Abstract
Clinical trials have provided conflicting results regarding whether epidermal growth factor receptor (EGFR) overexpression predicts poor survival in cervical cancer patients. In this study, we perform a meta-analysis of the association between EGFR expression and survival in cervical cancer patients. We searched clinical studies in the Medline, PubMed, Embase, and Web of Science databases. A total of 22 studies with 2,505 patients were included, and pooled hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated for each study. Heterogeneity was assessed using Higgins I2 to select a Mantel-Haenszel fixed effects model (I2 ≤50%) or a DerSimonian-Laird random effects model (I2 ≥50%). High EGFR levels predicted poor overall survival (OS) (HR: 1.40, 95% CI: 1.10–1.78) and disease-free survival (DFS) (HR: 1.84, 95% CI: 1.51–2.24). Stratified analyses showed that EGFR overexpression was significantly related to poor DFS in patients treated with chemoradiation or surgery. Moreover, the pooled odds ratios (ORs) revealed associations between EGFR expression and clinicopathological features, such as lymph node metastasis (OR: 1.72, 95% CI: 1.23–2.40) and tumor size ≥4 cm (OR: 1.64, 95% CI: 1.20–2.23). This meta-analysis demonstrates that EGFR overexpression is closely associated with reduced survival in patients with cervical cancer. These results may facilitate the individualized management of clinical decisions for anti-EGFR therapies in cervical cancer patients.
Introduction
Cervical cancer is the third most frequently diagnosed malignancy and represents the fourth leading cause of cancer-related death in females worldwide [1]. With the introduction of screening programs, the incidence of and mortality associated with cervical cancer in developed areas have dramatically declined in recent decades [2]. The standard treatment for locally advanced cervical cancer consists of concurrent platinum-based chemoradiation, which results in a 5-year survival rate of only 66% [3]. Tremendous efforts are still needed to improve the overall survival rate in patients with advanced-stage cervical cancer.
Epidermal growth factor receptor (EGFR) is a 170-kDa transmembrane glycoprotein receptor dimerizes to activate a tyrosine kinase domain that modulates multiple functions, including cell differentiation, growth, gene expression, and development [4]. Because EGFR is known to play a role in epithelial tumor biology, various EGFR-targeted cancer therapies are currently being developed. EGFR inhibitors have demonstrated efficacy in some clinical trials involving patients with colon, lung, head, and neck cancers [5–7]. However, the value of using EGFR inhibition to treat cervical cancer remains unknown. Several small-scale clinical trials of EGFR inhibitors have been completed in cervical cancer patients, but the effects of these drugs are not yet well established [8–12]. Numerous clinical trials have demonstrated that only a subset of patients respond to EGFR inhibitors. However, a practical predictor of a response to these drugs has not been identified for cervical cancer.
The overexpression of EGFR is thought to be negatively associated with survival in cervical cancer patients, and the relationship between EGFR overexpression and altered survival in patients with cervical cancer has therefore been studied for many years [13]. However, inconclusive results have been reported by different laboratories. A meta-analysis is needed to comprehensively evaluate the prognostic value of EGFR in this type of malignancy. Therefore, we performed a systematic meta-analysis to quantify the effects of EGFR overexpression on survival in patients with cervical cancer.
Materials and Methods
Search strategy
The Medline, PubMed, Embase, and Web of Science databases (through March 2014) were searched to identify articles that examined EGFR expression status and survival in patients with cervical cancer using combinations of the following terms: EGFR (or epidermal growth factor receptor, Her family, Her-1, Erb B family, or Erb B1), outcome (or surviv*, prognos*, or predict*), and cervical cancer (or cervical carcinoma, cervical neoplasm, or cervical tumor). The references of all resulting publications and reviews were manually searched to identify missing relevant publications. All studies were carefully evaluated to identify duplicate data.
Selection criteria
The following criteria for study eligibility were set before articles were collected: (1) EGFR was evaluated in primary cervical cancer tissues using immunohistochemistry (IHC) or by quantifying EGFR protein levels; (2) a hazard ratio (HR) and its confidence interval (CI) from a survival analysis were reported; (3) the median follow up time exceeded 2 years; (4) the investigated endpoints were overall survival (OS) and disease-free survival (DFS); and (5) when a single study was reported on multiple occasions, the latest or most informative article was selected.
Data extraction
Two authors (W-J Tian and M-L Huang) independently extracted information using predefined data abstraction forms. Further information from each study is shown in S1 Table. If a study reported the results of both univariate and multivariate analyses, the latter was selected because multivariate analyses consider confounding factors, which makes them more precise.
Quality assessment of primary studies
The quality of each study was independently assessed by two investigators (W-J Tian and M-L Huang) using the criteria developed by McShane et al. [14] and Hayes et al. [15] (S2 Table). The following eight items were assessed and scored on a scale ranging from 0 to 8: 1) the study reported inclusion and exclusion criteria, 2) the study design was prospective or retrospective, 3) the patient and tumor characteristics were sufficiently described, 4) the method or assay used to measure biomarker expression was sufficiently described, 5) a description of the study endpoint was provided, 6) the duration of the follow-up period in the study was provided, 7) the study reported the number of patients who dropped out during the follow-up period or for whom data was not available for statistical analysis, and 8) staining was evaluated by more than one observer. Studies with a total score of eight were considered to have used the highest quality methodology, whereas a score of zero was considered to indicate a study with the lowest quality methodology.
All disagreements were resolved by discussion with the third author (P-L Wang).
Statistical analysis
All statistical methods used in this study were performed using Stata statistical software (version 12.0; Stata Corporation, College Station, TX, USA). The HRs and associated 95% Cis that were obtained from original articles were directly extracted or estimated from available data using methods previously reported by Tierney et al. [16]. For the pooled analysis of the relationship between EGFR expression and clinicopathological parameters, odds ratios (ORs) and their 95% CIs were combined to determine effective values. The point estimate of the HR or OR was considered statistically significant at a level of P<0.05 if the 95% CI did not include the value “1”.
DerSimonian-Laird random effects analysis [17] and the Mantel-Haenszel fixed effects method [18] were used to calculate the pooled HRs/ORs. The heterogeneity assumption was tested using Cochran’s Q test and simultaneously quantified using the Higgins I2 statistic [19]. A value of P>0.1 was regarded as indicating a lack of heterogeneity, while I2>50% indicated substantial heterogeneity. Sensitivity analyses were conducted to evaluate the stability of the results. An evaluation of potential publication bias was performed using a funnel plot. The asymmetry of the funnel plot was also assessed using Egger’s test [20].
Results
Study characteristics
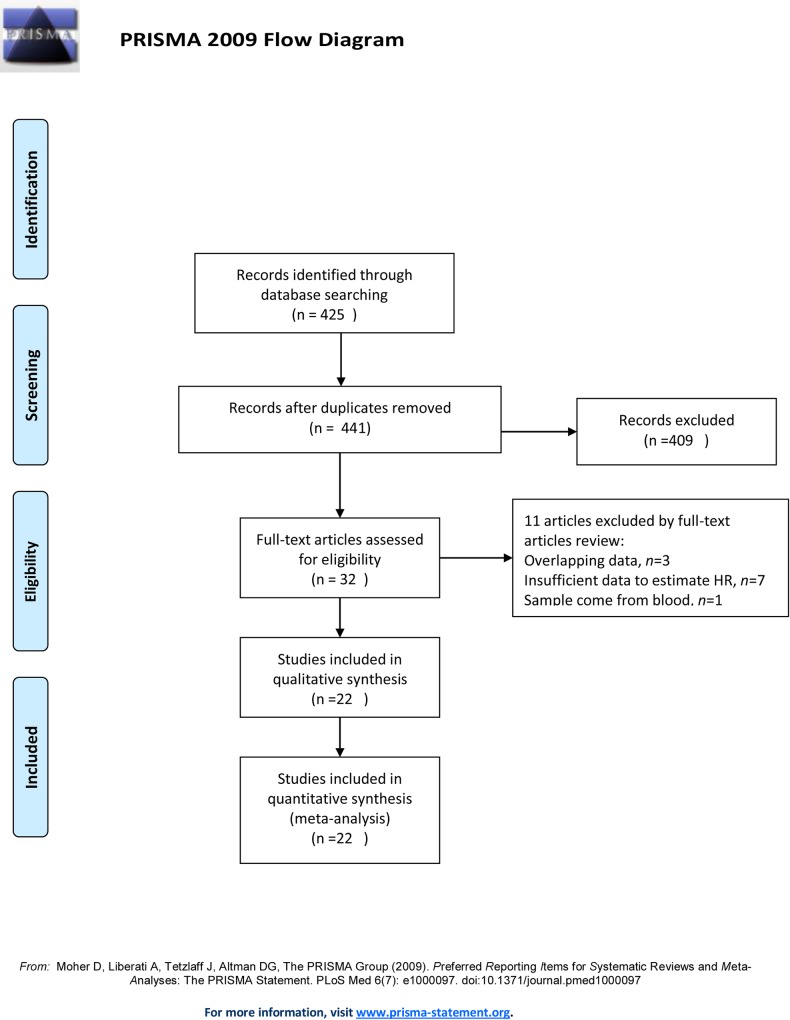
A total of 441 records were identified from a search of the primary literature that involved screening the title and abstract for the research results (Fig 1). Based on the inclusion criteria listed in a previous sections, 32 papers were eligible for further assessment. Overlap between patients was discovered among three studies [21–23]. The study [21] with the most recent data was selected for inclusion in the overall analysis. Seven studies were excluded because insufficient data were provided to estimate HRs (for an overview, see S3 Table). One study [24] that explored the relationship between circulating EGFR levels and cervical cancer was not excluded because it reduced the source of heterogeneity. In addition, two studies [25,26] involving data obtained from the same medical center had different inclusion criteria for the observed populations and treatments, and both of these studies were therefore included. One study [27] examined squamous cell carcinoma and adenocarcinoma separately, and the data from this study were therefore considered to represent two studies. Therefore, 22 studies [21,25–45] involving 2,505 patients were included in the final meta-analysis. The main characteristics of the 22 eligible studies are summarized in S1 Table. Among the studies included in this meta-analysis, 11 (52.4%) reported a significant association between EGFR overexpression and survival, including 10 (47.6%) that concluded that EGFR overexpression was associated with shorter survival and one (4.8%) that reported that EGFR expression was associated with longer survival. The remaining 11 reports (47.6%) yielded insignificant results.
Fig 1. Study Selection Flowchart.
The rate of positivity for EGFR overexpression in individual studies ranged from 18.0% to 87.3%. When subdivided according to the histological type of tissue, EGFR positivity was found to be 47.9% in squamous cell carcinomas and 38.2% in adenocarcinomas. The data were insufficient to analyze EGFR overexpression positivity in other histotypes. Overall, OS was extracted from 19 studies [21,26–28,30–39,41,42,44,45]. Among these studies, the predominant treatments included surgery in six studies and chemoradiation in nine studies. The remaining studies used mixed treatments or unknown methods. DFS was obtained from 11 studies [21,25,26,29,32,35,37,40,42,43,45]. Among these studies, the predominant treatment was surgery in four studies and chemoradiation in six studies.
Pooled analysis
EGFR expression and OS in cervical cancer patients
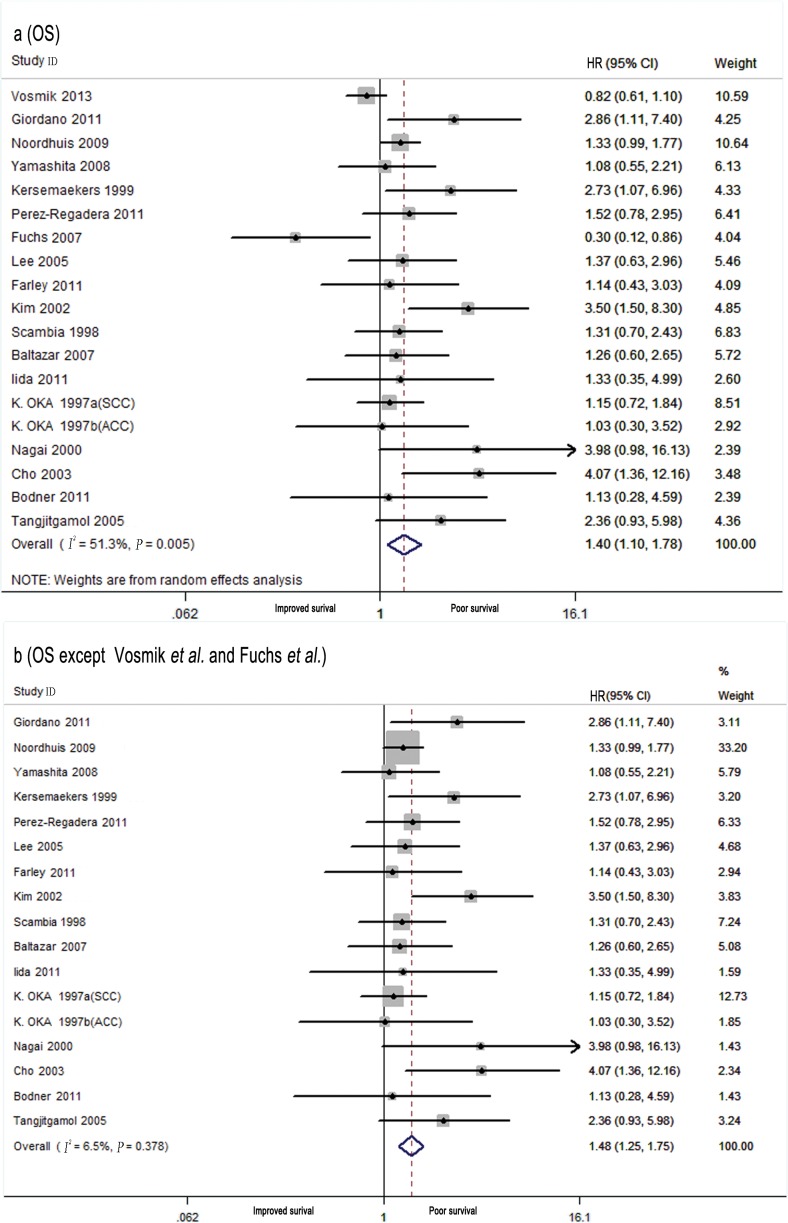
Table 1 demonstrates the main result of the meta-analysis results. Overall, in cervical patients, high EGFR levels in the primary tumor were significantly associated with poor OS in the random effects model (combined HR: 1.40, 95% CI: 1.10–1.78; Fig 2A) despite the presence of heterogeneity between the studies (I2 = 51.3%, Ph = 0.005). To identify the sources of this heterogeneity, subgroup analyses and meta-regressions were performed by treatment, histological type, quality rating score, number of patients, publication year, study design, and study location. Heterogeneity was found to be associated with study quality, the number of patients, the publication year, and the study design. When data related to these four characteristics were restricted (quality rating score ≥6, number of patients ≥100, publication year <2007, and prospective studies), the heterogeneity was substantially decreased and the pooled results remained practically unchanged (Table 1). Further meta-regression analyses showed that the publication year might account for part of the observed inter-study heterogeneity (P = 0.047). In studies performed after 2007, heterogeneity was mainly due to the results of studies by Vosmik et al. [28] and Fuchs et al. [39]. When these two studies were excluded from the meta-analysis, there was no heterogeneity (I2 = 6.5%, Ph = 0.378), and the pooled results remained practically unchanged (HR: 1.48, 95% CI: 1.25–1.75; Fig 2B) (Table 1). The subgroup analysis indicated the presence of an association between EGFR overexpression and OS in studies with a quality rating score ≥6 (HR: 1.42, 95% CI: 1.41–1.76), studies with ≥100 patients (HR: 1.40, 95% CI: 1.34–1.73), prospectively designed studies (HR: 1.49, 95% CI: 1.18–1.88), studies published before 2007 (HR: 1.69; 95% CI: 1.30–2.20), and studies performed in Asia (HR: 1.76, 95% CI: 1.09–2.82) (Table 1).
Table 1. Main Results of the Pooled Analysis.
| Analysis | N | References | Random-effects model | Fixed-effects model | Meta-regression P-value | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled HR | 95% CI of HR | Pooled HR | 95% CI of HR | I2 test (%) | P-value | ||||||
| Overall survival (OS) | 19 | 21, 26–28, 30–39, 41, 42, 44, 45 | 1.40 | 1.10–1.78 | 1.24 | 1.08–1.44 | 51.30% | 0.005 | |||
| OS (except Vosmik, M.; Fuchs, I.) | 17 | 21, 26–27, 30–38, 41, 42, 44, 45 | 1.51 | 1.26–1.81 | 1.48 | 1.25–1.75 | 6.50% | 0.378 | |||
| Subgroup 1: Treatment | 0.580 | ||||||||||
| Chemoradiation | 9 | 21, 26–28, 32, 37, 38, 41 | 1.19 | 0.94–1.51 | 1.14 | 0.96–1.34 | 33.60% | 0.149 | |||
| Surgery | 6 | 30, 31, 34, 35, 39, 45 | 1.53 | 0.67–3.48 | 1.51 | 0.96–2.37 | 68.10% | 0.008 | |||
| Subgroup 2:Quality rating score | 0.632 | ||||||||||
| Score ≥6 | 6 | 26, 31, 37, 32, 33, 36 | 1.42 | 1.14–1.76 | 1.42 | 1.41–1.76 | 0.00% | 0.53 | |||
| Score ≤5 | 13 | 21, 27, 28, 30, 34, 35, 38, 39, 41, 42, 44, 45 | 1.37 | 0.95–1.97 | 1.12 | 0.93–1.36 | 60.50% | 0.002 | |||
| Subgroup 3: Histological type | |||||||||||
| SCC | 8 | 27, 28, 30, 31, 32, 39, 41 | 1.17 | 0.73–1.87 | 0.99 | 0.80–1.23 | 68.50% | 0.004 | |||
| Other type | — | — | — | — | — | — | — | — | |||
| Subgroup 4: No. of patients | 0.590 | ||||||||||
| ≥100 | 7 | 26, 27, 31, 35, 37, 44 | 1.41 | 1.34–1.74 | 1.40 | 1.34–1.73 | 1.30% | 0.48 | |||
| <100 | 12 | 21, 28, 30, 32, 33, 34, 36, 38, 39, 41, 42, 45 | 1.35 | 0.94–1.96 | 1.12 | 0.92–1.36 | 59.40% | 0.003 | |||
| Subgroup 5: Publication year | 0.047 | ||||||||||
| ≥2007 | 10 | 26, 28, 30, 31, 32, 37, 38, 39, 44, 45 | 1.12 | 0.84–1.50 | 1.09 | 0.92–1.30 | 47.50% | 0.046 | |||
| <2007 | 9 | 21, 27, 33–36, 41, 42 | 1.86 | 1.31–2.63 | 1.69 | 1.30–2.20 | 35.10% | 0.137 | |||
| Subgroup 6: Study design | 0.295 | ||||||||||
| prospective | 5 | 26, 34, 36, 37, 42 | 1.66 | 1.52–2.38 | 1.49 | 1.18–1.88 | 38.20% | 0.166 | |||
| retrospective | 14 | 21, 27, 28, 30–33, 35, 38, 39, 41, 44, 45 | 1.29 | 0.95–1.75 | 1.11 | 0.92–1.33 | 51.20% | 0.014 | |||
| Subgroup 7: Study location | 0.132 | ||||||||||
| Europe | 8 | 26, 28, 31, 35–37, 45, 39 | 1.23 | 0.84–1.73 | 1.13 | 0.95–1.35 | 66.30% | 0.004 | |||
| Asia | 7 | 27, 30, 32, 34, 41, 42 | 1.76 | 1.09–2.82 | 1.54 | 1.13–2.10 | 46.80% | 0.08 | |||
| America | 4 | 21, 32, 33, 44 | 1.44 | 0.95–2.19 | 1.44 | 0.95–2.19 | 0.00% | 0.696 | |||
| Disease-free survival (DFS) | 11 | 21, 25, 26, 29, 32, 35, 37, 40, 42, 43, 45 | 1.91 | 1.50–2.44 | 1.84 | 1.51–2.24 | 23.90% | 0.216 | |||
| Subgroup 1: Treatment | |||||||||||
| Chemoradiation | 6 | 21, 26, 29, 32, 37, 40 | 1.70 | 1.30–2.23 | 1.69 | 1.32–2.15 | 10.80% | 0.346 | |||
| Surgery | 4 | 25, 35, 43, 45 | 2.15 | 1.27–2.63 | 2.02 | 1.37–2.96 | 40.20% | 0.17 | |||
| Subgroup 2: Quality rating score | |||||||||||
| Score ≥6 | 6 | 25. 26, 29, 32, 37, 40 | 1.67 | 1.29–2.15 | 1.65 | 1.31–2.07 | 13.70% | 0.327 | |||
| Score ≤5 | 5 | 21, 35, 42, 43, 45 | 2.55 | 1.72–3.77 | 2.55 | 1.72–3.77 | 0.00% | 0.435 | |||
| Subgroup 3: Histological type | |||||||||||
| SCC | 4 | 29, 32, 40, 43 | 2.22 | 1.30–3.49 | 2.06 | 1.43–3.01 | 37.60% | 0.186 | |||
| Other type | — | — | — | — | — | — | — | — | |||
| Subgroup 4: No. of patients | |||||||||||
| ≥100 | 6 | 25, 26, 29, 35, 37, 43 | 1.90 | 1.43–2.52 | 1.82 | 1.45–2.28 | 25.30% | 0.244 | |||
| <100 | 5 | 21, 32, 40, 42, 45 | 1.93 | 1.16–3.20 | 1.91 | 1.29–2.84 | 37.50% | 0.171 | |||
| Subgroup 5: Publication year | |||||||||||
| ≥2007 | 6 | 25, 26, 29, 32, 37, 45 | 1.59 | 1.26–2.00 | 1.59 | 1.26–2.00 | 0.00% | 0.594 | |||
| <2007 | 5 | 21, 35, 40, 42, 43 | 2.70 | 1.85–3.94 | 2.70 | 1.85–3.94 | 0.00% | 0.411 | |||
| Subgroup 6: Study design | |||||||||||
| prospective | 5 | 25, 26, 29, 37, 42 | 1.78 | 1.35–2.35 | 1.74 | 1.37–2.21 | 18.30% | 0.298 | |||
| retrospective | 6 | 21, 32, 35, 40, 43, 45 | 2.10 | 1.34–3.28 | 2.09 | 1.46–2.98 | 33.70% | 0.183 | |||
| Subgroup 7: Study location | |||||||||||
| Europe | 7 | 25, 26, 29, 35, 37, 43, 45 | 1.87 | 1.46–2.41 | 1.83 | 1.46–2.29 | 11.80% | 0.34 | |||
| Asia | 3 | 32, 40, 42 | 2.20 | 1.07–4.48 | 2.12 | 1.34–3.34 | 58.50% | 0.09 | |||
| America | 1 | 21 | — | — | — | — | — | — | |||
| Clinicopathological parameters | Pooled OR | 95% CI of OR | Pooled OR | 95% CI of OR | |||||||
| Age (old vs. young) | 4 | 26, 32, 36, 45 | 1.00 | 0.99–1.02 | 1.00 | 0.99–1.02 | 0.00% | 0.47 | |||
| Lymph node metastasis (Yes vs. No) | 7 | 26, 29, 32, 35, 36, 43, 45 | 1.72 | 1.23–2.40 | 1.72 | 1.23–2.40 | 0.00% | 0.862 | |||
| Tumor grade (grade 3 vs. grade 1 and grade 2) | 7 | 26, 35, 36, 40, 43, 45, 46 | 0.74 | 0.51–1.05 | 0.74 | 0.55–1 | 16.10% | 0.307 | |||
| Tumor size (size ≥4 cm vs. size <4 cm) | 7 | 26, 32, 35, 36, 40, 43, 45 | 1.64 | 1.20–2.23 | 1.64 | 1.20–2.23 | 0.00% | 0.935 | |||
| FIGO stage (stage III/IV vs. stage IB/II) | 6 | 26, 29, 35, 36, 45, 46 | 1.37 | 0.74–2.53 | 1.34 | 0.98–1.84 | 67.10% | 0.01 | |||
Fig 2. Meta-analysis of the Predictive and Prognostic Value of EGFR Expression for Determining Overall Survival (OS).
Each study is shown as the point estimate of its hazard ratio (HR) (the size of the square is proportional to the weight of each study) and the 95% confidence interval (CI) for the HR (horizontal bars). a, OS of all studies; b, OS of all studies except Vosmik et al. [28] and Fuchs et al. [39].
EGFR expression and DFS in cervical cancer
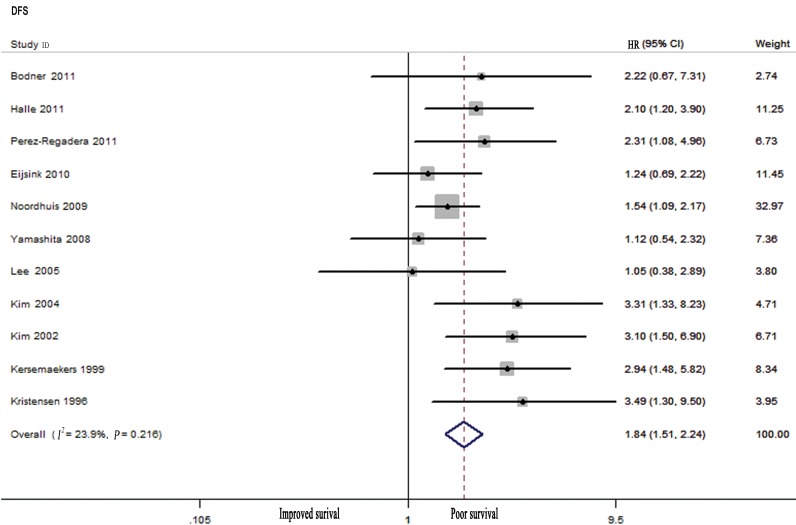
A meta-analysis of 11 studies revealed that high EGFR levels were associated with poor DFS (HR: 1.84, 95% CI: 1.51–2.24; Fig 3), and no significant heterogeneity was observed (I2 = 23.9%, Ph = 0.216). Subgroup analyses demonstrated that EGFR overexpression was related to poor DFS in cervical cancer patients who were treated with chemoradiation (HR: 1.69, 95% CI: 1.32–2.15) and surgery (HR: 2.02, 95% CI: 1.37–2.96). Other subgroup analyses performed by quality rating score, histological type, number of patients, publication year, study design, and study location also revealed associations between high EGFR levels and poor DFS in cervical cancer patients. There was no obvious heterogeneity in these data, except in studies that were conducted in Asia (Table 1).
Fig 3. Meta-analysis of the Predictive and Prognostic Value of EGFR Expression for Predicting Disease-free Survival (DFS).
EGFR expression and clinicopathological characteristics
Seven studies [26,29,32,35,36,43,45] assessed the relationship between EGFR overexpression and lymph node status. The pooled data showed that patients with lymph node metastasis exhibited significantly higher levels of EGFR expression than were observed in patients without metastasis, with a combined OR of 1.72 (95% CI: 1.23–2.40) and without heterogeneity (I2 = 0.00%, Ph = 0.862). After combining the seven studies [26,32,35,36,40,43,45] that examined tumor size, we also observed a statistically significant effect of high EGFR levels on tumor sizes ≥4 cm, with a combined OR of 1.64 (95% CI: 1.20–2.23) and no significant heterogeneity (I2 = 0.00%, Ph = 0.935). Furthermore, four studies [26,32,36,45] reported data on age, seven studies [26,35,36,40,43,45,46] reported the tumor grade, and six studies [26,29,35,36,45,46] reported the FIGO stage. The pooled results indicated that there was no significant association between high EGFR expression levels and age (OR: 1, 95% CI: 0.99–1.02), tumor grade (OR: 0.74, 95% CI: 0.51–1.05), or FIGO stage (OR: 1.37, 95% CI: 0.74–2.53) (Table 1).
Sensitivity analyses and publication bias
The sensitivity analysis indicated that omitting any single study did not significantly affect the combined HR, supporting the robustness of the HR estimates.
We constructed Begg’s funnel plots and conducted Egger’s tests to evaluate publication bias. The shape of the funnel plots revealed no significant asymmetry (figures not shown). Egger’s tests also demonstrated no any evidence of obvious publication bias among the studies included in the overall analyses of OS (P = 0.068) and DFS (P = 0.205).
Discussion
EGFR monoclonal antibodies have been applied as efficacious adjuvant treatments in solid tumors, such as colon and lung cancer. However, data related to the effect of EGFR inhibitors on cervical cancer remain inconclusive. Correctly evaluating the association between EGFR expression and survival in cervical cancer patients is essential to understanding the mechanisms of action underlying anti-EGFR therapies. The results that have been reported in other studies have been controversial. Therefore, we performed this meta-analysis to determine whether EGFR overexpression could predict prognoses in cervical cancer patients.
To our knowledge, this is the first meta-analysis to study the association between EGFR expression and OS, DFS, and clinicopathological characteristics in cervical cancer patients. Our combined analysis of 23 published studies that included 2,505 patients with cervical cancer yielded summary statistics demonstrating that in cervical cancer patients, high EGFR levels are associated with lower OS and DFS (Table 1).
In the studies included in this meta-analysis, significant heterogeneity was observed for OS. To explore the sources of this heterogeneity, we performed a sensitivity analysis, subgroup analysis, and meta-regression and found that the heterogeneity was mainly because of the studies by Vosmik et al. [28] and Fuchs et al. [39]. Further analysis revealed that both studies reported a favorable trend for cervical cancer patients with EGFR overexpression. This interesting result may have occurred because both studies simultaneously explored EGFR and other HER family members. Fuchs et al. [39] reported that the prognostic impact of HER1 (EGFR) or HER2 was often dependent on the correlation with HER3 or HER4. Therefore, further studies of the prognostic effects of HER family combinations are needed. Some other sources of heterogeneity might also exist in this meta-analysis. This include the method used to detect EGFR expression, the IHC scoring system, the cut-off values used for high EGFR expression, and differences in patients (e.g., differences in their ages, clinical stages, and physical conditions). Because of data limitations, we were unable to comprehensively analyze these aspects. The sources of heterogeneity that were identified indicate that using a consistent, standard study design and a larger sample size are necessary to obtain reliable results. Interestingly, no obvious evidence of data heterogeneity was found for DFS except for the studies conducted in Asia. Many of the included DFS studies met the criteria related to higher quality rating scores and larger numbers of patients. We also found that high EGFR levels were significantly correlated with poor OS and DFS in patients in studies performed in Asia (OS: HR: 1.76, 95% CI: 1.09–2.82; DFS: HR: 2.2, 95% CI: 1.07–4.48) but not in studies performed in other locations. This may be because the characteristics of cervical cancer might vary between geographical regions as a result of a variety of environmental factors and race-related genetic effects. However, significant heterogeneity was observed in both subgroup analyses. Thus, it is essential that more should be performed to determine whether EGFR overexpression is a prognostic factor for cervical cancer patients in Asia and other regions.
It has long been known that squamous cell carcinoma in cervical tumors differs in important ways from other histological subtypes [4]. In this study, we assessed the prognostic value of EGFR expression in squamous cell carcinoma. A significant association was observed between high EGFR levels and poor DFS in squamous cell cervical cancer patients (HR: 2.06, 95% CI: 1.43–3.01). However, the result for OS was not of prognostic value (HR: 1.17, 95% CI: 0.73–1.87), and significant heterogeneity was detected. Thus, these results should be interpreted with caution, and further studies are needed. Moreover, the number of studies of other histological subtypes is limited, and we were unable to conduct a subgroup analysis. Thus, further research regarding adenocarcinoma or adenosquamous cell carcinoma is encouraged.
EGFR inhibitors mainly target activating EGFR mutations in non-small-cell lung cancer (NSCLC), and EGFR expression and EGFR mutations have been investigated as potential predictors of responsiveness to EGFR tyrosine kinase inhibitors in NSCLC [47]. Sonobe et al. [48] reported that EGFR gene mutations were significantly associated with higher EGFR expression in patients with NSCLC. It is therefore reasonable to assume that there is a relationship between EGFR gene mutations and EGFR expression and that EGFR gene mutations may also be useful for predicting responses to EGFR inhibitors in patients with cervical cancer. However, because of EGFR mutations are very rare in cervical cancer [49] and data limitations, we were unable to analyze these hypotheses. They may be important areas for future work.
Some limitations were present in this meta-analysis. First, it has been shown that trials that provide negative results are usually only briefly reported or reported in non-English languages. This phenomenon was observed in this meta-analysis, although Begg’s funnel plots and Egger’s tests did not show any clear evidence of publication bias. Second, this meta-analysis was performed to analyze the results of observational trials, and more confounding factors are present in these types of trials than in randomized controlled trials. However, because of data limitations, we were unable to assess these factors in this study. Third, HRs that are extrapolated from survival curves or other relevant data might be less reliable than HRs that are obtained directly from articles. Hence, because of the limitations of this meta-analysis, the value of EGFR expression as a prognostic indicator in cervical cancer should be confirmed in future well-designed prospective clinical trials.
Despite its limitations, the results of the current stratified analysis, sensitivity analysis, and random and fixed effects models demonstrate the robustness of this meta-analysis.
Conclusions
This meta-analysis reveals that EGFR overexpression might be a predictive biomarker of reduced survival in patients with cervical cancer. This finding could potentially affect clinical decision-making and ultimately result in more effective targeted therapies for cervical cancer patients.
Supporting Information
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
All contributing authors are aware of and agree to the submission of this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by B2013118 (http://kyjj.gdwskj.cn/), Guangdong Province Medical Science Technology Grant (to MLH); and 2012D04 (http://www.sinopharmacy.com.cn/), Guangdong Pharmaceutical Association Fund (to MLH).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61: 69–90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam S, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62: 147–172. 10.3322/caac.21139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC). Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis. Cochrane Database Syst Rev. 2010: CD008285 10.1002/14651858.CD008285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soonthornthum T, Arias-Pulido H, Joste N, Lomo L, Muller C, Rutledge T, et al. Epidermal growth factor receptor as a biomarker for cervical cancer. Ann Oncol. 2011;22: 2166–2178. 10.1093/annonc/mdq723 [DOI] [PubMed] [Google Scholar]
- 5.Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26: 2311–2319. 10.1200/JCO.2007.13.1193 [DOI] [PubMed] [Google Scholar]
- 6.Rivera F, Garcia-Castano A, Vega N, Vega-Villegas ME, Gutierrez-Sanz L. Cetuximab in metastatic or recurrent head and neck cancer: the EXTREME trial. Expert Rev Anticancer Ther. 2009;9: 1421–1428. 10.1586/era.09.113 [DOI] [PubMed] [Google Scholar]
- 7.Johnson JR, Cohen M, Sridhara R, Chen YF, Williams GM, Duan J, et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res. 2005;11: 6414–6421. [DOI] [PubMed] [Google Scholar]
- 8.Sharma DN, Rath GK, Julka PK, Gandhi AK, Jagadesan P, Kumar S. Role of gefitinib in patients with recurrent or metastatic cervical carcinoma ineligible or refractory to systemic chemotherapy: first study from Asia. Int J Gynecol Cancer. 2013;23: 705–709. 10.1097/IGC.0b013e31828b1699 [DOI] [PubMed] [Google Scholar]
- 9.Goncalves A, Fabbro M, Lhomme C, Gladieff L, Extra JM, Floquet A, et al. A phase II trial to evaluate gefitinib as second- or third-line treatment in patients with recurring locoregionally advanced or metastatic cervical cancer. Gynecol Oncol. 2008;108: 42–46. [DOI] [PubMed] [Google Scholar]
- 10.Nogueira-Rodrigues A, Moralez G, Grazziotin R, Carmo CC, Small IA, Alves FV, et al. Phase 2 trial of erlotinib combined with cisplatin and radiotherapy in patients with locally advanced cervical cancer. Cancer. 2014;120: 1187–1193. 10.1002/cncr.28471 [DOI] [PubMed] [Google Scholar]
- 11.Schilder RJ, Sill MW, Lee YC, Mannel R. A phase II trial of erlotinib in recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Int J Gynecol Cancer. 2009;19: 929–933. 10.1111/IGC.0b013e3181a83467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santin AD, Sill MW, McMeekin DS, Leitao MM Jr, Brown J, Sutton GP, et al. Phase II trial of cetuximab in the treatment of persistent or recurrent squamous or non-squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;122: 495–500. 10.1016/j.ygyno.2011.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37 Suppl 4: S9–15. [DOI] [PubMed] [Google Scholar]
- 14.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes DF, Bast RC, Desch CE, Fritsche H Jr, Kemeny NE, Jessup JM, et al. Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst. 1996;88: 1456–1466. [DOI] [PubMed] [Google Scholar]
- 16.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8: 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7: 177–188. [DOI] [PubMed] [Google Scholar]
- 18.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22: 719–748. [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CM, Shrieve DC, Zempolich KA, Lee RJ, Hammond E, Handrahan DL, et al. Correlation between human epidermal growth factor receptor family (EGFR, HER2, HER3, HER4), phosphorylated Akt (P-Akt), and clinical outcomes after radiation therapy in carcinoma of the cervix. Gynecol Oncol. 2005;99: 415–421. [DOI] [PubMed] [Google Scholar]
- 22.Lee CM, Lee RJ, Hammond E, Tsodikov A, Dodson M, Zempolich K, et al. Expression of HER2neu (c-erbB-2) and epidermal growth factor receptor in cervical cancer: prognostic correlation with clinical characteristics, and comparison of manual and automated imaging analysis. Gynecol Oncol. 2004;93: 209–214. [DOI] [PubMed] [Google Scholar]
- 23.Gaffney DK, Haslam D, Tsodikov A, Hammond E, Seaman J, Holden J, et al. Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) negatively affect overall survival in carcinoma of the cervix treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56: 922–928. [DOI] [PubMed] [Google Scholar]
- 24.Oh MJ, Choi JH, Lee YH, Lee JK, Hur JY, Park YK, et al. Mutant p53 protein in the serum of patients with cervical carcinoma: correlation with the level of serum epidermal growth factor receptor and prognostic significance. Cancer Lett. 2004;203: 107–112. [DOI] [PubMed] [Google Scholar]
- 25.Eijsink JJ, Noordhuis MG, ten Hoor KA, Kok M, Hollema H, de Bock GH, et al. The epidermal growth factor receptor pathway in relation to pelvic lymph node metastasis and survival in early-stage cervical cancer. Hum Pathol. 2010;41: 1735–1741. 10.1016/j.humpath.2010.04.017 [DOI] [PubMed] [Google Scholar]
- 26.Noordhuis MG, Eijsink JJ, ten Hoor KA, Roossink F, Hollema H, Arts HJ, et al. Expression of epidermal growth factor receptor (EGFR) and activated EGFR predict poor response to (chemo)radiation and survival in cervical cancer. Clin Cancer Res. 2009;15: 7389–7397. 10.1158/1078-0432.CCR-09-1149 [DOI] [PubMed] [Google Scholar]
- 27.Oka K, Nakano T, Arai T. Expression of cathepsin D and epidermal growth factor receptor in stage III cervical carcinomas. Int J Gynecol Cancer. 1997;7: 122–126. [Google Scholar]
- 28.Vosmik M, Laco J, Sirak I, Beranek M, Hovorkova E, Vosmikova H, et al. Prognostic significance of human papillomavirus (HPV) status and expression of selected markers (HER2/neu, EGFR, VEGF, CD34, p63, p53 and Ki67/MIB-1) on outcome after (chemo-) radiotherapy in patients with squamous cell carcinoma of uterine cervix. Pathol Oncol Res. 2014;20: 131–137. 10.1007/s12253-013-9674-5 [DOI] [PubMed] [Google Scholar]
- 29.Halle C, Lando M, Svendsrud DH, Clancy T, Holden M, Sundfor K, et al. Membranous expression of ectodomain isoforms of the epidermal growth factor receptor predicts outcome after chemoradiotherapy of lymph node-negative cervical cancer. Clin Cancer Res. 2011;17: 5501–5512. 10.1158/1078-0432.CCR-11-0297 [DOI] [PubMed] [Google Scholar]
- 30.Iida K, Nakayama K, Rahman MT, Rahman M, Ishikawa M, Katagiri A, et al. EGFR gene amplification is related to adverse clinical outcomes in cervical squamous cell carcinoma, making the EGFR pathway a novel therapeutic target. Br J Cancer. 2011;105: 420–427. 10.1038/bjc.2011.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giordano G, D'Adda T, Dal Bello B, Brigati F, Bersiga A, Campanini N, et al. Clinicopathologic implications of the epidermal growth factor receptor, cyclooxygenase 2 expression, and human papillomavirus status in squamous cell carcinoma of the uterine cervix in the elderly. Int J Gynecol Cancer. 2011;21: 337–348. 10.1097/IGC.0b013e31820864b7 [DOI] [PubMed] [Google Scholar]
- 32.Yamashita H, Murakami N, Asari T, Okuma K, Ohtomo K, Nakagawa K. Correlation among six biologic factors (p53, p21(WAF1), MIB-1, EGFR, HER2, and Bcl-2) and clinical outcomes after curative chemoradiation therapy in squamous cell cervical cancer. Int J Radiat Oncol Biol Phys. 2009;74: 1165–1172. 10.1016/j.ijrobp.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 33.Tangjitgamol S, Ramirez PT, Sun CC, See HT, Jhingran A, Kavanagh JJ, et al. Expression of HER-2/neu, epidermal growth factor receptor, vascular endothelial growth factor, cyclooxygenase-2, estrogen receptor, and progesterone receptor in small cell and large cell neuroendocrine carcinoma of the uterine cervix: a clinicopathologic and prognostic study. Int J Gynecol Cancer. 2005;15: 646–656. [DOI] [PubMed] [Google Scholar]
- 34.Nagai N, Oshita T, Fujii T, Kioka H, Katsube Y, Ohama K. Prospective analysis of DNA ploidy, proliferative index and epidermal growth factor receptor as prognostic factors for pretreated uterine cancer. Oncol Rep. 2000;7: 551–559. [DOI] [PubMed] [Google Scholar]
- 35.Kersemaekers AM, Fleuren GJ, Kenter GG, Van den Broek LJ, Uljee SM, Hermans J, et al. Oncogene alterations in carcinomas of the uterine cervix: overexpression of the epidermal growth factor receptor is associated with poor prognosis. Clin Cancer Res. 1999;5: 577–586. [PubMed] [Google Scholar]
- 36.Scambia G, Ferrandina G, Distefano M, D'Agostino G, Benedetti-Panici P, Mancuso S. Epidermal growth factor receptor (EGFR) is not related to the prognosis of cervical cancer. Cancer Lett. 1998;123: 135–139. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Regadera J, Sanchez-Munoz A, De-la-Cruz J, Ballestin C, Lora D, Garcia-Martin R, et al. Impact of epidermal growth factor receptor expression on disease-free survival and rate of pelvic relapse in patients with advanced cancer of the cervix treated with chemoradiotherapy. Am J Clin Oncol. 2011;34: 395–400. 10.1097/COC.0b013e3181e84634 [DOI] [PubMed] [Google Scholar]
- 38.Farley J, Sill MW, Birrer M, Walker J, Schilder RJ, Thigpen JT, et al. Phase II study of cisplatin plus cetuximab in advanced, recurrent, and previously treated cancers of the cervix and evaluation of epidermal growth factor receptor immunohistochemical expression: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;121: 303–308. 10.1016/j.ygyno.2011.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuchs I, Vorsteher N, Buhler H, Evers K, Sehouli J, Schaller G, et al. The prognostic significance of human epidermal growth factor receptor correlations in squamous cell cervical carcinoma. Anticancer Res. 2007;27: 959–963. [PubMed] [Google Scholar]
- 40.Kim GE, Kim YB, Cho NH, Chung HC, Pyo HR, Lee JD, et al. Synchronous coexpression of epidermal growth factor receptor and cyclooxygenase-2 in carcinomas of the uterine cervix: a potential predictor of poor survival. Clin Cancer Res. 2004;10: 1366–1374. [DOI] [PubMed] [Google Scholar]
- 41.Cho NH, Kim YB, Park TK, Kim GE, Park K, Song KJ. P63 and EGFR as prognostic predictors in stage IIB radiation-treated cervical squamous cell carcinoma. Gynecol Oncol. 2003;91: 346–353. [DOI] [PubMed] [Google Scholar]
- 42.Kim YT, Park SW, Kim JW. Correlation between expression of EGFR and the prognosis of patients with cervical carcinoma. Gynecol Oncol. 2002;87: 84–89. [DOI] [PubMed] [Google Scholar]
- 43.Kristensen GB, Holm R, Abeler VM, Trope CG. Evaluation of the prognostic significance of cathepsin D, epidermal growth factor receptor, and c-erbB-2 in early cervical squamous cell carcinoma. An immunohistochemical study. Cancer. 1996;78: 433–440. [DOI] [PubMed] [Google Scholar]
- 44.Baltazar F, Filho AL, Pinheiro C, Moreira MA, Queiroz GS, Oton GJ, et al. Cyclooxygenase-2 and epidermal growth factor receptor expressions in different histological subtypes of cervical carcinomas. Int J Gynecol Pathol. 2007;26: 235–241. [DOI] [PubMed] [Google Scholar]
- 45.Bodner K, Laubichler P, Kimberger O, Czerwenka K, Zeillinger R, Bodner-Adler B. Expression of p16 protein and epidermal growth factor receptor in patients with adenocarcinoma of the uterine cervix: an immunohistochemical analysis. Arch Gynecol Obstet. 2011;283: 611–616. 10.1007/s00404-010-1464-7 [DOI] [PubMed] [Google Scholar]
- 46.Skomedal H, Kristensen GB, Lie AK, Holm R. Aberrant expression of the cell cycle associated proteins TP53, MDM2, p21, p27, cdk4, cyclin D1, RB, and EGFR in cervical carcinomas. Gynecol Oncol. 1999;73: 223–228. [DOI] [PubMed] [Google Scholar]
- 47.Roengvoraphoj M, Tsongalis GJ, Dragnev KH, Rigas JR. Epidermal growth factor receptor tyrosine kinase inhibitors as initial therapy for non-small cell lung cancer: focus on epidermal growth factor receptor mutation testing and mutation-positive patients. Cancer Treat Rev. 2013;39: 839–850. 10.1016/j.ctrv.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 48.Sonobe M, Nakagawa M, Takenaka K, Katakura H, Adachi M, Yanagihara K, et al. Influence of epidermal growth factor receptor (EGFR) gene mutations on the expression of EGFR, phosphoryl-Akt, and phosphoryl-MAPK, and on the prognosis of patients with non-small cell lung cancer. J Surg Oncol. 2007;95: 63–69. [DOI] [PubMed] [Google Scholar]
- 49.Arias-Pulido H, Joste N, Chavez A, Muller CY, Dai D, Smith HO, et al. Absence of epidermal growth factor receptor mutations in cervical cancer. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2008;18(4):749–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.