Abstract
Background
Prostaglandin (PG) D2 is an early-phase mediator in inflammation, but its action and the roles of the 2 D-type prostanoid receptors (DPs) DP1 and DP2 (also called chemoattractant receptor–homologous molecule expressed on TH2 cells) in regulating macrophages have not been elucidated to date.
Objective
We investigated the role of PGD2 receptors on primary human macrophages, as well as primary murine lung macrophages, and their ability to influence neutrophil action in vitro and in vivo.
Methods
In vitro studies, including migration, Ca2+ flux, and cytokine secretion, were conducted with primary human monocyte-derived macrophages and neutrophils and freshly isolated murine alveolar and pulmonary interstitial macrophages. In vivo pulmonary inflammation was assessed in male BALB/c mice.
Results
Activation of DP1, DP2, or both receptors on human macrophages induced strong intracellular Ca2+ flux, cytokine release, and migration of macrophages. In a murine model of LPS-induced pulmonary inflammation, activation of each PGD2 receptor resulted in aggravated airway neutrophilia, tissue myeloperoxidase activity, cytokine contents, and decreased lung compliance. Selective depletion of alveolar macrophages abolished the PGD2-enhanced inflammatory response. Activation of PGD2 receptors on human macrophages enhanced the migratory capacity and prolonged the survival of neutrophils in vitro. In human lung tissue specimens both DP1 and DP2 receptors were located on alveolar macrophages along with hematopoietic PGD synthase, the rate-limiting enzyme of PGD2 synthesis.
Conclusion
For the first time, our results show that PGD2 markedly augments disease activity through its ability to enhance the proinflammatory actions of macrophages and subsequent neutrophil activation.
Keywords: D-type prostanoid receptor 1, D-type prostanoid receptor 2/chemoattractant receptor–homologous molecule expressed on TH2 cells, prostaglandin D2, hematopoietic prostaglandin D synthase, macrophages, pulmonary inflammation, neutrophils
Prostaglandin (PG) D2, a lipid mediator from the arachidonic acid/COX pathway, has been shown to play complex and often opposing roles in the development and resolution of inflammation, which can be attributed to differential activation of its receptors. PGD2 activates 2 G protein–coupled receptors, the D-type prostanoid receptors (DPs) DP1 and DP2, with the latter also known as chemoattractant receptor–homologous molecule expressed on TH2 cells.1 At higher concentrations, PGD2 can also signal through the thromboxane A2 receptor.2,3 Although PGD2 exerts similar binding affinities toward the DP1 and DP2 receptors, its metabolites, formed rapidly by enzymatic and nonenzymatic pathways, can differentially induce DP2-mediated effects.1
In patients with allergic diseases, the role of PGD2 has mostly been associated with its release from activated mast cells and induction of vasodilation. More recently, however, PGD2 has been found to promote additional proinflammatory responses through activation of DP2 receptors reflected by increased eosinophilic infiltration into the lungs and skin of mice.4,5 Consequently, DP2 antagonists were shown to ameliorate eosinophilic pulmonary inflammation in murine ovalbumin-induced6 and house dust mite–induced7 models, a rat Alternaria species–induced model,8 and the setting of chronic allergic skin inflammation.9 Moreover, the DP2 antagonist CAY10471 ameliorated weight loss and intestinal inflammation in a dextran sodium sulfate–induced colitis model in mice.10 In asthmatic patients induction of hematopoietic prostaglandin D synthase (HPGDS), the rate-limiting enzyme of PGD2 synthesis, was observed in the epithelial compartment,11 and PGD2 levels in bronchoalveolar lavage (BAL) fluid correlated positively with the severity of the disease.12 DP2/chemoattractant receptor–homologous molecule expressed on TH2 cell antagonists was found to have some effects in allergic rhinitis,13 allergic conjunctivitis,14 eosinophilic esophagitis,15 and bronchial asthma.16,17
Macrophages are essential in pulmonary inflammatory diseases by maintaining tissue homeostasis and mounting rapid responses to exogenous and endogenous stimuli. Because they are the main source of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, their role in inflammation is pivotal.18 Acute lung injury (ALI) or its more severe clinical manifestation, acute respiratory distress syndrome (ARDS), is a pulmonary inflammatory disease that can lead to respiratory failure. Pulmonary complications in this disease are mainly attributed to rapid neutrophil infiltration into the alveolar space,19 subsequent edema formation, and dysfunction of the involved cell types, including epithelial cells,20 endothelial cells,21 and macrophages.22–24 Several studies have made it clear that macrophages orchestrate neutrophilic infiltration and thus strongly modulate the outcome of ARDS.23–27
Stimulated by the dominant physiologic role of PGD2 in the lung, we hypothesized that PGD2 might govern disease activity and progression by acting on lung macrophages. Previous studies revealed anti-inflammatory effects of the PGD2 metabolite 15d-PGJ2 acting through peroxisome proliferator–activated receptor γ in RAW 264.7 macrophages28 and demonstrated the expression of PGD2 receptors on human monocytes,29 whereas the role of PGD2 in regulation of macrophage function has not been assessed yet.
Methods
Detailed description of ethical permits, materials, and procedures is provided in the Methods section in this article’s Online Repository at www.jacionline.org.
Isolation of peripheral blood polymorphonuclear neutrophils and PBMCs
Human peripheral blood polymorphonuclear cells and PBMCs were isolated from healthy donors independent of sex and age, as described previously.30
Differentiation from monocytes to macrophages
Human peripheral blood monocytes isolated from healthy donors were differentiated to human monocyte-derived macrophages (MDMs) for 7 to 10 days with 20 ng/mL human recombinant macrophage colony-stimulating factor.
Live cell fluorescent Ca2+ imaging
Macrophages were loaded with 2 µmol/L Fura-2/AM. Fluorescence images were obtained with alternate excitation at 340 and 380 nm, and emitted light was collected at 510 nm. Intracellular calcium levels were calculated, as previously described.31
Monocyte Ca2+ flux
Ca2+ flux was measured by means of flow cytometry, as previously described.30
Neutrophil apoptosis
Neutrophil survival was assessed by using Annexin V/propidium iodide staining, as described previously.32
Neutrophil and macrophage chemotaxis
Neutrophils were placed in the upper compartment of a Transwell chamber (Corning, Inc, New York, NY) in the absence or presence of MDMs. After 1 hour, neutrophils that migrated to the bottom well were collected, suspended in 150 µL of fixative solution, and enumerated by means of flow cytometric analysis.33 Migration of human MDMs was assessed by using Transwell inserts, as described previously.30
Flow cytometric staining
The following antibodies and concentrations were used: DP2 (20 µg/mL), DP1 (20 µg/mL), anti-mouse MHC class II (2.5 µg/mL), anti-mouse Siglec-F (5 µg/mL), anti-mouse CD3ε (5 µg/mL), anti-mouse B220 (2 µg/mL), and anti-mouse CD11c (2 µg/mL) antibodies.
LPS-induced lung injury
Pulmonary inflammation was induced in 8- to 10-week-old BALB/c mice by means of intranasal application of 1 mg/kg LPS. Agonists or antagonists were applied 24 hours before LPS application subcutaneously every 12 hours. Unless stated otherwise, mice were killed 4 hours after LPS administration.
Myeloperoxidase assay
Myeloperoxidase (MPO) activity was determined, as described previously.34
BAL protein content
BAL protein concentrations were measured by using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Rockford, Ill), according to the manufacturer’s protocol.
Vascular permeability in lung tissue was assessed by using Evans blue dye extravasation after 60 minutes of circulation. Evans blue protein leak was determined, as described in the Methods section in this article’s Online Repository.
Murine lung histology
Paraffin-embedded murine lungs were cut (5-µm sections), deparaffinized, and immunostained with anti-Ly6G and hematoxylin.
Isolation of murine alveolar and interstitial macrophages
Alveolar and interstitial macrophages were isolated from BAL fluid, as described in the Methods section in this article’s Online Repository.
Depletion of murine lung macrophages
Three hundred micrograms (in 60 µL volume) of clodronate or control liposomes were intranasally applied to mice 24 hours before LPS challenge.35
Cytokine measurements
Cytokine levels were determined by using either a multianalyte immunoassay (Bender Medsystems, Vienna, Austria), ELISA (PeproTech, Rocky Hill, NJ), or the ProcartaPlex Mouse Cytokine Kit (eBioscience, San Diego, Calif).
Immunohistochemistry of human lung tissue
Human paraffin-embedded lung samples were stained with anti-DP2 (1:200), anti-DP1 (1:100), or anti-HPGDS (1:200) antibodies.
Lipid mediator analysis
PGD2, PGE2, TXB2, 12S-hydroxy-5Z,8E,10E-heptoadectrienoic acid, and 6-keto-PGF1α were analyzed by using liquid chromatography–tandem mass spectrometry, as previously published36 and as described in the Methods section in this article’s Online Repository.
Measurement of murine lung function
Decreased lung compliance caused by pulmonary edema and atelectasis is a hallmark of human ALI/ARDS and a preferred readout in mouse models.37 Therefore mouse lung function was determined by using the flexiVent system (SCIREQ, Montreal, Quebec, Canada), as described previously.38
Statistical analysis
Data were analyzed by using either the Student t test, 1-way ANOVA, or 2-way ANOVA, followed by the Bonferroni or Dunnett posttest, with GraphPad Prism software (version 5.04; GraphPad Software, La Jolla, Calif). In vitro experiments were performed in duplicates, with n numbers indicating biological replicates with cells from different donors. P values of less than .05 were considered significant.
Results
DP1 and DP2 are expressed on human macrophages and induce Ca2+ signaling
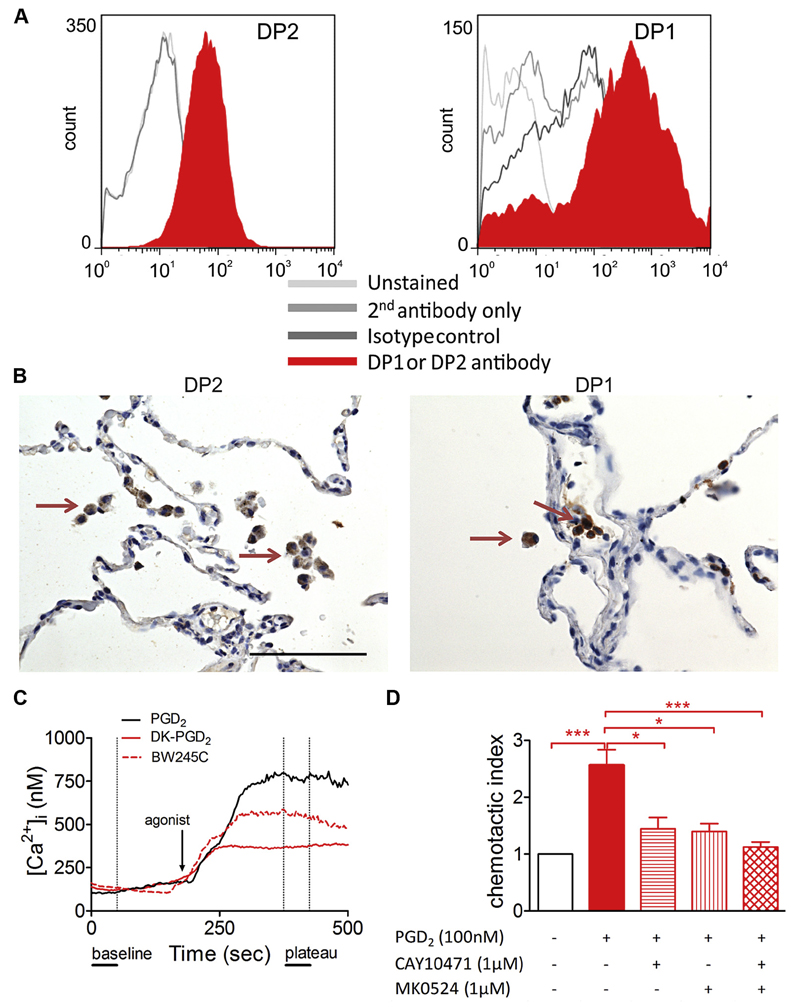
We first set out to elucidate whether PGD2 receptors are expressed on human monocytes and macrophages and found that both cell types express DP1 and DP2 on their cell surface (Fig 1, A, and see Fig E1 in this article’s Online Repository at www.jacionline.org). Interestingly, expression of both receptors increased during differentiation of MDMs, with DP2 expression being more abundant than DP1 expression in both monocytes and macrophages (see Fig E1). Next, we probed for DP1 and DP2 expression in human lung tissue with different pathologies (see Table E1 in this article’s Online Repository at www.jacionline.org). Both were highly and consistently expressed on human alveolar macrophages (Fig 1, B) because 60% to 75% of alveolar macrophages were positively stained for both DP1 and DP2 independent of the underlying disease (see Figs E2 and E3 in this article’s Online Repository at www.jacionline.org). This expression pattern suggested a possible role for the PGD2-DP1-DP2 axis in the modulation of macrophage biology in patients with pulmonary diseases.
Fig 1.
PGD2 receptors DP1 and DP2 are expressed on macrophages and induce Ca2+ flux and migration. A, Flow cytometric histograms of DP2 and DP1 staining (filled histograms) on MDMs, respectively. B, Immunohistochemistry of healthy human lung tissue showing DP2- and DP1-positive alveolar macrophages (arrows). C, Representative Ca2+ responses of MDMs over time. D, MDM migration toward PGD2 is blocked by DP1- and DP2-specific antagonists (n = 4-5). *P < .05 and ***P < .001.
Then we sought to determine the biological activities of DP1 and DP2 in vitro. Ca2+ imaging revealed that superfusion of human MDMs with 1 µmol/L PGD2 led to a strong release of intracellular calcium (Fig 1, C, and see Fig E4, A, in this article’s Online Repository at www.jacionline.org), whereas human monocytes showed no Ca2+ response to PGD2 stimulation (see Fig E5 in this article’s Online Repository at www.jacionline.org). Both DP2- and DP1-selective agonists (13,14-dihydro-15-keto PGD2, and BW245C, respectively; 1 µmol/L) were likewise able to elicit Ca2+ flux, although to a lesser extent than PGD2 (Fig 1, C, and see Fig E4, A). In agreement with this observation, blockade of either DP2 (CAY10471, 10 µmol/L) or DP1 (MK0524, 10 µmol/L) slightly decreased, whereas simultaneous antagonism of both receptors abolished the PGD2-induced Ca2+ signal (see Fig E4, B and C). Ca2+ release induced by both PGD2 and 13,14-dihydro-15-keto prostaglandin D2 (DK-PGD2) was prevented by overnight incubation with pertussis toxin (100 ng/mL), indicating involvement of Gαi heterotrimers in this process (see Fig E4, D and E).
PGD2 induces migration of macrophages through DP1 and DP2 and enhances TNF-α secretion
We next evaluated the chemotactic potential of PGD2 toward MDMs by using Transwell inserts, followed by enumeration of fluorescently labeled cell nuclei of the migrated cells on the lower surface of the filter (see Fig E6, B). We found that PGD2 exerted a concentration-dependent chemotactic activity toward MDMs, with the highest responses observed at 100 nmol/L (Fig 1, D, and see Fig E6, A, in this article’s Online Repository at www.jacionline.org). This could be partially blocked by either the DP2- or DP1-specific antagonist (CAY10471 or MK0524, 1 µmol/L, respectively; Fig 1, D). Inhibition of both receptors completely abolished the migratory activity of MDMs toward PGD2 (Fig 1, D). Because PGD2 was shown to be critical in mediating macrophage migration toward LPS in a murine model,39 we hypothesized that PGD2 could alter the LPS-induced cytokine secretion from MDMs. Although almost no TNF-α was detectable in the supernatants of vehicle-treated cells, LPS stimulation (100 ng/mL) induced a strong release of the proinflammatory cytokine. Indeed, this response was markedly enhanced in cells pretreated with PGD2 or DK-PGD2 (see Fig E6, C). Although the DP1-specific agonist BW245C was not able to induce changes in TNF-α secretion, involvement of both DP1 and DP2 receptors in the PGD2 response is still likely because only antagonism of both receptors was able to completely block the PGD2-mediated increase in TNF-α secretion.
Endotoxin-induced lung injury is aggravated by PGD2
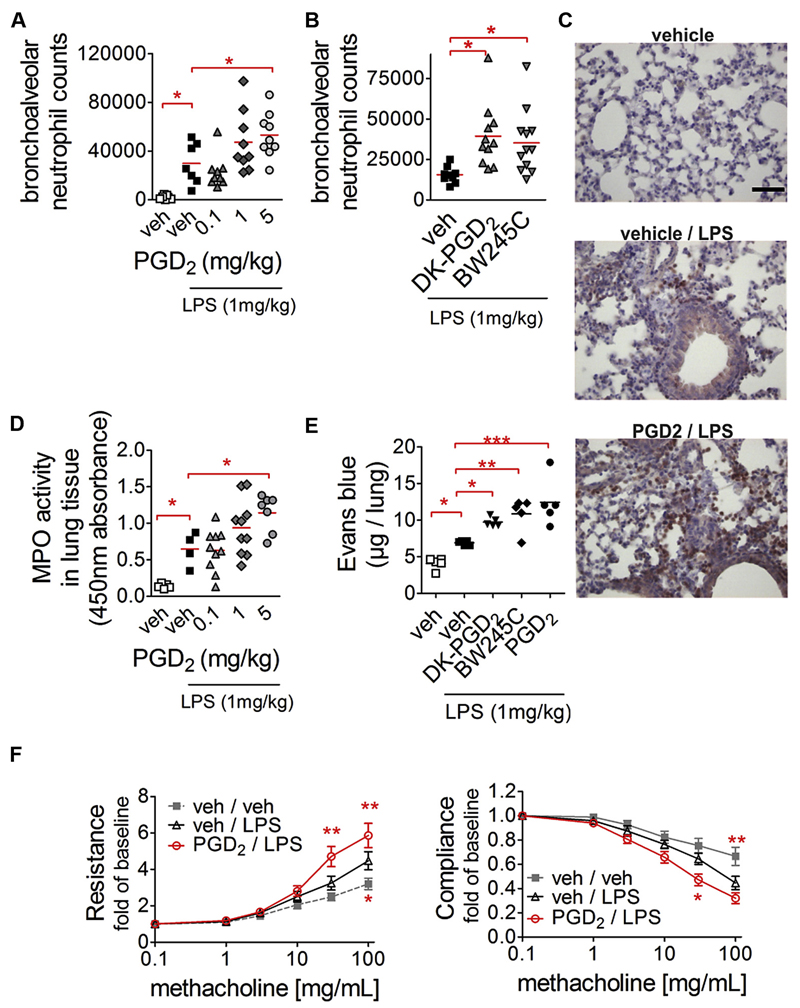
Prompted by these observations, we investigated the effect of PGD2 in an in vivo model of pulmonary inflammation. Here neutrophil infiltration is a main pathogenic feature that essentially depends on macrophage function.23 LPS significantly increased neutrophil influx into the bronchoalveolar space after 4 hours of treatment. Of particular interest, this effect was almost doubled when PGD2 (5 mg/kg) was administered subcutaneously before LPS challenge (Fig 2, A). Selective DP1 and DP2 agonists induced an even more pronounced response; in detail, the selective DP2 agonist DK-PGD2 (5 mg/kg) enhanced neutrophil influx by 2.5-fold and the DP1 agonist BW245C (5 mg/kg) enhanced neutrophil influx by 2.3-fold (Fig 2, B). Histologically, the inflammatory state of the lungs was indicated by dense Ly6G-positive neutrophil infiltrates in the bronchiolar region, with disturbed alveolar morphology in LPS-treated animals. When PGD2 was administered before LPS treatment, even more Ly6G-positive neutrophils were found around the bronchioli accompanied by neutrophil infiltrates in the alveolar space, which was largely absent in vehicle/LPS-treated mice (Fig 2, C, and see Fig E7, A, in this article’s Online Repository at www.jacionline.org). MPO activity, a marker for lung inflammation, was likewise increased depending on the applied dose of PGD2 (Fig 2, D). These observations were reflected by increased protein content in the BAL fluid in PGD2-treated animals (see Fig E7, B), as well as enhanced Evans blue extravasation in the lung tissue that depended on both DP1 and DP2 (Fig 2, E). It is noteworthy that the capacity of PGD2 to increase neutrophil recruitment was sustained after 24 hours (see Fig E7, C). Only minor alterations were found in the number of other cell types, such as monocytes, macrophages, lymphocytes, and eosinophils (see Fig E8 in this article’s Online Repository at www.jacionline.org). The observation that PGD2 aggravated pulmonary inflammation was also reflected by a decrease in lung function. In these experiments we found that LPS alone caused hyperresponsiveness to methacholine with respect to airway resistance and compliance (Fig 2, F), which was further accentuated after combined treatment of mice with PGD2 and LPS (Fig 2, F). In all experimental readouts, including cell infiltration into the alveolar space, MPO activity, and lung function, PGD2 treatment in the absence of LPS had no effect (data not shown).
Fig 2.
PGD2 acting through DP1 and DP2 promotes neutrophil influx into lungs and aggravates airway hyperreactivity. A and B, Neutrophil infiltration is increased in animals pretreated with PGD2 (Fig 2, A) or DP1- and DP2-selective agonists (Fig 2, B). C-F, PGD2 pretreatment increases Ly6G-positive neutrophil infiltration in the peribronchial and alveolar space (representative pictures, scale bar = 50µm; Fig 2, C), MPO activity (Fig 2, D), Evans blue dye extravasation (Fig 2, E), and airway hyperreactivity (vs vehicle/LPS; Fig 2, F). *P < .05, **P < .01, and ***P < .001.
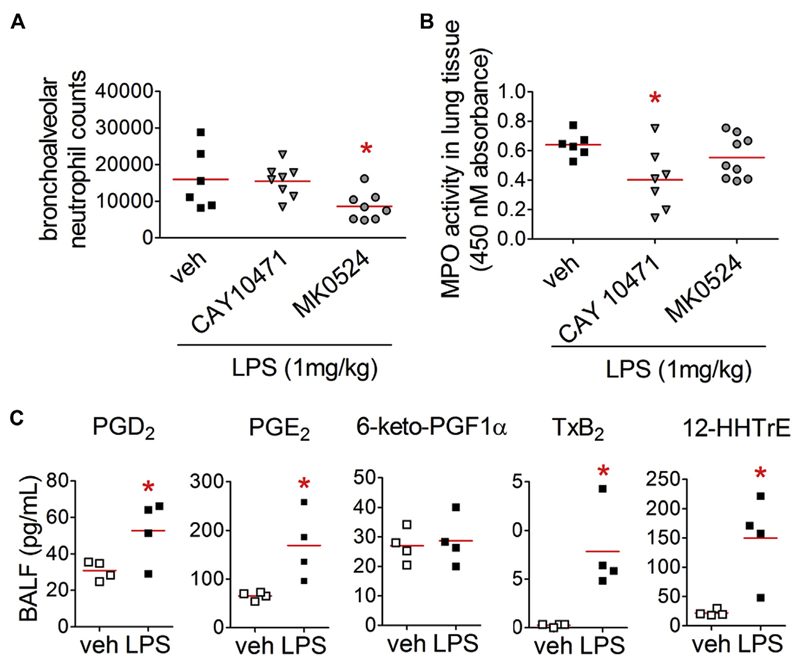
Blocking of endogenous PGD2 reduces neutrophilic infiltration into the lungs
Mice were pretreated with the PGD2 receptor antagonists MK0524 or CAY10471 (5 mg/kg) followed by LPS challenge to delineate the pathogenic role of endogenous PGD2. Analysis of BAL cells revealed that the DP1 antagonist MK0524 reduced endotoxin-induced neutrophilia (Fig 3, A), whereas the DP2 antagonist CAY10471 reduced MPO activity (Fig 3, B). Next, we compared lipid mediator levels in the BAL fluid of vehicle- and LPS-treated animals and found that there was a marked increase in PGD2 levels in the latter (Fig 3, C). In agreement with previous findings, PGE2 levels also increased, as did TXB2 and 12S-hydroxy-5Z,8E,10E-heptoadectrienoic acid levels. We could not detect any significant changes in PGI2 levels (estimated through its metabolite 6-keto-PGF1α).
Fig 3.
Blocking of endogenous PGD2 ameliorates LPS-induced neutrophil influx into the alveolar space and pulmonary tissue. A and B, DP1 antagonist reduced neutrophil counts in the bronchoalveolar space (Fig 3, A), whereas DP2 antagonist reduced MPO activity (Fig 3, B; n = 6-9). *P < .05 versus vehicle. C, Lipid mediators in BAL fluid 4 hours after vehicle or LPS treatment were quantified by using liquid chromatography– tandem mass spectrometry (n = 4). HHTrE, 12S-hydroxy-5Z,8E,10E-heptoadectrienoic acid. *P < .05 versus vehicle.
PGD2 treatment enhances proinflammatory cytokine release in vivo and in vitro
In addition to prostanoids, increased keratinocyte-derived chemokine (KC) and monocyte chemotactic protein 1 levels were observed in the cell-free BAL fluid of LPS-treated mice. Importantly, the increase in levels of these chemokines was significantly stronger in PGD2-treated animals (see Fig E9, A, in this article’s Online Repository at www.jacionline.org). To determine whether macrophages were responsible for the increased release of these chemokines, we isolated alveolar and interstitial macrophages and mimicked the in vivo model. Both alveolar and interstitial pulmonary macrophages increased secretion of the neutrophil chemoattractant KC when pretreated with PGD2 (see Fig E9, B and C), implicating macrophages as the cell type responsible for the enhanced neutrophil recruitment in vivo. Interestingly, monocyte chemotactic protein 1 secretion by alveolar and interstitial macrophages was influenced by neither LPS nor PGD2 treatment (see Fig E9, B and C).
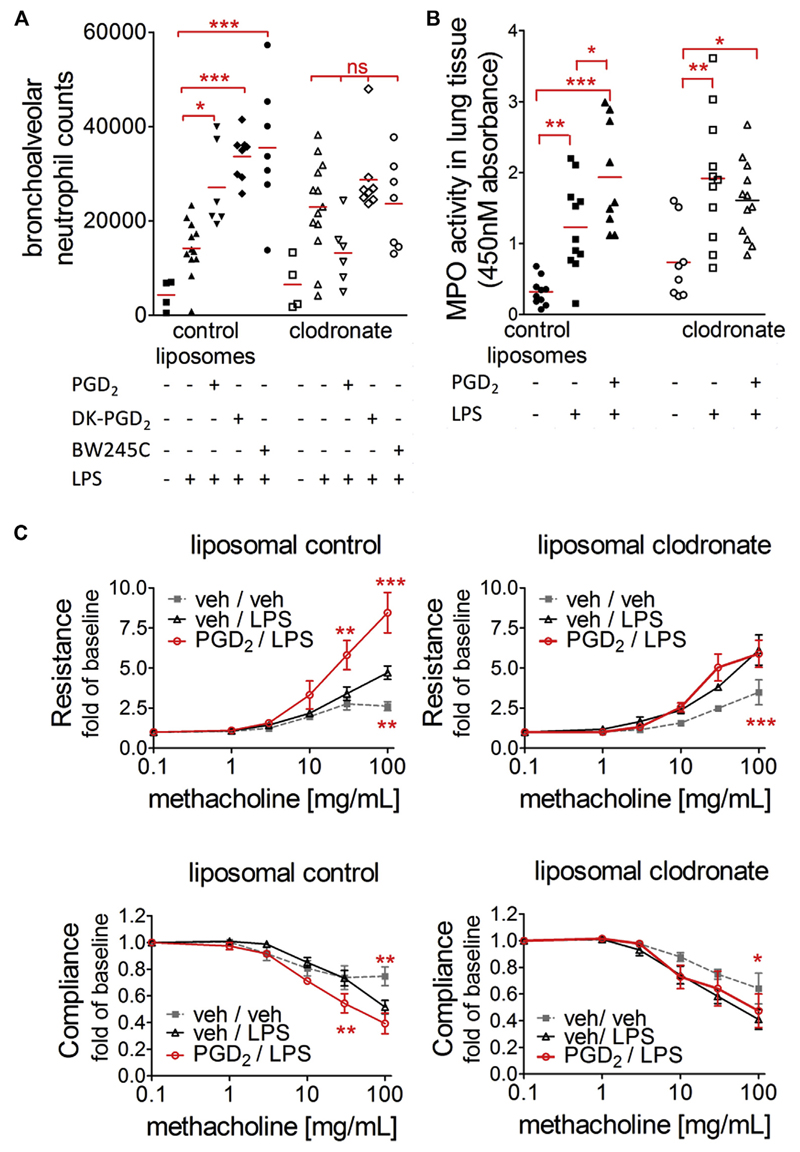
Macrophage depletion prevents the increased inflammatory response mediated by PGD2
To highlight the role of macrophages in the PGD2-induced enhancement of pulmonary inflammation, we next selectively reduced alveolar macrophage counts in the pulmonary LPS model. Intranasal application of 300 µg of clodronate led to a marked reduction of macrophage counts by approximately 70% throughout the groups (see Fig E10 in this article’s Online Repository at www.jacionline.org). This dose was chosen to avoid proinflammatory responses caused by clodronate and/or liposomes themselves, which were observed at higher doses (data not shown). In animals that had received control liposomes, LPS treatment led to neutrophil recruitment into the bronchoalveolar space, which was again further enhanced when the animals were pretreated with PGD2 or any of the specific agonists for DP1 and DP2. After reduction of alveolar macrophage counts, LPS still enhanced neutrophil counts in the lungs, suggesting that 30% residual macrophages were sufficient to induce lung neutrophilia, but no further increase in neutrophil counts was seen in PGD2-, DK-PGD2–, or BW245C-treated animals (Fig 4, A, and see Fig E11 in this article’s Online Repository at www.jacionline.org). Concomitant to decreased alveolar neutrophil counts, the PGD2-induced increase in MPO activity, as well as compromised lung function, were reversed by alveolar macrophage reduction (Fig 4, B and C).
Fig 4.
Macrophage depletion prevents the proinflammatory effect of PGD2 on neutrophil recruitment. A, Neutrophil numbers in BAL fluid. B, MPO activity in lung tissue. *P < .05, **P < .01, and ***P < .001. C, Macrophage depletion prevents reduction in lung function induced by PGD2 (n = 6-8). *P < .05, **P < .01, and ***P < .001 versus vehicle/LPS.
PGD2 receptor activation on macrophages modulates neutrophil function
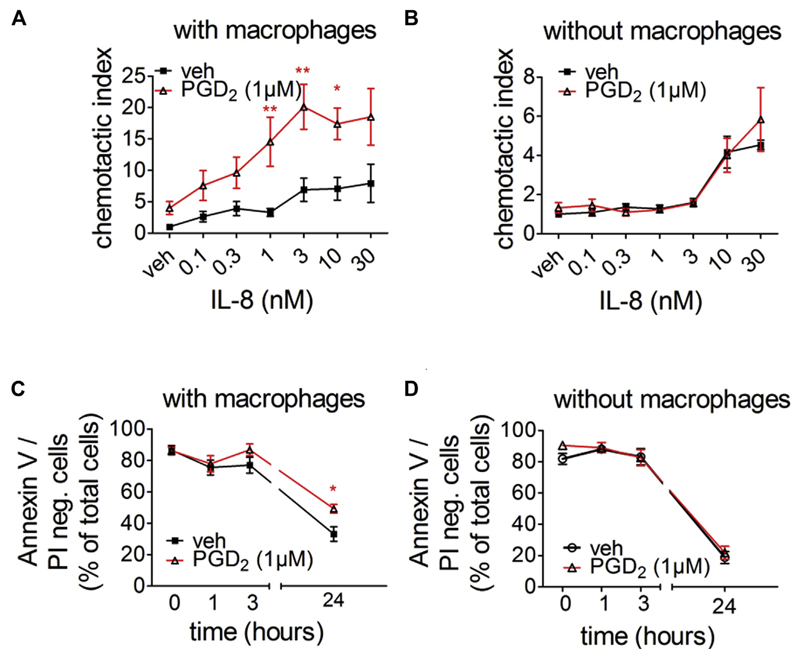
In vitro neutrophil migration assays were performed in the presence of human MDMs treated with vehicle or PGD2 to further clarify the mechanisms through which PGD2 receptor activation on macrophages augmented the LPS-induced neutrophil recruitment. In this coculture experiment PGD2-treated macrophages enhanced the migration of neutrophils when compared with basal neutrophil migration in the presence of untreated MDMs (Fig 5, A). This effect did not depend on IL-8 concentrations because PGD2 treatment of MDMs enhanced the basal migratory capacity and IL-8–induced migration of neutrophils alike. Importantly, neutrophil migration was unaffected by PGD2 when macrophages were absent (Fig 5, B). Therefore PGD2 does not exert its effect through direct stimulation of neutrophils but rather through a macrophage-dependent pathway. In addition to migration, we monitored neutrophil survival in further coculture experiments. MDMs grown on 48-well plates were treated with vehicle or PGD2 24 hours before neutrophils were added. Neutrophil apoptosis was monitored over 24 hours. The portion of viable neutrophils (Annexin V/propidium iodide negative) was higher when macrophages were treated with PGD2 (Fig 5, C). Importantly, this effect was not due to enhanced macrophage phagocytosis of apoptotic/necrotic neutrophils because neutrophil numbers did not change throughout the experiment (see Fig E12 in this article’s Online Repository at www.jacionline.org). Furthermore, neutrophils cultivated without macrophages did not react to PGD2 and showed the same survival rate as in the presence of vehicle (Fig 5, D). These data show that PGD2 receptor activation on macrophages profoundly influences neutrophil function by enhancing their migratory capacity and survival.
Fig 5.
PGD2 receptor activation on MDMs increases neutrophil migration toward IL-8 (A and B) and prolongs neutrophil survival (C and D) in vitro. Fig 5, A, C, and D: n = 5-10; Fig 5, B: n = 3-5. *P < .05 and **P < .01 versus vehicle. PI, Propidium iodide.
Cells expressing hematopoietic PGD2 synthase are abundant in lungs of patients with ARDS
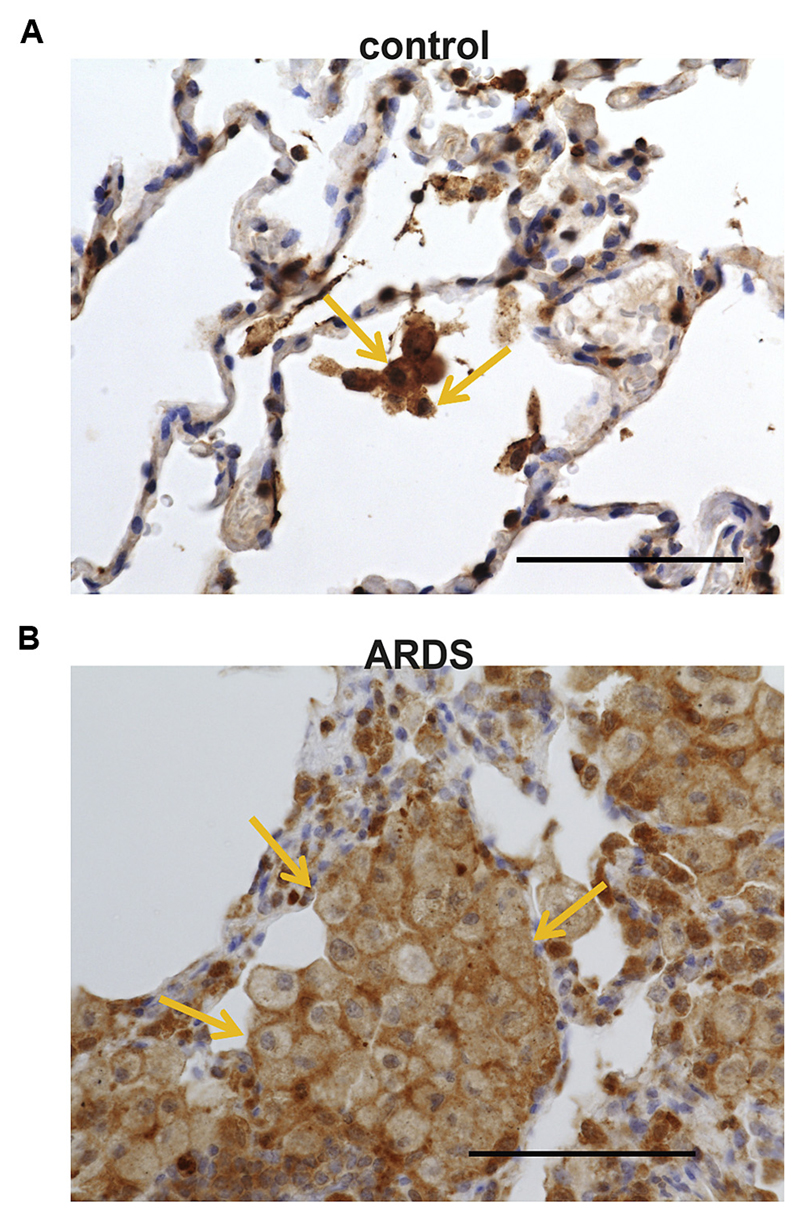
We finally examined whether levels of HPGDS, the rate-limiting enzyme responsible for the production of PGD2, differed in patients with ARDS and control subjects. Indeed, immunohistochemistry revealed that, compared with control lung samples (Fig 6, A), there was a clear increase in the number of cells highly expressing HPGDS in patients with ARDS (Fig 6, B). Moreover, although 50% to 60% of macrophages, as identified by means of morphology, were positive for HPGDS, this value increased to 85% to 95% in lung sections from patients with ARDS.
Fig 6.
Increased numbers of HPGDS-expressing cells in lungs of patients with ARDS. Representative immunohistochemical staining of human lung samples showing positive cells for HPGDS (brown) in a control subject (A) and a patient with ARDS (B). Stainings are representative pictures of 5 patients and control subjects. Note the high amount of HPGDS found in alveolar macrophages. Bar = 100 µm.
Discussion
In this study we propose a novel role for PGD2 and its corresponding receptors in pulmonary inflammation. Here, for the first time, we show that human macrophages express active DP1 and DP2, which are associated with alterations in cytokine profiles and migratory responses, factors that contribute greatly to inflammation in pulmonary diseases. First, we confirmed basal expression of PGD2 receptors on monocytes,29 but we also showed an upregulation that was more pronounced in the expression of DP2 than DP1 during differentiation to macrophages. Furthermore, we were able to demonstrate that both PGD2 receptors are functional on human macrophages: not only did macrophages themselves migrate toward PGD2, but the PG also stimulated the interaction of macrophages with neutrophils, thereby supporting neutrophil migration and survival. This finding might be explained by the increased production of cytokines after PGD2 stimulation of macrophages prompted by DP1/DP2-mediated Ca2+ flux.
Previous reports suggested that DP1- and DP2-mediated actions oppose each other, being anti-inflammatory and proinflammatory, respectively.10,40 Such divergent actions of PGD2 have not been observed here because we could show that on human MDMs, selective activation of both receptors increases intracellular free Ca2+ levels and induces migration. Furthermore, these responses were only partially inhibited by either selective antagonist, and only blockade of DP1 along with DP2 was sufficient to abolish the PGD2-induced activation of macrophages. In this respect PGD2 receptors on macrophages differed from other cell types, such as eosinophils, where these receptors engage in a crosstalk and both are needed for the complete functional response,41 and bronchial epithelial cells, which express DP2 but not DP1.11
We also observed DP1 and DP2 expression on human macrophages in the lung, both in the healthy state and in several pathologic conditions, from organizing pneumonia to diffuse alveolar damage. Moreover, it was mainly the macrophages themselves that were positive for the PGD2-synthesizing enzyme HPGDS in the lungs of patients with ARDS. Although upregulation of HPGDS has up to now mostly been described as a consequence of increased numbers of epithelial and submucosal mast cells,42 our finding proposes a new important role for PGD2 in the regulation of macrophages in the lung.
Next, we investigated the biological role of PGD2 in lung pathophysiology in vivo and used a murine, LPS-induced lung injury model that relies largely on functional macrophages.22,23 As predicted by our in vitro data, systemic application of PGD2, acting through both DP1 and DP2, aggravated LPS-induced lung pathology in several ways by (1) increasing neutrophil influx into the airways, (2) promoting MPO activity in lung tissue, (3) increasing cytokine levels, and (4) impairing lung function. Thus PGD2 enhances neutrophilic inflammatory responses in the presence of disease-activating triggers. In parallel with increased levels of PGD2 in the BAL fluid of LPS-treated animals, TXB2 levels also increased. This upregulation of the TX pathway can further substantiate our finding of DP2 activation in pulmonary inflammation because we previously identified the TX metabolite 11-dehydro-TXB2 as a full agonist for DP2.43 Finally, we provided evidence that these PGD2 responses essentially depended on macrophages because murine alveolar macrophages were a rich source of the neutrophil chemoattractant KC and macrophage depletion reversed the ability of PGD2 to enhance lung inflammation. Two types of macrophages can be found in the lungs: alveolar and interstitial macrophages. Both are long-lived resident cells that orchestrate tissue homeostasis and can react rapidly to endogenous and exogenous stimuli, thus forming a first line of defense,22 and both seemed to respond to PGD2 when KC secretion was analyzed. Human cell studies, which we conducted in parallel, identified 2 novel mechanisms of how PGD2 receptor activation on macrophages can regulate neutrophil function, namely enhanced migratory capacity and survival of the latter. A recent study showed that activated TH2 cells in response to PGD2 treatment react with secretion of IL-6 and GM-CSF at levels that are able to modulate neutrophil functions.44 Our data strongly suggest that this mechanism is not restricted to TH2 cells but also applies to macrophages. One could speculate that type 2 activated macrophages would be even more susceptible in this respect.
Finally, using the same mouse model, we could also demonstrate a clear proinflammatory role of endogenous PGD2. Blocking of endogenous PGD2 either by DP1- or DP2-specific antagonists ameliorated the inflammatory response, although in a differential manner. On the one hand, the DP1-specific antagonist MK0524 decreased neutrophilia in the bronchoalveolar space. On the other hand, the DP2-specific antagonist CAY10471 markedly reduced MPO activity measured in lung tissue. One reason for that discrepancy might be that here we are looking at 2 different lung compartments. Neutrophils migrate from the blood through the interstitium into the alveolar space, and the 2 receptors might play different roles in this process, such as DP2 regulating neutrophil recruitment from the blood to the tissue and DP1 promoting alveolar evasion of neutrophils. Along with the increase in PGD2 levels in the BAL fluid, these observations clearly highlight endogenous PGD2 in the development of experimental pulmonary inflammation.
Recently, it was shown that mice lacking the DP1 receptor display aggravated neutrophil influx and increased mortality in experimental ALI.45 Although this study seemingly contradicts our results, several experimental details make a direct comparison difficult. First, the authors used almost 4 times higher doses of LPS and observed the ensuing effects for 3 days. Second, DP1 was knocked out unconditionally, so that mice might have experienced compensational mechanisms, such as an upregulation of DP2 receptors, thereby driving inflammation. A significant advantage of our study is the use of pharmacologic approaches, which lends our findings clear translational potential toward clinical application.
Collectively, we propose that the PGD2-DP1-DP2 axis on macrophages is of pivotal importance in regulating inflammatory responses, and thus also in tissue damage, by triggering and maintaining a proinflammatory environment. Given the involvement of both PGD2 receptors in macrophage regulation, recently developed dual DP1/DP2 antagonists46 appear to be a promising approach to treating distinct inflammatory diseases that involve macrophage activation and neutrophil accumulation in the lung.
Supplementary Material
Key messages.
DP1 and DP2 are expressed and functionally active on isolated MDMs and pulmonary macrophages.
PGD2 receptor activation of macrophages promotes their interaction with neutrophils and enhances neutrophil function.
The presence of hematopoietic PGD synthase, DP1, and DP2 in macrophages highlights a novel role for PGD2 in pulmonary inflammation.
Acknowledgments
Supported by the Austrian Science Fund FWF (grant P22521-B18 to A.H., P25531-B23 to V.K., P26185-B19 to R.S., and P27070 to D.K.), the Austrian National Bank (grant 14263 to A.H. and 14446 to E.M.S.), and the Swedish Heart-Lung Foundation. K.J., J.M., and M.P. were funded by the PhD Program DK-MOLIN (FWF-W1241).
Abbreviations used
- ALI
Acute lung injury
- ARDS
Acute respiratory distress syndrome
- BAL
Bronchoalveolar lavage
- DK-PGD2
13,14-Dihydro-15-keto prostaglandin D2
- DP
D-type prostanoid receptor
- HPGDS
Hematopoietic prostaglandin D synthase
- KC
Keratinocyte-derived chemokine
- MDM
Monocyte-derived macrophages
- MPO
Myeloperoxidase
- PG
Prostaglandin
- TX
Thromboxane
Footnotes
Disclosure of potential conflict of interest: E. M. Sturm has received research support from the Austrian National Bank (grant #14446). I. Aringer is employed by the Medical University of Graz. V. Konya has received research support from Austrian Science Fund FWF (P25531-B23) and from the European Union’s Horizon 2020 (Marie Sklodowska-Curie grant 655677). S.-E. Dahlen has received research support from the Swedish MRC, Heart-Lung Foundations, and many other foundations; is a board member for, has received consultancy fees from, and has received lecture fees from AstraZeneca, Hydra, RSPR Pharma, and Chugai Pharmaceuticals; and has received lecture fees from Novartis and GlaxoSmithKline. R. Schuligoi has received research support from the Austrian Science Fund FWF (P26185-B19). A. Heinemann has received research support from Austrian Science Funds FWF, Austrian National Bank OeNB, AstraZeneca, 7TM Pharma, and Almirall; has received consultancy fees from AstraZeneca; and is a board member for Amgen and Bayer. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Schuligoi R, Sturm E, Luschnig P, Konya V, Philipose S, Sedej M, et al. CRTH2 and D-type prostanoid receptor antagonists as novel therapeutic agents for inflammatory diseases. Pharmacology. 2010;85:372–82. doi: 10.1159/000313836. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RA, Sheldrick RL. Prostanoid-induced contraction of human bronchial smooth muscle is mediated by TP-receptors. Br J Pharmacol. 1989;96:688–92. doi: 10.1111/j.1476-5381.1989.tb11869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liston TE, Roberts LJ. Transformation of prostaglandin D2 to 9 alpha, 11 beta-(15S)-trihydroxyprosta-(5Z,13E)-dien-1-oic acid (9 alpha, 11 beta-prostaglandin F2): a unique biologically active prostaglandin produced enzymatically in vivo in humans. Proc Natl Acad Sci U S A. 1985;82:6030–4. doi: 10.1073/pnas.82.18.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiraishi Y, Asano K, Nakajima T, Oguma T, Suzuki Y, Shiomi T, et al. Prostaglandin D2-induced eosinophilic airway inflammation is mediated by CRTH2 receptor. J Pharmacol Exp Ther. 2005;312:954–60. doi: 10.1124/jpet.104.078212. [DOI] [PubMed] [Google Scholar]
- 5.Spik I, Brénuchon C, Angéli V, Staumont D, Fleury S, Capron M, et al. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005;174:3703–8. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- 6.Uller L, Mathiesen JM, Alenmyr L, Korsgren M, Ulven T, Högberg T, et al. Antagonism of the prostaglandin D2 receptor CRTH2 attenuates asthma pathology in mouse eosinophilic airway inflammation. Respir Res. 2007;8:16. doi: 10.1186/1465-9921-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stebbins KJ, Broadhead AR, Correa LD, Scott JM, Truong YP, Stearns BA, et al. Therapeutic efficacy of AM156, a novel prostanoid DP2 receptor antagonist, in murine models of allergic rhinitis and house dust mite-induced pulmonary inflammation. Eur J Pharmacol. 2010;638:142–9. doi: 10.1016/j.ejphar.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Gil MA, Caniga M, Woodhouse JD, Eckman J, Lee H-H, Salmon M, et al. Anti-inflammatory actions of chemoattractant receptor-homologous molecule expressed on Th2 by the antagonist MK-7246 in a novel rat model of Alternaria alternata elicited pulmonary inflammation. Eur J Pharmacol. 2014;743:106–16. doi: 10.1016/j.ejphar.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Satoh T, Moroi R, Aritake K, Urade Y, Kanai Y, Sumi K, et al. Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol. 2006;177:2621–9. doi: 10.4049/jimmunol.177.4.2621. [DOI] [PubMed] [Google Scholar]
- 10.Sturm EM, Radnai B, Jandl K, Stančić A, Parzmair GP, Höogenauer C, et al. Opposing roles of prostaglandin d2 receptors in ulcerative colitis. J Immunol. 2014;193:827–39. doi: 10.4049/jimmunol.1303484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stinson SE, Amrani Y, Brightling CE. D prostanoid receptor 2 (chemoattractant receptor-homologous molecule expressed on TH2 cells) protein expression in asthmatic patients and its effects on bronchial epithelial cells. J Allergy Clin Immunol. 2014;35:395–406. doi: 10.1016/j.jaci.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, et al. Prostaglandin D2 pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;131:1504–12. doi: 10.1016/j.jaci.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krug N, Gupta A, Badorrek P, Koenen R, Mueller M, Pivovarova A, et al. Efficacy of the oral chemoattractant receptor homologous molecule on TH2 cells antagonist BI 671800 in patients with seasonal allergic rhinitis. J Allergy Clin Immunol. 2014;133:414–9. doi: 10.1016/j.jaci.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Horak F, Zieglmayer P, Zieglmayer R, Lemell P, Collins LP, Hunter MG, et al. The CRTH2 antagonist OC000459 reduces nasal and ocular symptoms in allergic subjects exposed to grass pollen, a randomised, placebo-controlled, double-blind trial. Allergy. 2012;67:1572–9. doi: 10.1111/all.12042. [DOI] [PubMed] [Google Scholar]
- 15.Straumann A, Hoesli S, Bussmann C, Stuck M, Perkins M, Collins LP, et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68:375–85. doi: 10.1111/all.12096. [DOI] [PubMed] [Google Scholar]
- 16.Barnes N, Pavord I, Chuchalin A, Bell J, Hunter M, Lewis T, et al. A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin Exp Allergy. 2012;42:38–48. doi: 10.1111/j.1365-2222.2011.03813.x. [DOI] [PubMed] [Google Scholar]
- 17.Singh D, Cadden P, Hunter M, Pearce Collins L, Perkins M, Pettipher R, et al. Inhibition of the asthmatic allergen challenge response by the CRTH2 antagonist OC000459. Eur Respir J. 2013;41:46–52. doi: 10.1183/09031936.00092111. [DOI] [PubMed] [Google Scholar]
- 18.Dayer J-M. The process of identifying and understanding cytokines: from basic studies to treating rheumatic diseases. Best Pract Res Clin Rheumatol. 2004;18:31–45. doi: 10.1016/j.berh.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23:243–52. doi: 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito Y, Correll K, Schiel JA, Finigan JH, Prekeris R, Mason RJ. Lung fibroblasts accelerate wound closure in human alveolar epithelial cells through hepatocyte growth factor/c-Met signaling. Am J Physiol Lung Cell Mol Physiol. 2014;307:L94–105. doi: 10.1152/ajplung.00233.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maniatis NA, Orfanos SE. The endothelium in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care. 2008;14:22–30. doi: 10.1097/MCC.0b013e3282f269b9. [DOI] [PubMed] [Google Scholar]
- 22.Herold S, Mayer K, Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol. 2011;2:65. doi: 10.3389/fimmu.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck-Schimmer B, Schwendener R, Pasch T, Reyes L, Booy C, Schimmer RC. Alveolar macrophages regulate neutrophil recruitment in endotoxin-induced lung injury. Respir Res. 2005;6:61. doi: 10.1186/1465-9921-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doherty DE, Downey GP, Worthen GS, Haslett C, Henson PM. Monocyte retention and migration in pulmonary inflammation. Requirement for neutrophils. Lab Investig J Tech Methods Pathol. 1988;59:200–13. [PubMed] [Google Scholar]
- 25.Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–71. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhaliwal K, Scholefield E, Ferenbach D, Gibbons M, Duffin R, Dorward DA, et al. Monocytes control second-phase neutrophil emigration in established lipopolysaccharide-induced murine lung injury. Am J Respir Crit Care Med. 2012;186:514–24. doi: 10.1164/rccm.201112-2132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubota Y, Iwasaki Y, Harada H, Yokomura I, Ueda M, Hashimoto S, et al. Role of alveolar macrophages in Candida-induced acute lung injury. Clin Diagn Lab Immunol. 2001;8:1258–62. doi: 10.1128/CDLI.8.6.1258-1262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 29.Gosset P, Bureau F, Angeli V, Pichavant M, Faveeuw C, Tonnel A-B, et al. Prostaglandin D2 affects the maturation of human monocyte-derived dendritic cells: consequence on the polarization of naive Th cells. J Immunol. 2003;170:4943–52. doi: 10.4049/jimmunol.170.10.4943. [DOI] [PubMed] [Google Scholar]
- 30.Konya V, Blättermann S, Jandl K, Platzer W, Ottersbach PA, Marsche G, et al. A biased non-Gαi OXE-R antagonist demonstrates that Gαi protein subunit is not directly involved in neutrophil, eosinophil, and monocyte activation by 5-Oxo-ETE. J Immunol. 2014;192:4774–82. doi: 10.4049/jimmunol.1302013. [DOI] [PubMed] [Google Scholar]
- 31.Bálint Z, Zabini D, Konya V, Nagaraj C, Végh AG, Váró G, et al. Double-stranded RNA attenuates the barrier function of human pulmonary artery endothelial cells. PLoS One. 2013;8:e63776. doi: 10.1371/journal.pone.0063776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinemann A, Sturm GJ, Ofner M, Sturm EM, Weller C, Peskar BA, et al. Stem cell factor stimulates the chemotaxis, integrin upregulation, and survival of human basophils. J Allergy Clin Immunol. 2005;116:820–6. doi: 10.1016/j.jaci.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Luschnig-Schratl P, Sturm EM, Konya V, Philipose S, Marsche G, Frohlich E, et al. EP4 receptor stimulation down-regulates human eosinophil function. Cell Mol Life Sci. 2011;68:3573–87. doi: 10.1007/s00018-011-0642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149–55. doi: 10.1159/000336871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang F-F, Barnes PF, Feng Y, Donis R, Chroneos ZC, Idell S, et al. GM-CSF in the lung protects against lethal influenza infection. Am J Respir Crit Care Med. 2011;184:259–68. doi: 10.1164/rccm.201012-2036OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundström SL, Saluja R, Adner M, Haeggström JZ, Nilsson G, Wheelock CE. Lipid mediator metabolic profiling demonstrates differences in eicosanoid patterns in two phenotypically distinct mast cell populations. J Lipid Res. 2013;54:116–26. doi: 10.1194/jlr.M030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–38. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nemmar A, Al-Salam S, Zia S, Marzouqi F, Al-Dhaheri A, Subramaniyan D, et al. Contrasting actions of diesel exhaust particles on the pulmonary and cardiovascular systems and the effects of thymoquinone. Br J Pharmacol. 2011;164:1871–82. doi: 10.1111/j.1476-5381.2011.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tajima T, Murata T, Aritake K, Urade Y, Hirai H, Nakamura M, et al. Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2) J Pharmacol Exp Ther. 2008;326:493–501. doi: 10.1124/jpet.108.137992. [DOI] [PubMed] [Google Scholar]
- 40.Sarashina H, Tsubosaka Y, Omori K, Aritake K, Nakagawa T, Hori M, et al. Opposing immunomodulatory roles of prostaglandin D2 during the progression of skin inflammation. J Immunol. 2014;192:459–65. doi: 10.4049/jimmunol.1302080. [DOI] [PubMed] [Google Scholar]
- 41.Sedej M, Schröder R, Bell K, Platzer W, Vukoja A, Kostenis E, et al. D-type prostanoid receptor enhances the signaling of chemoattractant receptor-homologous molecule expressed on T(H)2 cells. J Allergy Clin Immunol. 2012;129:492–500. e1–9. doi: 10.1016/j.jaci.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Balzar S, Fajt ML, Comhair SAA, Erzurum SC, Bleecker E, Busse WW, et al. Mast cell phenotype, location, and activation in severe asthma. Am J Respir Crit Care Med. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Böhm E, Sturm GJ, Weiglhofer I, Sandig H, Shichijo M, McNamee A, et al. 11-Dehydro-thromboxane B2, a stable thromboxane metabolite, is a full agonist of chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2) in human eosinophils and basophils. J Biol Chem. 2004;279:7663–70. doi: 10.1074/jbc.M310270200. [DOI] [PubMed] [Google Scholar]
- 44.Xue L, Fergusson J, Salimi M, Panse I, Ussher JE, Hegazy AN, et al. Prostaglandin D2 and leukotriene E4 synergize to stimulate diverse TH2 functions and TH2 cell/neutrophil crosstalk. J Allergy Clin Immunol. 2015;135:1358–66. e11. doi: 10.1016/j.jaci.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murata T, Aritake K, Tsubosaka Y, Maruyama T, Nakagawa T, Hori M, et al. Anti-inflammatory role of PGD2 in acute lung inflammation and therapeutic application of its signal enhancement. Proc Natl Acad Sci U S A. 2013;110:5205–10. doi: 10.1073/pnas.1218091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu JJ, Wang Y, Johnson MG, Li A-R, Shen W, Wang X, et al. Optimization of phenylacetic acid derivatives for balanced CRTH2 and DP dual antagonists. Bioorg Med Chem Lett. 2012;22:1686–9. doi: 10.1016/j.bmcl.2011.12.107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.