Abstract
RAF kinases play a prominent role in cancer. Their mode of activation is complex, but critically requires dimerization of their kinase domains. Unexpectedly, several ATP-competitive RAF inhibitors were recently found to promote dimerization and transactivation of RAF kinases in a RAS-dependent manner and as a result undesirably stimulate RAS/ERK-mediated cell growth. The mechanism by which these inhibitors induce RAF kinase domain dimerization remains unclear. Here we describe BRET-based biosensors for the extended RAF family enabling the detection of RAF dimerization in living cells. Notably, we demonstrate the utility of these tools for profiling kinase inhibitors that selectively modulate RAF dimerization as well as for probing structural determinants of RAF dimerization in vivo. Our findings, which appear generalizable to other kinase families allosterically regulated by kinase domain dimerization, suggest a model whereby ATP-competitive inhibitors mediate RAF dimerization by stabilizing a rigid closed conformation of the kinase domain.
Introduction
Dysregulation of the RAS-RAF-MEK-ERK pathway is conducive to tumor formation1, 2. While activating mutations in the RAS genes (H-, K- and NRAS) are the most recurrent lesions driving oncogenic RAS/ERK signaling, gain-of-function mutations in BRAF are arguably the second leading cause3, 4. Under normal conditions, RAF activation is initiated at the plasma membrane by binding to growth factor-stimulated RAS GTPases. This triggers the sequential phosphorylation and activation of MEK and ERK. Active ERK then phosphorylates a diverse set of substrates eliciting various cell-specific responses, including proliferation and survival.
Mammals express three RAF paralogs (A-, B- and CRAF) and two distantly related proteins (KSR1 and 2) herein referred to as RAF family members5. A recently discovered feature of RAS-mediated RAF activation involves the homo- or heterodimerization of the kinase domain of RAF family members through a conserved side-to-side interface6–9. The mechanism by which dimerization induces catalytic activity has not been elucidated, but likely involves allosteric switching of the respective protomers7.
Given its involvement in tumorigenesis, several inhibitors of RAF have been developed 10. Selective inhibitors of BRAFV600E (a frequent BRAF oncogenic variant) are now available and clinical activity against BRAFV600E-dependent metastatic melanomas has been observed with vemurafenib (PLX4032)11, 12. Regrettably, two shortcomings have emerged. Firstly, virtually all inhibitors tested to date promote RAS-dependent RAF dimerization, and in a dose-dependent manner increase ERK signaling and cell growth13–15. Apparently, drug-bound RAF protomers dimerize with and transactivate drug-free protomers leading to enhanced signaling16. This situation warns against using current RAF inhibitors to treat RAS-dependent cancers. Secondly, resistance to vemurafenib invariably develops within a year and one frequent mechanism driving resistance involves RAF dimerization17, 18. Clearly, RAF dimerization is a critical parameter to consider when designing compounds targeting RAS/ERK-dependent tumors.
Current methods for monitoring RAF dimerization are based on low-throughput assays 6–9 that are ill-adapted for surveying numerous samples/conditions or for screening large libraries. Here, we developed bioluminescence resonance energy transfer (BRET)-based biosensors enabling quantitative detection of kinase domain dimerization of each RAF family member in living cells. The system recapitulates known genetic and pharmacological perturbations of RAF dimerization with high specificity, sensitivity and robustness. Pairwise assays revealed discrete dimerization capabilities for each RAF family member. In drug profiling experiments, the biosensors provided a snapshot of the complex and the varied effects that inhibitors have on the RAF dimerization network and therefore informed on the potential in vivo consequences of an inhibitor. In a high-throughput setting, these biosensors unveiled unforeseen off-target effects of diverse ATP-competitive kinase inhibitors on RAF dimerization. Based on biophysical characterization of a subset of these inhibitors and crystallographic data, we propose that ATP-competitive RAF inhibitors directly promote dimerization by stabilizing a closed conformation of the kinase domain.
Results
Engineering RAF dimerization biosensors
RAF dimerization biosensors were developed using the BRET2 system, which allows real-time monitoring of protein-protein interactions in living cells19. Isolated RAF kinase domains have the propensity to form dimers in solution in a RAS-independent manner7. We thus used the CRAF kinase domain (CRAFKD) as a starting point, which we fused to the N or C terminus of Renilla luciferase variant II (RlucII; donor moiety) or GFP10 (acceptor moiety)20, 21. These constructs produced relatively weak BRET signals when tested by transient transfections in HEK293T cells (not shown). To improve signal output, we added a membrane-targeting CAAX box to the C terminus of the fusion proteins to increase the effective concentration of the interacting pairs in a bidimensional space. CAAX box-containing CRAFKD constructs with N-terminal donor and acceptor fusions led to higher BRET signals that were saturable in titration experiments, unlike noninteracting probes, which served as a reference for nonspecific interactions (Fig. 1a). Membrane-targeted BRAFKD constructs also produced saturable BRET signals (Figs. 1b,c; for simplicity, the term CAAX is omitted in the construct names described hereafter) that clearly depended on membrane targeting (Supplementary Results, Supplementary Fig. 1a,b) and did not fluctuate linearly in response to the total amount of the interacting probes (Supplementary Fig. 1c) as generally observed for nonspecific interactors22.
Figure 1. Development of BRET-based RAF dimerization biosensors.
(a) BRET titration curves of membrane-targeted (CAAX box) CRAFKD biosensor. The RlucII and GFP10 moieties are inserted at the N-terminus of CRAFKD. The blue open square denotes the RlucII donor construct, whereas the green open square denotes the GFP10 acceptor construct. The noninteracting RlucII-KRASG12V--GFP10-CRAFKD-CAAX pair was used as a reference for nonspecific BRET signals. (b) Titration curves of wild-type (WT) versus BRAFKD_R509H BRET probes. The BRAFKD BRET probes used the same configuration as the one shown for CRAFKD in (a). The R509H mutation, which impairs side-to-side dimerization, augments BRET50 values and reduces BRETmax values. Double asterisks (**) denote F-test p-values smaller than 1×10−3. (c) Modulation of BRAFKD biosensor signals upon addition (333 nM) of the indicated RAF inhibitors as assessed in titration experiments. Single asterisks (*) denote F-test p-values smaller than 1×10−3 and double asterisks (**) smaller than 1×10−4. (d) Mutations of the kinase domain gatekeeper residue (T529M) or the side-to-side dimerization interface (R509H) impede GDC-0879-induced BRET signals. Dose-response experiments were conducted using the indicated drug concentrations. (e) Induction kinetics of the BRAFKD BRET signal using 33 nM of GDC-0879. The R509H mutant was insensitive to the drug at this concentration. (f) The BRAFKD homodimerization BRET assay exhibits highly reproducible signal induction (Z factor = 0.72) upon GDC-0879 treatment (33 nM). Each experiment was repeated at least two times. Where error bars are presented, they correspond to mean values ± s.d. of biological triplicates.
RAF biosensors detect perturbations of dimerization
We next sought to ascertain whether the dimerization signature detected with the BRET assay depended on the side-to-side dimer interface7, 13, 15. Mutation of R509, which lies on the BRAFKD dimerization surface, was shown to reduce dimer formation and to lower kinase activity7, 17, 23. Introduction of the R509H mutation in GFP10-BRAFKD did not affect protein expression levels (Supplementary Figs. 1d and 2a) but, as expected, impeded kinase activity (Supplementary Fig. 2a) and significantly reduced BRET output (Fig. 1b). Furthermore, it significantly increased the BRET50 (a proxy for affinity) and reduced the BRETmax (a proxy for total number of dimers; Online Methods), which together are consistent with impaired dimer formation (Fig. 1b). Binding of 14-3-3 proteins to a C-terminal site on RAF proteins has been suggested to promote and/or stabilize RAFKD dimer formation7–9, 24. Consistent with this, mutagenesis of a key residue within the 14-3-3 C-terminal binding site of BRAF (S729A) significantly elevated the BRET50 and reduced the BRETmax (Supplementary Fig. 2b).
Given the induction of RAF dimerization by specific ATP-competitive inhibitors, we examined whether the BRET assay could detect the influence of a Type I (GDC-0879) and a Type II inhibitor (AZ-628) that had previously been shown to promote RAF dimerization by co-immunoprecipitation13–15. Type I inhibitors bind their kinase target in a DFG-in, “active” configuration, whereas Type II inhibitors bind in a DFG-out, “inactive” configuration25. Consistent with the ability of the BRET assay to detect drug-induced dimer formation, both compounds significantly reduced the BRET50 and augmented the BRETmax in titration experiments (Fig. 1c), while leaving total luciferase and GFP10 intensities unchanged (Supplementary Fig. 3a,b). To evaluate the potency of GDC-0879 in our system, we selected construct ratios producing BRET80 signals (80% of BRETmax) and tested a range of GDC-0879 concentrations. A half-maximal effective concentration (EC50) of 12 nM was obtained from these experiments (Fig. 1d), which is in the same range as the IC50 (34 nM) obtained by in vitro kinase assays13. To demonstrate that the compound-promoted BRET changes depended specifically on drug-binding to the BRAF kinase domain, we tested the “gatekeeper” mutation (T529M) in BRAFKD, which reduces drug access to the catalytic cleft and prevents drug-induced RAF dimerization15, 26. Consistent with this model, GDC-0879 increased the BRET signal with an EC50 80-fold higher for BRAFKD_T529M than for wild-type protein (Fig. 1d). An intact dimer interface was also required as the R509H mutation increased the EC50 of GDC-0879 by 50-fold (Fig. 1d). In agreement with previous kinetic data13, GDC-0879 activity could be detected as early as 5 minutes upon drug treatment and plateaued by 60 minutes, while the R509H mutant was insensitive at the concentration tested (33 nM; Fig. 1e). Finally, the GDC-0879-induced BRET signal was highly reproducible and yielded a Z-factor of 0.72 in 384-well format (Fig. 1f)27. Together, these findings indicated that our BRET assay detects genuine dimerization of the RAF kinase domain in vivo and that it can identify compounds impinging on BRAF dimerization in a specific and sensitive manner.
RAF inhibitors distinctly affect the RAF dimer network
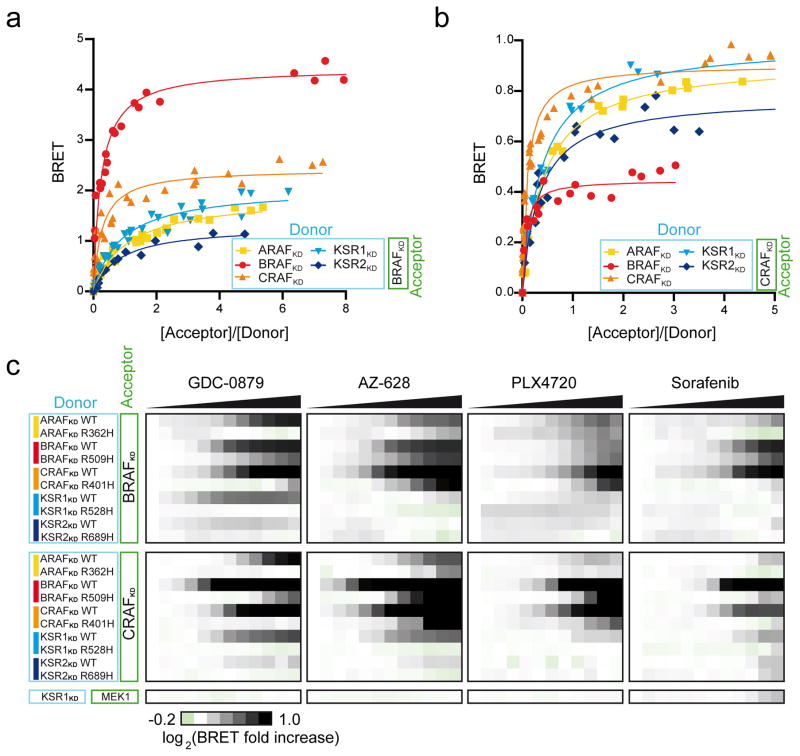
The ability of RAF family members to dimerize and the impact of inhibitors on dimers remains poorly understood. To investigate these issues, we generated CAAX box-containing BRET probes for RAF family members (ARAF, KSR1 and KSR2). In contrast to the BRAFKD and CRAFKD constructs, these constructs displayed no significant activity towards MEK (Supplementary Fig. 4). We tested all bidirectional pairs in titration experiments and identified GFP10-BRAFKD as the best BRET acceptor in terms of BRETmax for any RAFKD donor (Fig. 2a, Supplementary Table 1, and data not shown). Although lower BRETmax values were obtained with GFP10-CRAFKD, this construct also gave significant BRET signals with each donor probe (Fig. 2b and Supplementary Table 1). In contrast, the remaining combinations gave weak and unreliable BRET signals even though the fusions expressed to similar levels. Various reasons could explain these observations such as missing factors in HEK293T cells or perturbations imposed by the GFP and/or luciferase moieties. Nevertheless, these results suggest that BRAF and CRAF have the capacity to engage in dimer formation with any member of the RAF family. Finally, distinct BRETmax and BRET50 values were observed for each pair. While these parameters are useful for comparing the dimerization potential of a given pair as a consequence of amino acid changes or upon drug treatment (Fig. 1), they cannot be used to compare altogether different pairs.
Figure 2. Profiling RAF inhibitors using RAF dimerization biosensors.
(a–b) The indicated BRET donor probes were systematically tested in titration experiments using (a) BRAFKD or (b) CRAFKD as acceptor probes. BRET50 and BRETmax values are shown in Supplementary Table 1. (c) Dose-response curves conducted with increasing concentrations of GDC-0879, AZ-628, PLX4720 and Sorafenib (Supplementary Fig. 5 and Supplementary Table 2) were log2-transformed and converted into heatmaps. Black saturation represents positive effects on BRET signals, whereas green color denotes negative impacts. The residue homologous to R509 of BRAF was mutated in each family member and used as negative control. Each experiment was repeated at least two times.
Using the full donor panel of RAF biosensors, we verified whether other dimers could form upon drug treatment and thus could predict their occurrence in vivo. We evaluated the impact of GDC-0879, AZ-628, and two other RAF inhibitors, namely, PLX4720 (a Type I inhibitor28) and Sorafenib (a Type II inhibitor29) on each RAFKD pair using the BRAFKD and CRAFKD acceptor probes (Fig. 2c, Supplementary Fig. 5a,b, Supplementary Table 2). Interestingly, the inhibitors showed distinct induction profiles that depended on an intact dimer interface (Fig. 2c). Further demonstrating the specificity of the effect, the four compounds did not modulate an unrelated interacting pair (Fig. 2c and Supplementary Fig. 5c,d). As summarized in Supplementary Figure 6a, PLX4720 and Sorafenib were relatively weak RAFKD dimer inducers. In contrast, both GDC-0879 and AZ-628 were strong and broad inducers of BRAF-containing dimers and yet, each displayed differences in their ability to promote specific RAF dimers. Notably, while GDC-0879 strongly induced BRAF/KSR1 dimers, AZ-628 did not. Conversely, AZ-628 (but not GDC-0879) strongly promoted CRAF homodimers. To rule out the possibility that BRET signal variations reflected protein conformational changes rather than dimerization, we conducted titration experiments on three pairs, namely, ARAFKD-BRAFKD, CRAFKD-BRAFKD and KSR1KD-BRAFKD ± drug treatment. In every case, a significant reduction of the BRET50 value was observed, supporting the notion that the inhibitors promoted RAF dimer formation (Supplementary Fig. 6b–d). Together, these data suggest that RAF inhibitors modulate the dimerization landscape of the RAF family in a complex and selective manner. Differences in induction profiles likely reflect differences in 1) compound affinity for and structural impact on RAF proteins; 2) compound pharmacokinetics; and 3) the inherent propensity of RAF dimerization surfaces to pair among themselves.
RAFKD biosensors behave as RAS-induced full-length RAF
The induction of RAF dimerization by kinase inhibitors was shown to depend on RAS activity13–15. We were thus intrigued that our CAAX-boxed RAFKD biosensors could detect dimerization in the absence of overt RAS activity. We reasoned that the CAAX box on our RAFKD biosensors mimicked the recruitment of RAF to the plasma membrane triggered by RAS activation2. Moreover, RAS binding to RAF is thought to release an inhibitory interaction between the N-terminal regulatory region of RAF and the kinase domain, enabling kinase domain dimerization2. By using isolated kinase domains, the propensity of our biosensors to dimerize would therefore be increased thereby bypassing the need for upstream inputs.
To verify whether our RAFKD biosensors simulate a RAS-mediated context, we generated full-length (FL) BRAF and CRAF BRET biosensors and characterized their ability to form dimers in a RAS-dependent manner and compared their dimerization profiles upon RAF inhibitor treatment. In the absence of co-expressed RASG12V, the CRAFFL-BRAFFL pair produced titration curves that fit a low confidence hyperbolic function, suggestive at best of weak dimerization (Fig. 3a and Supplementary Table 3). In contrast, co-expression of mCherry-tagged activated KRAS (KRASG12V or KRASQ61H) strongly stimulated CRAFFL/BRAFFL dimerization in a dose-dependent manner (Fig. 3a and Supplementary Fig. 7). Demonstrating the specificity of the RAFFL dimerization assay, a dominant-negative KRASS17N as well as a RAF RBD mutation (R188L) in BRAF did not support CRAFFL/BRAFFL dimerization (Fig. 3a). In addition, the R509H mutation in the BRAF side-to-side interface weakened dimerization as evidenced by an increased BRET50 value (Fig. 3a and Supplementary Table 3).
Figure 3. Development of a RAS-dependent CRAF/BRAF dimerization biosensor.
(a) Titration experiment using full-length CRAF (CRAFFL) donor (RlucII) probe and WT or mutant full-length BRAF (BRAFFL) acceptor (GFP10) probe ± mCherry-tagged KRASG12V, KRASQ61H or KRASS17N. The mCherry tag was used for monitoring KRAS expression. Its excitation and emission spectra does not overlap with that of our BRET donor or acceptor constructs20. BRET50 and BRETmax values are shown in Supplementary Table 3. (b) Drug-induced BRET signals for the CRAFFL/BRAFFL biosensor depends on an intact dimerization interface. ND, not determined. (c) Co-expression of mCherry-tagged KRASQ61H potentiates drug-induced dimerization as measured by a decrease of the EC50 for each RAF inhibitor tested. Dose-response experiments with the indicated RAF inhibitors on the CAAX-boxed CRAFKD/BRAFKD biosensor produced EC50s nearly identical to those obtained with the RAS-induced CRAFFL/BRAFFL biosensor. Because of their distinct intensities (for instance, biosensors in the presence of co-expressed KRASQ61H yield significantly higher signals), BRET signals were normalized from 0.0 (vehicle-treated cells) to 1.0 (maximal effect of a given compound). This facilitated the comparison of the response of distinct BRET pairs to specific compounds. Each experiment was repeated at least two times. Where error bars are presented, they correspond to mean values ± s.d. of biological triplicates.
We next conducted RAF inhibitor dose-response experiments on the CRAFFL/BRAFFL probes ± RAS and compared the responses to those obtained with the CRAFKD/BRAFKD biosensors. Intriguingly, each inhibitor induced the CRAFFL/BRAFFL BRET signals in the absence of co-expressed RAS (Figs. 3b,c), suggesting that basal RAS activity in HEK293T cells supports drug-induced CRAFFL/BRAFFL dimerization. Alternatively, it is possible that the compounds promote full-length RAF dimerization in a RAS-independent manner, a phenomenon not detected previously, possibly due to the low sensitivity of dimerization detection methods used. At any rate, the induction was dependent on a functional side-to-side interface since the R509H mutation in the BRAFFL probe increased the EC50 of the CRAFFL/BRAFFL biosensors to GDC-0879 by approximately 30-fold (Fig. 3b). Importantly, constitutive RAS activity systematically reduced the EC50 of each compound for the CRAFFL/BRAFFL pair to a level nearly identical to that obtained with the CRAFKD/BRAFKD probes (Fig. 3c) consistent with the notion that the N-terminal region of RAF represses the dimerization potential of the kinase domain in the absence of RAS activity. Together, these findings further support the importance of RAS function for the ability of RAF inhibitors to promote RAF dimerization and provide evidence that our CAAX-boxed RAFKD biosensors mimic a RAS-induced state.
A screen for modulators of CRAF/BRAF dimers
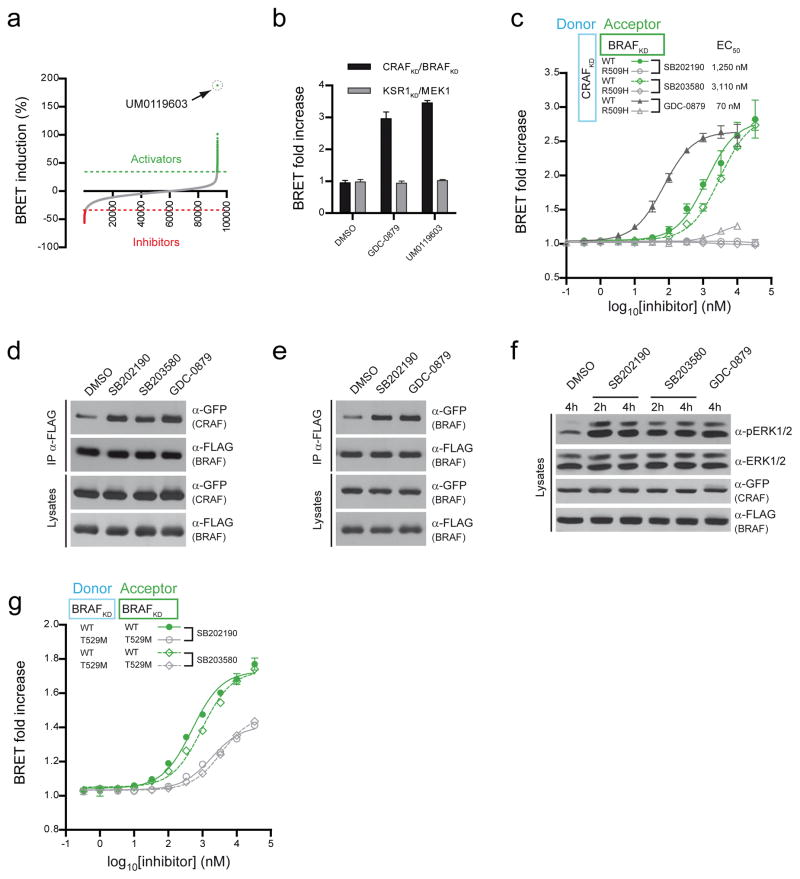
We exploited the robustness and scalability of our RAF dimerization assay in a high-throughput screen to identify compounds that selectively modulate RAF dimerization. We selected the CRAFKD/BRAFKD biosensor pair and screened a library of ~115,000 small molecules assembled primarily from commercial sources. Compounds affecting RlucII luminescence or intrinsic GFP10 fluorescence by greater than 2-fold were not considered further. We identified 503 primary hits (249 activators and 254 inhibitors) using a cut off of three standard deviations from controls (Fig. 4a). Retesting confirmed >95% of the hits. Two alternate BRET-based interaction assays (KSR1KD/MEK1 and KRASG12V/BRAFFL) were then carried out to narrow down the hit list to molecules selective for the CRAFKD/BRAFKD biosensor. This eliminated ~75% of the primary hits resulting in 8 inducers and 65 suppressors of dimerization.
Figure 4. A high-throughput chemical screen using the CRAFKD/BRAFKD biosensor identifies novel modulators of RAF dimerization.
(a) Distribution of the compound activities in a HTS performed on 110,000 drug-like compounds. UM0119603 was the most potent inducer of CRAFKD/BRAFKD dimerization. (b) Like GDC-0879, UM0119603 induces the CRAFKD/BRAFKD BRET signal (10 μM), but does not alter the BRET produced by the KSR1KD/MEK1 pair. (c) Dose-response experiments performed with a range of SB202190 and SB203580 concentrations. Mutations of the side-to-side dimerization interface (R509H) abolished the effect of each inhibitor. GDC-0879 was used as an internal standard. EC50 value calculated for each compound is shown. (d–e) The two p38 inhibitors (SB202190 and SB203580) induce the formation of full-length BRAF/CRAF and BRAF/BRAF dimers as demonstrated by co-immunoprecipitation (SB202190 and SB203580 10 μM; GDC-0879 1 μM). (f) p38 inhibitors induce ERK phosphorylation in RAF-expressing HEK293T cells (SB202190 and SB203580 2 μM; GDC-0879 1 μM). (g) Dose-response experiments show that a T529M gatekeeper mutant of BRAF reduces BRAFKD homodimerization induced either by SB202190 or SB203580. Each experiment was repeated at least two times. Where error bars are presented, they correspond to mean values ± s.d. of biological triplicates.
We initially focused our attention on the most potent inducer, UM0119603 (Fig. 4a,b). This compound, also known as SB202190, was developed as a specific ATP-competitive inhibitor of p38 MAPK30 (Supplementary Figs. 8 and 9). Interestingly, a close structural analogue (SB203580; Supplementary Fig. 8) was previously recognized as a p38-independent inducer of RAF activity31, 32 but the mechanism of action was not known. The identification of SB202190 as an inducer of the CRAFKD/BRAFKD BRET signal suggested that this class of molecules stimulates RAF by promoting dimerization. Consistent with this notion, SB203580 also induced CRAFKD/BRAFKD BRET signal and both SB203580 and SB202190 required an intact RAF dimerization surface to show an effect (Fig. 4c). The higher EC50 values of SB203580 and SB202190 compared to GDC-0879 likely reflect their weaker affinity for RAF proteins. To further validate their capacity to promote RAF dimerization, we conducted co-immunoprecipitation experiments using full-length GFP-CRAF and Flag-BRAF. Both SB203580 and SB202190 increased BRAF/CRAF dimerization comparable to GDC-0879 stimulation (Fig. 4d). The effect of the compounds was not restricted to CRAF/BRAF heterodimers as SB202190 also induced BRAF homodimerization (Fig. 4e). Finally, consistent with their ability to promote dimerization, both compounds stimulated ERK activity in RAF transfected cells (Fig. 4f).
We noticed a striking structural similarity between SB202190, SB203580 and two other RAF inhibitors, L779450 and SB590885, that were previously shown to induce RAF dimerization14, 33 (Supplementary Fig. 8). The binding mode of SB590885 in the catalytic cleft of BRAF34 shows the same general orientation and conformation as its structural analogue, SB203580 in its co-crystal with the p38 MAP kinase35 (Supplementary Fig. 8b,c). This implies that the p38 inhibitors may interact with RAF in a manner similar to with p38. Given its predicted binding mode in the BRAF catalytic cleft, we surmised that the gatekeeper mutation would impair inhibitor binding and thereby weaken the induction of RAF dimerization by these compounds. Indeed, the T529M_BRAFKD biosensor pair showed an EC50 roughly four-times higher than wild type (Fig. 4g). These data provide compelling evidence that our biosensors can identify selective RAF dimerization modulators from compound libraries and that p38 inhibitors selectively induce RAF dimerization and downstream signaling in a manner related to known RAF inhibitors.
Diverse kinase inhibitors induce RAF dimerization
The observation that two p38 inhibitors can induce RAF dimerization prompted us to investigate the effect of other ATP-competitive kinase inhibitors on our panel of RAF dimer biosensors. We assembled a collection of 184 compounds targeting a broad spectrum of kinases, most of which had been profiled for inhibition of in vitro kinase activity against approximately 300 kinases, including RAF proteins36. Our analysis identified several compounds that reproducibly induced dimerization as measured by BRET (Fig. 5a and Supplementary Data Set 1). In general, the dimer-inducing activity of the kinase inhibitors correlated with their reported ability to inhibit the in vitro kinase activity of BRAF or CRAF (Fig. 5a and Supplementary Data Set 1). Notably, in addition to retrieving all RAF inhibitors present in the library, we identified multiple inhibitors of three distinct kinases, namely, six inhibitors of p38, three inhibitors of BCR-ABL, and four inhibitors of VEGFR as significant inducers of RAF dimerization (Fig. 5a and Supplementary Figs. 9 and 10). Supporting our findings, the same three BCR-ABL inhibitors (Imatinib, Nilotinib and Dasatinib) were reported to promote RAF dimerization in co-IP assays and to stimulate ERK signaling in leukemic cells37.
Figure 5. Screening of a kinase inhibitor library reveals widespread off-target effects on RAF dimerization.
(a) Unsupervised clustering of the response of a panel of RAF dimerization BRET biosensors tested against a library of kinase inhibitors (left panel; see Supplementary Data Set 1). For comparison, a heatmap depicting previously published in vitro RAF kinase inhibition36 is shown. Gray bars denote data not available. The right panel shows enlarged areas comprising BRET inducers. (b) Confirmation of the dimerization-inducing potential of selected kinase inhibitors. FLAG-BRAF was co-expressed with CRAF-RlucII and luciferase activity was monitored in anti-Flag immunoprecipitates. Data were from triplicates and normalized to DMSO. Error bars correspond to standard deviations. (c) HCT-116 cells were treated (2 hrs) with increasing concentrations of the indicated compounds. pERK levels (Supplementary Fig. 11) were normalized to DMSO, log2-transformed and converted into heatmaps. (d) Correlation between concentrations causing maximal pERK levels (Fig. 5c and Supplementary Fig. 11) and the BRET EC50s for the same compounds against the CRAFKD/BRAFKD pair (Supplementary Fig. 10b). (e) Analytical ultracentrifugation demonstrates the ability of type I and type II p38 inhibitors to promote BRAFKD dimerization in vitro. AMP-PNP inhibits BRAFKD dimerization. The respective position of monomeric and dimeric BRAFKD is indicated by a red and a blue line. Peaks at or below 2 svedbergs represent artifacts of the refractive index detector system that is evident at low protein concentrations. Peak heights are not protein concentration-dependent.
To establish that the BRET-inducing kinase inhibitors genuinely promoted RAF dimerization, we evaluated the activity of the 14 strongest inhibitors (Supplementary Fig. 9) in co-immunoprecipitation experiments using the LUMIER assay38. As shown in Figure 5b, all inhibitors promoted BRAF/CRAF co-immunoprecipitation. Moreover, as predicted from their RAF dimerizing properties, the same compounds induced ERK phosphorylation in KRASG13D mutant cells (HCT-116; Fig. 5c and Supplementary Fig. 11), but reduced ERK activation in KRAS WT; BRAFV600E mutant cells (COLO205; Supplementary Fig. 11). We observed a strong correlation between the concentration of inhibitor causing maximal ERK activation and its associated CRAFKD/BRAFKD BRET EC50 (Fig. 5d). To verify that the BRET-inducing kinase inhibitors acted by direct binding to the RAF catalytic cleft, we used an in vitro time resolved (TR)-FRET-based assay to monitor the ability of the compounds to compete with a fluorescent kinase tracer bound to the BRAF orthosteric site. All compounds with off-target effects on RAF dimerization effectively displaced the tracer (Supplementary Fig. 12). Interestingly, with the exception of PLX4720, the IC50 for these inhibitors closely correlated with their EC50 in the BRAFKD/BRAFKD BRET assay, suggesting that the BRET assay can also predict the affinity of small-molecule inhibitors for RAF (Supplementary Fig. 12f).
Kinase inhibitors induce RAF dimerization in vitro
To test if induced dimerization was mediated directly by the kinase domain of RAF as a consequence of inhibitor binding, we established a sedimentation velocity analytical ultracentrifugation (AUC) assay using the purified BRAF kinase domain. We first characterized the isolated kinase domain of human BRAF (residues 444 to 723) in its apo state and found a weak ability to dimerize with a Kd greater than 25 μM (Fig. 5e and Supplementary Fig. 13). This was considerably weaker than observed previously for a shorter BRAF kinase domain construct (residues 448 to 723, Kd <6.25 μM) analysed by sedimentation equilibrium7 and may reflect either a real difference in dimerization propensity or a difference in experimental conditions.
We then tested the impact of GDC-0879 at saturating inhibitor concentration (40 μM)13. We observed a drastic enhancement in the ability to form dimers (Kd ⋘ 0.78 μM) such that no evidence of a monomer state was observed even at the lowest detectable concentration of BRAF protein (0.78 μM) (Supplementary Fig. 13). Analysis of the RAF inhibitor AZ-628 revealed a similar enhancement of dimerization (Kd ⋘0.78 μM) (Supplementary Fig. 13) as did Sorafenib, but the limited solubility of Sorafenib in aqueous solution hampered a comparable full analysis (not shown).
We next examined the effect of other kinase inhibitors predicted by BRET to have off-target effects on RAF dimerization. Consistent with their effects on BRET, SB202190, BIRB796, Dasatinib, Nilotinib and Tivozanib promoted dimerization relative to the apo state (Fig. 5e and Supplementary Fig. 13). Interestingly, ADP and the ATP mimetic AMP-PNP in contrast, inhibited dimer formation with no dimer species detected even at the highest protein concentration tested (25 μM; Fig. 5e and Supplementary Fig. 13). Together, these findings demonstrate that kinase inhibitors promote RAF dimerization directly through effects on the kinase domain.
Model for inhibitor-induced RAF dimerization
Available co-structures for RAF dimer-promoting kinase inhibitors bound to RAF or to other protein kinase domains (Supplementary Figs. 9 and 14) did not reveal any obvious feature in either small-molecule structure or binding mode that could readily explain why these diverse molecules commonly promote RAF dimerization. The only comparable characteristic of all co-structures was that the kinase domains adopted a closed conformation of the N- and C-lobes. Protein kinases are dynamic, possessing a large degree of flexibility between N and C lobes and within the N lobe itself (Fig. 6a). With respect to the latter, helix αC is tenuously tethered to a five strand β-sheet, which provides great opportunity for regulatory control of phosphotransfer function39, 40. Interestingly, the side-to-side dimerization surface of RAF kinases spans both N and C lobes7 (Fig. 6a). Furthermore, the N lobe portion of the contact surface spans both the β-sheet and helix αC components. Thus dimerization through the side-to-side surface would require a great restriction in flexibility of the RAF kinase domain. We posited that the binding of dimer promoting compounds to the catalytic cleft of the RAF kinase domains, irrespective of their variable modes of association, commonly stabilize the kinase domain in the closed state, thereby promoting dimerization.
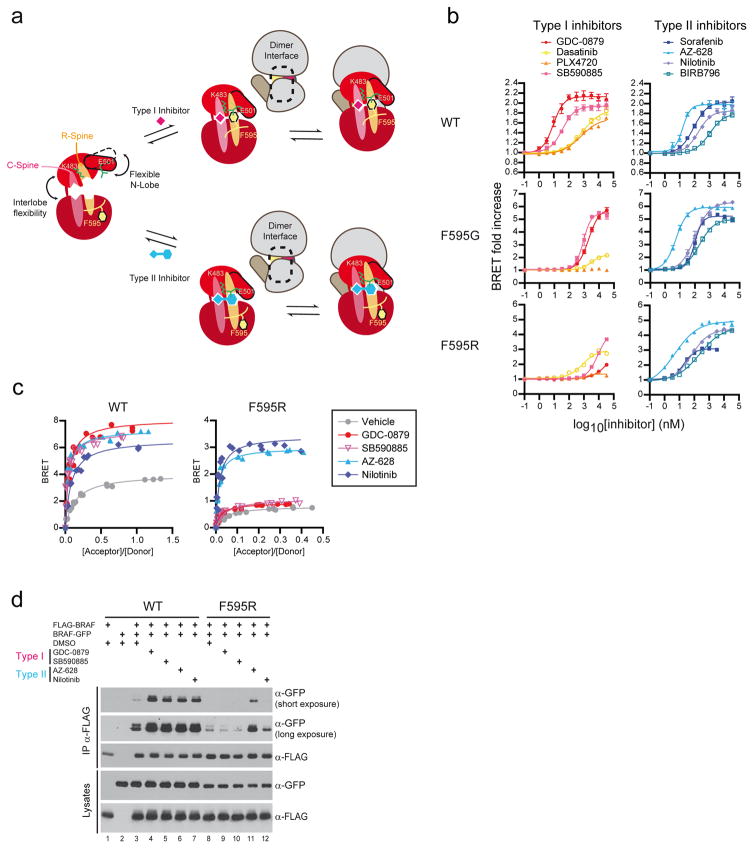
Figure 6. Probing the binding mode of RAF dimer inducers with BRAF mutant biosensors.
(a) Models for type I (top) and type II (bottom) kinase inhibitor-induced RAF dimerization. (b) BRAFKD R spine (F595R or F595G) mutants can distinguish type I from type II inhibitors by the capacity of the latter to selectively induce the dimerization of R spine mutant variants as monitored by BRET. (c) BRET saturation curves also demonstrate the distinct ability of type II inhibitors (AZ-628 and Nilotinib) over type I inhibitors (GDC-0879 and SB590885) to promote dimerization of a BRAF R spine mutant (F595R). (d) Co-immunoprecipitation of full-length BRAF_F595R is selectively induced by type II, but not by type I inhibitors. Each experiment was repeated at least two times. Where error bars are presented, they correspond to mean values ± s.d. of biological triplicates.
The alignment of the hydrophobic regulatory (R) and catalytic (C) spines, each of which traverses both N and C lobes of the kinase domain, serves as a diagnostic feature of low-energy closed conformations of the kinase domain41,42 (Fig. 6a and Supplementary Figs. 14 and 15). All inhibitors found to induce RAF dimerization preserve spine alignment (Supplementary Fig. 14) through related but distinct mechanisms. Type I inhibitors achieve spine alignment by binding within the ATP-binding pocket to bridge the N and C lobes along the C spine (Fig. 6a and Supplementary Fig. 15). The R spine in contrast is composed strictly of hydrophobic side chains of the kinase domain, including the phenylalanine of the DFG-motif. Type II inhibitors bind the ATP-binding pocket and similarly bridge the N and C lobes along the C spine but also occupy the DFG-out hydrophobic pocket (as a surrogate phenylalanine) to directly bridge the N and C lobes along the R spine (Fig. 6a and Supplementary Fig. 15). The end result of either inhibitor-binding mode is a closed rigid conformation of the kinase domain that presents a relatively static outer surface conducive to dimerization.
If lobe closure is essential for creating a productive dimerization interface, we hypothesized that interfering with it should reduce dimerization. To verify this, we mutagenized the DFG phenylalanine (F595) in BRAF to a glycine or arginine as these changes have been reported to induce a constitutive DFG-out-like conformation in p38α43. Consistent with our model, DFG mutant BRAFKD biosensors showed a significant reduction in dimerization-dependent BRET signals (Supplementary Fig. 16a) and similar loss of dimerization was observed by co-immunoprecipitation (Fig. 6).
To further test the model, we took advantage of the distinct binding modes of Type I and Type II inhibitors (schematized in Supplementary Fig. 16b). Type I inhibitors should be sensitive to DFG mutations since the DFG-in configuration would be unattainable. In addition, their affinity for DFG mutants might be reduced since Type I inhibitors are selected on the basis of binding to DFG-in configurations. In contrast, given that Type II inhibitors provide a surrogate phenylalanine, they should bind DFG mutants similar to WT and promote a closed configuration enabling dimerization. We first tested the binding of both classes of inhibitors by TR-FRET and indeed our predictions were confirmed (Supplementary Fig. 16c,d). Next, we examined the ability of both classes to promote dimerization of WT versus DFG BRAF mutants. As shown in Figure 6b, four distinct Type I inhibitors were highly sensitive to DFG mutations, whereas four Type II inhibitors were less so (Supplementary Table 4). Co-crystal structures confirmed that BIRB796 bound to BRAF in a DFG-out conformation (Supplementary Fig. 17 and Supplementary Table 5). We then characterized two representative inhibitors from each class for dimerization potential using BRET titration and BRAF co-immunoprecipitation assays (Fig. 6c,d). Again we observed that Type I inhibitors were more sensitive to the DFG mutation than Type II inhibitors. Together, these findings are consistent with a model whereby kinase inhibitors promote RAF dimerization by stabilizing a rigid closed conformation of the kinase domain.
PERK kinase domain shows inhibitor-induced dimerization
We next examined if other protein kinase families regulated by kinase domain dimerization would respond similarly to the binding of kinase inhibitors. Recently a nanomolar inhibitor, GSK2606414, was reported for PERK44, a member of the eIF2α kinase family that is regulated through a distinct mode of dimerization48,49. Crystallographic analysis revealed a Type I inhibitor-binding mode with unconventional features resembling a Type II inhibitor-binding mode44; while the DFG-out allosteric binding pocket remained occupied by DFG, the compound displaces another C spine residue L642. Nevertheless, contacts between the lobes are still maintained along the C spine through GSK2606414, allowing a rigid closed conformation that we reasoned would favor dimerization in solution as observed in the crystal environment. AUC analysis showed that the apo form of PERK was predominantly a monomer at all kinase domain concentrations tested (Supplementary Fig. 18). Saturating concentrations of ADP weakly promoted dimerization while saturating levels of a close structural analog of GSK2606414 45 strongly promoted dimerization. Since the mode of dimerization of the eIF2α kinase and RAF family kinases are entirely unrelated, and the inhibitors that promote RAF and PERK dimer formation are chemically distinct, these results support the notion that dimer promoting inhibitors may act through a common ability to restrict kinase domain dynamics.
Discussion
The observation that dimerization is critical for RAF activation and that ATP-competitive inhibitors exert paradoxical effects by stimulating RAF dimerization has raised caution regarding their clinical use and has created a need for methods enabling fast and reliable detection of RAF dimerization. The BRET-based assay presented here offers high sensitivity and reproducibility as well as quantitative real-time monitoring of RAF dimerization in living cells. The flexibility and simplicity of the assay make possible exhaustive surveys aimed at characterizing the dimerization properties of any RAF family member combination under a vast range of conditions and enables assessment of how mutations or small molecules impinge on RAF activity.
Despite the high level of conservation across RAF kinase domain isoforms, our data shows that kinase inhibitors can exert selective effects on their dimerization. This underscores the need to assess the overall impact of lead compounds on the RAF dimerization landscape to minimize unanticipated adverse biological consequences. Moreover, in addition to bona fide RAF inhibitors, our data and those from others groups37, 46 indicate that other kinase inhibitors can modulate RAF oligomeric status and thereby influence RAF activity. This would argue for a need to assess the RAF dimer inducing potential of all kinase inhibitors in development for therapeutic use.
In contrast to protein kinase inhibitors, we found that ADP and AMP-PNP inhibit BRAF dimerization relative to the weakly dimerizing apo state. Consistent with these findings, previous work also observed reduced BRAF-CRAF interaction by co-immunoprecipitation on purified proteins in the presence of an ATP analog13. While structures of dimer-promoting inhibitors bound to BRAF or CRAF are plentiful, structures bound to nucleotide and/or structures of RAF in a monomeric state are still lacking. This might reflect the fact that ATP and ADP binding to RAF disfavors the side-to-side dimer configuration. One possible explanation for this unexpected difference is that the binding mode of nucleotides precludes a rigid clamping of the kinase domain. If true, this might reflect the importance of conformational dynamics in the catalytic mechanism of phosphotransfer. A second possibility relates to the issue of affinity. ATP/ADP binding affinity to active state kinase domains typically lies in the micromolar range, while inhibitors commonly bind to their targets in the picomolar to nanomolar range. By selecting for high binding affinity, kinase inhibitors might be inescapably better at rigidifying the kinase domain and hence promoting RAF dimerization. This could suggest that it may be difficult to optimize inhibitor affinity independent of dimer-promoting ability. If true, one possible solution would be to identify inhibitors that induce a rigid conformation of the kinase domain but one that is sufficiently distorted to preclude dimerization. Alternatively, it may be possible to engineer high-affinity inhibitors that do not restrict the intrinsic flexibility of the kinase domain by binding to sites remote from the catalytic cleft. An obvious site would be the dimer interface itself.
Like the RAF family, the eIF2α kinases (PERK, Gcn2, PKR and HRI) are also regulated through a dimer-induced allosteric mechanism47–49. We have shown that the PERK-specific inhibitor GSK2606414 potently promotes PERK kinase domain dimerization. Whether GSK2606414 binding to PERK can promote the formation of active heterodimers between PERK and Gcn2, PKR or HRI remains to be determined. Based on the unintended consequences of RAF kinase inhibitors currently in clinical use, it would be advantageous to verify this possibility for all protein kinase families regulated by kinase domain dimerization.
Online Methods
Plasmids
All constructs were introduced in pCDNA3.1-based vectors (Invitrogen). The CAAX box of human KRAS (last 20 amino acids) was added to ARAF301-606, BRAF448-766, CRAF340-648, KSR1602-921 or KSR2657-950 kinase domains by PCR and BRET fusions were generated by inserting these kinase domains or full-length BRAF and CRAF proteins between KpnI and XbaI in a pCDNA3.1/Hygro plasmid already containing a N- or C-terminal cassette containing either the GFP10 or Renilla luciferase II cDNA20 (oligonucleotides used are listed in Supplementary Table 6). FLAG-tagged BRAF was generated by PCR and cloned in the same plasmid backbone between KpnI and XbaI sites. KRAS, HRAS and NRAS mCherry fusions were also cloned between KpnI and XbaI and were site-directed mutagenized by PCR using standard procedures. BRAF444-723, used in AUC analysis and crystallography was cloned with 16 solubilization mutations28 (I543A, I544S, I551K, Q562R, L588N, K630S, F667E, Y673S, A688R, L706S, Q709R, S713E, L716E, S720E, P722S, K723G), referred to as BRAF16mut, into pPROEX-HTa (Invitrogen) between NcoI and NotI sites. Murine PERK577-1082 with flexible loop residues 661–875 removed (here after referred to as PERK) was cloned into a SUMO-cleavable GST fusion vector.
Cell culture, transfection and preparation for BRET assays
HEK293T and COLO-205 cells were maintained in DMEM supplemented with 10% FBS and Penicillin/Streptomycin. HCT-116 cells were cultured in McCoy’s medium with 10% FBS and Penicillin/Streptomycin. For titration curves, 3 × 105 cells were seeded in 6-well plates and transfected the next day with polyethylenimine (PEI)50 at 1 μg/μl (see Supplementary Note 1). For dose-response curves, 2.5×106 cells were seeded in 100 mm plates and transfected with a total of 4 μg of DNA. 48 hours post-transfection, cells were washed, resuspended in Tyrode’s buffer (10 mM Hepes, 137 mM NaCl, 2.68 mM KCl, 0.42 mM NaH2PO4, 1.7 mM MgCl2, 11.9 mM NaHCO3, 5 mM glucose), counted and transferred to white opaque microtiter plates (BD Biosciences). A similar procedure was conducted for the high-throughput chemical screening except that cells were cultured in CellStacks (Corning) (see Supplementary Note 1).
BRET measurements
BRET signals and luciferase activity were read 15 minutes post-addition of 2.5 μM Coelenterazine 400a (Biotium) using a Mithras LB940 plate reader (Berthold Technologies) equipped with BRET1 emission filter set (donor: 480nm ± 20 nm; acceptor: 530 nm ± 20 nm). BRET signals emitted by RlucII/GFP10 pairs (BRET2 probes) can be read with either BRET1 or BRET2 filter sets20. The main difference is that the calculated BRET ratio is higher with BRET1 filters than with BRET2 filters (donor: 400 nm ± 20 nm; acceptor: 510 nm ± 20 nm) since only the shoulder of the RlucII emission spectrum is captured, while the full peak of RlucII would be detected with a 400 nm filter. In addition, the BRET1 filter set produced slightly better Z-factors. BRET signals correspond to the light emitted by the GFP10 acceptor constructs (530 nm ± 20 nm) upon addition of Coelenterazine 400a divided by the light emitted by the RlucII donor constructs (480 nm ± 20 nm). Specific BRET signals referred to as BRET in the text and figures correspond to total BRET signals measured from donor/acceptor-transfected samples minus background BRET signals measured from donor-transfected (RlucII constructs) alone samples. Total GFP10 or mCherry levels were detected on a FlexStation II (Molecular Devices) with excitation and emission peaks set at 400 and 510 nm, and 580 and 635 nm, respectively. Total intrinsic GFP10 (expressed as Relative Fluorescence Unit; RFU) and RlucII (Relative Luminescence Unit; RLU) signals were used as proxy to ensure that similar protein expression levels between comparable probes were used in titration experiments. In titration experiments whereby GFP10 acceptor constructs are titrated in, BRET signals (Y-axis) were plotted in relation to the increasing ratio of total GFP10 signal (RFU)/total luciferase signal (RLU) (X-axis: [Acceptor]/[Donor]). BRET-based dose-response experiments were expressed as BRET fold increase and were calculated by dividing the BRET of compound-treated cells by the BRET of control DMSO-treated cells. Finally, for high-throughput chemical screening (see Supplementary Table 7), BRET measurements were acquired using a SpectramaxL luminometer (Molecular Devices) and GFP10 signals were read on an Envision (Perkin Elmer) plate reader. Data for the chemical screen were expressed as percent of BRET induction and were calculated as follows: (100 × (BRETCOMPOUND/BRETDMSO)) − 100; where BRETCOMPOUND corresponds to the BRET signals obtained for the compound-treated cells and BRETDMSO corresponds to the BRET signals obtained for control DMSO-treated cells. Detailed BRET procedures are described in Supplementary Note 1.
Co-immunoprecipitation, Western blotting and AlphaScreen® assays
Co-immunoprecipitation and western blotting procedures were essentially conducted as follows. To prepare cell lysates, cells were washed once in cold 1X phosphate-buffered saline (PBS) and then directly lysed on plates by adding 1ml of Igepal lysis buffer (20 mM Tris at pH 8.0, 137 mM NaC1,10% glycerol, 1% Igepal CA-630, 2 mM EDTA, 1X phosphatase inhibitor cocktail (Sigma), 1 mM sodium vanadate, 20 μM leupeptin, aprotinin (0.15 U/ml), 1 mM phenylmethylsulfonyl fluoride (PMSF)). Lysing cells were incubated for 10 min at 4°C with gentle rocking, collected and spun at 14,000g, 4°C for 10 min. For co-immunoprecipitations, primary antibodies were added to fresh cell lysates and incubated at 4°C for 1 h. Protein A/G agarose beads (Santa Cruz Biotechnology) were then added, and gently rocked at 4°C for an additional 3 h. Immunoprecipitates were washed three times with cold lysis buffer. FLAG-tagged complexes were eluted with 3XFLAG peptide (Sigma) prior to gel electrophoresis. Cell lysates or immunoprecipitated proteins were resolved on 8% SDS-PAGE, transferred to nitrocellulose membranes (Dupont) and probed using appropriate primary antibodies. All antibodies were diluted in Tris-buffered saline (TBS) supplemented with 0.2% Tween. Anti-Renilla luciferase 5B11.2 (Millipore), anti-GFP clones 7.1/13.1 (Roche), anti-phosphoERK1/2 (Sigma), anti-phosphoMEK (Cell Signaling Technology) and anti-MEK1 (610121; BD Biosciences) were used at a 1:2000 dilutions. Anti-FLAG M2 (Sigma) was used at a 1:5000 dilution. Secondary anti-mouse and anti-rabbit-HRP (Santa Cruz Biotechnology) were used at a 1:10000 dilution in TBS-0.2%Tween. Phospho-ERK analysis was conducted on 40,000 cells cultured overnight in 96-well plates and treated with the indicated compound concentrations for two hours. Phospho-ERK1/2 AlphaScreen® (PerkinElmer) assays were performed according to the manufacturer’s specifications.
Inhibitors used in this study
PLX4720, GDC-0879 and AZ-628 were obtained from Axon Medchem. Sorafenib, Imatinib, Dasatinib, Nilotinib, Pazopanib and Tivozanib were purchased from LC laboratories. SB202190 and SB203580, BIRB796, SB590885 were from Selleck Chemicals. TWS119, MNK1 inhibitor and VEGFR inhibitor II were from Calbiochem. All compounds were at least 95% pure as evaluated by HPLC (Supplementary Table 8). For dose-response experiments, serial dilutions of all drugs were prepared in DMSO and 1:100 dilution were prepared in Tyrode’s buffer prior to addition to 90 μl of cell suspensions in Tyrode’s buffer (1×106 cells/ml) at a 1:10 dilution for the indicated time. GGTI-298 and FTI-277 (Sigma-Aldrich) were also prepared in DMSO. The compound library used for high-throughput screening is available through the IRIC HTS facility and was obtained from various sources (http://www.iric.ca/en/research/core-facilities/high-throughput-screening/?section=technologies). The focused kinase inhibitor library was assembled from the EMD chemicals InhibitorSelect library and various other inhibitors obtained from LC laboratories (Supplementary Data Set 1 and Supplementary Table 7). The GSK2606414 analog referred to as Compound 39 (5-(1-{[3-Fluoro-5-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine) was kindly provided by Dr. David Uehling (OICR, Toronto).
Protein purification
TEV-cleavable 6XHis-tagged BRAF16mut was expressed in BL21(DE3)-RIL bacterial expression cells, purified with nickel affinity chromatography, TEV-cleaved overnight and purified through gel filtration chromatography into a final buffer of 15 mM Hepes pH 7.5, 200 mM NaCl, 10 mM DTT and 5% glycerol. SUMO protease-cleavable GST tagged PERK was expressed in BL21(DE3)-RIL cells, purified by glutathione affinity chromatography, treated with SUMO protease overnight and purified through gel filtration chromatography into a final buffer of 100 mM Hepes pH 7, 100 mM NaCl and 1 mM TCEP. Following gel filtration, protein fractions corresponding to greater than 95% purity were pooled and concentrated to 20 mg/mL, then flash frozen in liquid nitrogen.
Protein crystallization and Structural Analysis
156 μM (5mg/mL) BRAF16mut was co-crystallized with 230 μM BIRB796 at 4°C in 0.1M BisTris propane pH 8 and 30% PEG 3350 using the hanging drop method. X-ray diffraction data was collected on a flash frozen crystal cryo protected in mother liquor containing 22% glycerol at the Advanced Photon Source (NECAT beamline 24-ID). Data reduction was performed using HKL2000 (HKL Research Inc.). The BRAF16mut-BIRB796 co-structure was solved by molecular replacement using PDB 3C4C as a search model in Phaser51. Model refinement was performed using Phenix 1.7.151.
Analytical ultracentrifugation (AUC)
Sedimentation velocity analytical ultracentrifugation was performed with a Beckman ProteomeLab XL-I at 42,000 rpm. Data was obtained after 7.5 h of centrifugation at 20°C by monitoring the relative refractive index between sample and blank. Various concentrations of BRAF16mut, ranging from 0.78 μM to 25 μM, were tested minimally in duplicate in AUC buffer (BRAF16mut: 15 mM Hepes pH 7, 200 mM NaCl, 3 mM DTT; PERK: 20 mM Hepes pH 7.5, 150 mM NaCl, 1 mM TCEP) in the presence or absence of 40 μM inhibitor compound, or 200 μM AMP-PNP with 200 μM MgCl2, or 200 μM ADP with 200 μM MgCl2. AUC analyses of PERK employed identical conditions as per BRAF16mut with the exception that ADP analyses were performed with 500 μM ADP with 2 mM MgCl2. The AUC experimental conditions for BRAF16mut, were extensively optimized to maximize protein stability for the duration of the AUC analysis, and differed from those employed previously for a shorter BRAF construct7 in temperature (4°C), duration of centrifugation (14 days per sedimentation equilibrium analysis) and buffer composition (20 mM Tris pH 7.0, 200 mM NaCl, 5% glycerol and 1.5 mM TCEP).
Drug-binding assay by TR-FRET
For drug-binding assays, a procedure similar to the LanthaScreen™ Eu Kinase Binding Assay for BRAF (Invitrogen) was used. Purified 6XHis-tagged BRAF444-723 kinase domain (50 nM final concentration) was co-incubated with 2 nM LANCE® Europium-coupled anti-His antibody (PerkinElmer), 60 nM Alexa Fluor® 647-labeled kinase tracer (Invitrogen) and varying concentrations of kinase inhibitors for 1h at room temperature in Kinase Buffer (50 mM Hepes pH7.5, 100 mM NaCl, 3mM DTT, 10 mM MgCl2, 1mM EDTA, 0.01% Brij-35). Each experiment included control wells (triplicates) containing the LANCE® antibody and Alexa Fluor® 647-labeled kinase tracer alone; the average signal of the blank wells was subtracted from each data point. TR-FRET was read on an Envision (PerkinElmer) plate reader with a 340±30 nm excitation filter. The emission of Alexa Fluor® 647 signal was monitored with a 665±10 nm filter and the Europium emission signal was acquired using a 615±10 nm filter. The TR-FRET signal was calculated by dividing the emission signal at 665nm by the emission at 615 nm. The relative reduction in TR-FRET signal was calculated by normalizing each data point to the DMSO vehicle-treated wells.
Data analysis and structure rendering
BRET titration curves allow extrapolation of two key parameters, namely BRET50 and BRETmax, which can be used to assess pharmacological or genetic alterations of interacting proteins. The BRET50 corresponds to the ratio of acceptor construct over donor construct required to attain 50% of the maximum BRET signal. It is essentially dictated by the relative affinity of interacting BRET pairs and their propensity to co-localize. On the other hand, the BRETmax represents the maximum BRET signal strength obtained with saturating amounts of the acceptor probe. This parameter depends on the distance between BRET pairs, their relative orientation as well as the proportion of donor proteins engaged in dimerization19, 52.
Raw data was analysed using the Prism 5.04 software (GraphPad Software). BRETmax and BRET50 parameters were derived from a one-site binding hyperbolic fitting of the data and EC50s were calculated using a log(agonist) versus response fitting. Significance in BRET50 and BRETmax differences between BRAF mutants and after drug treatments was assessed using an F-test. Heatmap displays were generated using the Treeview program (http://rana.lbl.gov/EisenSoftware.htm). All protein structure representations were prepared using PyMol (Schrödinger). AUC data was processed using SEDFIT (National Institute of Health) to calculate a continuous c(s) distribution. Solute partial specific volume, buffer density and buffer viscosity were calculated using Sednterp (Thomas Laue).
Data deposition
BRAF-BIRB796 coordinates have been submitted to the PDB under ID:4JVG.
Supplementary Material
Acknowledgments
We thank B. Breton and M. Audet for advice with the BRET2 system, D. Uehling and co-workers at the Ontario Institute for Cancer Research for GSK2606414, and the IRIC HTS platform. IRIC is supported by the Canadian Center of Excellence in Commercialization and Research, the Canada Foundation for Innovation and by the Fonds de Recherche du Québec en Santé. HL is a recipient of Cancer Research Society and Canadian Institutes for Health Research (CIHR) Banting postdoctoral fellowships. NT is a recipient of the CIHR Canadian Graduate Scholarship. MB, FS and MT hold Canada Research Chairs. This work was supported by operating funds from the Canadian Cancer Society to MT (018046) and from the CIHR to MT (MOP119443) and FS (MOP36399).
Footnotes
Author contributions
HL, NT, FS, and MT designed the experiments and wrote the manuscript. MB contributed to the theoretical framework surrounding BRET assay development, participated to the analysis of the BRET data and in the revision of the manuscript. HL with assistance from GG, AP, SG, and JD, conducted cell-based BRET analyses, the HTS chemical screen, and TR-FRET in vitro binding assays. NT with assistance from DM, JL and HL, performed AUC and X-ray structure analyses.
Competing financial interests
HL, FS, and MT have filed a patent covering the BRET-based method described in this study for detecting RAF dimerization.
References
- 1.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 2.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 3.Dhomen N, Marais R. New insight into BRAF mutations in cancer. Current opinion in genetics & development. 2007;17:31–39. doi: 10.1016/j.gde.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- 5.Claperon A, Therrien M. KSR and CNK: two scaffolds regulating RAS-mediated RAF activation. Oncogene. 2007;26:3143–3158. doi: 10.1038/sj.onc.1210408. [DOI] [PubMed] [Google Scholar]
- 6.Garnett MJ, Rana S, Paterson H, Barford D, Marais R. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol Cell. 2005;20:963–969. doi: 10.1016/j.molcel.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Rajakulendran T, Sahmi M, Lefrancois M, Sicheri F, Therrien M. A dimerization-dependent mechanism drives RAF catalytic activation. Nature. 2009;461:542–545. doi: 10.1038/nature08314. [DOI] [PubMed] [Google Scholar]
- 8.Rushworth LK, Hindley AD, O’Neill E, Kolch W. Regulation and role of Raf-1/B-Raf heterodimerization. Mol Cell Biol. 2006;26:2262–2272. doi: 10.1128/MCB.26.6.2262-2272.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber CK, Slupsky JR, Kalmes HA, Rapp UR. Active Ras induces heterodimerization of cRaf and BRaf. Cancer Res. 2001;61:3595–3598. [PubMed] [Google Scholar]
- 10.Halilovic E, Solit DB. Therapeutic strategies for inhibiting oncogenic BRAF signaling. Curr Opin Pharmacol. 2008;8:419–426. doi: 10.1016/j.coph.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Bollag G, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph EW, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci U S A. 2010;107:14903–14908. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatzivassiliou G, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 14.Heidorn SJ, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poulikakos PI, Rosen N. Mutant BRAF melanomas--dependence and resistance. Cancer Cell. 2011;19:11–15. doi: 10.1016/j.ccr.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Poulikakos PI, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solit DB, Rosen N. Resistance to BRAF inhibition in melanomas. The New England journal of medicine. 2011;364:772–774. doi: 10.1056/NEJMcibr1013704. [DOI] [PubMed] [Google Scholar]
- 19.Bacart J, Corbel C, Jockers R, Bach S, Couturier C. The BRET technology and its application to screening assays. Biotechnol J. 2008;3:311–324. doi: 10.1002/biot.200700222. [DOI] [PubMed] [Google Scholar]
- 20.Breton B, et al. Multiplexing of multicolor bioluminescence resonance energy transfer. Biophys J. 2010;99:4037–4046. doi: 10.1016/j.bpj.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kocan M, See HB, Seeber RM, Eidne KA, Pfleger KD. Demonstration of improvements to the bioluminescence resonance energy transfer (BRET) technology for the monitoring of G protein-coupled receptors in live cells. J Biomol Screen. 2008;13:888–898. doi: 10.1177/1087057108324032. [DOI] [PubMed] [Google Scholar]
- 22.James JR, Oliveira MI, Carmo AM, Iaboni A, Davis SJ. A rigorous experimental framework for detecting protein oligomerization using bioluminescence resonance energy transfer. Nat Methods. 2006;3:1001–1006. doi: 10.1038/nmeth978. [DOI] [PubMed] [Google Scholar]
- 23.Roring M, et al. Distinct requirement for an intact dimer interface in wild-type, V600E and kinase-dead B-Raf signalling. EMBO J. 2012;31:2629–2647. doi: 10.1038/emboj.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritt DA, Monson DM, Specht SI, Morrison DK. Impact of feedback phosphorylation and Raf heterodimerization on normal and mutant B-Raf signaling. Mol Cell Biol. 2010;30:806–819. doi: 10.1128/MCB.00569-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dar AC, Shokat KM. The evolution of protein kinase inhibitors from antagonists to agonists of cellular signaling. Annu Rev Biochem. 2011;80:769–795. doi: 10.1146/annurev-biochem-090308-173656. [DOI] [PubMed] [Google Scholar]
- 26.Whittaker S, et al. Gatekeeper mutations mediate resistance to BRAF-targeted therapies. Sci Transl Med. 2010;2:35ra41. doi: 10.1126/scitranslmed.3000758. [DOI] [PubMed] [Google Scholar]
- 27.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 28.Tsai J, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan PT, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 30.Lee JC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 31.Hall-Jackson CA, Goedert M, Hedge P, Cohen P. Effect of SB 203580 on the activity of c-Raf in vitro and in vivo. Oncogene. 1999;18:2047–2054. doi: 10.1038/sj.onc.1202603. [DOI] [PubMed] [Google Scholar]
- 32.Kalmes A, Deou J, Clowes AW, Daum G. Raf-1 is activated by the p38 mitogen-activated protein kinase inhibitor, SB203580. FEBS Lett. 1999;444:71–74. doi: 10.1016/s0014-5793(99)00034-4. [DOI] [PubMed] [Google Scholar]
- 33.McKay MM, Ritt DA, Morrison DK. RAF Inhibitor-Induced KSR1/B-RAF Binding and Its Effects on ERK Cascade Signaling. Curr Biol. 2011;21:563–568. doi: 10.1016/j.cub.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King AJ, et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res. 2006;66:11100–11105. doi: 10.1158/0008-5472.CAN-06-2554. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, et al. Structural basis of inhibitor selectivity in MAP kinases. Structure. 1998;6:1117–1128. doi: 10.1016/s0969-2126(98)00113-0. [DOI] [PubMed] [Google Scholar]
- 36.Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1039–1045. doi: 10.1038/nbt.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Packer LM, et al. Nilotinib and MEK inhibitors induce synthetic lethality through paradoxical activation of RAF in drug-resistant chronic myeloid leukemia. Cancer Cell. 2011;20:715–727. doi: 10.1016/j.ccr.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrios-Rodiles M, et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science. 2005;307:1621–1625. doi: 10.1126/science.1105776. [DOI] [PubMed] [Google Scholar]
- 39.Jeffrey PD, et al. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 40.Sicheri F, Kuriyan J. Structures of Src-family tyrosine kinases. Curr Opin Struct Biol. 1997;7:777–785. doi: 10.1016/s0959-440x(97)80146-7. [DOI] [PubMed] [Google Scholar]
- 41.Kornev AP, Haste NM, Taylor SS, Eyck LF. Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc Natl Acad Sci U S A. 2006;103:17783–17788. doi: 10.1073/pnas.0607656103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kornev AP, Taylor SS. Defining the conserved internal architecture of a protein kinase. Biochim Biophys Acta. 2010;1804:440–444. doi: 10.1016/j.bbapap.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bukhtiyarova M, Karpusas M, Northrop K, Namboodiri HV, Springman EB. Mutagenesis of p38alpha MAP kinase establishes key roles of Phe169 in function and structural dynamics and reveals a novel DFG-OUT state. Biochemistry. 2007;46:5687–5696. doi: 10.1021/bi0622221. [DOI] [PubMed] [Google Scholar]
- 44.Axten JM, et al. Discovery of 7-Methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a Potent and Selective First-in-Class Inhibitor of Protein Kinase R (PKR)-like Endoplasmic Reticulum Kinase (PERK) J Med Chem. 2012;55:7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 45.Korennykh AV, et al. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457:687–693. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sen B, et al. Kinase-impaired BRAF mutations in lung cancer confer sensitivity to dasatinib. Sci Transl Med. 2012;4:136ra170. doi: 10.1126/scitranslmed.3003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dar AC, Dever TE, Sicheri F. Higher-order substrate recognition of eIF2alpha by the RNA-dependent protein kinase PKR. Cell. 2005;122:887–900. doi: 10.1016/j.cell.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 48.Dey M, et al. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2alpha substrate recognition. Cell. 2005;122:901–913. doi: 10.1016/j.cell.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 49.Taylor SS, Haste NM, Ghosh G. PKR and eIF2alpha: integration of kinase dimerization, activation, and substrate docking. Cell. 2005;122:823–825. doi: 10.1016/j.cell.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Boussif O, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta crystallographica. Section D, Biological crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciruela F. Fluorescence-based methods in the study of protein-protein interactions in living cells. Curr Opin Biotechnol. 2008;19:338–343. doi: 10.1016/j.copbio.2008.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.