Abstract
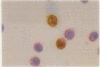
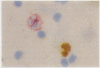
AIMS: To investigate the numbers, morphology, and lineage assignment of Ki-67 positive cells in peripheral blood from normal subjects. METHODS: Single and double immunoenzymatic staining procedures, immunoperoxidase, and immunoalkaline phosphatase were used with Ki-67, a monoclonal antibody that recognises a nuclear antigen present in proliferating cells, with markers expressed in B and T lymphocytes and monocytes. RESULTS: In the five healthy donors 2.1% (range 1.6-3.7%) cells of the blood mononuclear fraction and 2.7% (range 2.3-3.9%) lymphocytes were Ki-67 positive. Of these, 88% (range 85-90%) were small cells and 12% (range 10-15%) were medium sized. Forty one per cent of the Ki-67 positive cells were CD3 positive by double immunoenzymatic staining and corresponded to T lymphocytes, and 11.4% were mature B cells expressing kappa or lambda light chains. Monocytes detected by the anti-lysozyme antibody were consistently Ki-67 negative. Half of the Ki-67 positive lymphocytes could not be accounted for by B or T cells with the markers used. Most Ki-67 positive cells were of small size; the B lymphocytes in cycle showed abundant cytoplasm and features suggestive of lymphoplasmacytic differentiation. CONCLUSIONS: The methodology described is useful for the simultaneous detection of nuclear and cytoplasmic antigens. The demonstration that a proportion of normal blood lymphocytes are in cell cycle raises the issue of whether immunophenotypic analysis of Ki-67 positive cells in haemopoietic malignancies with peripheral blood disease should be carried out to define more precisely the proportion of normal and neoplastic cells in cycle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard N. J., Hall P. A., Lemoine N. R., Kadar N. Proliferative index in breast carcinoma determined in situ by Ki67 immunostaining and its relationship to clinical and pathological variables. J Pathol. 1987 Aug;152(4):287–295. doi: 10.1002/path.1711520407. [DOI] [PubMed] [Google Scholar]
- Campana D., Coustan-Smith E., Janossy G. Double and triple staining methods for studying the proliferative activity of human B and T lymphoid cells. J Immunol Methods. 1988 Feb 24;107(1):79–88. doi: 10.1016/0022-1759(88)90012-9. [DOI] [PubMed] [Google Scholar]
- Campana D., Janossy G. Proliferation of normal and malignant human immature lymphoid cells. Blood. 1988 May;71(5):1201–1210. [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Oka M. S., Steinkamp J. A. Rapid staining methods for analysis of deoxyribonucleic acid and protein in mammalian cells. J Histochem Cytochem. 1976 Jan;24(1):64–71. doi: 10.1177/24.1.56392. [DOI] [PubMed] [Google Scholar]
- Falini B., Abdulaziz Z., Gerdes J., Canino S., Ciani C., Cordell J. L., Knight P. M., Stein H., Grignani F., Martelli M. F. Description of a sequential staining procedure for double immunoenzymatic staining of pairs of antigens using monoclonal antibodies. J Immunol Methods. 1986 Nov 6;93(2):265–273. doi: 10.1016/0022-1759(86)90199-7. [DOI] [PubMed] [Google Scholar]
- Franklin W. A., McDonald G. B., Stein H. O., Gatter K. C., Jewell D. P., Clarke L. C., Mason D. Y. Immunohistologic demonstration of abnormal colonic crypt cell kinetics in ulcerative colitis. Hum Pathol. 1985 Nov;16(11):1129–1132. doi: 10.1016/s0046-8177(85)80181-7. [DOI] [PubMed] [Google Scholar]
- Gatter K. C., Dunnill M. S., Gerdes J., Stein H., Mason D. Y. New approach to assessing lung tumours in man. J Clin Pathol. 1986 Jun;39(6):590–593. doi: 10.1136/jcp.39.6.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Dallenbach F., Lennert K., Lemke H., Stein H. Growth fractions in malignant non-Hodgkin's lymphomas (NHL) as determined in situ with the monoclonal antibody Ki-67. Hematol Oncol. 1984 Oct-Dec;2(4):365–371. doi: 10.1002/hon.2900020406. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lelle R. J., Pickartz H., Heidenreich W., Schwarting R., Kurtsiefer L., Stauch G., Stein H. Growth fractions in breast cancers determined in situ with monoclonal antibody Ki-67. J Clin Pathol. 1986 Sep;39(9):977–980. doi: 10.1136/jcp.39.9.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Schwarting R., Stein H. High proliferative activity of Reed Sternberg associated antigen Ki-1 positive cells in normal lymphoid tissue. J Clin Pathol. 1986 Sep;39(9):993–997. doi: 10.1136/jcp.39.9.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangaspero F., Doglioni C., Rivano M. T., Pileri S., Gerdes J., Stein H. Growth fraction in human brain tumors defined by the monoclonal antibody Ki-67. Acta Neuropathol. 1987;74(2):179–182. doi: 10.1007/BF00692849. [DOI] [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Richards M. A., Gregory W. M., d'Ardenne A. J., Lister T. A., Stansfeld A. G. The prognostic value of Ki67 immunostaining in non-Hodgkin's lymphoma. J Pathol. 1988 Mar;154(3):223–235. doi: 10.1002/path.1711540305. [DOI] [PubMed] [Google Scholar]
- Klein G., Steiner M., Wiener F., Klein E. Human leukemia-associated anti-nuclear reactivity. Proc Natl Acad Sci U S A. 1974 Mar;71(3):685–689. doi: 10.1073/pnas.71.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. Y., Cordell J. L., Abdulaziz Z., Naiem M., Bordenave G. Preparation of peroxidase: antiperoxidase (PAP) complexes for immunohistological labeling of monoclonal antibodies. J Histochem Cytochem. 1982 Nov;30(11):1114–1122. doi: 10.1177/30.11.6183312. [DOI] [PubMed] [Google Scholar]
- Miyachi K., Fritzler M. J., Tan E. M. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978 Dec;121(6):2228–2234. [PubMed] [Google Scholar]
- Sasaki K., Matsumura K., Tsuji T., Shinozaki F., Takahashi M. Relationship between labeling indices of Ki-67 and BrdUrd in human malignant tumors. Cancer. 1988 Sep 1;62(5):989–993. doi: 10.1002/1097-0142(19880901)62:5<989::aid-cncr2820620525>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Traganos F., Darzynkiewicz Z., Sharpless T., Melamed M. R. Simultaneous staining of ribonucleic and deoxyribonucleic acids in unfixed cells using acridine orange in a flow cytofluorometric system. J Histochem Cytochem. 1977 Jan;25(1):46–56. doi: 10.1177/25.1.64567. [DOI] [PubMed] [Google Scholar]