Abstract
The biofilm degradation of Aggregatibacter actinomycetemcomitans is essential as a complete periodontal disease therapy, and here we show the effects of potential probiotic bacteria such as Lactobacillus spp. for the biofilm of several serotypes of A. actinomycetemcomitans strains. Eight of the 13 species showed the competent biofilm degradation of ≥ 90% reduction in biofilm values in A. actinomycetemcomitans Y4 (serotype b) as well as four of the seven species for the biofilm of A. actinomycetemcomitans OMZ 534 (serotype e). In contrast, the probiotic bacteria did not have a big impact for the degradation of A. actinomycetemcomitans SUNY 75 (serotype a) biofilm. The dispersed A. actinomycetemcomitans Y4 cells through the biofilm detachment were still viable and plausible factors for the biofilm degradation were not due to the lactic acid and low pH conditions. The three enzymes, protease, lipase, and amylase may be responsible for the biofilm degradation; in particular, lipase was the most effective enzyme for the biofilm degradation of A. actinomycetemcomitans Y4 along with the protease activity which should be also important for the other serotypes. Remarkable lipase enzyme activities were detected from some of the potential probiotics and a supporting result using a lipase inhibitor presented corroborating evidence that lipase activity is one of the contributing factors for biofilm degradation outside of the protease which is also another possible factor for the biofilm of the other serotype of A. actinomycetemcomitans strains. On the other hand, the biofilm of A. actinomycetemcomitans SUNY 75 (serotype a) was not powerfully degraded by the lipase enzyme because the lipase inhibitor was slightly functional for only two of potential probiotics.
Introduction
Aggregatibacter actinomycetemcomitans is a gram-negative, non-motile, pathogenic oral bacterium that contributes to periodontal disease [1]. It is localized in the dental plaque, gingival crevices, and the buccal mucosa of up to 36% of the normal population [2, 3]. A. actinomycetemcomitans is one of the causative agents of periodontal disease such as juvenile localized periodontitis and the early onset of periodontitis [1] and might sometimes be accompanied by alveolar bone loss associated with bone defects and probing attachment loss [4]. In fact, the pathogen can express several virulence factors to survive in the oral cavity; these include leukotoxin, cytolethal distending toxin (Cdt) [5, 6], lipopolysaccharide (LPS), bone resorption-inducing toxins [7], and epitheliotoxin, which are known to be involved in the interaction between host cells [8]. Furthermore, A. actinomycetemcomitans and other members of the HACEK group of bacteria (Haemophilus influenzae, H. parainfluenzae, Aggregatibacter aphrophilus, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae) are primarily associated with infective endocarditis [9, 10]. It was proposed in a review article that periodontitis influences the host`s susceptibility to cardiovascular disease and preterm labor in three ways: 1) by shared risk factors, 2) via the periodontium acting as a reservoir for inflammatory mediators, and 3) by the subgingival biofilm acting as a reservoir for gram-negative bacteria (10). A. actinomycetemcomitans biofilm has been reported to form tenacious attachments on surfaces due to an existence of fimbriae [1], which are mediated by the tight-adherence (tad) gene loci flp, rcpA, and rcpB [11]. However, the true mechanism of this biofilm formation is not yet fully understood. For instance, a colony variant with very few fimbriae is still able to form a robust biofilm and adhere to surfaces [12]. It has become increasingly clear that bacterial activities in the oral cavity might ease the propagation of this pathogenic organism to other body parts, particularly in immunocompromised patients, such as those with diabetes, rheumatoid arthritis, or those receiving immunosuppressive treatment [13]. Antibiotics or nonsurgical therapies such as scaling and root planning are used to manage periodontal disease [14]. Unfortunately, systemic antibiotic usage definitely suppresses the periodontal microflora and has a limited effect against the targeted bacteria.
Furthermore, A. actinomycetemcomitans biofilm exhibits a higher resistance against antibiotic applications than planktonic cells [15]. Established biofilms can even be tolerant to antimicrobial agents at concentrations 10–1000-times higher than those needed to completely kill planktonic bacteria [16]; they are almost impossible to be phagocytized by immune cells due to the restricted penetration of immunity factors by extracellular polysaccharides [17], and they are sometimes not recognized by the hosts cells [18]. Phagocytes that attempt an assault on the biofilm might cause more harm to the surrounding tissues than to the biofilm itself [19]. By targeting biofilm degradation, an optimum effect may be achieved by the use of antimicrobials and the cell defense of the host. A bacteriotherapy approach using probiotic cells to counteract this activity has been extensively studied [20–23]. A probiotic, as defined by the World Health Organization (WHO, 2001), is a “live microorganism which, when administered in adequate amounts, confers a health benefit on the host” [24]. We chose probiotic bacteria for this study due to their wide spectrum of different effects including direct antagonism against a pathogen, improving gut health and enhancing the immunity response in humans [20]. Another salient property of probiotics is their ability to aggregate with another organism. For example, a pathogen, which might provide great advantages over non-aggregating microorganisms, can be easily removed from the intestinal environment [25]. These properties of probiotic bacteria make them a smart choice to promote the natural killing of a pathogen via bacterial interactions without affecting the normal flora at the site of infection. Furthermore, several research groups have reported a positive role of probiotics in enhancing the immune response by inducing cytokine production, such as interleukin-6 and interleukin-12 [26], as well as TNF-alpha secretion [27]. Recently, a research group reported that lipoteichoic acid from L. plantarum suppressed the production of proinflammatory cytokines and nitric oxide in LPS-stimulated cells that had infiltrated the atherosclerotic plaque in mice [28]. Conversely, A. actinomycetemcomitans produced immunosuppressive factors that were capable of impairing the human lymphocyte function by distracting cell cycle progression [29]. Thus, probiotics have several advantages that make them the promising candidates against A. actinomycetemcomitans. The aims of this study were to evaluate the potential of probiotic bacteria as a degrading agent against periodontal pathogenic A. actinomycetemcomitans and to elucidate the mechanisms underlying the observations made.
Materials and Methods
Strains and Culture Conditions
A. actinomycetemcomitans strains (smooth colony type) and probiotic strains are given in Table 1. A. actinomycetemcomitans strains and Actinomycetes naeslundii JCM 8349 were grown in a BHI broth (Wako) containing brain heart (8 g/L), peptic digested animal tissue (5 g/L), pancreatic digested casein (16 g/L), sodium chloride (5 g/L), 0.2% glucose (w/v), and disodium phosphate (2.5 g/L) with 1% yeast extract. The cultures were shaken at 120 rpm and incubated at 37°C and 5% CO2 for 48 h. Whereas for probiotic strains, all were grown in De Man, Rogosa, and Sharpe (MRS) broth supplemented with l mL/L Tween 80 (Fluka, Sigma-Aldrich) under anaerobic conditions by using anaerobic container and gas generators for anaerobic culture (Mitsubishi Gas Chemical co.Inc) at 37°C for 48 h.
Table 1. List of bacterial strains used in this study.
| Name | Source | |
|---|---|---|
| Periodontal bacteria | A. actinomycetemcomitans strains | |
| Y4 (serotype b) | Kyushu Dental University, Japan | |
| SUNY 75 (serotype a) | Kyushu Dental University, Japan | |
| OMZ 534 (serotype e) | Kyushu Dental University, Japan | |
| Actinomycetes naeslundii JCM 8349 | Japan Collection of Microorganism | |
| Probiotic strains | ||
| L. acidophilus JCM 1021 | Japan Collection of Microorganism | |
| L. casei subsp. rhamnosus NBRC 3831 | National Biological Research Center, Japan | |
| L. delbrueckii subsp. casei JCM 1012 | Japan Collection of Microorganism | |
| L. fermentum JCM 1137 | Japan Collection of Microorganism | |
| L. fermentum NBRC 15885 | National Biological Research Center, Japan | |
| Lactococcus lactis NBRC 12007 | National Biological Research Center, Japan | |
| L. casei NBRC 15883 | National Biological Research Center, Japan | |
| Leuconostoc fructosum NBRC 3516 | National Biological Research Center, Japan | |
| Leuconostoc mesenteroides IAM 1046 | Institute of Molecular and Cellular Bioscience (IAM), Japan | |
| L. plantarum NBRC 15891 | National Biological Research Center, Japan | |
| L. johnsonii NBRC 13952 | National Biological Research Center, Japan | |
| L. sake NBRC 3541 | National Biological Research Center, Japan | |
| L. paracasei subsp. paracasei NBRC 3533 | National Biological Research Center, Japan | |
Biofilm Degradation Assay
The biofilm assay was performed following the technique of Pratt and Kolter [30], with some modifications. A. actinomycetemcomitans strains were grown overnight in BHI broth supplemented with 1% yeast extract and incubated at 37°C and 5% CO2. In order to develop the biofilm, the overnight cultures of A. actinomycetemcomitans strains were diluted to an optical density (OD) of 0.05 at 600 nm in fresh BHI medium supplemented with 1% yeast extract. This was to prepare the planktonic stage prior to developing a mature biofilm. A 200-μL aliquot of the suspension was assayed into each well of a 96-well flat bottom plate (Costar, Corning NY). All plates were incubated under static anaerobic conditions at 37°C for 72 h and formation of the biofilm was evaluated periodically by visual inspection. After a 72 h incubation period, a mature and tenacious biofilm had formed on the surface of the 96-well plate. In order to determine the effect of probiotic strains against the matured A. actinomycetemcomitans biofilm, the biofilm culture medium was removed using an aspirator and 200 μL of cell cultures of probiotic strains in fresh MRS broth (adjusted to OD 0.05 at 600 nm) were added directly onto the A. actinomycetemcomitans biofilm. As a negative control, A. naeslundii JCM 8349 cell cultures in fresh BHI broth (adjusted to OD 0.05 at 600 nm) were added directly onto the A. actinomycetemcomitans biofilm. All samples were incubated for another 24 h under static anaerobic conditions at 37°C prior to measuring the biofilm degradation. The percentage of biofilm degradation was calculated using following formula:
Where C is the average absorbance per well for untreated biofilm and T is the average absorbance per well with the addition of probiotic cells or a cell-free supernatant.
Crystal Violet Staining and Biofilm Quantification
Biofilm degradation was determined by 0.1% crystal violet staining, as previously described [30]. The microtiter plate with biofilm was gently washed by submerging the plate in a small container of distilled water three times. The plates were then dried by patting them on a piece of paper towel. This cleaning procedure removed any loosely attached cells or media that otherwise might be stained in the next step. In order to determine the total mass of biofilm, 200 μL of 0.1% crystal violet (w/v) were added into each well and dissolved for 30 min [31]. The plate was then gently rinsed using distilled water and allowed to air-dry in an incubator at 37°C for 15 min. The remaining biofilm with or without the addition of probiotic cells was visualized by photograph. Afterwards, the stained biofilm was dissolved over 30 min by a 200 μL addition of 95% ethanol into each well. The plates were read using an ELISA microplate reader (Tecan, Waco) at an absorbance of 492 nm.
Biofilm Degradation by Co-Aggregation Assay
Based on our laboratory experiments, Lactobacillus spp. cells used in this study have poor biofilm formation on the microtiter plate (S1 Fig). Due to this, we suspect if the lactobacilli have poor adhesion or auto-aggregation ability between cells which might influence the degradation of biofilm. Thus, a biofilm degradation assay using a co-aggregation buffer was developed to assist the co-aggregation ability of the viable or dead lactobacilli cell pellets only with the pre-formed A. actinomycetemcomitans biofilm. Instead of degradation, co-aggregation between cells might promote the propagation of biofilm formation. Thus, this assay might reconfirm if biofilm degradation ability will be effected by co-aggregation activity. Briefly, probiotic strains were cultured overnight in MRS broth up to the mid-exponential growth phase (OD 1.4 at 600 nm). The cell pellets were extracted by centrifugation at 10 000 g for 15 min, washed twice, and suspended in a sterile co-aggregation buffer containing 0.05 M-Tris/HCl and 0.005 M-CaCI2 at a final pH of 7.0. The buffer was then used to stabilize the co-aggregation activity [32, 33] between cells by indirectly promoting a stronger cell attachment during biofilm activity. Dead probiotic cells were prepared by autoclaving the washed cells at 121°C for 20 min. Cells viability were reconfirmed by a spread plate on MRS agar. A 200 μL of viable or dead cell pellets in the co-aggregation buffer were then added into each well containing A. actinomycetemcomitans Y4 biofilm, except the control, and co-incubated for another 24 h under static anaerobic conditions at 37°C. Biofilm degradations were determined by 0.1% crystal violet staining.
Cell Viability of Degraded Biofilm
Ideally, a degraded biofilm will cause a release of cells into the supernatant. To determine cell viability from the degraded biofilm, a 100 μL sample was collected by gently aspirating the supernatant without contacting the biofilm which had formed at the bottom of the well. The sample was serially diluted in sterile PBS and filtered using a 0.8 μm non-pyrogenic sterilized filter (Sartorius Stedim Biotech, Goettingen, Germany) to separate the A. actinomycetemcomitans Y4 cells from probiotic cells, and spread on BHI agar supplemented with 1% yeast extract. All plates were incubated at 5% CO2 for 48 h and colony counts were performed within the range of 30 to 300 colonies.
pH Adjustment in Culture Supernatant
Probiotic strains were grown in MRS broth for 24 h at 37°C in an anaerobic jar to the mid-log growth phase (OD 1.4 at 600 nm). The cell-free culture supernatant was collected via centrifugation at 10 000 g for 15 min at 4°C, followed by filter sterilization using a 0.2 μm cellulose acetate filter (Sartorius Stedim, Germany). The supernatants were adjusted to pH 6.5 using 1M HCI and concentrated 10-fold by a centrifugal evaporator (VC-L5SP TAITEC). In order to evaluate the influence of a low pH of the culture supernatant of probiotic strains on biofilm degradation, the pH was adjusted to 6.5 and this cell-free supernatants were added to wells containing biofilm. Supernatants without a pH adjustment were used as a control. All samples were incubated under static anaerobic conditions at 37°C for 24 h. Activities associated with the adjusted pH and control were compared. pH was measured by a LAQUAtwin compact pH meter (Horiba Ltd, Kyoto, Japan).
Effect of Lactic Acid on Biofilm Degradation
The concentration of lactic acid was quantified because it was one of the main compounds produced by probiotic strains in this study. After 24 h incubation following the addition of probiotic cells, the spent supernatant was collected and analyzed for its lactic acid concentration by high performance liquid chromatography (HPLC, Shimadzu LC-10AD) [34]). The range of lactic acid concentrations produced was used as a reference to determine the effect of exogenously added lactic acid on biofilm degradation. The biofilm assay was performed as previously described. On incubation day three, 200 μL of lactic acid at final concentrations of 100, 150, 200 and 250 mM in BHI broth were added to wells containing A. actinomycetemcomitans Y4 biofilm, except the control well. The plate was incubated at 37°C under anaerobic conditions for 24 h prior to biofilm measurements.
Effect of Enzymes on Biofilm Degradation
To investigate the factors that contribute to biofilm degradation, we performed an enzyme treatment on the formed biofilm. Enzymatic solutions with a final concentration of 5 μg/mL of proteinase K (Nacalai Tesque), α-amylase (Wako Pure Chemical Industries Co. Ltd.), or lipase (Nacalai Tesque) in Tris-HCI buffer pH 7.0 were prepared. Each enzymatic solution was then added to wells containing biofilm, as a single or combination enzyme mixture and incubated under static anaerobic conditions at 37°C for 24 h. Biofilm measurements were performed as previously described. Biofilm without this treatment was used as a control.
Effect of Lipase Inhibitor on Biofilm Degradation
The potent degradation effect by the lipase enzymes was reconfirmed by using a lipase inhibitor (Tetrahydrolipstatin,Nacalai Tesque). Briefly, a stock solution of lipase inhibitor was prepared in 50% DMSO. Subsequently, the solution was diluted to 250 μg/mL using sterilized distilled water and then mixed with each probiotic cell-free supernatant, with the final concentration of 5 μg/mL. Subsequently, each supernatant with or without the lipase inhibitor was added into wells having the biofilm of A. actinomycetemcomitans strains, and incubated anaerobically under a static condition at 37°C for 24 h. As a negative control, A. naeslundii JCM 8349 cell-free supernatant was used and tested with or without the lipase inhibitor. Biofilm measurements were performed as previously described.
Lipase Assay
To determine the lipase activity profile of probiotic cells and A. naeslundii JCM 8349 an overnight culture supernatants of those bacteria were collected by centrifugation at 10 000 g for 10 min at 4°C, filter sterilized using a 0.2 μm cellulose acetate filter and applied to a lipase assay using a Lipase kit S (DS Pharma Biomedical), following the manufacturer’s instructions. A lipase enzyme level of 0.01 mg/mL was used as a positive control, and sterilized distilled water was used as negative control.
Statistical Analysis
Data from at least three independent experiments represented as mean ± SD (standard deviation). Comparisons were performed by the means of Student t-test using GraphPad Software and P ˂ 0.05 was considered as significant.
Results
Biofilm Degradation by Probiotic Bacteria
We investigated the potential of probiotic bacteria to degrade the formed biofilm of a periodontal pathogen A. actinomycetemcomitans in vitro. Application of nutrient rich medium as medium growth for biofilm assay facilitates the optimum growth of the probiotic cells and produces active enzymes that may be contribute to the biofilm degradation.
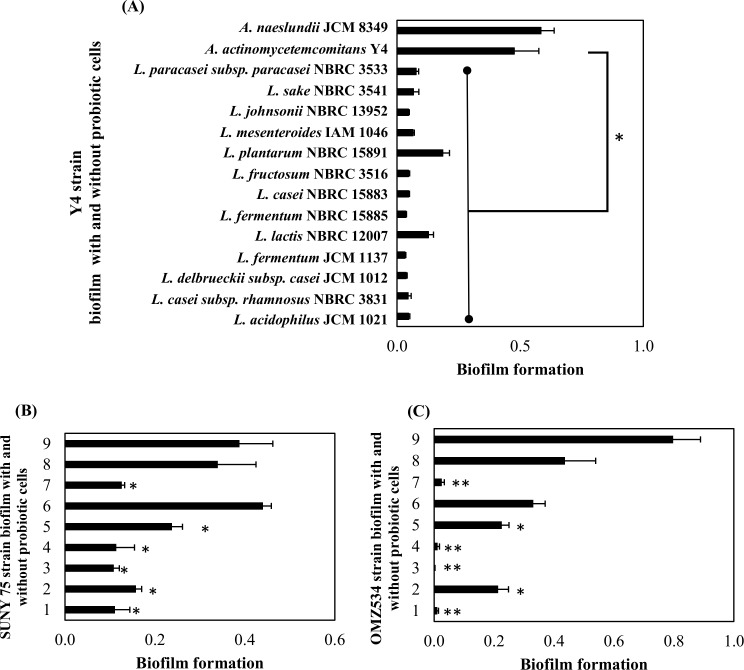
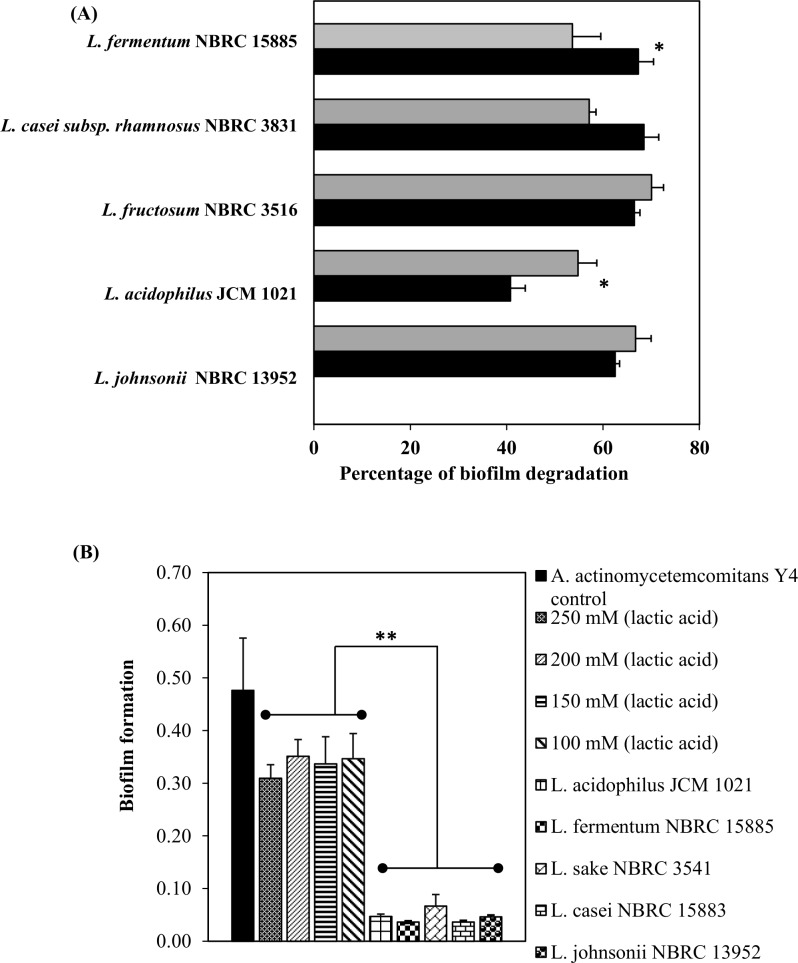
Interestingly, after 24 h incubation, a significant biofilm degrading activity was observed for all the probiotic strains against the Y4 strain, with a different ratio of degradation (Fig 1A). Six of the seven probiotic strains showed a significant biofilm degradation against SUNY 75 (Fig 1B). In addition, for OMZ 534, four of the seven probiotic strains showed a high significant ability of biofilm degradation (Fig 1C). The probiotic strains did not have a big effect against the biofilm of SUNY 75 strain compared to Y4 and OMZ 534 strains in which more than 90% of the biofilm was degraded by several probiotic strains. The range of biofilm degradation for SUNY 75 was approximately 18 to 42% (Table 2).
Fig 1. Biofilm values of A. actinomycetemcomitans strains with addition of probiotic bacteria on pre-formed biofilm.
(A) represents Y4 strain (serotype b). Whereas (B and C) represents SUNY75 strain (serotype a) and OMZ534 strain (serotype e) respectively. Probiotic species and controls were labelled with number as follows; 1: L. fermentum JCM 1137, 2: L. acidophilus JCM 1021, 3: L. fermentum NBRC 15885, 4: L. fructosum NBRC 3516, 5: L. plantarum NBRC 15891, 6: L. casei subsp. rhamnosus NBRC 3831, 7: L. johnsonii NBRC 13952. Positive controls (8) are A. actinomycetemcomitans biofilm without probiotic addition, and A. naeslundii JCM 8349 was used as a negative control (9). Bars represent the mean, error bars represent standard deviation and significance was measured using paired T-test (* = P< 0.05, ** = P< 0.001).
Table 2. Degradation percentage after the addition of probiotic cells on pre-formed A. actinomycetemcomitans strains.
| Potential Probiotic strains | Percentage of A. actinomycetemcomitans biofilm degradation (%) | ||
|---|---|---|---|
| Y4 | SUNY 75 | OMZ 534 | |
| L. fermentum JCM 1137 | 93 ± 1 | 65 ± 13 | 97 ± 1 |
| L. delbrueckii subsp. casei JCM 1012 | 92 ± 1 | N/A | N/A |
| L. fermentum NBRC 15885 | 92 ± 1 | 65 ± 12 | 99 ± 0 |
| L. casei subsp. rhamnosus NBRC 3831 | 90 ± 3 | -29 ± 5 | 24 ± 10 |
| L. johnsonii NBRC 13952 | 90 ± 1 | 60 ± 11 | 94 ± 2 |
| L. fructosum NBRC 3516 | 90 ± 1 | 65 ± 11 | 97 ± 1 |
| L. acidophilus JCM 1021 | 90 ± 1 | 53 ± 3 | 51 ± 8 |
| L. casei NBRC 15883 | 90 ± 1 | N/A | N/A |
| L. sake NBRC 3541 | 86 ± 5 | N/A | N/A |
| L. mesenteroides IAM 1046 | 86 ± 4 | N/A | N/A |
| L. paracasei subsp. paracasei NBRC 3533 | 84 ± 2 | N/A | N/A |
| L. lactis NBRC 12007 | 73 ± 4 | N/A | N/A |
| L. plantarum NBRC 15891 | 61 ± 6 | 30 ± 6 | 48 ± 5 |
Y4 (serotype b), SUNY 75 (serotype a) and OMZ 534 (serotype e) strains of A. actinomycetemcomitans were used for this study. The ± is referring to standard deviation (SD) from at least three independent experiments. NA represent not available data from that particular strains.
Degradation Ability of Probiotic Bacteria Cells and Supernatant
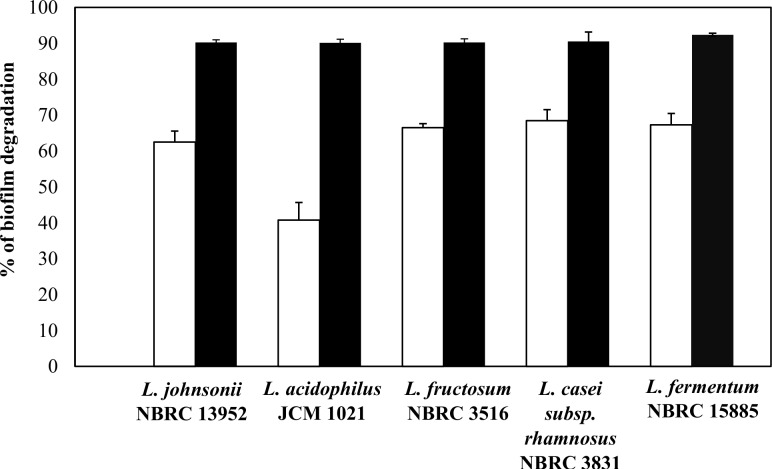
Regardless of the species, all probiotic bacteria demonstrated their ability to degrade A. actinomycetemcomitans Y4 biofilm. Five probiotic species were randomly selected to determine whether biofilm degradation was driven by the cells or extracellular compounds produced in the supernatant. We observed that both the concentrated supernatant and cells promoted the biofilm degradation activity at different levels of intensity (Fig 2). Interestingly, the probiotic cells showed higher biofilm degradation activity compared with a 10 times concentrated cell-free supernatants. The phenomena gain our interest to explore the potential of the cells and what is the possible factors contributing to the biofilm degradation.
Fig 2. Percentage of biofilm degradation by probiotic cells or cell-free supernatant (concentrated) against A. actinomycetemcomitans Y4 biofilm.
Black bars represent biofilm degradation by cells and white columns represent biofilm degradation by a cell-free supernatant. Bars represent the mean, error bars represent standard deviation.
Probiotic Cell Pellet and Degradation Activity
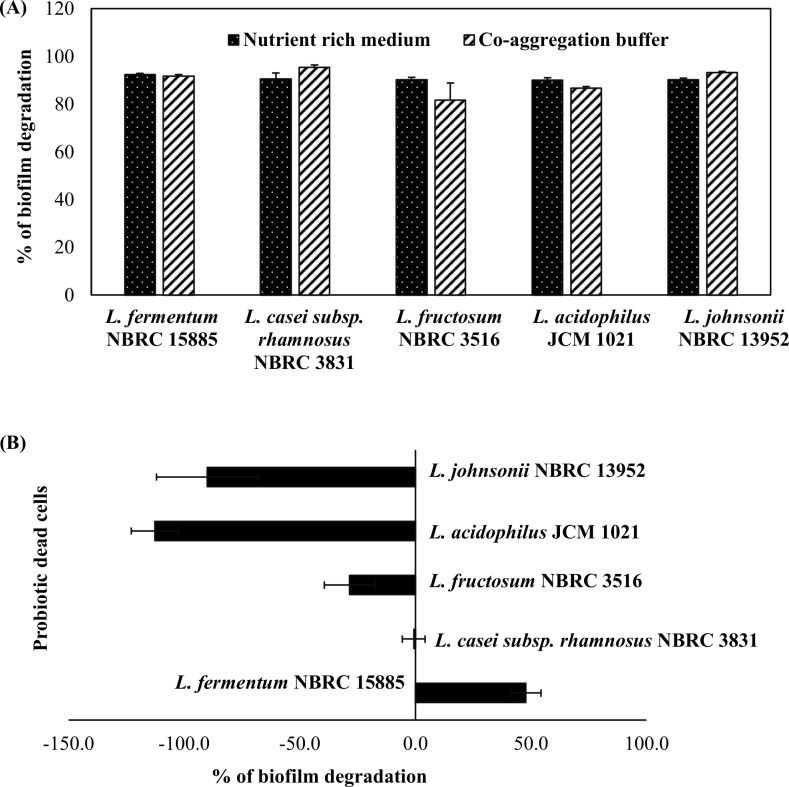
The influence of probiotic cell pellets on the degradation activity was reconfirmed by a coaggregation assay. This assay might facilitate direct cell attachment between the probiotic cells and the biofilm, without the influence of nutrients or the low pH of the culture medium. The co-aggregation buffer may also promote and stabilize the aggregation activity that occurs between cells.
In this assay, the A. actinomycetemcomitans Y4 biofilm was propagated to be two-fold higher (biofilm value: 1.04) than in the BHI medium (0.48). Corresponding this, the percent biofilm degradation by cell pellets was shown similar for cells in a nutrient rich medium (MRS) or co-aggregation buffer (Fig 3A). This is an important finding as it demonstrates that probiotic cells have an effective and continual impact against formed biofilm. The potential of cell pellets was further investigated using dead probiotic cells as a degrading agent. Our findings showed that only dead cells of L. fermentum NBRC 15885 had the ability to affect the pre-biofilm, at half the rate compared to viable cells. Conversely, other strains of dead probiotic cells lost their ability to degrade the biofilm. In spite of the degradation, dead cells of L. johnsonii NBRC 13952, L. acidophilus JCM 1021, and L. fructosum NBRC 3516 did propagate in the biofilm and contributed to the degradation values (Fig 3B). These results indicate that a factor for the biofilm degradation should be produced by the viable probiotic strains.
Fig 3. Influence of culture medium nutrient and cell viability on biofilm degradation activity.
Fig 3A represents a comparison of biofilm degradation by probiotic cells in a nutrient rich medium and a co-aggregation buffer. The nutrient rich medium contained a low density of probiotic cells (OD 0.05 at 600 nm absorbance). Washed cell pellets from an overnight culture were co-incubated with pre-formed A. actinomycetemcomitans Y4 biofilm to form the co-aggregation buffer. Fig 3B represents biofilm degradation by dead probiotic cells (autoclaved). Bars represent the mean, error bars represent standard deviation.
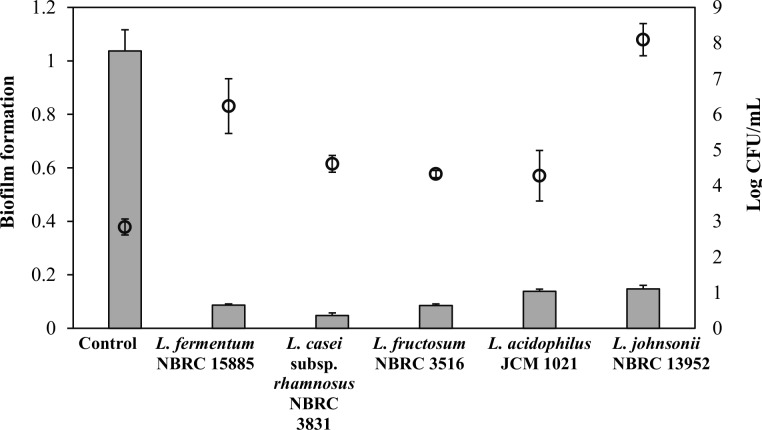
Viability of A. actinomycetemcomitans Y4 from Degraded Biofilm
In theory, degraded biofilm dispersed as live or dead cells into the culture medium. Therefore, viability of the degraded biofilm following the addition of probiotic cells was assessed. As expected, the degraded biofilm showed a higher cell number compared with the control (Fig 4). However, the number of viable bacteria in the culture supernatant was not proportional to the percentage of biofilm degradation. An existence of a high number of viable cells from a biofilm assay with L. fermentum NBRC 15885 and L. johnsonii 13952 might suggest that the degradation activity was possibly not due to an inhibitory or killing effect of the probiotic cells against A. actinomycetemcomitans Y4.
Fig 4. Co-incubation of pre-formed A. actinomycetemcomitans Y4 biofilms with probiotic cells leading to biofilm degradation.
A. actinomycetemcomitans Y4 biofilm was allowed to form under static anaerobic conditions for 72 h before a further 24 h co-culture with probiotic cells in a co-aggregation buffer. Biofilm with the addition of a co-aggregation buffer was used as a control. Grey columns represent biofilm formation and empty circles represent viable A. actinomycetemcomitans Y4 from the biofilm supernatant. Bars and circle represent the mean, error bars represent standard deviation.
Effect of Low pH and Lactic Acid on Biofilm Degradation
Any of the probiotic species used in this study could have contributed to a low pH of the culture supernatant due to metabolic activity and production of organic acids. To assess whether a low pH had contributed to biofilm degradation, the activity was confirmed using an adjusted pH (6.5) cell-free supernatant. Initial pH range of the untreated cell-free culture supernatant was from pH 4.4 to pH 5.2. We observed that the percent biofilm degradation was relatively the same between the untreated and adjusted pH cell-free supernatant (Fig 5A). Except for L. fermentum NBRC 15885 and L. acidophilus JCM 1021 which show a significant different after pH changes. This result suggests that a low pH condition did not contribute to the degradation activity of the biofilm.
Fig 5. Influence of probiotic bacteria supernatant and lactic acid on biofilm growth.
Percent biofilm degradation following (A) the addition of an untreated cell-free supernatant (black column) or an adjusted pH (6.5) cell-free supernatant (gray column). (B) Biofilm growth of A. actinomycetemcomitans Y4 with lactic acid at various concentrations compared with the addition of probiotic cells. Biofilm of A. actinomycetemcomitans Y4 was allowed to pre-form under static anaerobic conditions for 72 h prior to the addition of lactic acid or probiotic cells. Bars represent the mean, error bars represent standard deviation and significance was measured using paired T-test (* = P< 0.05, ** = P< 0.001).
Because lactic acid is one of potent metabolites produced by probiotic species, effect of lactic acid was determined using the same method. The concentrations of lactic acid used ranged from 100 to 250 mM (S2 Fig), which might represent the same amount produced by probiotics in the actual biofilm assay. All probiotic cells significantly caused a higher degradation activity compared to lactic acid (Fig 5B). Lower values of biofilm formation compared to the A. actinomycetemcomitans Y4 control indicated that a higher degradation activity had occurred. This finding indicates lactic acid has vice-versa effect toward biofilm degradation. Thus, other factors have roles the biofilm degradation activity.
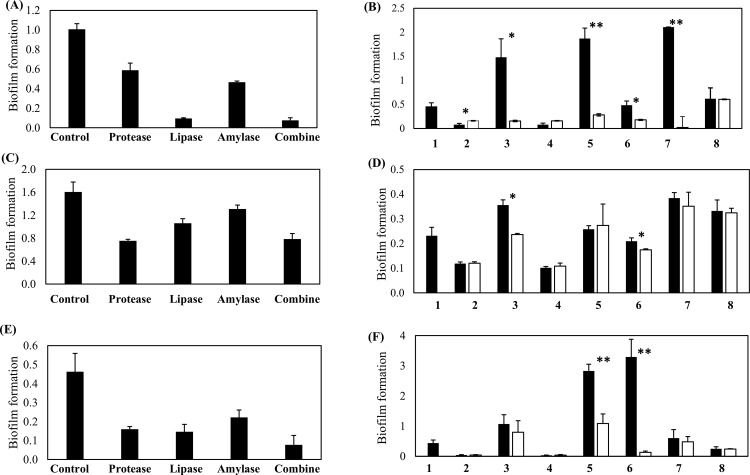
Disruption of Biofilm Growth by Enzymes Activity
The bacterial biofilm matrix contains proteins, nucleic acid, polysaccharides, lipids, mineral ions, and cell debris [35]. To examine the effect of enzymes on biofilm activity, proteinase, amylase, lipase, and all enzymes in combination were applied to the pre-formed biofilm using the same method for the addition of probiotic cells. Our findings showed that all enzymes have a degrading effect against the A. actinomycetemcomitans Y4 biofilm (Fig 6A). A potent degradation effect was demonstrated by lipase and enzymes in combination with a 90.5% and 92.4% reduction of biofilm, respectively. Proteinase K and amylase showed a moderate effect with a 41.2% and 53.6% reduction of biofilm, respectively. The influence of lipase enzyme on biofilm degradation in Y4 strain was reconfirmed by adding a lipase inhibitor to the probiotic cell-free supernatant (Fig 6B). The activity of biofilm degradation significantly reduced by the addition of the lipase inhibitor into the probiotic cell-free supernatants (Fig 6B). This might suggest that lipase plays an important role in the biofilm degradation of Y4 strain. In contrast, L. fermentum NBRC 15885 and L. fructosum NBRC 3516 showed no influence for biofilm degradation in the presence of the lipase inhibitor, meaning that the biofilm degradation by these bacteria may be due to another mechanism. A. naeslundii JCM 8349 (as a negative control) showed no difference with or without the lipase inhibitor.
Fig 6.
Effect of enzymes and influence of lipase inhibitor on the formed biofilm (A, C and E) show degradation activity by the presence of proteinase K, amylase, lipase, or a combination of all enzymes against Y4, SUNY 75, and OMZ 534 respectively. (B, D and F) are the influence of lipase inhibitor on biofilm degradation against Y4, SUNY 75 and OMZ 534 respectively. Probiotic strains and controls were labelled as follows. 1: Positive control (A. actinomycetemcomitans biofilm without the addition of probiotic cells), 2: L. fermentum NBRC 15885, 3: L. casei subsp. rhamnosus NBRC 3831, 4: L. fructosum NBRC 3516, 5: L. acidophilus JCM 1021, 6: L. johnsonii NBRC 13952, 7: L. plantarum NBRC 15891, 8: A. naeslundii JCM 8439 (negative control species). Bars represent the mean, error bars represent standard deviation and significance was measured using paired T-test (* = P< 0.05, ** = P< 0.001).
The biofilm of the other serotype of A. actinomycetemcomitans SUNY 75 was moderately affected by the three enzymes (protease, lipase, and amylase), of which the highest biofilm degradation activity was shown in the presence of protease (Fig 6C). The addition of lipase inhibitor to the probiotic cell-free supernatant showed an inhibitory effect for the biofilm degradation by two probiotic strains, L. casei subsp. rhamnosus NBRC 3831 and L. johnsonii NBRC 13952; however, no/less effect was observed for other potential probiotics (Fig 6D). On the other hand, the OMZ 534 biofilm was greatly affected by the three enzymes as shown in a result that more than 50% of biofilm was degraded by protease, lipase, or amylase (Fig 6E). In addition, the biofilm degradation by L. acidophilus JCM 1021 and L. johnsonii NBRC 13952 was remarkably inhibited by a lipase inhibitor (Fig 6F). Other probiotic strains also showed an inhibitory effect of biofilm degradation but the effect was not significant in conditions with or without the lipase inhibitor. A. naeslundii JCM 8349 (used as a negative control) showed no difference with or without the lipase inhibitor.
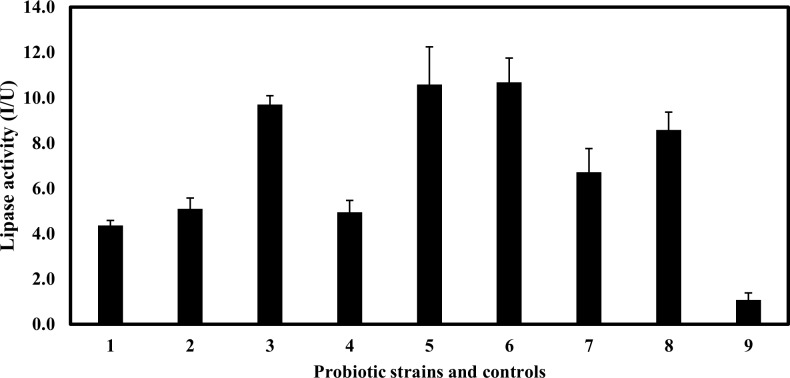
In order to verify the importance of lipase in the biofilm degradation, lipase activities in all the strains used in this study were measured. As a result, the lipase activities of all probiotic strains cell-free supernatants were higher than the positive control (Fig 7). The relatively-high lipase activities were detected from the supernatants of L. acidophilus JCM 1021, L. johnsonii 13952, and L. casei subsp. rhamnosus NBRC 3831. In addition, although the negative control, A. naeslundii JCM 8349 did not have the ability of biofilm degradation, a high lipase activity was detected from the strain. We hypothesize that lipase enzymes derived from the probiotic strains may be highly specific to the biofilm of A. actinomycetemcomitans strains due to the substrate specificity as there have been some literatures that describe the diversity of lipase enzymes [36–38]. Furthermore, the biofilm degradation by L. fermentum NBRC 15885 and L. fructosum NBRC 3516 might be due to another mechanism because they did not have a high lipase activity despite the cell-free supernatants from both species had great biofilm degradation abilities for A. actinomycetemcomitans strains tested.
Fig 7. Profile of lipase activity from an overnight culture in a supernatant of probiotic bacteria.
Probiotic strains and controls were labelled was follows. 1: Positive control (lipase enzyme with final concentration 0.01 mg/mL), 2: L. fermentum NBRC 15885, 3: L. casei subsp. rhamnosus NBRC 3831, 4: L. fructosum NBRC 3516, 5: L. acidophilus JCM 1021, 6: L. johnsonii NBRC 13952, 7: L. plantarum NBRC 15891, 8: A. naeslundii JCM 8439 and 9: negative control (sterilized distilled water). Bars represent the mean, error bars represent standard deviation.
Discussion
A biofilm is a group of bacteria which stick together, adhere to surfaces, are phenotypically resistant, and very difficult to eradicate from a living host. Various diseases are initiated by or are associated with biofilm formation such as cystic fibrosis, otitis media, and chronic prostatitis [39]. Diseases specific to A. actinomycetemcomitans are infective endocarditis and periodontitis [9, 13, 40].
Antibiotic applications to counteract these infections are not promising because their high bacterial load facilitates antibiotic resistance. During scaling, root planning, or periodontal surgery, the administration of antibiotics is to ensure that A. actinomycetemcomitans is eliminated from periodontal lesions. Unfortunately, at the biofilm stage, almost all species are protected by their EPS thus, reducing the effectiveness of antibiotics. For example, ampicillin and cephalexin have been shown to inhibit A. actinomycetemcomitans Y4 biofilm formation during the first 24 h of incubation, but an inverse effect was observed for matured biofilm at 48 h incubation with a significant increase in adenosine triphosphate levels [14]. This phenomenon indirectly demonstrates the continuous challenges faced when treating an infection. Various studies have discussed the issue of how to tackle biofilm formation by pathogens. Several of them highlighted the promising effect of probiotic strains including L. rhamnosus, L. salivarius, L. reuteri, and W. cibaria as potential candidates for the treatment of oral diseases by suppressing the growth of periodontal pathogens [41–44]. Probiotic bacteria are a good alternative due to several advantages that these organisms have that are believed to counteract pathogenesis by periodontal pathogens. Furthermore, their ability to induce an immunomodulatory response by an increase in cytokine production [45], an antiviral response against vescular stomatitis via interaction with macrophages [46], and induction of nitric oxide synthesis [47] might provide a powerful effect against pathogens which virulence toward immune cells, as previously reported for A. actinomycetemcomitans [6, 48–50].
Using 13 species of probiotic bacteria, one valuable finding in this study was their high degradation activity against an A. actinomycetemcomitans biofilm. Eight of the 13 strains and four of the seven strains had a more than 90% biofilm degradation efficiency against the strains, Y4 and OMZ 534, respectively. A. naeslundii JCM 8349 which was used as a negative control strain is implicating in various tooth cavities [51, 52] and the results indicate that potential probiotic bacteria have surely a great ability of biofilm degradation. Effective biofilm degradation by probiotic cells directly to the pre-formed biofilm might suggest another novel mechanism. However, given that the determination of biofilm degradation using the crystal violet assay does present certain limitations (for example, it does not give a measure of biofilm viability as it stains both live and dead bacteria cells, EPS, and extracellular DNA), assessment of the relative proportion of dead and living biofilm cells might provide useful information regarding the mechanism underlying the observed effects.
An overnight incubation of A. actinomycetemcomitans Y4 biofilm (control) in a co-aggregation buffer showed an approximate 2-fold higher propagation than in a nutrient-rich medium. A co-aggregation buffer might induce a higher auto-aggregation between cells which later assembled into a bigger biofilm. In addition, environmental and pH changes might also influence A. actinomycetemcomitans biofilm formation [53]. Otherwise, an addition of probiotic cells and co-aggregation of those cells with the biofilm would be expected to contribute to a higher biofilm. However, the degradation of biofilm activity was robust and there was almost no difference between the co-aggregation buffer and the nutrient-rich medium. This suggests that the activity was not nutrient-dependent and that direct cell contact was possibly a physical factor that contributed to biofilm degradation. In theory, a co-aggregation between cells will contribute to a higher biofilm formation [54, 55]. This phenomenon was observed with dead probiotic cells against the pre-formed biofilm. A negative biofilm degradation activity associated with dead probiotic cells indicated that viable cells were potent agents and that active compounds were possibly produced by the viable cells. A reduced biofilm showed that the addition of probiotics onto the biofilm might suggest a low co-aggregation activity between probiotic cells and the biofilm. The higher number of viable cells detected in the supernatant of degraded biofilm compared to the control proves that there was a disassociation between the cells in the biofilm. Because the number of viable cells was not correlated with the rate of biofilm degradation, various factors such as strain-specific inhibitory or non-inhibitory effects might also play a role. A. actinomycetemcomitans biofilm contains an assemblage of cells which are extremely tenacious on surfaces [1] and are resistant to removal agents such as detergents, proteases, heat, sonication, and vortex agitation [56]. Biofilms are always enclosed in a complex matrix which is primarily structured by microbial cells and extracellular polymeric substances (EPS) [39]. The composition and structure of EPS varies widely among bacterial species [18]. In addition, established biofilms have been reported to be variably susceptible against enzymatic treatments. For example, protease, amylase, and pectinase enzymes from Aspergillus clavatus degrades the biofilms of Pseudomonas aeruginosa, Bacillus subtilis, and Staphylococcus aureus [35]. Another study reported that trypsin significantly reduced biofilm formation and, by contrast, proteinase K enhanced biofilm formation of a Rhodococcus ruber C208 biofilm [57]. Our findings demonstrate that the lipase enzyme and the mixed enzyme of lipase, protease, and amylase provided a powerful degradation biofilm activity against the A. actinomycetemcomitans Y4 and OMZ 534 strain, whereas partially in SUNY 75. Lipase activities with the potency of biofilm degradation ability against the Y4 shows a certain relationship between the L. casei subsp. rhamnosus NBRC 3831, L. acidophilus JCM 1021, L. johnsonii NBRC 13952, and L. plantarum NBRC 15891. However, the relationship is not highly corresponded to the other serotypes, SUNY 75 and OMZ 534 which showed no/less effect of biofilm degradation in the presence of lipase enzyme. Variations in the effect of enzymes for the biofilm degradation were observed in A. actinomycetemcomitans strains (three serotypes) because A. actinomycetemcomitans strains are with the large genetic variations [58–61], by which the property of each A. actinomycetemcomitans strain can be diverse. In fact, other factors such as LPS and EPS components which are varied between A. actinomycetemcomitans serotypes [62, 63] may be influential in the degradation of biofilm [64]. The genetic variation of A. actinomycetemcomitans may be one of the reasons why the biofilm degradation of SUNY 75 was harder than that of other serotypes. Also, instead of lipase enzyme, in SUNY 75 the treatment by proteases showed the highest biofilm degradation activity among the three enzymes (protease, lipase, and amylase), indicating that the composition of biofilm in SUNY 75 may be different with the other serotypes of A. actinomycetemcomitans strains. In addition, L. fermentum NBRC 15885 and L. fructosum NBRC 3516 which have a low lipase activity, able to sustain their biofilm degradation ability against Y4 and OMZ 534, might have another mechanism of biofilm degradation independent of the lipase activity. Thus, the biofilm degradation using potential probiotics may have a variety of strategies because A. actinomycetemcomitans strains are with the large genetic variations [58–61]. There is a possibility that the high biofilm degradation effect by the lipase enzyme was due to the digested lipoprotein in the A. actinomycetemcomitans Y4 biofilm matrix. Paul-Satyaseela et al. [65] reported that the outer-membrane proteins of A. actinomycetemcomitans contained peptidoglycan associated lipoprotein, which has a strong immunoreactivity. This was also supported by another study which identified the proteins from an A. actinomycetemcomitans strain D7S biofilm by LC-MS/MS [66]. Their findings showed that a relatively high abundance of the protein predicted that it was either periplasmic or located in the outer membrane. In an analysis of extracellular proteins of a single strain, it was found that 250 proteins were grouped into lipoproteins and outer membrane proteins [65]. Another study researched the amyloid-like fiber formation in rough and smooth phenotypes of A. actinomycetemcomitans strains [67] using Congo red (CR) as a binding assay, and confirmed that this species binds to CR. Congo red is a hydrophobic diazo dye which binds to lipids, lipoproteins, and a variety of amyloid proteins [67]. Amyloid-like fibers are abundant in natural biofilms [67] and are described as highly organized protein aggregates which are resistant to chemical or temperature denaturation and proteases digestion [68]. In the recent study by Chalabaev et al. (52), it was reported that the biofilm of gram-negative bacteria was associated with an increased level of lipid A palmitoylation, which influenced their antimicrobial resistance and inflammatory response. An abundance of lipids or lipoproteins reported by previous studies support the role of the lipase enzyme as a plausible key factor in biofilm degradation.
In conclusion, probiotic bacteria demonstrate a robust degradation activity on A. actinomycetemcomitans Y4 and OMZ 534 strain, and a moderate effect against SUNY 75 strain. Lipase enzyme from probiotic strains might be an influential factor in the biofilm degradation against A. actinomycetemcomitans Y4 and OMZ 534 strains. This novel property can be utilized as a starting point on the usage of probiotic cells as agents that can directly interact with biofilms at a clinical level. However, further research and more specific analyses need to be conducted in order to exemplify the mechanisms underlying this activity. Contrasting roles of probiotic bacteria and the periodontal pathogen towards host cells contribute to promising techniques to control infections in vivo.
Supporting Information
Initial OD of each sample of cell culture suspensions were 0.05 at 600nm. All samples were incubated in anaerobic condition at 37°C for 24 hour. Bars represent the mean and error bars represent standard deviation.
(TIFF)
Numbers represent strains as follows; 1: Lactococcus lactic NBRC 12007, 2: L. johnsonii NBRC 13952, 3: L. casei subsp. rhamnosus NBRC 3831, 4: Lactobacillus paracasei subsp paracasei 3533, 5: Leuconostoc mesenteroides IAM 1046, 6: L. sake NBRC 3541, 7: L. fermentum NBRC 15885, 8: L. casei NBRC 15883, 9: L. plantarum NBRC 15891 and 10: Leuconostuc fructosum NBRC 3516. Bars represent the mean and error bars represent standard deviation.
(TIFF)
(PDF)
Acknowledgments
This research was supported by the Pfizer Health Research Foundation. NJ received a scholarship from the University of Sultan Zainal Abidin (UniSZA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the Pfizer Health Research Foundation. NJ received a scholarship from the University of Sultan Zainal Abidin (UniSZA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kaplan JB, Meyenhofer MF, Fine DH. Biofilm growth and detachment of Actinobacillus actinomycetemcomitans. J Bacteriol. 2003;185(4):1399–404. Epub 2003/02/04. ; PubMed Central PMCID: PMCPmc142852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown SA, Whiteley M. A novel exclusion mechanism for carbon resource partitioning in Aggregatibacter actinomycetemcomitans. J Bacteriol. 2007;189(17):6407–14. 10.1128/JB.00554-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zambon JJ. Actinobacillus actinomycetemcomitans in human periodontal disease. Journal of clinical periodontology. 1985;12(1):1–20. Epub 1985/01/01. . [DOI] [PubMed] [Google Scholar]
- 4.Aberg CH, Kelk P, Johansson A. Aggregatibacter actinomycetemcomitans: virulence of its leukotoxin and association with aggressive periodontitis. Virulence. 2015;6(3):188–95. 10.4161/21505594.2014.982428 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matangkasombut O, Wattanawaraporn R, Tsuruda K, Ohara M, Sugai M, Mongkolsuk S. Cytolethal distending toxin from Aggregatibacter actinomycetemcomitans induces DNA damage, S/G2 cell cycle arrest, and caspase- independent death in a Saccharomyces cerevisiae model. Infect Immun. 2010;78(2):783–92. 10.1128/IAI.00857-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shenker BJ, Besack D, McKay T, Pankoski L, Zekavat A, Demuth DR. Actinobacillus actinomycetemcomitans Cytolethal Distending Toxin (Cdt): Evidence That the Holotoxin Is Composed of Three Subunits: CdtA, CdtB, and CdtC. The Journal of Immunology. 2003;172(1):410–7. 10.4049/jimmunol.172.1.410 [DOI] [PubMed] [Google Scholar]
- 7.Slots J, Genco RJ. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984;63(3):412–21. Epub 1984/03/01. . [DOI] [PubMed] [Google Scholar]
- 8.Wang PL, Azuma Y, Shinohara M, Ohura K. Effect of Actinobacillus actinomycetemcomitans protease on the proliferation of gingival epithelial cells. Oral diseases. 2001;7(4):233–7. Epub 2001/09/29. . [PubMed] [Google Scholar]
- 9.Wang C-Y, Wang H-C, Li J-M, Wang J-Y, Yang K-C, Ho Y-K, et al. Invasive Infections of Aggregatibacter (Actinobacillus) Actinomycetemcomitans. Journal of Microbiology, Immunology and Infection. 2010;43(6):491–7. 10.1016/s1684-1182(10)60076-x [DOI] [PubMed] [Google Scholar]
- 10.Norskov-Lauritsen N, Kilian M. Reclassification of Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus, Haemophilus paraphrophilus and Haemophilus segnis as Aggregatibacter actinomycetemcomitans gen. nov., comb. nov., Aggregatibacter aphrophilus comb. nov. and Aggregatibacter segnis comb. nov., and emended description of Aggregatibacter aphrophilus to include V factor-dependent and V factor-independent isolates. International journal of systematic and evolutionary microbiology. 2006;56(Pt 9):2135–46. Epub 2006/09/08. 10.1099/ijs.0.64207-0 . [DOI] [PubMed] [Google Scholar]
- 11.Saito T, Ishihara K, Ryu M, Okuda K, Sakurai K. Fimbriae-associated genes are biofilm-forming factors in Aggregatibacter actinomycetemcomitans strains. Bull Tokyo Dent Coll. 2010;51(3):145–50. Epub 2010/09/30. . [DOI] [PubMed] [Google Scholar]
- 12.Inouye T, Ohta H, Kokeguchi S, Fukui K, Kato K. Colonial variation and fimbriation of Actinobacillus actinomycetemcomitans. FEMS Microbiol Lett. 1990;57(1–2):13–7. Epub 1990/05/01. . [DOI] [PubMed] [Google Scholar]
- 13.Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic Diseases Caused by Oral Infection. Clinical microbiology reviews. 2000;13(4):547–58. PMC88948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi N, Ishihara K, Kato T, Okuda K. Susceptibility of Actinobacillus actinomycetemcomitans to six antibiotics decreases as biofilm matures. J Antimicrob Chemother. 2007;59(1):59–65. 10.1093/jac/dkl452 . [DOI] [PubMed] [Google Scholar]
- 15.Fine DH, Furgang D, Barnett ML. Comparative antimicrobial activities of antiseptic mouthrinses against isogenic planktonic and biofilm forms of Actinobacillus actinomycetemcomitans. Journal of clinical periodontology. 2001;28(7):697–700. Epub 2001/06/26. . [DOI] [PubMed] [Google Scholar]
- 16.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45(4):999–1007. Epub 2001/03/21. 10.1128/aac.45.4.999-1007.2001 ; PubMed Central PMCID: PMCPmc90417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis K. Programmed Death in Bacteria. Microbiology and Molecular Biology Reviews. 2000;64(3):503–14. PMC99002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansch GM. Host Defence against Bacterial Biofilms: Mission Impossible? ISRN Immunology. 2012;2012:17 10.5402/2012/853123 [DOI] [Google Scholar]
- 19.Jefferson KK. What drives bacteria to produce a biofilm? FEMS Microbiology Letters. 2004;236(2):163–73. [DOI] [PubMed] [Google Scholar]
- 20.Caglar E, Kargul B, Tanboga I. Bacteriotherapy and probiotics' role on oral health. Oral diseases. 2005;11(3):131–7. Epub 2005/05/13. 10.1111/j.1601-0825.2005.01109.x . [DOI] [PubMed] [Google Scholar]
- 21.Free RH, Busscher HJ, Elving GJ, van der Mei HC, van Weissenbruch R, Albers FW. Biofilm formation on voice prostheses: in vitro influence of probiotics. The Annals of otology, rhinology, and laryngology. 2001;110(10):946–51. Epub 2001/10/20. . [DOI] [PubMed] [Google Scholar]
- 22.Saha S, Tomaro-Duchesneau C, Tabrizian M, Prakash S. Probiotics as oral health biotherapeutics. Expert opinion on biological therapy. 2012;12(9):1207–20. Epub 2012/06/14. 10.1517/14712598.2012.693474 . [DOI] [PubMed] [Google Scholar]
- 23.Sashihara T, Sueki N, Ikegami S. An Analysis of the Effectiveness of Heat-Killed Lactic Acid Bacteria in Alleviating Allergic Diseases. Journal of Dairy Science. 89(8):2846–55. 10.3168/jds.S0022-0302(06)72557-7 [DOI] [PubMed] [Google Scholar]
- 24.Health ECoEo, Bacteria NPoPiFiPMwLLA. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria: Report of a Joint FAO WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, American Córdoba Park Hotel, Córdoba, Argentina, 1–4 October 20012001.
- 25.Collado MC, Meriluoto J, Salminen S. Adhesion and aggregation properties of probiotic and pathogen strains. European Food Research and Technology. 2007;226(5):1065–73. 10.1007/s00217-007-0632-x [DOI] [Google Scholar]
- 26.Chise S, Hiromi K- N, Miho K, Masaru N, Keisuke S, Ayako Y, et al. Immunomodulatory Effects of Lactococcus lactis Strains. JARQ. 2013;47(3):249–55. [Google Scholar]
- 27.Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, et al. Lipoteichoic Acids from Lactobacillus Strains Elicit Strong Tumor Necrosis Factor Alpha-Inducing Activities in Macrophages through Toll-Like Receptor 2. Clinical and Diagnostic Laboratory Immunology. 2003;10(2):259–66. 10.1128/CDLI.10.2.259-266.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JY, Kim H, Jung BJ, Kim N- R, Park JE, Chung DK. Lipoteichoic Acid Isolated from Lactobacillus plantarum Suppresses LPS-Mediated Atherosclerotic Plaque Inflammation. Molecules and Cells. 2013;35(2):115–24. 10.1007/s10059-013-2190-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruce J. Shenker, Terrry McKay, Sugandha Datar, Mark Miller, Chowhan R, Demuth D. Actinobacillus actinomycetemcomitans Immunosuppressive Proteins is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. The Journal of Immunology. 1999;(162):4773–80. [PubMed] [Google Scholar]
- 30.Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30(2):285–93. Epub 1998/10/29. . [DOI] [PubMed] [Google Scholar]
- 31.Sol A, Ginesin O, Chaushu S, Karra L, Coppenhagen-Glazer S, Ginsburg I, et al. LL-37 opsonizes and inhibits biofilm formation of Aggregatibacter actinomycetemcomitans at subbactericidal concentrations. Infect Immun. 2013;81(10):3577–85. Epub 2013/07/10. 10.1128/iai.01288-12 ; PubMed Central PMCID: PMCPmc3811755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collado MC, Meriluoto J, Salminen S. Measurement of aggregation properties between probiotics and pathogens: in vitro evaluation of different methods. J Microbiol Methods. 2007;71(1):71–4. 10.1016/j.mimet.2007.07.005 . [DOI] [PubMed] [Google Scholar]
- 33.Handley PS, Harty DW, Wyatt JE, Brown CR, Doran JP, Gibbs AC. A comparison of the adhesion, coaggregation and cell-surface hydrophobicity properties of fibrillar and fimbriate strains of Streptococcus salivarius. Journal of general microbiology. 1987;133(11):3207–17. Epub 1987/11/01. 10.1099/00221287-133-11-3207 . [DOI] [PubMed] [Google Scholar]
- 34.Mohd Yusoff MZ, Maeda T, Sanchez-Torres V, Ogawa HI, Shirai Y, Hassan MA, et al. Uncharacterized Escherichia coli proteins YdjA and YhjY are related to biohydrogen production. International Journal of Hydrogen Energy. 2012;37(23):17778–87. 10.1016/j.ijhydene.2012.08.115. [DOI] [Google Scholar]
- 35.Singh V, Verma N, Banerjee B, Vibha K, Haque S, Tripathi CKM. Enzymatic degradation of bacterial biofilms using Aspergillus clavatus MTCC 1323. Microbiology. 2015;84(1):59–64. 10.1134/s0026261715010130 [DOI] [Google Scholar]
- 36.Yuhong Z, Shi P, Liu W, Meng K, Bai Y, Wang G, et al. Lipase diversity in glacier soil based on analysis of metagenomic DNA fragments and cell culture. Journal of microbiology and biotechnology. 2009;19(9):888–97. [DOI] [PubMed] [Google Scholar]
- 37.Jaeger K- E, Ransac S, Dijkstra BW, Colson C, van Heuvel M, Misset O. Bacterial lipases. FEMS Microbiology Reviews. 1994;15(1):29–63. 10.1016/0168-6445(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 38.Arpigny JL, Jaeger KE. Bacterial lipolytic enzymes: classification and properties. Biochemical Journal. 1999;343(Pt 1):177–83. . [PMC free article] [PubMed] [Google Scholar]
- 39.D R M. Biofilms: Microbial Life on Surfaces. Emerging Infectious Diseases. 2002;8(No. 9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paturel L, Casalta JP, Habib G, Nezri M, Raoult D. Actinobacillus actinomycetemcomitans endocarditis. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2004;10(2):98–118. Epub 2004/02/05. . [DOI] [PubMed] [Google Scholar]
- 41.Kang MS, Kim BG, Chung J, Lee HC, Oh JS. Inhibitory effect of Weissella cibaria isolates on the production of volatile sulphur compounds. Journal of clinical periodontology. 2006;33(3):226–32. 10.1111/j.1600-051X.2006.00893.x . [DOI] [PubMed] [Google Scholar]
- 42.Krasse P, Carlsson B, Dahl C, Paulsson A, Nilsson A, Sinkiewicz G. Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri. Swedish dental journal. 2006;30(2):55–60. Epub 2006/08/02. . [PubMed] [Google Scholar]
- 43.Nikawa H, Makihira S, Fukushima H, Nishimura H, Ozaki Y, Ishida K, et al. Lactobacillus reuteri in bovine milk fermented decreases the oral carriage of mutans streptococci. Int J Food Microbiol. 2004;95(2):219–23. Epub 2004/07/30. 10.1016/j.ijfoodmicro.2004.03.006 . [DOI] [PubMed] [Google Scholar]
- 44.Samot J, Badet C. Antibacterial activity of probiotic candidates for oral health. Anaerobe. 2013;19:34–8. 10.1016/j.anaerobe.2012.11.007 . [DOI] [PubMed] [Google Scholar]
- 45.Habil N, Al-Murrani W, Beal J, Foey AD. Probiotic bacterial strains differentially modulate macrophage cytokine production in a strain-dependent and cell subset-specific manner. Benef Microbes. 2011;2(4):283–93. 10.3920/BM2011.0027 . [DOI] [PubMed] [Google Scholar]
- 46.Ivec M, Botic T, Koren S, Jakobsen M, Weingartl H, Cencic A. Interactions of macrophages with probiotic bacteria lead to increased antiviral response against vesicular stomatitis virus. Antiviral Res. 2007;75(3):266–74. 10.1016/j.antiviral.2007.03.013 . [DOI] [PubMed] [Google Scholar]
- 47.Korhonen R, Korpela R, Saxelin M, Maki M, Kankaanranta H, Moilanen E. Induction of nitric oxide synthesis by probiotic Lactobacillus rhamnosus GG in J774 macrophages and human T84 intestinal epithelial cells. Inflammation. 2001;25(4):223–32. Epub 2001/10/03. . [DOI] [PubMed] [Google Scholar]
- 48.Baker PJ, Busby WF, Wilson ME. Subinhibitory concentrations of cefpodoxime alter membrane protein expression of Actinobacillus actinomycetemcomitans and enhance its susceptibility to killing by neutrophils. Antimicrob Agents Chemother. 1995;39(2):406–12. Epub 1995/02/01. ; PubMed Central PMCID: PMCPmc162551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boesze-Battaglia K, Walker LP, Zekavat A, Dlakic M, Scuron MD, Nygren P, et al. The Aggregatibacter actinomycetemcomitans cytolethal distending toxin active subunit, CdtB, contains a cholesterol recognition sequence required for toxin binding and subunit internalization. Infect Immun. 2015. Epub 2015/07/29. 10.1128/iai.00788-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shenker BJ, McKay T, Datar S, Miller M, Chowhan R, Demuth† D. Actinobacillus actinomycetemcomitans Immunosuppressive Protein Is a Member of the Family of Cytolethal Distending Toxins Capable of Causing a G2 Arrest in Human T Cells1,2. The Journal of Immunology. 1999. [PubMed] [Google Scholar]
- 51.Dige I, Raarup MK, Nyengaard JR, Kilian M, Nyvad B. Actinomyces naeslundii in initial dental biofilm formation. Microbiology. 2009;155(7):2116–26. [DOI] [PubMed] [Google Scholar]
- 52.Morou-Bermudez E, Burne RA. Genetic and physiologic characterization of urease of Actinomyces naeslundii. Infection and immunity. 1999;67(2):504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haase EM, Bonstein T, Palmer RJ Jr, Scannapieco FA. Environmental influences on Actinobacillus actinomycetemcomitans biofilm formation. Archives of oral biology. 2006;51(4):299–314. 10.1016/j.archoralbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 54.de Toledo A, Nagata E, Yoshida Y, Oho T. Streptococcus oralis coaggregation receptor polysaccharides induce inflammatory responses in human aortic endothelial cells. Molecular oral microbiology. 2012;27(4):295–307. Epub 2012/07/05. 10.1111/j.2041-1014.2012.00646.x . [DOI] [PubMed] [Google Scholar]
- 55.Karched M, Bhardwaj RG, Asikainen SE. Coaggregation and biofilm growth of Granulicatella spp. with Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans. BMC Microbiology. 2015;15(1):1–10. 10.1186/s12866-015-0439-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fine DH, Furgang D, Kaplan J, Charlesworth J, Figurski DH. Tenacious adhesion of Actinobacillus actinomycetemcomitans strain CU1000 to salivary-coated hydroxyapatite. Archives of oral biology. 1999;44(12):1063–76. Epub 2000/02/11. . [DOI] [PubMed] [Google Scholar]
- 57.Gilan I, Sivan A. Effect of proteases on biofilm formation of the plastic-degrading actinomycete Rhodococcus ruber C208. FEMS Microbiology Letters. 2013;342(1):18–23. 10.1111/1574-6968.12114 [DOI] [PubMed] [Google Scholar]
- 58.Kaplan JB, Schreiner HC, Furgang D, Fine DH. Population Structure and Genetic Diversity of Actinobacillus actinomycetemcomitans Strains Isolated from Localized Juvenile Periodontitis Patients. Journal of Clinical Microbiology. 2002;40(4):1181–7. 10.1128/JCM.40.4.1181-1187.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DiRienzo JM, Slots J, Sixou M, Sol MA, Harmon R, McKay TL. Specific genetic variants of Actinobacillus actinomycetemcomitans correlate with disease and health in a regional population of families with localized juvenile periodontitis. Infect Immun. 1994;62(8):3058–65. Epub 1994/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paju S, Carlson P, Jousimies-Somer H, Asikainen S. Heterogeneity of Actinobacillus actinomycetemcomitans strains in various human infections and relationships between serotype, genotype, and antimicrobial susceptibility. J Clin Microbiol. 2000;38(1):79–84. Epub 2000/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zambon JJ, Slots J, Genco RJ. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect Immun. 1983;41(1):19–27. Epub 1983/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoover C. Electrophoretic heterogeneity of lipopolysaccharides of Actinobacillus actinomycetemcomitans. Journal of dental research. 1988;67(3):574–6. [DOI] [PubMed] [Google Scholar]
- 63.Wilkinson SG. Bacterial lipopolysaccharides—themes and variations. Progress in lipid research. 1996;35(3):283–343. [DOI] [PubMed] [Google Scholar]
- 64.Amarasinghe JJ, Scannapieco FA, Haase EM. Transcriptional and translational analysis of biofilm determinants of Aggregatibacter actinomycetemcomitans in response to environmental perturbation. Infect Immun. 2009;77(7):2896–907. Epub 2009/05/13. 10.1128/iai.00126-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paul-Satyaseela M, Karched M, Bian Z, Ihalin R, Boren T, Arnqvist A, et al. Immunoproteomics of Actinobacillus actinomycetemcomitans outer-membrane proteins reveal a highly immunoreactive peptidoglycan-associated lipoprotein. J Med Microbiol. 2006;55(Pt 7):931–42. 10.1099/jmm.0.46470-0 . [DOI] [PubMed] [Google Scholar]
- 66.Zijnge V, Kieselbach T, Oscarsson J. Proteomics of Protein Secretion by Aggregatibacter actinomycetemcomans. PLoS ONE. 2012;7(7):e41662 10.1371/journal.pone.0041662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kimizuka R, Kato T, Hashimoto S, Yamanaka-Okada A, Okuda K, Ishihara K. Congo Red-binding Protein in Rough-phenotype Aggregatibacter actinomycetemcomitans is Amyloid-like Fiber. The Bulletin of Tokyo Dental College. 2009;50(1):23–9. 10.2209/tdcpublication.50.23 [DOI] [PubMed] [Google Scholar]
- 68.Nordstedt C, Naslund J, Tjernberg LO, Karlstrom AR, Thyberg J, Terenius L. The Alzheimer A beta peptide develops protease resistance in association with its polymerization into fibrils. The Journal of biological chemistry. 1994;269(49):30773–6. Epub 1994/12/09. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Initial OD of each sample of cell culture suspensions were 0.05 at 600nm. All samples were incubated in anaerobic condition at 37°C for 24 hour. Bars represent the mean and error bars represent standard deviation.
(TIFF)
Numbers represent strains as follows; 1: Lactococcus lactic NBRC 12007, 2: L. johnsonii NBRC 13952, 3: L. casei subsp. rhamnosus NBRC 3831, 4: Lactobacillus paracasei subsp paracasei 3533, 5: Leuconostoc mesenteroides IAM 1046, 6: L. sake NBRC 3541, 7: L. fermentum NBRC 15885, 8: L. casei NBRC 15883, 9: L. plantarum NBRC 15891 and 10: Leuconostuc fructosum NBRC 3516. Bars represent the mean and error bars represent standard deviation.
(TIFF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.