Abstract
The yeast fatty acid synthase (M(r) = 2.5 x 10(6)) is organized in an alpha 6 beta 6 complex. In these studies, the synthase structure has been examined by negative-stain and cryo-electron microscopy. Side and end views of the structure indicate that the molecule, shaped similar to a prolate ellipsoid, has a high-density band of protein bisecting its major axis. Stained and frozen-hydrated average images of the end views show an excellent concordance and a hexagonal ring having three each alternating egg- and kidney-shaped features with low-protein-density protrusions extending outward from the egg-shaped features. Images also show that the barrel-like structure is not hollow but has a Y-shaped central core, which appears to make contact with the three egg-shaped features. Numerous side views of the structure give good evidence that the beta subunits have an archlike shape. We propose a model for the synthase that has point-group symmetry 32 and six equivalent sites of fatty acid synthesis. The protomeric unit is alpha 2 beta 2. The ends of each of the two archlike beta subunits interact with opposite sides of the two dichotomously arranged disclike alpha subunits. Three such protomeric units form the ring. We propose that the six fatty acid synthesizing centers are composed of two complementary half-alpha subunits and a beta subunit, an arrangement having all the partial activities of the multifunctional enzyme required for fatty acid synthesis.
Full text
PDF
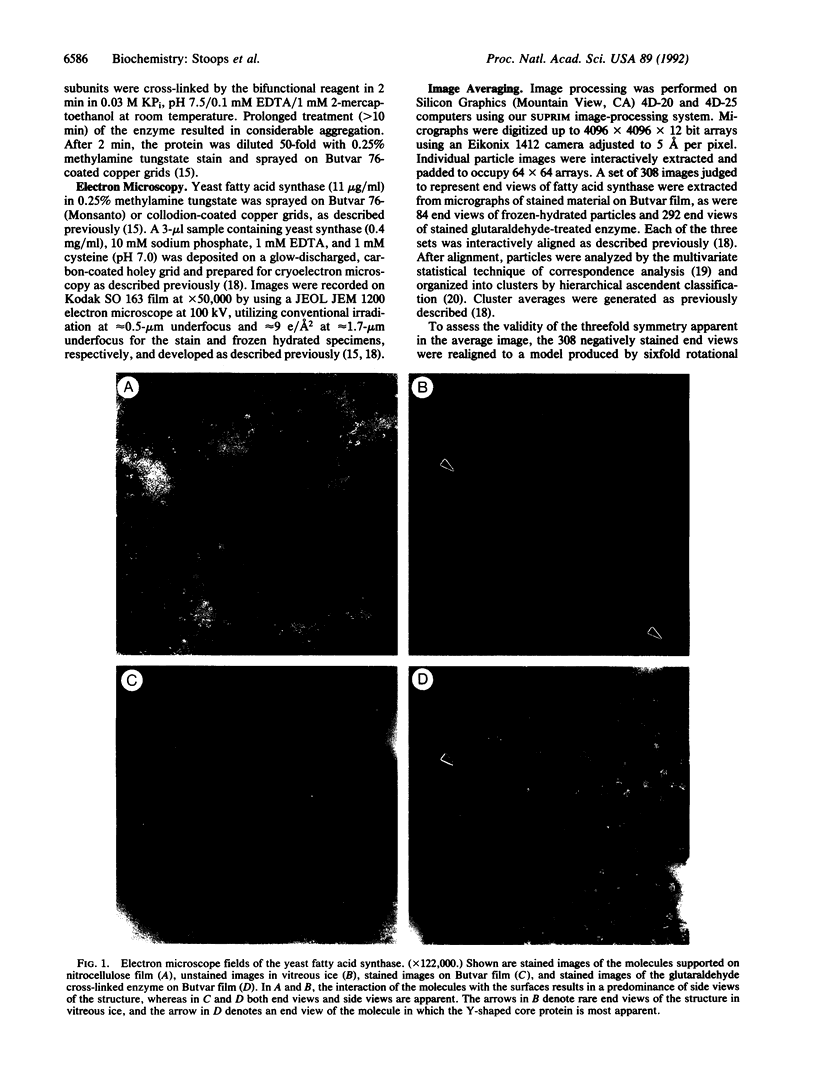


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretaudiere J. P., Tapon-Bretaudiere J., Stoops J. K. Structure of native alpha 2-macroglobulin and its transformation to the protease bound form. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1437–1441. doi: 10.1073/pnas.85.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirala S. S., Kuziora M. A., Spector D. M., Wakil S. J. Complementation of mutations and nucleotide sequence of FAS1 gene encoding beta subunit of yeast fatty acid synthase. J Biol Chem. 1987 Mar 25;262(9):4231–4240. [PubMed] [Google Scholar]
- Dubochet J., Adrian M., Chang J. J., Homo J. C., Lepault J., McDowall A. W., Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988 May;21(2):129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- Hackenjos W. A., Schramm H. J. Electron microscopical structure analysis of yeast fatty-acid synthase at low resolution. Biol Chem Hoppe Seyler. 1987 Jan;368(1):19–36. doi: 10.1515/bchm3.1987.368.1.19. [DOI] [PubMed] [Google Scholar]
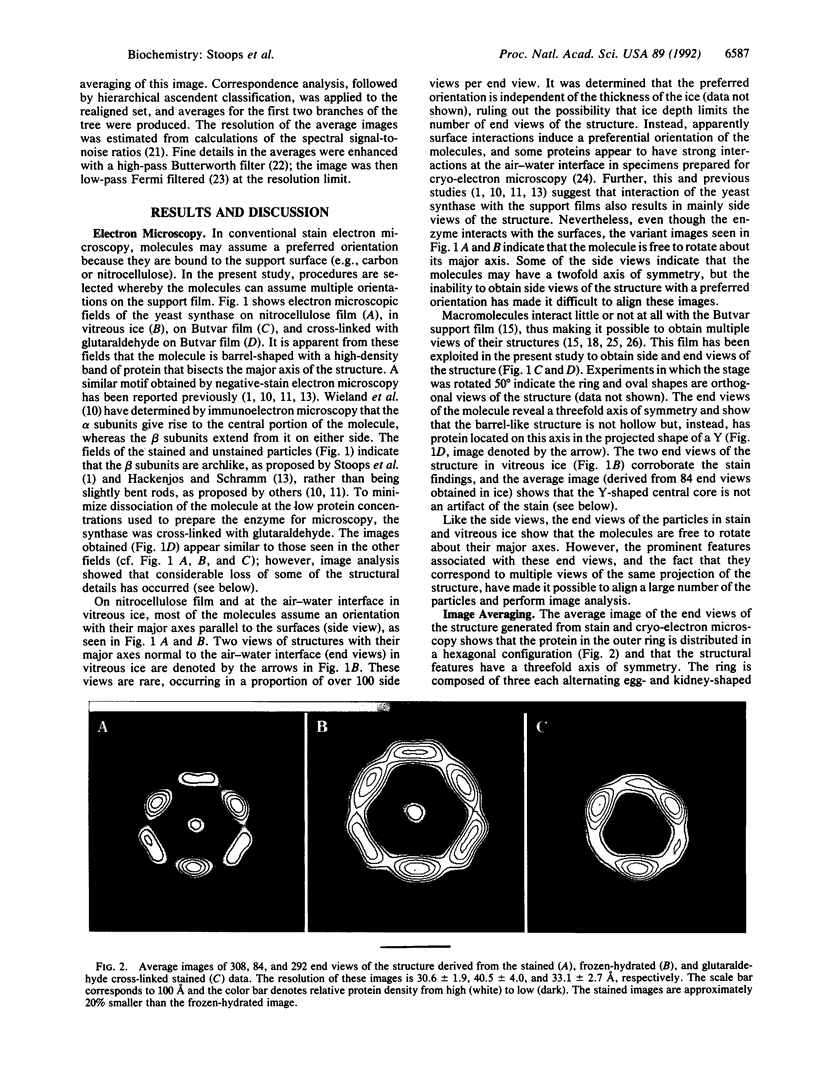
- Lynen F. On the structure of fatty acid synthetase of yeast. Eur J Biochem. 1980 Dec;112(3):431–442. doi: 10.1111/j.1432-1033.1980.tb06105.x. [DOI] [PubMed] [Google Scholar]
- Mohamed A. H., Chirala S. S., Mody N. H., Huang W. Y., Wakil S. J. Primary structure of the multifunctional alpha subunit protein of yeast fatty acid synthase derived from FAS2 gene sequence. J Biol Chem. 1988 Sep 5;263(25):12315–12325. [PubMed] [Google Scholar]
- Oesterhelt D., Bauer H., Kresze G. B., Steber L., Lynen F. Reaction of yeast fatty acid synthetase with iodoacetamide. 1. Kinetics of inactivation and extent of carboxamidomethylation. Eur J Biochem. 1977 Sep 15;79(1):173–180. doi: 10.1111/j.1432-1033.1977.tb11795.x. [DOI] [PubMed] [Google Scholar]
- Olson N. H., Baker T. S. Magnification calibration and the determination of spherical virus diameters using cryo-microscopy. Ultramicroscopy. 1989 Jul-Aug;30(3):281–297. doi: 10.1016/0304-3991(89)90057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz I., Herbst M., Kratky O., Oesterhelt D., Lynen F. Röntgenkleinwinkel-Untersuchungen an der Fettsäuresynthetase aus Hefe. Eur J Biochem. 1970 Mar 1;13(1):55–64. doi: 10.1111/j.1432-1033.1970.tb00898.x. [DOI] [PubMed] [Google Scholar]
- Singh N., Wakil S. J., Stoops J. K. Yeast fatty acid synthase: structure to function relationship. Biochemistry. 1985 Nov 5;24(23):6598–6602. doi: 10.1021/bi00344a044. [DOI] [PubMed] [Google Scholar]
- Stoops J. K., Arslanian M. J., Aune K. C., Wakil S. J. Further evidence for the multifunctional enzyme characteristic of the fatty acid synthetases of animal tissues. Arch Biochem Biophys. 1978 Jun;188(2):348–359. doi: 10.1016/s0003-9861(78)80019-8. [DOI] [PubMed] [Google Scholar]
- Stoops J. K., Arslanian M. J., Oh Y. H., Aune K. C., Vanaman T. C., Wakil S. J. Presence of two polypeptide chains comprising fatty acid synthetase. Proc Natl Acad Sci U S A. 1975 May;72(5):1940–1944. doi: 10.1073/pnas.72.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops J. K., Awad E. S., Arslanian M. J., Gunsberg S., Wakil S. J., Oliver R. M. Studies on the yeast fatty acid synthetase. Subunit composition and structural organization of a large multifunctional enzyme complex. J Biol Chem. 1978 Jun 25;253(12):4464–4475. [PubMed] [Google Scholar]
- Stoops J. K., Bretaudiere J. P., Strickland D. K. Electron microscope studies of human alpha 2-macroglobulin-chymotrypsin complex: demonstration that the two structures assigned to native and proteolyzed alpha 2-macroglobulin represent two views of the proteolyzed molecule. Biochem Biophys Res Commun. 1989 May 30;161(1):216–220. doi: 10.1016/0006-291x(89)91583-0. [DOI] [PubMed] [Google Scholar]
- Stoops J. K., Momany C., Ernst S. R., Oliver R. M., Schroeter J. P., Bretaudiere J. P., Hackert M. L. Comparisons of the low-resolution structures of ornithine decarboxylase by electron microscopy and X-ray crystallography: the utility of methylamine tungstate stain and Butvar support film in the study of macromolecules by transmission electron microscopy. J Electron Microsc Tech. 1991 Jun;18(2):157–166. doi: 10.1002/jemt.1060180210. [DOI] [PubMed] [Google Scholar]
- Stoops J. K., Schroeter J. P., Bretaudiere J. P., Olson N. H., Baker T. S., Strickland D. K. Structural studies of human alpha 2-macroglobulin: concordance between projected views obtained by negative-stain and cryoelectron microscopy. J Struct Biol. 1991 Apr;106(2):172–178. doi: 10.1016/1047-8477(91)90086-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops J. K., Singh N., Wakil S. J. The yeast fatty acid synthase. Pathway for transfer of the acetyl group from coenzyme A to the Cys-SH of the condensation site. J Biol Chem. 1990 Oct 5;265(28):16971–16977. [PubMed] [Google Scholar]
- Stoops J. K., Wakil S. J. The yeast fatty acid synthetase. Structure-function relationship and the role of the active cysteine-SH and pantetheine-SH. J Biol Chem. 1981 Aug 25;256(16):8364–8370. [PubMed] [Google Scholar]
- Unser M., Trus B. L., Steven A. C. A new resolution criterion based on spectral signal-to-noise ratios. Ultramicroscopy. 1987;23(1):39–51. doi: 10.1016/0304-3991(87)90225-7. [DOI] [PubMed] [Google Scholar]
- Wieland F., Siess E. A., Renner L., Verfürth C., Lynen F. Distribution of yeast fatty acid synthetase subunits: three-dimensional model of the enzyme. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5792–5796. doi: 10.1073/pnas.75.12.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heel M., Frank J. Use of multivariate statistics in analysing the images of biological macromolecules. Ultramicroscopy. 1981;6(2):187–194. doi: 10.1016/0304-3991(81)90059-0. [DOI] [PubMed] [Google Scholar]