Abstract
Aim:
Our preliminary study shows that a bibenzyl compound isolated from Gastrodia elata, 2-[4-hydroxy-3-(4-hydroxybenzyl)benzyl]-4-(4-hydroxybenzyl)phenol (designated 20C), protects PC12 cells against H2O2-induced injury. In this study we investigated whether 20C exerted neuroprotective action in a cell model of Parkinson's disease.
Methods:
A cell model of Parkinson's disease was established in PC12 cells by exposure to rotenone (4 μmol/L) for 48 h. Cell viability and apoptosis were assessed, and intracellular ROS level and the mitochondrial membrane potential (MMP) were detected. The expression of apoptosis-related proteins Bax, Bcl-2, cytochrome c, cleaved caspase-3, and oxidative stress-related proteins Nrf2, HO-1 and NQO1 were examined using Western blotting. The mRNA levels of HO-1 and NQO1 were determined with RT-PCR. The nuclear translocation of Nrf2 was observed with immunofluorescence staining.
Results:
Treatment with rotenone significantly increased the number of apoptotic cells, accompanied by marked increases in the Bax/Bcl-2 ratio, cytochrome c release and caspase-3 activation. Rotenone also increased ROS accumulation, reduced MMP, and increased the nuclear translocation of Nrf2 as well as the mRNA and protein levels of the Nrf2 downstream target genes HO-1 and NQO1 in PC12 cells. Co-treatment with 20C (0.01–1 μmol/L) dose-dependently attenuated rotenone-induced apoptosis and oxidative stress in PC12 cells. Nrf2 knockdown by siRNA partially reversed the protective effects of 20C in rotenone-treated PC12 cells.
Conclusion:
The bibenzyl compound 20C protects PC12 cells from rotenone-induced apoptosis, at least in part, via activation of the Nrf2/ARE/HO-1 signaling pathway.
Keywords: bibenzyl compound, Gastrodia elata, rotenone, apoptosis, oxidative stress, Nrf2, neuroprotection, Parkinson's disease, PC12 cells
Introduction
Parkinson's disease (PD) is the second most common age-related neurodegenerative disorder after Alzheimer's disease1. Neuropathologically, it is characterized by the progressive loss of dopaminergic neurons and the associated depletion of postsynaptic dopamine within the striatum2. However, the molecular mechanism underlying dopaminergic neuron death is still not completely understood. One of the major hypotheses posits that mitochondrial dysfunction and the subsequent oxidative stress are the main contributors to neuronal cell death3,4. The overproduction of reactive oxygen species (ROS) in response to oxidative stress induces the peroxidation of lipids and proteins, the oxidation of nucleic acids, and DNA breakdown, thus promoting further cell damage5.
Rotenone is one of the most widely used pesticides. As a neurotoxin and mitochondrial complex I inhibitor, rotenone has been shown to enhance the formation of intracellular ROS and lead to mitochondrial dysfunction, culminating in apoptotic cell death and is used to create models of PD in vivo and in vitro6. Recently, a considerable number of reports have shown that different exogenous herbal antioxidants attenuate the oxidative damage caused by rotenone, which emphasizes that these promising herbal drugs should be explored as PD therapies7.
Gastrodia elata (Tianma, GE) is a commonly used traditional Chinese medicine with numerous therapeutic applications, such as for treating vertigo and epilepsy8. A number of studies suggest that extracts from GE exert antioxidant activity9,10. 20C is a novel bibenzyl compound isolated from Gastrodia elata. Our previous data indicated that 20C could protect PC12 cells against H2O2-induced injury (data not published). It has not been determined if 20C could inhibit the neurotoxicity of rotenone.
The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) is a key protein that regulates the redox state of cells in response to oxidative stress11. Under physiological, unstressed conditions, Nrf2 is anchored by the Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm and degraded by the ubiquitin proteasome pathway12. Once cells encounter an oxidative insult, Nrf2 is released from Keap1 and translocates into the nucleus where it binds to the antioxidant response element (ARE) to activate the transcription of a battery of antioxidant and cytoprotective genes13. The antioxidant enzymes include hemeoxygenase-1 (HO-1), nicotinamide adenine dinucleotide phosphate: quinine oxidoreductase-1 (NQO1), superoxide dismutase, and glutathione peroxidase14.
The Nrf2/ARE/HO-1 signaling pathway is considered to be a protective molecular mechanism in several pathological processes, particularly in oxidative stress, and this pathway is also involved in several central nervous system diseases, including PD15,16. In this study, we investigated the anti-apoptotic effects of 20C on PC12 cells intoxicated by rotenone as well as the role of the Nrf2/ARE/HO-1 signaling pathway in this process.
Materials and methods
Chemicals and reagents
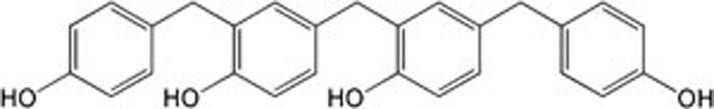
20C was provided by the Department of Chemosynthesis, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China). The chemical structure is shown in Figure 1. 20C was dissolved in dimethyl sulfoxide (DMSO), aliquoted and frozen at −80 °C until further use. Rotenone was purchased from Sigma-Aldrich (St Louis, MO, USA) and freshly dissolved in DMSO prior to each experiment. The Annexin V-FITC/PI staining kit was purchased from Beijing Biosea Biotechnology Company (Beijing, China), and JC-1 and dichlorofluorescin diacetate (DCFH-DA) were obtained from the Beyotime Institute of Biotechnology (Beijing, China).
Figure 1.
The chemical structure of 20C.
Cell culture and treatment
PC12 cells, a rat pheochromocytoma cell line, were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 5% fetal bovine serum, 10% heat-inactivated horse serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mmol/L L-glutamine (Invitrogen, Grand Island, NY, USA). The cells were maintained at 37 °C in a humidified atmosphere with 5% CO2 and 95% air. The media were replaced every two or three days, and the cells were passaged twice per week. The cells were plated in Poly-L-lysine (PLL)-coated plates at an appropriate density, according to the requirements of each experiment. After a 24-h subculture, the cells were switched to fresh medium for treatment. The attached cells were then exposed to 4 μmol/L rotenone in the presence or absence of 0.01, 0.1, 1 μmol/L 20C (added immediately before rotenone). For the viability assay and morphological observations, the cells were seeded in 96-well culture plates at a density of 5×104 cells/mL. For immunocytochemical staining and the ROS and mitochondrial membrane potential measurements, the cells were plated on 24-well culture plates at a density of 3×105 cells/mL. For the other experiments, the cells were plated on 6-well culture plates at a density of 3×105 cells/mL. For the MTT (Methyl Thiazolyl Tetrazolium) assay, the cells were treated with 20C and rotenone for 48 h; for the other experiments, the cells were treated with 20C and rotenone for 24 h.
Morphological observations and cell viability
The morphological changes in the different groups of PC12 cells were observed using a phase-contrast microscope (Olympus, Tokyo, Japan). Cell viability was assessed by the MTT assay. The cells were incubated with 10 μL of a 5 mg/mL MTT solution (Sigma-Aldrich) for 4 h at 37 °C, and then the media were carefully removed. The formazan crystals were dissolved in 100 μL of DMSO and the optical density (OD) was measured using a Microplate Reader (Thermo, Waltham, MA, USA) at a wavelength of 570 nm. The cell viability was expressed as a percentage of the OD value of the control cultures.
Analysis of apoptosis by flow cytometry
The apoptosis rate was measured by flow cytometry, as previously reported17. After exposure to the indicated doses of rotenone or rotenone plus 20C, the cells were harvested by centrifugation (800×g, 5 min) and washed with ice-cold phosphate-buffered saline (PBS) three times. The apoptosis rate was detected by flow cytometry using an Annexin V-FITC/PI apoptosis detection kit, as previously described18.
Intracellular ROS measurement
The intracellular ROS levels were examined using DCFH-DA, and assayed according to the manufacturer's instructions. DCFH-DA, which is oxidized to the fluorescent product DCF in the presence of ROS, was used to measure the relative cellular peroxide levels. Briefly, the cells were washed once with serum-free medium and incubated with 10 μmol/L DCFH-DA for 20 min at 37 °C in the dark. Then, the cells were washed with serum-free medium three times and visualized using a fluorescent microscope (Nikon Eclipse Ti-U, Tokyo, Japan). The cells were located under bright-field optics and then scanned with the laser (488 nm for excitation and 525 nm for emission). The fluorescent density was analyzed by Image-Pro Plus software version 6.0 (Media Cybemetics, Bethesda, MD, USA).
Measurement of the mitochondrial membrane potential (MMP)
The changes in the inner MMP were determined by incubating the cells with 10 μg/mL JC-1, a cationic dye used to measure the change in the inner MMP at 37 °C. JC-1 accumulates in the mitochondria, forming red fluorescent aggregates at high membrane potential; however, at low membrane potential, JC-1 is mainly in the form of green fluorescent monomers. After a 20-min incubation, the cells were rinsed with PBS three times and immediately submitted to fluorescence microscopy analysis. The JC-1-loaded cells were excited at 488 nm, and the emission was detected at 590 nm (JC-1 aggregates) and 525 nm (JC-1 monomers). Mitochondrial depolarization was indicated by an increase in the green/red fluorescence ratio, which was calculated with Image-Pro Plus software version 6.0.
Western blotting
The PC12 cells were harvested and lysed in lysis buffer (50 mmol/L Tris-HCl, pH 7.5, 150 mol/L NaCl, 1 % NP-40, and 1 mol/L EDTA) for 30 min in the presence of a protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitors (Thermo) on ice. The lysate was centrifuged for 30 min at 12 000 revolutions/min, and the supernatant was collected and boiled for 10 min at 100 °C. The nuclear and cytoplasmic proteins were extracted from the cells with the Nuclear-Cytosol Extraction Kit (Applygen Technologies Inc, Beijing, China). The cytoplasmic proteins and mitochondrial proteins were extracted from the cells with the Cell Mitochondria Isolation Kit (Beyotime Institute of Biotechnology, Beijing, China). The protein concentrations were determined using the bicinchoninic acid protein assay (Applygen Technologies Inc). Twenty micrograms of protein from each sample were separated by SDS-PAGE and then transferred to a PVDF membrane (Millipore, Boston, MA, USA). The membrane was blocked with 3% BSA (Sigma-Aldrich) and incubated with the following primary antibodies overnight at 4 °C: anti-Bcl-2, anti-Bax, anti-cytochrome C, anti-caspase-3, anti-Nrf2, anti-HO-1, anti-NQO1 (1:500 dilutions; all from Santa Cruz Biotechnology, Dallas, TX, USA), anti-PCNA (1:1000 dilution, Cell Signaling Technology, Danvers, MA, USA), and anti-β-actin (1:5000 dilution, Sigma-Aldrich). The blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000 dilution, all from KPL, Gaithersburg, MD, USA) for 2 h at room temperature, and the bands were detected with the enhanced chemiluminescence plus detection system (ImageQuant LAS4000mini, GE, Fairfield, CT, USA). The signals from specific protein brands were quantified using the Gel-Pro Analyzer software (Media Cybernetics).
Immunofluorescence staining
The PC12 cells were grown on PLL-coated (0.1 mg/mL) Lab-Tek chambered cover glasses (Nunc, IL, USA). The cell culture media was removed and the cells were fixed in 4% paraformaldehyde for 20 min at room temperature and washed three times with PBS. The cells were permeabilized in 0.1% Triton-X-100 (Sigma-Aldrich) for 10 min and blocked in 3% goat serum for 1 h at room temperature.
After an overnight incubation with the anti-Nrf2 antibody at 4 °C, the cells were washed three times with PBS and then incubated with secondary antibody (AlexaFluor 546-conjugated goat anti-rabbit antibody, 1:200 dilution, Invitrogen, New York, USA) for 1 h at room temperature. Afterwards, the cells were washed three times with PBS and stained with Hoechst for 10 min at room temperature. The images were acquired with a laser scanning confocal microscope fluorescence microscope (Leica TCS SP2, Solms, Germany).
Reverse transcription polymerase chain reaction
The total RNA was extracted from the PC12 cells in the presence or absence of 20C. One microgram of RNA from each sample was reversed transcribed to cDNA using the reverse transcription kit (Invitrogen) according to the manufacturer's instructions, and amplified by polymerase chain reaction (PCR) with primers specific for HO-1 (forward primer: 5′-ATGGAGCGCCCACAGTCGAC-3′, reverse primer: 5′-GGTAGCGGGTATATGCGTGGG-3′), NQO1 (forward primer: 5′-ATGGCGGTGAGAAGAGCCCTGC-3′, reverse primer: 5′-ACCCTTGTCATACATGGTGGC-3′), and GAPDH (forward primer: 5′-CATCACCATCTTCCAGGAGCG-3′, reverse primer: 5′-TGACCTTGCCCACAGCCTTG-3′). PCR was performed using the following parameters: 95 °C for 5 min; then, for HO-1: 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s; for NQO1: 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; and for GAPDH: 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. After 30 cycles of amplification, a final extension step was performed at 72 °C for 10 min. The amplified cDNAs were separated by electrophoresis on a 1 % agarose gel, stained with ethidium bromide, and visualized using an electrophoresis gel imaging analysis system and Quantity One software (Bio-Rad, Hercules, CA, USA).
Nrf2-siRNA knockdown
The PC12 cells were grown to 60%–80% confluence in culture media. The cells were transfected with Nrf2-siRNA or control-siRNA using an siRNA transfection reagent (Santa Cruz Biotechnology), according to the manufacturer's protocol. The final concentration of the siRNA was 60 nmol/L. The knockdown efficiency was validated by Western blotting. To confirm the specificity of the interference, a nontargeting siRNA was used as a negative control. After 24 h of transfection, the transfection solution was removed and the cells were rinsed with PBS and treated with rotenone in the presence or absence of 20C. The cells were collected for the cell viability assay.
Statistical analysis
The data were expressed as the means±SD. Significant differences between the experimental and control groups were assessed by Student's t-test (Figure 6A) and one-way ANOVA using GraphPad Prism 5 software (La Jolla, CA, USA). P<0.05 was considered statistically significant.
Results
20C protected PC12 cells against the rotenone-induced apoptosis
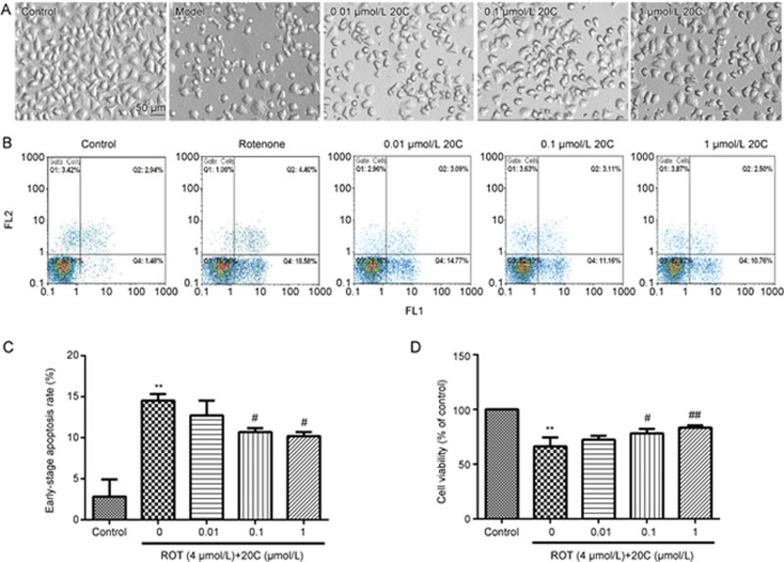
As shown in Figure 2A, the rotenone-treated PC12 cells exhibited morphological alterations that are normally associated with apoptosis, such as cell shrinkage and membrane blebbing. The 20C treatment alleviated these morphological features of damaged cells. Using the MTT assay, we showed that rotenone induced remarkable cytotoxicity in the PC12 cells, with only 66.2% of the cells remaining viable (Figure 2D). When the cells were treated with 0.01, 0.1 and 1 μmol/L 20C, the cell viability was restored to 72.4%, 78.2% and 83.3%, respectively.
Figure 2.
Effects of 20C on rotenone-induced apoptosis. (A) Phase-contrast micrographs of PC12 cells exposed to 4 μmol/L rotenone for 48 h in the presence or absence of 20C. (B, C) Flow cytometry analysis of rotenone-induced apoptosis in PC12 cells. (B) The graphs of apoptosis were obtained from the flow cytometry analysis. (C) Quantitative analysis of the ratio of early apoptotic cells. (D) Cell viability was measured by the MTT assay. The absorbance of the control cells was set as 100%. The data were obtained from three independent experiments. **P<0.01 vs the control group, #P<0.05, ##P<0.01 vs the model group (n=3). ROT, rotenone.
The anti-apoptosis effect of 20C in the PC12 was further studied using the Annexin V-FITC/PI assay. As shown in Figure 2B and C, the percentage of PC12 cells undergoing early apoptosis following rotenone treatment was significantly increased (P<0.01). Moreover, the 0.1 and 1 μmol/L doses of 20C reduced the rotenone-induced apoptosis (P<0.05, P<0.05).
20C suppressed the pro-apoptotic effect of rotenone by inhibiting the increase in the Bax/Bcl-2 ratio, cytochrome c release, and caspase-3 cleavage
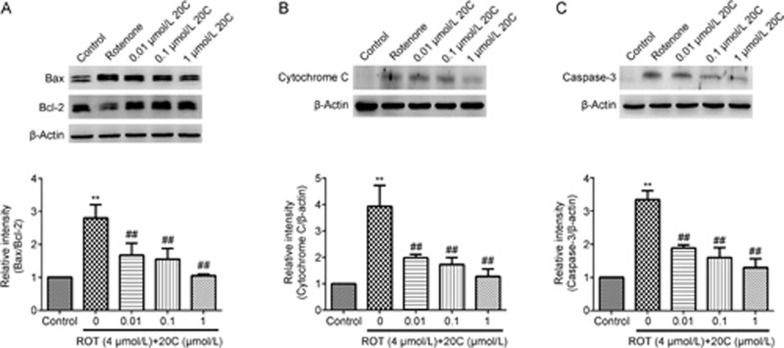
In this study, we demonstrated that 20C (0.01, 0.1 and 1 μmol/L) inhibited the rotenone-induced up-regulation of Bax and down-regulation of Bcl-2, thus decreasing the Bax/Bcl-2 ratio, as shown in Figure 3A (P<0.01). Additionally, the cytoplasmic cytochrome C release was decreased in the cells that were treated with various concentrations of 20C (0.01, 0.1 and 1 μmol/L; P<0.01) compared with the rotenone-treated group (Figure 3B). Furthermore, we assessed the caspase-3 cleavage by determining the concentration of cleaved caspase-3 (17 kD). As shown in Figure 3C, the rotenone-induced increase in cleaved caspase-3 was reversed by co-treatment with 20C at doses of 0.01, 0.1 and 1 μmol/L (P<0.01).
Figure 3.
Effects of 20C on the expression of apoptosis-related proteins. (A) Western blotting analysis of the levels of the Bax and Bcl-2 proteins in PC12 cells exposed to rotenone in the presence or absence of various concentrations of 20C. (B) Western blotting analysis of the levels of the cytoplasmic cytochrome C protein in PC12 cells exposed to rotenone in the presence or absence of various concentrations of 20C. (C) Western blotting analysis of the levels of the cleaved caspase-3 protein in PC12 cells exposed to rotenone in the presence or absence of various concentrations of 20C. **P<0.01 vs the control group, ##P<0.01 vs the model group (n=3).
20C suppressed the accumulation of intracellular ROS and the collapse of the mitochondrial membrane potential
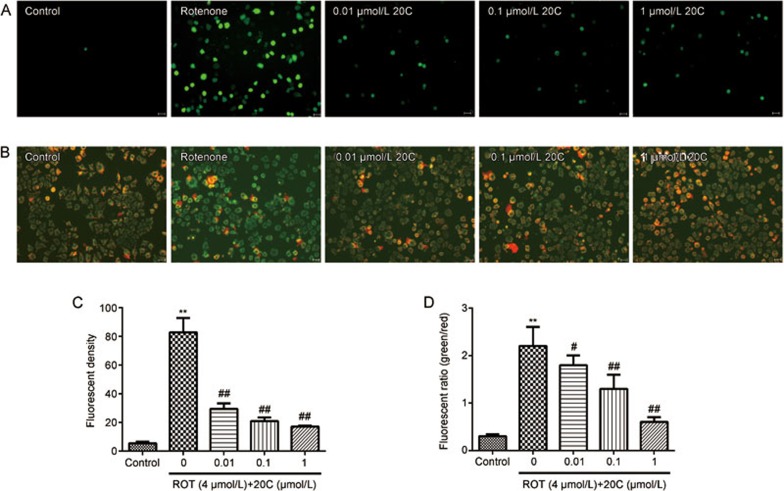
To further study the mechanisms underlying the protective effect of 20C, the intracellular ROS levels were determined using DCFH-DA and fluorescence microscopy. As shown in Figure 4A, normal PC12 cells exhibited weak green fluorescence, and the green fluorescence was remarkably enhanced following rotenone exposure (P<0.01). In the 20C treatment group, the intensity of the green fluorescence was significantly reduced (Figure 4C, P<0.01).
Figure 4.
Effects of 20C on rotenone-induced oxidative stress. (A, B) The ROS levels (A) and MMP (B) of PC12 cells exposed to rotenone in the presence or absence of 20C were determined using DCFH-DA (A) and JC-1 (B). The scale bar represents 20 μm. (C, D) Quantitative analysis of the ROS levels (C) and MMP (D). **P<0.01 vs the control group, #P<0.05, ##P<0.01 vs the model group (n=3).
The MMP was identified using the mitochondria-specific fluorescent dye JC-1. Normal PC12 cells stained with the JC-1 dye emitted a mitochondrial orange-red fluorescence, with a small amount of green fluorescence, as shown in Figure 4B. These JC-1 aggregates within the normal mitochondria were dispersed into the monomeric form (green fluorescence) upon addition of rotenone to the culture medium. After treatment with 0.01, 0.1 and 1 μmol/L 20C, the ratio of green/red fluorescence was significantly decreased (Figure 4D, P<0.05, P<0.01, P<0.01).
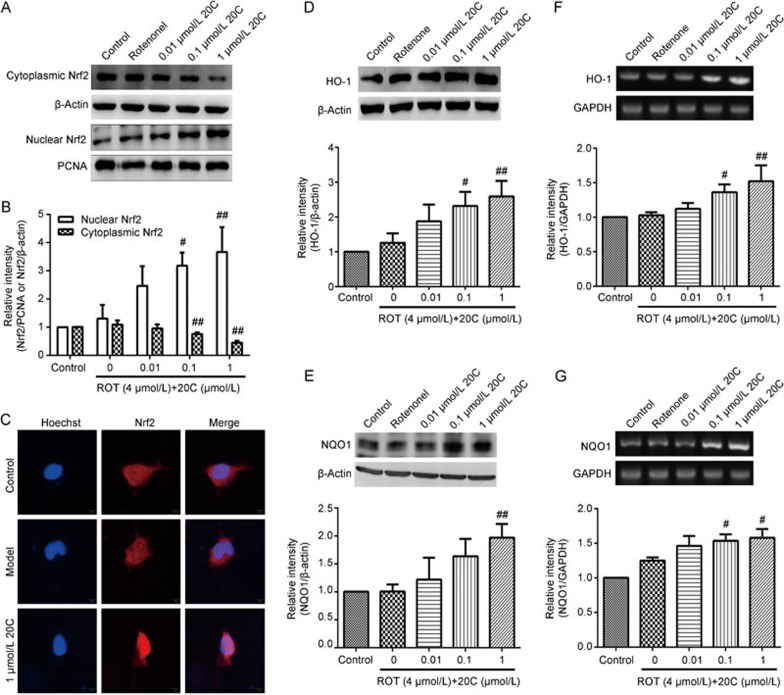
20C promoted Nrf2 translocation from the cytoplasm to the nucleus and the expression of its downstream factors
To gain further insights into the molecular mechanisms underlying the anti-apoptosis effect of 20C in PC12 cells, the transcription factor Nrf2 was examined as a potential upstream regulator of the cellular antioxidant system. The well-established, classical activation pattern of Nrf2 involves its translocation from the cytoplasm to the nucleus. Therefore, we first investigated the nuclear accumulation of Nrf2 protein in the cells treated with 20C. The results obtained from the Western blotting analysis showed that treatment with 0.1 and 1 μmol/L 20C resulted in a significant accumulation of Nrf2 in the nucleus (P<0.05, P<0.01) and a decrease in cytoplasmic Nrf2 in a dose-dependent manner (Figure 5A and B, P<0.01). The nuclear translocation of Nrf2 was also confirmed by immunofluorescence. In the control and rotenone-treated groups, Nrf2 was predominantly located in the cytoplasm, whereas Nrf2 translocated from the cytoplasm to the nucleus in the cells treated with 1 μmol/L 20C (Figure 5C).
Figure 5.
Effects of 20C on the Nrf2/ARE/HO-1 signaling pathway. (A) Western blotting analysis of the levels of the Nrf2 protein in the cytoplasm and nucleus of PC12 cells exposed to rotenone in the presence or absence of various concentrations of 20C. (B) Quantitative analysis of the density of Nrf2 in the cytoplasm and nucleus. (C) Confocal microscopy images of immunofluorescence with an anti-Nrf2 antibody showed the nuclear translocation of Nrf2 in the 20C-treated PC12 cells. The scale bar represents 10 μm. (D, E) Western blotting analysis of the levels of the HO-1 and NQO1 proteins in PC12 cells exposed to rotenone in the presence or absence of 20C. (F, G) RT-PCR analysis of the mRNA levels of HO-1 and NQO1 in PC12 cells exposed to rotenone in the presence or absence of 20C. #P<0.05, ##P<0.01 vs the model group (n=3).
The above observations showed that 20C promoted Nrf2 nuclear translocation and then activated Nrf2. Thus, we hypothesized that 20C might regulate Nrf2 downstream target genes. Therefore, we investigated the expression of the downstream factors in this pathway. As shown in Figure 5D and 5E, the HO-1 proteins were up-regulated after the 0.1 μmol/L 20C treatment (P<0.05), while both the HO-1 and NQO1 proteins were up-regulated after the 1 μmol/L 20C treatment (P<0.01, P<0.01). Moreover, the mRNA levels were consistent with the changes in the protein levels; 0.1 μmol/L and 1 μmol/L 20C enhanced the transcription of the HO-1 (P<0.05, P<0.05) and NQO1 mRNAs (P<0.01, P<0.05) compared with the rotenone-treated group (Figure 5F and 5G). These results indicated that 20C induced the expression of the mRNAs and proteins of Nrf2 downstream factors by activating the Nrf2 signaling pathway.
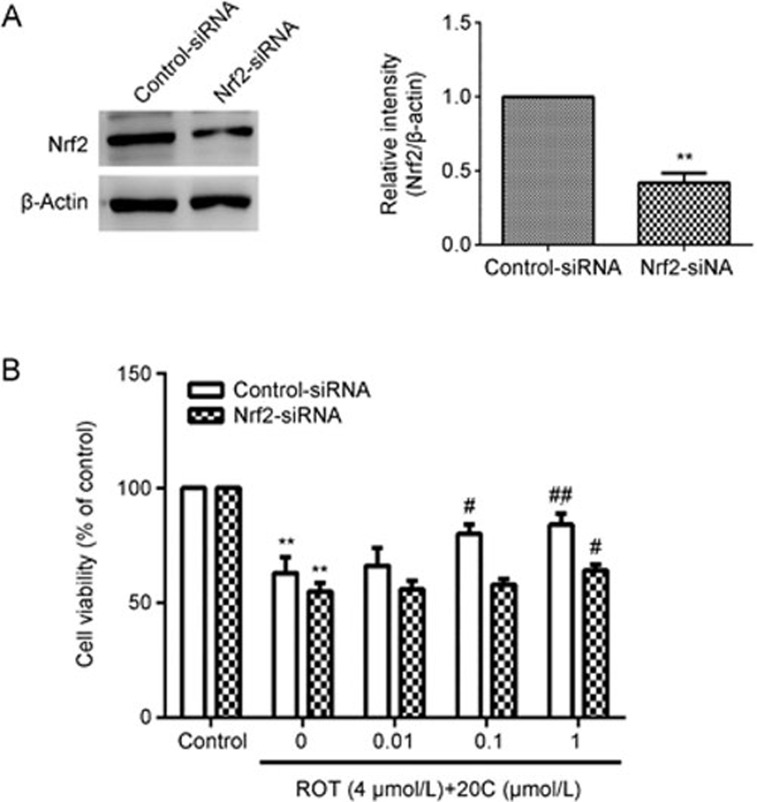
The protective effect of 20C is involved the activation of Nrf2
To provide direct evidence for the involvement of Nrf2 activation in 20C-mediated cytoprotection, we transfected PC12 cells with either Nrf2-siRNA or control-siRNA for 24 h, followed by treatment with 4 μmol/L rotenone in the presence or absence of 0.01, 0.1, 1 μmol/L 20C for an additional 48 h. The efficiency of the Nrf2-siRNA was verified by Western blotting. As shown in Figure 6A, the Nrf2-siRNA treatment significantly decreased the level of Nrf2 (P<0.01). We subsequently exposed the Nrf2-siRNA- and control-siRNA-transfected PC12 cells to rotenone with or without 20C. As expected, 0.1 and 1 mol/L 20C protected the control-siRNA-treated cells against rotenone-induced cell death (P<0.05, P<0.01), but only the 1 μmol/L 20C dose exerted a protective effect on the Nrf2-siRNA-treated cells (Figure 6B, P<0.05). Taken together, these results suggested that 20C partially protected the cells against rotenone-induced death by activating Nrf2.
Figure 6.
Nrf2 activation is involved in the protective effect of 20C. (A) Transient transfection of PC12 cells with Nrf2-siRNA inhibited the expression of the Nrf2 protein. Western blotting analysis of the levels of the Nrf2 protein in PC12 cells 24 h after transfection. (B) The PC12 cells were transfected with the control-siRNA or Nrf2-siRNA for 24 h, and then subjected to 4 μmol/L rotenone treatment for an additional 48 h in the presence or absence of 20C. Cell survival was assessed by the MTT assay. **P<0.01 vs the control group, #P<0.05, ##P<0.01 vs the model group (n=3).
Discussion
Accumulating evidence suggests that exposure to environmental toxins, such as pesticides, could be an underlying risk factor for the development of many neurodegenerative diseases, including Parkinson's disease. Rotenone, a pesticide and specific inhibitor of mitochondrial complex I, has consistently been reported to be associated with the development of PD in human populations. Several studies have shown that mitochondrial dysfunction, oxidative stress, and cell death are important factors in the pathogenesis of PD19. In fact, exposure to rotenone induces critical damage to mitochondria, enhances ROS formation and provokes oxidative damage, resulting in cell death20. The generation of ROS is an essential metabolic process for maintaining homeostasis. The imbalance between ROS production and the antioxidative stress defense systems can be highly deleterious to the cells. Nrf2 is known to be an important transcription factor that is involved in the cellular antioxidant response, as it binds to antioxidant response elements (ARE) in the genes encoding detoxification enzymes, such as HO-1 and NQO121. Moreover, it has also been reported that Nrf2 activation provides insights into the treatment of PD15. In this study, we demonstrated that 20C protected PC12 cells against rotenone-induced toxicity. The underlying mechanisms were associated with the inhibition of rotenone-induced oxidative stress by activating Nrf2 and its downstream target genes.
PC12 cells, which share many properties with primary sympathetic neurons and chromaffin cell cultures, were used to establish the Parkinsonism cell model22. Consistent with previous reports, our results showed that rotenone primarily induced PC12 cell death through apoptosis23. 20C exerted protective effects against rotenone-induced neurotoxicity, as evidenced by the findings that the rotenone-induced morphological alterations, decreased cell viability and elevated apoptosis were ameliorated by 20C. These results demonstrated that 20C protected PC12 cells against rotenone-induced apoptosis.
The Bcl-2 family of proteins is an important endogenous regulator of cellular activity in response to a variety of physiological and pathological insults, including the mitochondrial pathway of apoptosis. The Bcl-2 family is composed of anti-apoptotic proteins such as Bcl-2 and pro-apoptotic proteins, including Bax. During apoptosis, cytochrome C is translocated from the mitochondrial membrane to the cytosol, where it is required for the activation of caspase-3. Overexpression of Bcl-2 has been shown to prevent the translocation of cytochrome C, thereby blocking the apoptotic process. Caspase-3 is a critical executioner of apoptosis and one of the critical enzymes in rotenone-induced apoptosis in neuronal cells24. In the present study, 20C regulated the levels of Bcl-2 family proteins, and then inhibited cytochrome C release and the production of active caspase-3 (17 kD) in the rotenone-treated PC12 cells. In summary, 20C prevented rotenone-induced apoptosis, as determined by the Bax/Bcl-2 ratio, cytochrome C release and caspase-3 cleavage.
Mitochondria are involved in cell survival and play a central role in apoptosis through the control of cellular energy metabolism, the generation of ROS, and the release of apoptotic factors into the cytoplasm. Previous evidence showed that ROS was involved in the apoptotic mechanism of rotenone-induced neurotoxicity25. In addition to the intracellular ROS levels, the changes in MMP during apoptosis are also consistent with mitochondrial dysfunction26. Our data showed that rotenone significantly aggravated MMP collapse and induced the over production of intracellular ROS. However, 20C could attenuate the rotenone-induced MMP collapse and intracellular ROS overload, which indicated that 20C contributed to the recovery of mitochondrial function.
Considering the positive effect of 20C on intracellular ROS production and H2O2-induced injury (data unpublished), we speculated that 20C might participate in the cellular antioxidant response. Nrf2 is an essential mediator of the expression of antioxidant enzymes and stress-inducible proteins. The activation of Nrf2 in cells provides an indirect way to enhance their antioxidant capacity, thereby preventing cellular dysfunction in response to free radicals. Under non-oxidizing conditions, Nrf2 binds to Keap1, forming a complex that remains sequestered in the cytoplasm27. Following an oxidative insult, Nrf2 dissociates from the Keap1-Nrf2 complex and translocates to the nucleus, binding to ARE and potentiating the cellular response against oxidative stress28. Among the target genes regulated by Nrf2, both HO-1 and NQO1 participate in the antioxidant response. Numerous evidence has showed that HO-1 plays an important role in neuroprotective function against oxidative injury29. In addition, the expression of antioxidative enzyme HO-1 exerts a neuroprotective effect by protecting the dopaminergic neurons30. NQO1 is a cytoplasmic two electron reductase that catalyzes the reduction of a wide range of substrates, including quinones, quinone-imines, and nitro-compounds31. Thus, the up-regulation of HO-1 and NQO1 in response to oxidative stresses provides an effective endogenous antioxidant defense mechanism in rotenone-induced PC12 cells32,33. To determine whether the Nrf2/ARE/HO-1 signaling pathway is involved in the neuroprotective effect of 20C, we investigated the changes in the Nrf2/ARE/HO-1 signaling pathway in the 20C-treated cells. The present data showed that 20C significantly promoted the translocation of Nrf2 from the cytoplasm to the nucleus, which indicated that Nrf2 was activated. Meanwhile, the up-regulated mRNA and protein levels of HO-1 and NQO1 in the 20C-treated PC12 cells were associated with Nrf2 activation. Collectively, these results demonstrated that 20C improved the antioxidant capacity of PC12 cells against rotenone-induced damage as a result of Nrf2/ARE/HO-1 activation.
Because the regulation of antioxidant response involves a number of signaling pathways, we hypothesized that the mechanism of the protective effect of 20C treatment was associated with the downstream pathways activated by Nrf2. RNAi-mediated Nrf2 knockdown diminished the cytoprotective effects of 20C, which strongly suggests that the Nrf2/HO-1/NQO1 pathway was activated. These results are similar to previous studies demonstrating that Nrf2 plays a key role in protecting cells against oxidative stress34,35,36. However, in the MTT assay, 1 μmol/L 20C had a beneficial effect against rotenone-induced apoptosis in Nrf2-knockdown PC12 cells, which indicated that, 20C might activate other targets, in addition to Nrf2/ARE/HO-1 signaling pathway, to exert its cytoprotective effect. We have demonstrated that 20C regulated the levels of the Bcl-2 family proteins, reduced ROS accumulation and stabilized the mitochondrial membrane potential, which suggested that 20C inhibited the mitochondria-dependent cell death pathway. We speculated that in addition to Nrf2/ARE/HO-1 signaling pathway, 20C might have direct or indirect effects on the mitochondria to inhibit the mitochondria-dependent cell death pathway. Further research is required to explore the effect of 20C on the mitochondria. Taken together, these results demonstrated that 20C protected PC12 cells against rotenone-induced apoptosis, and the mechanism involved the activation of the Nrf2/ARE/HO-1 signaling pathway.
In conclusion, our present observations identified a beneficial effect from treatment with 20C, a brand new bibenzyl compound, against rotenone-induced apoptosis in PC12 cells, which operates, at least in part, via the activation of the Nrf2/ARE/HO-1 signaling pathway. The study provides further evidence of the beneficial effects of 20C in the prevention of rotenone-induced toxicity in PC12 cells, which suggested that 20C could be a leading candidate for the treatment of Parkinson's disease.
Author contribution
Ju-yang HUANG and Nai-hong CHEN proposed the study concepts; Ju-yang HUANG designed and performed the experiments and performed the data analysis; Yu-he YUAN, Jia-qing YAN, and Shi-feng CHU prepared the manuscript; Ya-nan WANG, Cheng-gen ZHU, Qing-lan GUO, and Jian-gong SHI assisted in preparing the chemical reagents.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81274122, No 81102831, and No 81273629), the National Key Sci-Tech Major Special Item (No 2012ZX09301002-004 and No 2012ZX09103101-006), the National High-Tech R&D Programme (863 Program) (No 2012AA020303), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) (No IRT1007), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No 20121106130001), Beijing Natural Science Foundation (No 7131013 and No 7142115), Beijing Key Laboratory of New Drug Mechanisms and Pharmacological Evaluation Study (No BZ0150).
References
- Dauer W, Przedborski S. Parkinson's Disease: mechanisms and models. Neuron 2003; 39: 889–909. [DOI] [PubMed] [Google Scholar]
- Ozansoy M, Basak AN. The central theme of Parkinson's disease: alpha-synuclein. Mol Neurobiol 2013; 47: 460–5. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondria in the etiology and pathogenesis of Parkinson's disease. Lancet Neurol 2008; 7: 97–109. [DOI] [PubMed] [Google Scholar]
- Trushina E, Mcmurray CT. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience 2007; 145: 1233–48. [DOI] [PubMed] [Google Scholar]
- Perier C, Bove J, Vila M, Przedborski S. The rotenone model of Parkinson's disease. Trends Neurosci 2003; 26: 345–6. [DOI] [PubMed] [Google Scholar]
- Li N, Ragheb KE, Lawler G, Sturgis J, Rajwa B, Melendez JA, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 2003; 278: 8516–25. [DOI] [PubMed] [Google Scholar]
- Girish C, Muralidhara. Propensity of Selaginella delicatula aqueous extract to offset rotenone-induced oxidative dysfunctions and neurotoxicity in Drosophila mela-nogaster: implications for Parkinson's disease. Neurotoxicology 2012; 33: 444–56. [DOI] [PubMed] [Google Scholar]
- Ojemann LM, Nelson WL, Shin DS, Rowe AO, Buchanan RA. Tian ma, an ancient Chinese herb, offers new options for the treatment of epilepsy and other conditions. Epilepsy Behav 2006; 8: 376–83. [DOI] [PubMed] [Google Scholar]
- Shin EJ, Bach JH, Nguyen TT, Nguyen XK, Jung BD, Oh KW, et al. Gastrodia elata bl attenuates methamphetamine-induced dopaminergic toxicity via inhibiting oxidative burdens. Curr Neuropharmacol. 2011; 9: 118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Mori A. Antioxidant and free radical scavenging activities of Gastrodia elata Bl. and Uncaria rhynchophylla (Miq.) Jacks. Neuropharmacology 1992; 31: 1287–98. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Yamamoto M. Nrf2–Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul 2006; 46: 113–40. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 2004; 24: 7130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, et al. Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med 2008; 45: 1375–83. [DOI] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Debiase A, Yamamoto M, Kleeberger SR. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic Biol Med 2005; 38: 325–43. [DOI] [PubMed] [Google Scholar]
- Barone MC, Sykiotis GP, Bohmann D. Genetic activation of Nrf2 signaling is sufficient to ameliorate neurodegenerative phenotypes in a Drosophila model of Parkinson's disease. Dis Model Mech 2011; 4: 701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufekci KU, Civi Bayin E, Genc S, Genc K. The Nrf2/ARE pathway: a promising target to counteract mitochondrial dysfunction in Parkinson's disease. Parkinsons Dis 2011; 314082. [DOI] [PMC free article] [PubMed]
- Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 1991; 139: 271–9. [DOI] [PubMed] [Google Scholar]
- Song XY, Hu JF, Chu SF, Li ZP, Wu DH, Ji HJ, et al. IMM-H004, a novel coumarin derivative compound, protects against amyloid beta-induced neurotoxicity through a mitochondrial-dependent pathway. Neuroscience 2013; 242: 28–38. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006; 443: 787–95. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 2000; 3: 1301–6. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Fabio G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol 2011; 44: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A 1976; 73: 2424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BY, Yuan YH, Hu JF, Zhao Q, Zhang DM, Chen NH. Protective effect of Bu-7, a flavonoid extracted from Clausena lansium, against rotenone injury in PC12 cells. Acta Pharmacol Sin 2011; 32: 1321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei W, Liou AK, Chen J. Two caspase-mediated apoptotic pathways induced by rotenone toxicity in cortical neuronal cells. FASEB J 2003; 17: 520–2. [DOI] [PubMed] [Google Scholar]
- Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. J Cell Biol 1998; 141: 1423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WH, Park WB, Gao B, Jung MH. Critical role of reactive oxygen species and mitochondrial membrane potential in Korean mistletoe lectin-induced apoptosis in human hepatocarcinoma cells. Mol Pharmacol 2004; 66: 1383–96. [DOI] [PubMed] [Google Scholar]
- Velichkova M, Hasson T. Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol Cell Biol 2005; 25: 4501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, et al. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A 2004; 101: 2040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J 1999; 13: 1800–9. [DOI] [PubMed] [Google Scholar]
- Quesada A, Ogi J, Schultz J, Handforth A. C-terminal mechano-growth factor induces heme oxygenase-1-mediated neuroprotection of SH-SY5Y cells via the protein kinase C/Nrf2 pathway. J Neurosci Res 2011; 89: 394–405. [DOI] [PubMed] [Google Scholar]
- Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact 2000; 129: 77–97. [DOI] [PubMed] [Google Scholar]
- Lin HY, Yeh WL, Huang BR, Lin C, Lai CH, Lin H, et al. Desipramine protects neuronal cell death and induces heme oxygenase-1 expression in Mes23.5 dopaminergic neurons. PLoS One 2012; 7: e 50138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineli A, Conte C, Grottelli S, Bellezza M, Cacciatore I, Bolanos JP. Cyclo(His-Pro) promotes cytoprotection by activating Nrf2-mediated up-regulation of antioxidant defence. J Cell Mol Med 2009; 13: 1149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Hu Y, Liu L, Cai J, Peng C, Li Q. Gastrodin protects against MPP(+)-induced oxidative stress by up regulates heme oxygenase-1 expression through p38 MAPK/Nrf2 pathway in human dopaminergic cells. Neurochem Int 2014; 75: 79–88. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, et al. The NRF2/ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci 2008; 1147: 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Chen M, Jiang Y, Chen M, Zhou T, Wang Y, et al. Polyhydroxylated fullerene attenuates oxidative stress-induced apoptosis via a fortifying Nrf2-regulated cellular antioxidant defence system. Int J Nanomedicine 2014; 9: 2073–87. [DOI] [PMC free article] [PubMed] [Google Scholar]