysplasia ossificance progressiva (FOP) is a genetic disease, which is characterized by progressive heterotopic ossification in skeletal muscle and caused by a mutation that results in constitutive activation of BMP-type I receptors (ALK2).38 We speculated that the gene change suppressed by the conversion of muscle into bone might give us a clue to find muscle-derived humoral bone anabolic factors. We therefore extracted osteoglycin and FAM5C based on comprehensive microarray analysis between FOP-causing mutation-transfected mouse myoblastic C2C12 cells and controls. Osteoglycin is the seventh member of the small leucin-rich proteoglycan family. It belongs to class III of the small leucin-rich proteoglycans. Osteoglycin is included in the mechanosensitive genes that mediate the bone anabolic response to mechanical loading in mice.39 In addition, osteoglycin is one of the secretome components that is differentially upregulated during skeletal myogenesis in mouse myoblastic C2C12 cells.40 We reported that osteoglycin decreased the expression of early-stage osteoblast differentiation genes, such as Runx2 and Osterix in mouse osteoblasts, but enhanced mineralization and the expression of late-stage osteoblast phenotype-related genes, such as alkaline phosphatase, type I collagen and osteocalcin.41 A reduction in endogenous osteoglycin using siRNA exerted the opposite effects to those of osteoglycin overexpression and recombinant osteoglycin addition in osteoblasts. Moreover, osteoglycin suppressed osteoblastic differentiation induced by BMP-2 in mouse mesenchymal C2C12 cells. These findings suggest that osteoglycin is a factor that inhibits osteoblast differentiation of premature osteoblasts and enhances osteoblast phenotype in well-differentiated osteoblasts. The conditioned medium from osteoglycin-overexpressed myoblasts enhanced late osteoblast differentiation, and osteoglycin was detected in cultured supernatant from myoblasts and human serum.41 We further revealed that osteoglycin enhances TGF-β-specific Smad3/4-responsive transcriptional activity, as well as the expression of type I collagen independently of TGF-β as one of the mechanism of osteoglycin effects in osteoblasts.41 These findings suggest that osteoglycin is one humoral bone anabolic factor produced from muscle. Our recent study revealed that active vitamin D and its derivative eldecarcitriol increase the production of osteoglycin in myoblasts, and then osteoglycin increased osteoblast differentiation as a muscle-derived humoral factor in vitro.42
FAM5C is associated with cell proliferation, migration and atherosclerosis. In our study, FAM5C overexpression, as well as the conditioned medium from FAM5C-overexpressed myoblasts, enhanced the osteoblast phenotype of differentiated osteoblasts, but suppressed osteoblastic differentiation induced by BMP-2 in mouse myoblastic cells.43 FAM5C is also detected in human serum. These findings suggested that FAM5C might be a muscle-derived humoral factor.
Interleukins
Numerous interleukins and chemokines are produced from muscles following exercise. IL-6 is abundantly expressed in muscle, and muscle cells produce and release IL-6 in response to exercise and muscle contraction.44 It is well-known that IL-6 affects bone metabolism, as well as glucose and energy metabolism. IL-6 stimulates bone resorption in concurrence with the soluble IL-6 receptor, and it might be related to the pathogenesis of postmenopausal osteoporosis.45 Mechanically loaded myotubes enhance osteoclast formation via IL-6 release in vitro.46 On the other hand, IL-6 enhances osteoblast differentiation at the early stage and IL-6-deficient mice exhibit low bone mass,44 suggesting that IL-6 may partly be a positive regulator of osteoblastic bone formation. However, controversy exists about the effects of IL-6 on osteoblast differentiation. Although IL-6 is expressed in various cells, including hematopoietic cells, stromal cells and osteoblasts, it may be related to bone metabolism by an enhanced release from muscle tissues in response to exercise and inflammation pathologically.
LIF is released from muscle tissue and regulates bone formation, although the controversy exists about the effects of LIF on osteoblast differentiation.47 Local effects may be greater on bone cells, compared with its effects as a myokine.
IL-7 influences both osteoblasts and osteoclasts.48 Although muscle-derived IL-7 might modulate bone metabolism, Aguila et al. reported that osteoblast-specific overexpression of IL-7 rescues osteopenia in IL-7-deficient female mice.48 These findings suggest that osteoblast-derived IL-7 is important for the regulation of bone mass.
IL-15 overexpression in muscle tissue, as well as systemic elevation of IL-15 levels, leads to an increase in bone mass, although it decreases visceral and body fat masses,49 suggesting that IL-15 may be a myokine, which affects both bone and fat metabolism.
CNTF is part of the IL-6 family of cytokines. CNTF-deficient female mice exhibit larger bone mass and activated osteoblast activity.50 Johnson et al.51 reported that CNTF, abundantly expressed in mouse muscle tissues, suppresses osteoblast differentiation in vitro. Therefore, CNTF may be a negative regulator of bone formation as a myokine. The existence of its soluble receptor, sCNTFR, is necessary for the full suppressive action of CNTF in osteoblast differentiation, similar to the action of IL-6, and the level of CNTFR is elevated as the differentiation of osteoblast progresses.50
A cytokine array of mouse myotube (C2C12)-conditioned medium identified MCP-1 (CCL-2) as one of the most abundant myokines. MCP-1 is a chemokine and a chemoattractant for osteoclast precursors and monocytes/macrophage. A recent study indicated that MCP-1 is involved in bone anabolic action by parathyroid hormone in mice.52 Although the significance of MCP-1 in its physiological state in bone is unknown, MCP-1 may have significant roles in altering bones, in the pathological and pharmacological states.
IGF-I and FGF-2
IGF-I, which is highly expressed in muscle, is a crucial growth factor for muscle growth, as well as bone development and the preservation of bone mass. The application of mechanical loading stimulates the expression of IGF-I in skeletal muscle. IGF-I enhances bone remodeling through the acceleration of osteoblastic bone formation and osteoclastic bone resorption.2,53 Several studies indicated that serum IGF-1 levels are positively associated with BMD and a predictive factor for osteoporotic fractures. Thus, the IGF-I pathway is crucial for the maintenance of bone metabolism. Although both circulating and bone-derived IGF-I proteins are physiologically necessary for bone metabolism, muscle-derived IGF-I may also have a role in the regulation of bone remodeling for a large volume of muscle in the body. IGF-binding proteins (IGFBPs) are related to the action of IGF-I. IGFBPs, such as IGFBP-2 and IGFBP-5, are produced in muscle tissues. There are conflicting reports about the effects of IGFBP-2 and IGFBP-5 on bone metabolism. Although IGFBP-2 exerts negative effects on IGF-I-mediated bone anabolic action dependently and independently of IGF-I, a human cohort study revealed that serum IGFBP-2 levels were the most robust negative predictors of muscle mass, when muscle mass was positively related to trabecular BMD.54 IGFBP-5 also supports bone anabolic action dependently and independently of IGF-I, although the roles of muscle-derived IGFBP-5 in bone remain unknown.
FGFs exert various cellular functions such as cell proliferation. FGF-2, an effective growth factor to enhance bone formation, fracture repair, bone/cartilage regeneration and chondrogenesis, is released from muscle tissues after injury and strong exercise.55 FGF-2 is abundantly produced in various tissues, including muscle and bone. Although muscle-derived FGF-2 might have some role in bone metabolism physiologically, bone-derived FGF-2 is more important as a paracrine factor.
Other Myokines
Osteonectin, a 43-KDa extracellular noncollagenous bone matrix glycoprotein, is involved in cell differentiation, vessel formation and growth factor binding. A previous study revealed that osteonectin levels were increased in plasma and muscle tissue of human and mice after exercise.56 Osteonectin is abundantly expressed in bone, and osteonectin-deficient mice exhibit severe osteopenia with decreased trabecular connectivity and bone mineral content, suggesting that osteonectin is a positive regulator of bone formation.57
MMP-2 is abundant in both muscle and bone. Its secretion from muscle cells is elevated with exercise and mechanical load,55 and MMP-2-deficient mice exhibit osteopenia and delayed fracture healing.58 However, the role of muscle-derived MMP-2 in bone metabolism remains unknown.
Mechanically loaded myotubes secrete soluble factors, which affect osteoclast formation.59 Although osteocytes have a central role during mechanical stress in bone during the linkage of bone to muscle, muscle produces unknown factors that preserve osteocyte viability in response to glucocorticoid treatment.60
Prospects
Muscle secretes various myokines, which regulate bone metabolism. The search for physiological and pathological roles of muscle-derived humoral factors is an interesting research topic. However, as numerous factors affect both muscle and bone in the same manner, and muscle mass change greatly affects bone metabolism, it will be difficult to distinguish myokine-specific effects from local paracrine action and other organ-derived effects scientifically. Identification of muscle-specific myokines may provide efficacious targets for diagnostic biomarkers and the development of therapeutic drug. Indeed, myostatin has been noted as a target for the treatment of muscle and bone disorders, especially sarcopenia and osteoporosis. Moreover, anti-myostatin signaling may provide a strategy for the treatment of inflammatory disease. In the near future, progress in myokine research, and the discovery of novel muscle-specific myokines, may increase the efficacy of treatments for skeletal disorders. To my knowledge, there is no evidence that demonstrates the direct contribution of myokines, discussed in this review, in bone discriminating muscle mass-mediated effects on bone, as those myokines affect both muscle and bone cells.
Footnotes
The author declares no conflict of interest.
References
- Cooper C, Dere W, Evans W, Kanis JA, Rizzoli R, Sayer AA et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int 2012; 23: 1839–1848. [DOI] [PubMed] [Google Scholar]
- Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care 2013; 16: 272–277. [DOI] [PubMed] [Google Scholar]
- Nakaoka D, Sugimoto T, Kaji H, Kanzawa M, Yano S, Yamauchi M et al. Determinants of bone mineral density and spinal fracture risk in postmenopausal Japanese women. Osteoporos Int 2001; 12: 548–554. [DOI] [PubMed] [Google Scholar]
- Kawao N, Kaji H. Interactions between muscle tissues and bone metabolism. J Cell Biochem 2015; 116: 687–695. [DOI] [PubMed] [Google Scholar]
- Armamento-Villareal R, Sadler C, Napoli N, Shah K, Chode S, Sinacore DR et al. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res 2012; 27: 1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause A, Speacht T, Govey P, Zhang Y, Steiner J, Jang C et al. Sarcopenia and increased body fat in sclerostin deficient mice. J Bone Miner Res 2014; 29(Suppl 1): S8–S9. [Google Scholar]
- Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol 2007; 103: 1093–1098. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 1997; 387: 83–90. [DOI] [PubMed] [Google Scholar]
- Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN et al. Induction of cachexia in mice by systemically administered myostatin. Science 2002; 296: 1486–1488. [DOI] [PubMed] [Google Scholar]
- Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab 2005; 288: E1110–E1119. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Samaddar T, Pennington C, McCormick J. Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res 2006; 21: 477–483. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Shi X, Zhang W, Pennington C, Thakore H, Haque M et al. Loss of myostatin (GDF-8) function increases osteogenic differentiation of bone marrow-derived stem cells but the osteogenic effect is ablated with unloading. Bone 2007; 40: 1544–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankbar B, Fennen M, Brunert D, Hayer S, Frank S, Wehmeyer C et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med 2015; 9: 1085–1090. [DOI] [PubMed] [Google Scholar]
- Zhang ZL, He JW, Qin YJ, Hu YQ, Li M, Zhang H et al. Association between myostatin gene polymorphisms and peak BMD varitation in Chinese nuclear families. Osteoporos Int 2008; 19: 39–47. [DOI] [PubMed] [Google Scholar]
- Karasik D, Cohen-Zinder M. The genetic pleiotropy of musculoskeletal aging. Front Physiol 2012; 3: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y, Brown M, Pearsall RS, Phillips C. The effects of activin receptor type IIB fusion protein (ActRIIB-Fc) on hindlimb skeletal muscles and femoral properties of osteogenesis imperfecta model (oim) mouse. J Bone Miner Res 2014; 29: S72–S73. [Google Scholar]
- Ruckle J, Jacobs M, Kramer W, Pearsall AE, Kumar R, Underwood KW et al. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J Bone Miner Res 2009; 24: 744–752. [DOI] [PubMed] [Google Scholar]
- Bonewald LF, Kiel DP, Clemens TL, Esser K, Orwoll ES, O'Keefe RJ et al. Forum on bone and skeletal muscle interactions: summary of the proceedings of an ASBMR workshop. J Bone Miner Res 2013; 28: 1857–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnounleut P, Bialek P, Liang LF, Upadhyay S, Fulzele S, Johnson M et al. A myostatin inhibitor (propeptide-Fc) increases muscle mass and muscle fiber size in aged mice but does not increase bone density or bone strength. Exp Gerontol 2013; 48: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum E, Starr H, Arounleut P, Immel D, Fulzele S, Wenger K et al. Myostatin (GDF-8) deficiency increases fracture callus size, Sox-5 expression, and callus bone volume. Bone 2009; 44: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori R, Sandri M. BMPs and the muscle-bone connection. Bone 2015; 80: 37–42. [DOI] [PubMed] [Google Scholar]
- Sugatani T, Alvarez UM, Hruska KA. Activin A stimulates IκB-α/NFκB and RANK expression for osteoclast differentiation, but not AKT survival pathway in osteoclast precursors. J Cell Biochem 2003; 90: 59–67. [DOI] [PubMed] [Google Scholar]
- Goh B, Singhal V, Herrera A, Clemens T, Lee SJ, DiGirolamo D. Activin receptor IIA (ACVR2A) functions directly in osteoblasts as a negative regulator of bone mass. J Bone Miner Res 2015; 30(Suppl 1): S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaden BC, Croy JE, Wang Y, Wilson JM, Datta-Mannan A, Shetler P et al. Follistatin: a novel therapeuitic for the improvement of muscle regeneration. J Pharmacol Exp Ther 2014; 349: 355–371. [DOI] [PubMed] [Google Scholar]
- Evans SF, Parent JB, Lasko CE, Zhen X, Knothe UR, Lemaire T et al. Periosteum, bone's "smart" bounding membrane, exhibits direction-dependent permeability. J Bone Miner Res 2013; 28: 608–617. [DOI] [PubMed] [Google Scholar]
- Fitts RH, Trappe SW, Costill DL, Gallagher PM, Creer AC, Colloton PA et al. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J Physiol 2010; 588: 3567–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwoll ES, Adler RA, Amin S, Binkley N, Lewiecki EM, Petak SM et al. Skeletal health in long-duration astronauts: nature, assessment, and management recommendations from the NASA Bone Summit. J Bone Miner Res 2013; 28: 1243–1255. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KH, Zaichenko L, Brinkoetter M, Thakkar B, Sahin-Efe A, Joung KE et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab 2013; 98: 4899–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianni G, Cuscito C, Mongelli T, Oranger A, Mori G, Brunetti G et al. Irisin enhances osteoblast differentiation in vitro. Int J Endocrinol 2014; 2014: 902186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao XY, Nie Y, Ma Y, Chen Y, Cheng R, Yin W et al. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci Rep 2016; 6: 18732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA 2015; 112: 12157–12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasilakis AD, Polyzos SA, Makras P, Gkiomisi A, Bisbinas I, Katsarou A et al. Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos Int 2014; 25: 1633–1642. [DOI] [PubMed] [Google Scholar]
- Loffler D, Muller U, Sheuermann K, Friebe D, Gesing J, Bielitz J et al. Serum irisin levels are regulated by acute strenous exercise. J Clin Endocrinol Metab 2015; 100: 1289–1299. [DOI] [PubMed] [Google Scholar]
- Albrecht E, Norheim F, Thiede B, Holen T, Ohashi T, Schering L et al. Irisin - a myth rather than an exercise-inducible myokine. Sci Rep 2015; 5: 8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM et al. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab 2015; 22: 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianni G, Grano M. Role of Irisin on the bone-muscle function unit. BoneKEy Rep 2015; 4: 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Kaplan FS. Insights from a rare genetic disorder of extra-skeletal bone formation, fibrodysplasia ossificans progressiva (FOP). Bone 2008; 43: 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MJ, Liu W, Sykes MC, Ward NE, Risin SA, Risin D et al. Identification of mechanosensitive genes in osteoblasts by comparative microarray studies using the rotating wall vessel and the random positioning machine. J Cell Biochem 2007; 101: 587–599. [DOI] [PubMed] [Google Scholar]
- Chan CY, Masui O, Krakovska O, Belozerov VE, Voisin S, Ghanny S et al. Indentification of differentially regulated secretome components during skeletal myogenesis. Mol Cell Proteomics 2011; 10: 004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Matsuomoto E, Higashimaki Y, Katagiri T, Sugimoto T, Seino S et al. Role of osteoglycin in the linkage between muscle and bone. J Biol Chem 2012; 287: 11616–11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Kanazawa I, Yamaguchi T, Yano S, Kaji H, Sugimoto T. Active vitamin D possesses beneficial effects on the interaction between muscle and bone. Biochem Biophys Res Commun 2014; 450: 482–487. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Matsumoto E, Higashimaki Y, Sugimoto T, Seino S, Kaji H. FAM5C is a soluble osteoblast differentiation factor linking muscle to bone. Biochem Biophys Res Commun 2012; 418: 134–139. [DOI] [PubMed] [Google Scholar]
- Hiscock N, Chan MH, Biscci T, Darby IA, Febbraio MA. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during constraction: evidence of fiber type specificity. FASEB J 2004; 18: 992–994. [DOI] [PubMed] [Google Scholar]
- Bakker AD, Jaspers RT. IL-6 and IGF-I signaling within and between muscle and bone: how important is the mTOR pathway for bone metabolism. Curr Osteoporos Rep 2015; 13: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juffer P, Jaspers RT, Klein-Nulend J, Bakker AD. Mechanically loaded myotubes affect osteoclast formation. Calcif Tissue Int 2014; 94: 319–326. [DOI] [PubMed] [Google Scholar]
- Walker EC, McGregor NE, Poulton IJ, Solano M, Pompolo S, Fernandes TJ et al. Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J Clin Invest 2010; 120: 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguila HL, Mun SH, Kalinowski J, Adams DJ, Lorenzo JA, Lee SK. Osteoblast-specific overexpression of human interleukin-7 rescues the bone mass phenotype of interleukin 7-deficient female mice. J Bone Miner Res 2012; 27: 1030–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn LS, Anderson BG, Strait-Bodey MM, Straud AM, Argiles JM. Ovesecretion of inteleukin-15 from skeletal muscle reduces adiposity. Am J Physiol Endocrinol Metab 2009; 296: E191–E202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor NE, Poulton IJ, Walker EC, Pompolo S, Quinn JM, Martin TJ et al. Ciliary neurotrophic factor inhibits bone formation and plays a sex-specific role in bone growth and remodeling. Calcif Tissue Int 2010; 86: 261–270. [DOI] [PubMed] [Google Scholar]
- Johnson RW, White JD, Walker EC, Martin TJ, Sims NA. Myokines (muscle-derived cytokines and chemokines) including ciliary neurotrophic factor (CNTF) inhibit osteoblast differentiation. Bone 2014; 64: 47–56. [DOI] [PubMed] [Google Scholar]
- Tamasji JA, Vasilov A, Shimizu E, Benton N, Johnston J, Bitel CL et al. Monocyte chemoattractant protein-1 is a mediator of the anabolic action of parathyroid hormone on bone. J Bone Miner Res 2013; 28: 1975–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Tahimic C, Chang W, Wang Y, Philippou A, Barton ER. Role of IGF-I signaling in muscle bone interactions. Bone 2015; 80: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrasseur NK, Achenbach SJ, Melton LJ 3rd, Amin S, Khosla S. Skeletal muscle mass is associated with bone geometry and microstructure and serum insulin-like growth factor binding protein-2 levels in adult women and men. J Bone Miner Res 2012; 27: 2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick MW. The skeletal muscle secretome: an emerging player in muscle-bone cross talk. BoneKEy Rep 2012; 1: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 2013; 62: 882–889. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Robey PG. The composition of bone. In: Rosen CJ (ed.)Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism Wiley-Blackwell2013; pp 49–58. [Google Scholar]
- Lieu S, Hansen E, Dedini R, Behonick D, Werb Z, Miclau T et al. Impaired remodeling phase of fracture repair in the absence of matrix metalloproteinase-2. Dis Model Mech 2011; 4: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juffer P, Jaspers RT, Lips P, Bakker AD, Klein-Nulend J. Expression of muscle anabolic and metabolic factors in mechanically loaded MLO-Y4 osteocytes. Am J Physiol Endocrinol Metab 2012; 302: E385–E395. [DOI] [PubMed] [Google Scholar]
- Jähn K, Lara-Castillo N, Brotto L, Mo CL, Johnson ML, Brotto M et al. Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of β-catenin. Eur Cell Mater 2012; 24: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]


