Abstract
We demonstrate derivation of induced pluripotent stem cells (iPSCs) from terminally differentiated mouse cells in serum- and feeder-free stirred suspension cultures. Temporal analysis of global gene expression revealed high correlations between cells reprogrammed in suspension and cells reprogrammed in adhesion-dependent conditions. Suspension (S) reprogrammed iPSCs (SiPSCs) could be differentiated into all three germ layers in vitro and contributed to chimeric embryos in vivo. SiPSC generation allowed for efficient selection of reprogramming factor expressing cells based on their differential survival and proliferation in suspension. Seamless integration of SiPSC reprogramming and directed differentiation enabled the scalable production of functionally and phenotypically defined cardiac cells in a continuous single cell- and small aggregate-based process. This method is an important step towards the development of a robust PSC generation, expansion and differentiation technology.
Introduction
The derivation of mouse and human induced pluripotent stem cells (iPSCs) from terminally differentiated somatic cells opened up new avenues to address important fundamental questions in developmental biology1–3. iPSC technology also sparks hope for therapeutic advances by enabling the generation of high quality disease models, derivation of patient-specific iPSC lines, improvements in the predictability of drug action or as a source of cells for regenerative medicine. One of the biggest challenges in the application of this technology has been the robustness of cell generation, including the ability to produce cells at scale4–6.
Despite the rapid progress in molecular tools for iPSC generation, the development of culture methods providing controlled microenvironments devoid of animal components at scale have lagged behind, primarily because of the dependence of PSC on adhesion or aggregation for propagation7–11. Current protocols typically reprogram somatic cells to pluripotency by serial passage under adherent culture conditions on feeder cells or on extracellular matrix components12. These approaches risk contamination by pathogens, require separation of feeder cells from the cell type of interest, increase costs, and are prone to variability. Both mouse and human ESC can be maintained and expanded in a pluripotent state as floating aggregates in the absence of feeder cells13–16., however, all suspension cultures reported to date require serial dissociation and reaggregation steps (manipulations that typically limit cell yields).
In this work we derive mouse iPSCs in a continuous adherence- and matrix-free suspension system. We take advantage of inducible secondary mouse embryonic fibroblasts to compare reprogramming in suspension culture to that in routine adherent culture. Gene expression analysis showed a high correlation between the two processes with regard to hallmark reprogramming genes. Differentially expressed transcripts mainly belonged to gene products involved in interactions with extracellular matrix components or cell adhesion, suggesting that these proteins may not be critical for reprogramming. We show directed differentiation of primary SiPSCs to cardiac progenitor cells, demonstrating a method whereby somatic cells are reprogrammed, expanded and differentiated in a continuous suspension culture. This system should prove useful for fundamental studies into iPSC reprogramming processes and for investigating the impact of media and the microenvironment on cellular reprogramming. Furthermore, it represents an important step towards the robust scalable production of iPSC for a variety of applications.
Results
Reprogramming enables fibroblast propagation in suspension
Fibroblast-like cells are typically anchorage dependent and stop dividing and apoptose when shifted from adherent to suspension culture conditions. To quantitatively study the effect of reprogramming on fibroblast survival in suspension we used secondary inducible fibroblasts derived from chimeric embryos generated from reprogrammed primary fibroblasts. These cells are a mixed population of wild type (control) cells and GFP positive (reprogrammable) cells; the latter reprogram at high frequencies11. We observed that these secondary fibroblasts started to proliferate when cultured in suspension in the presence, but not in the absence, of doxycycline (Fig. 1a). We observed an initial lag phase, where overall cell densities decreased, before cultures entered a phase of rapid cell division (Fig. 1a).
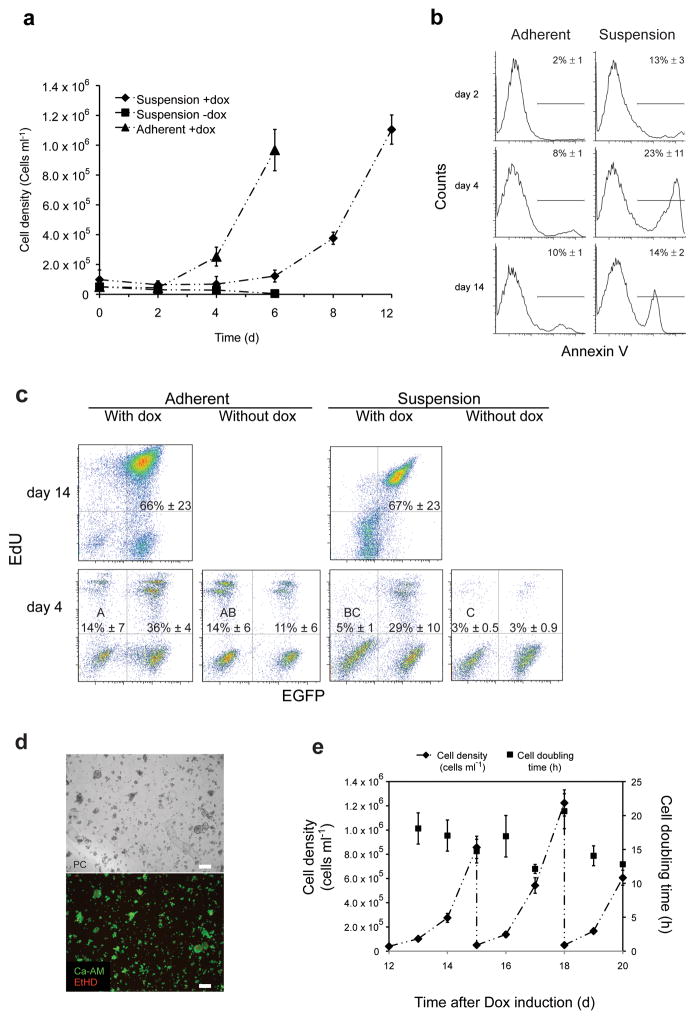
Figure 1.
Secondary fibroblasts inducibly expressing reprogramming factors survive and proliferate in suspension. (a) Growth kinetics of inducible secondary fibroblasts cultured under the indicated conditions. Error bars s.d. (n = 3). (b) Fluorescence-activated cell sorting (FACS) analysis of AnnexinV surface localization for secondary fibroblasts cultured under the indicated conditions. Values are means ± s.d. (n ≥ 3). (c) FACS analysis of 5-ethynyl-2′-deoxyuridine (EdU) incorporation by secondary fibroblasts cultured under the indicated conditions. Percentages of GFP−/EdU+ populations that do not share a letter are significantly different (ANOVA, P = 0.003; and Tukey post-hoc with P = 0.0113) Values are means ± s.d. (n = 5). (d) Live/dead staining of secondary fibroblasts cultured in suspension in the presence of doxycycline at day six of culture. PC, phase contrast; red, ethidium homodimer-1 (dead); green, Calcein acetoxymethyl ester (live). Scale bars: 100 μm (e) Growth kinetics of doxycycline-induced secondary fibroblasts growing in mouse embryonic stem cell (mESC) medium from day 12–20. Suspension cultures inoculated with d12 cells (5 × 104cells ml−1) were serially expanded with 20-fold media dilution every three days. Error bars s.d. (n = 3).
Secondary fibroblasts cultured in suspension exhibited increased AnnexinV staining during the first six days compared to adherent conditions (Fig. 1b). We assayed both adherent- and suspension-cultured cells for active cell division by tracing incorporation of EdU (Fig. 1c). In the case of adherent cells, both GFP positive (cells harboring the reprogramming factors) and negative populations showed strong incorporation of EdU, indicating actively dividing cells, regardless of doxycycline (Fig. 1c). With increased culture time in the presence of doxycycline, GFP positive cells exhibited an increased cell division rate compared to the GFP negative population. In contrast, suspension cultured cells exhibited substantially weaker incorporation of EdU into GFP− cells in the presence and absence of doxycycline, indicating decreased proliferation rates for this subpopulation compared to adherent conditions (Fig. 1c). In the presence of doxycycline, a rapidly proliferating GFP+/Edu+ double-positive subpopulation emerged (Fig. 1c), indicating actively reprogramming cells in suspension. These data suggest that suspension culture preferentially supports cells undergoing reprogramming via differential survival and proliferation.
Surprisingly, doxycycline-induced cells grew in suspension as a mixed culture of aggregates and viable single cells, as confirmed by calcein acetoxymethyl ester (Calcein-AM) staining (Fig. 1d). The cells entered a rapid growth phase and showed sustained growth during later stages (after day 12) of suspension reprogramming (Fig. 1e). These observations demonstrate that secondary fibroblasts are capable of surviving and proliferating in suspension upon induction of reprogramming factors.
Serum-free suspension reprogramming to pluripotency
To assess whether secondary fibroblasts can be reprogrammed to pluripotency in suspension, we assayed for SSEA-1 expression over 15 days by flow cytometry (Fig. 2a). SSEA-1 expression was detectable as early as two days after doxycycline induction and reached levels of > 70% by day 15 of culture. Cells reprogramming in adherent conditions and in suspension cultures ± serum exhibited strikingly similar SSEA-1 expression kinetics (Fig. 2b). We observed NANOG expression in a substantial proportion of the population eight days after induction (Fig. S1).
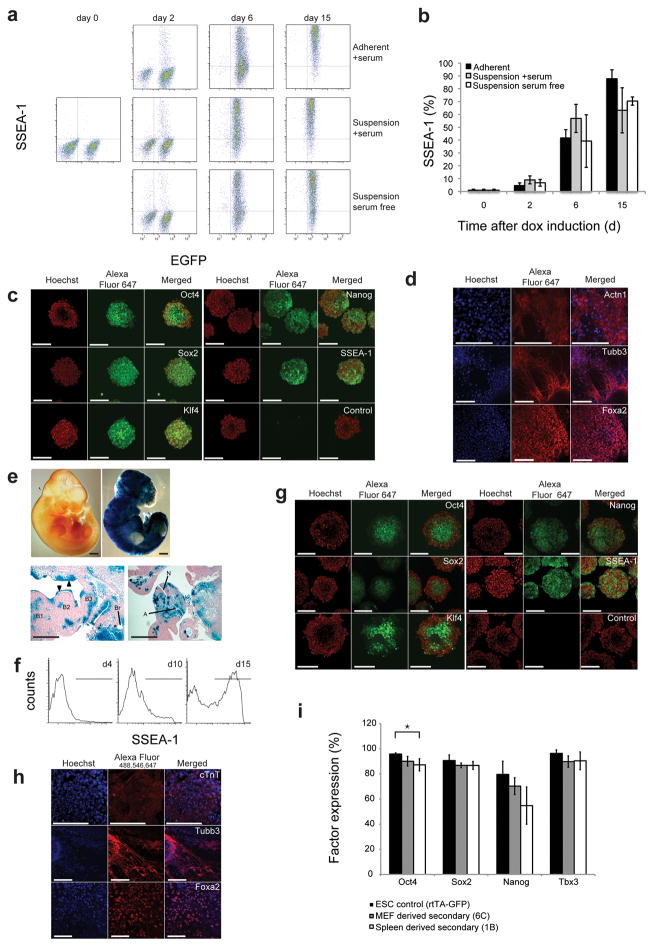
Figure 2.
Suspension reprogramming of multiple cell types to pluripotency. (a) FACS analysis showing SSEA-1 expression in inducible secondary fibroblasts reprogrammed under the indicated conditions. (b) Quantification of SSEA-1 expression data in (a). There was no significant difference between protocols at any time point (ANOVA: day 2: P = 0.115, day 6: P = 0.312, day 15: P = 0.122). Error bars s.d. (n ≥ 3). (c) Micrographs show immunocytochemistry of secondary MEF derived SiPSCs stained with antibodies against pluripotency factors. (d) Micrographs show staining of in vitro differentiated, suspension-reprogrammed, doxycycline-independent iPS cells for markers of mesoderm (ACTN1, top), ectoderm (TUBB3, middle) and endoderm (FOXA2, bottom). (e) Whole mount LacZ staining (top) and paraffin sections (bottom) of day 10.5 embryos chimeric for suspension-reprogrammed, lacZ-positive iPSCs. Positive staining demonstrates expression from the β-geo reporter (co-cistronic with the reprogramming factors) in the secondary fibroblast-derived embryo. A lacZ negative littermate is shown (top left). Scale bars: 400 μm. A: dorsal aorta (mesoderm); B: branchial arches (mesoderm and ectoderm); Br: left bronchus (endoderm); N: caudal neuropore (ectoderm); arrowheads indicate strong contribution to ectoderm (epithelium). (f) FACS analysis of SSEA-1 activation during suspension reprogramming of spleen cells derived from an adult secondary chimera. Error mean ± s.d. (n = 2) (g) Immunocytochemistry analysis of pluripotency marker expression of spleen derived doxycycline-independent SiPSC aggregates. (h) Fluorescence micrographs of in vitro differentiated spleen derived SiPS cells to mesoderm (cTNT), ectoderm (TUBB3) and endoderm (FOXA2). (i) The plot shows FACS analysis of gene expression levels in the indicated cell populations. Significant differences are depicted by horizontal brackets (OCT4: ANOVA P = 0.023 and Tukey post-hoc comparisons with P < 0.022). Scale bars: 100 μm for fluorescence images. Results are expressed as mean ± s.d., n = 3.
We assayed whether SiPSCs become independent of exogenous factor expression by removing doxycycline at d15 after induction. Upon doxycycline removal, cells aggregated (Fig. S2) and a trypsin dissociation step had to be included to passage cells. Doxycycline-independent aggregates exhibited nuclear staining for the transcription factors OCT4 (POU5F1), SOX2, KLF4, NANOG and membrane staining for SSEA-1, indicating stable expression of endogenous pluripotency factors (Fig. 2c). Doxycycline-independent SiPSCs expressing NANOG and SSEA-1 could also be obtained after more than 40 days in culture, indicating that suspension reprogramming is suitable for the long term growth of pluripotent cells (Fig. S3). Pluripotency factor expression was additionally confirmed by western blot analysis (Fig. S4). Doxycycline-independent SiPSCs were capable of differentiating into all three germ layers in vitro (Fig. 2d). Finally, we injected secondary fibroblast derived SiPSCs into mouse blastocysts and observed chimerism and contribution to all three germ layers at day e10.5 by lacZ expression (Fig. 2e).
In order to assay whether other cell types can be reprogrammed in suspension, we isolated spleen cells and T-cell progenitors from adult doxycycline-inducible secondary chimeric mice. Spleen-derived cells displayed robust SSEA-1 induction and became doxycycline independent after 15 days of exogenous factor expression (Fig. 2f). Spleen-derived SiPSCs stably expressed pluripotency factors (Fig. 2g), were capable of differentiating into all three germ layers in vitro (Fig. 2h), and showed a pluripotency-related gene expression profile closely related to that of mouse embryonic stem cell controls (Fig. S5). We analyzed stable pluripotency factor expression by FACS in MEF- and spleen-derived SiPSCs. We observed small variations in OCT4 expression for spleen derived SiPSCs compared to rtTA-GFP control ES cells; MEF-derived SiPSCs did not exhibit any notable differences (Fig. 2i). Purified T-cell progenitors could also be reprogrammed in suspension into doxycycline-independent SSEA-1 and NANOG expressing cells (Fig. S6). This demonstrates that multiple mouse cell types (adult and embryonic, as well as adherent and suspension cell types) can be reprogrammed in suspension.
Whole-genome expression analysis of suspension reprogramming
We collected samples of adherent and suspension cells undergoing reprogramming at different timepoints after doxycycline induction and analyzed them for expression of a total of 22,348 transcripts. We selected genes that changed more than three-fold at any of the four time-points, in either adherent or suspension conditions, relative to the un-induced MEF controls (3,801 transcripts) and examined Pearson correlation coefficients (PCCs) for all conditions. Cells cultures under adherent and suspension conditions showed similar trends for PCCs throughout the reprogramming process (Fig. 3a). Unsupervised hierarchical clustering analysis on the 3,801 genes that changed more than three-fold during the reprogramming process suggested that similar molecular events occur over the reprogramming process under the two conditions (Fig. 3b).
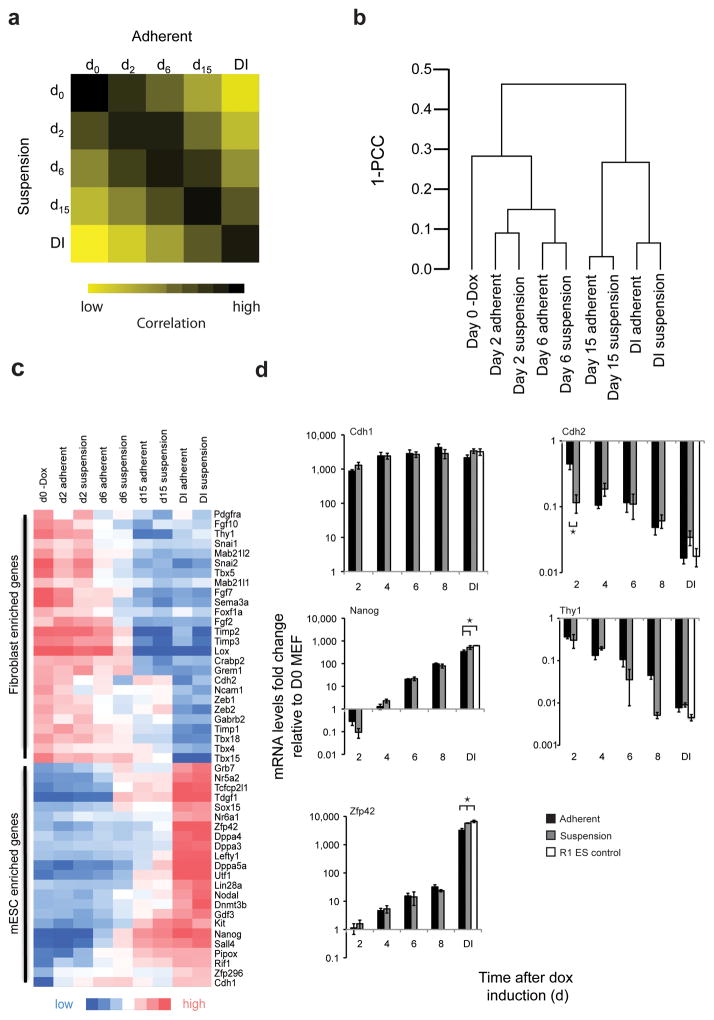
Figure 3.
Whole genome analysis reveals high similarity between reprogramming under adherent and suspension conditions. (a) Pearson correlation comparison between cells reprogrammed in suspension and adherent conditions of the 3,801 transcripts that changed more than three-fold relative to the parental MEF population at any time during the reprogramming process. DI, doxycycline independent state (b) Unsupervised hierarchical clustering of transcripts by Pearson correlation. PCC, Pearson Correlation Coefficient. (c) Heat map depicting expression profiles of subsets of selected fibroblast-enriched (top) and mESC enriched (bottom) genes during the reprogramming process under suspension and adherent conditions. (d) Expression profiles over the course of the reprogramming process for the indicated genes determined by quantitative PCR. Fold-changes relative to day zero MEFs are depicted for adherent and suspension conditions. R1 cells serve as an embryonic stem cell control. Expression levels that differ significantly at matching timepoints were depicted by horizontal brackets (ANOVA P < 0.001 and Tukey post-hoc with P = 0.0014 for each significant difference). Error bars s.d., (n = 3).
Fibroblast- and pluripotency-specific hallmark genes17 exhibited similar expression changes over the course of reprogramming for both adherent and suspension conditions (Fig. 3c). Several of the analyzed pluripotency factors showed different expression levels in cells on day 15 under doxycycline and cells in the doxycycline-independent state (for both adherent and suspension cells). This suggests that exogenous factor expression is repressed to establish endogenous expression levels of pluripotency genes or that further selection steps lead to the establishment of doxycycline independent cultures with expression profiles similar to ESC.
We next selected factors that are known to be up- or down-regulated at different phases of the reprogramming process17 and followed expression levels of these factors in two-day time intervals by quantitative RT PCR. Early (Cdh1), intermediate (Nanog) and late (Zfp1) induction was observed under adherent conditions, in accordance with results reported earlier17 (Fig. 3d). Cells reprogramming in suspension showed comparable induction profiles for these factors (Fig. 3d). Factors known to be down-regulated during reprogramming, such as Cdh2 and Thy1, were also down-regulated during suspension reprogramming (Fig. 3d). Small differences between adherent and suspension reprogrammed iPSC, and R1 ESC controls, were found for Nanog and Zfp42 expression in stably reprogrammed cell populations (Fig. 3d). Overall the two reprogramming protocols did not show notable expression differences for the analyzed factors over the entire length of the reprogramming process (with the exception of Cdh2, a cell adhesion marker that was down-regulated more quickly in suspension conditions compared to adherent conditions) (Fig. 3d).
We did however find some genes that were differentially expressed between cells reprogrammed under suspension and adherent conditions. To investigate these differences we selected genes which exhibited a > 2-fold expression change between the two conditions at paired time points during the reprogramming process. Analysis of the differentially expressed genes by GO enrichment analysis (Table 1) showed a considerable overrepresentation of GO terms associated with extracellular compartments, cell adhesion and extracellular matrix (ECM) components (Table 1, Table S1a and S1b). The absence of highly ranked enrichment terms for stem cell maintenance, nuclear compartment and transcription factor binding suggests that differences are mainly centered around the cell environment and not on the transcriptional network. Our data suggests that reprogramming under suspension conditions is similar to anchorage dependent reprogramming for inducible secondary fibroblasts.
Table 1.
GO term enrichment analysis for genes that vary more than two-fold between adherent and suspension conditions. Terms are ordered according to their P-values. Percentages correspond to fractions of differentially expressed genes in corresponding GO terms.
| Day 2 | Day 6 | ||||
|---|---|---|---|---|---|
|
| |||||
| GO TERM | % of input genes in GO-Term | P -value | GO TERM | % of input genes in GO-Term | P -value |
| Extracellular region | 24.9 | 1.6 × 10−36 | Extracellular region | 27.1 | 3.0 × 10−27 |
| Extracellular region part | 14.3 | 8.5 × 10−28 | Extracellular region part | 16.7 | 6.8 × 10−23 |
| Extracellular space | 8.9 | 8.4 × 10−16 | Extracellular matrix | 8.5 | 1.7 × 10−14 |
| Cell adhesion | 8.7 | 1.6 × 10−15 | Proteinaceous extracellular matrix | 7.8 | 9.7 × 10−13 |
| Biological adhesion | 8.7 | 1.9 × 10−15 | Extracellular space | 10.1 | 2.3 × 10−12 |
| Extracellular matrix | 6.6 | 5.3 × 10−15 | Growth factor activity | 4.0 | 2.2 × 10−7 |
| Proteinaceous extracellular matrix | 5.9 | 1.6 × 10−12 | Response to wounding | 6.1 | 6.0 × 10−7 |
| Insulin-like growth factor binding | 1.6 | 1.6 × 10−10 | Positive regulation of cell-substrate adhesion | 1.9 | 2.1 × 10−6 |
| Calcium ion binding | 9.6 | 3.1 × 10−10 | Regulation of cell substrate adhesion | 2.1 | 3.2 × 10−6 |
| Pattern binding | 3.2 | 9.8 × 10−10 | Calcium ion binding | 9.9 | 7.5 × 10−6 |
| Day 15 | Doxycycline independent | ||||
|---|---|---|---|---|---|
|
| |||||
| GO TERM | % of input genes in GO-Term | P -value | GO TERM | % of input genes in GO-Term | P -value |
| Extracellular matrix binding | 2.6 | 8.9 × 10−5 | Extracellular region | 14.1 | 5.0 × 10−7 |
| Epithelial cell differentiation | 3.7 | 9.9 × 10−4 | Extracellular region part | 8.5 | 7.3 × 10−7 |
| Regulation of smoothened signaling pathway | 2.1 | 1.1 × 10−3 | Basement membrane | 2.3 | 7.5 × 10−6 |
| Intermediate filament cytoskeleton organization | 2.1 | 1.1 × 10−3 | Proteinaceous extracellular matrix | 4.2 | 3.7 × 10−5 |
| Calcium ion binding | 10 | 1.1 × 10−3 | Extracellular matrix part | 2.3 | 4.9 × 10−5 |
| Intermediate filament-based process | 2.1 | 1.5 × 10−3 | Basolateral plasma membrane | 2.8 | 6.0 × 10−5 |
| Regulation of cell adhesion | 3.2 | 1.8 × 10−3 | Extracellular matrix | 4.2 | 6.1 × 10−5 |
| Embryonic organ development | 4.7 | 1.8 × 10−3 | Regulation of cell proliferation | 6.1 | 1.6 × 10−4 |
| Positive regulation of cell-substrate adhesion | 2.1 | 1.0 × 10−3 | Contractile fiber part | 2.1 | 1.8 × 10−4 |
| Keratinization | 2.1 | 2.2 × 10−3 | Regulation of angiogenesis | 1.6 | 3.2 × 10−4 |
Primary cell reprogramming in suspension
We delivered doxycycline-inducible reprogramming factors (Fig. S7) by lentiviral transduction into MEFs derived from mice harboring an rtTA-IRES-GFP cassette in the Rosa26 locus. Cultures displayed increasing percentages of SSEA-1 positive cells and reached levels of > 70% SSEA-1 by day 14 (Fig. 4a). Similar to inducible secondary fibroblasts, reprogrammed primary SiPSCs formed compact aggregates when doxycycline was withdrawn. Aggregates displayed expression of pluripotency-associated transcription factors at levels comparable to those in ESC controls and surface staining for SSEA-1 (Fig. 4b, c). Expression of OCT4, NANOG and DPPA3 was confirmed by western blot (Fig. S4). SiPSCs derived from primary fibroblasts could differentiate in vitro into all three germ layers (Fig. 4d). We also observed induction of SSEA-1 in MKOS-transduced adult fibroblasts during suspension reprogramming (Fig. S8), although this was delayed relative to observations in MEFs. Flow cytometry analysis of pluripotency factor expression demonstrated that while fibroblast-derived SiPSCs exhibited slightly lower Nanog expression than rtTA-GFP ESC controls, no considerable differences were seen for OCT4, SOX2 and TBX3 (Fig. 4e). These studies confirm that primary embryonic and adult cells can be reprogrammed to pluripotency in suspension culture.
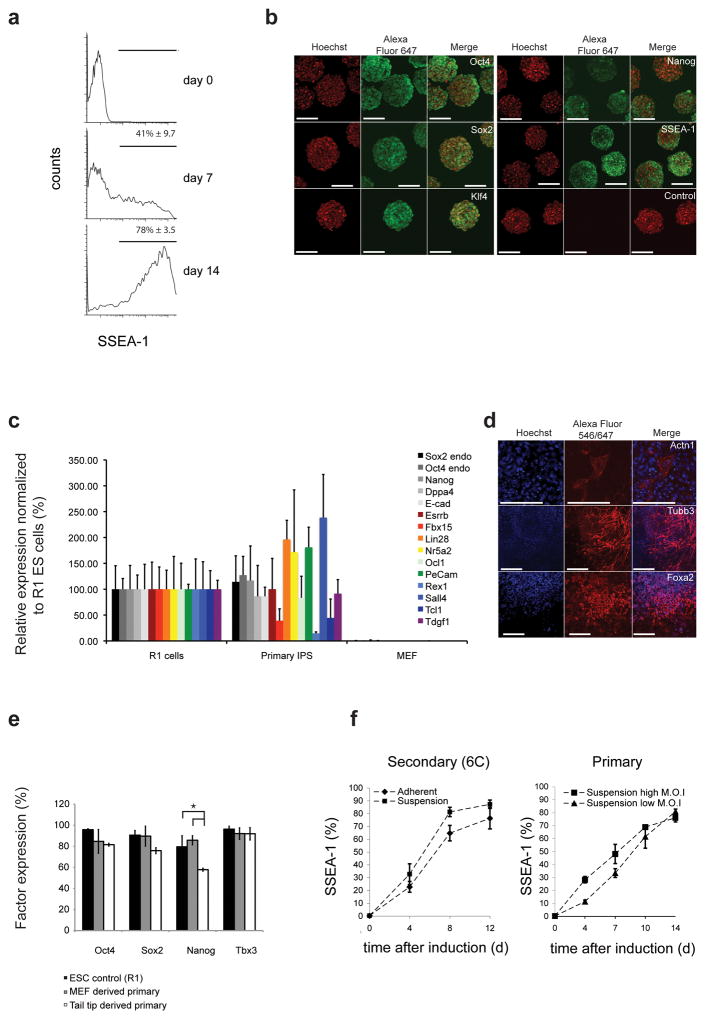
Figure 4.
Primary MEFs reprogrammed under suspension conditions. (a) FACS analysis of SSEA-1 activation in inducible reprogramming factor-transduced fibroblasts reprogrammed in suspension. (b) Fluorescence micrographs of pluripotency marker expression in doxycycline independent suspension reprogrammed primary iPS cells. (c) Quantification of expression levels of pluripotency genes in primary suspension reprogrammed SiPSCs and parental MEFs. Values are normalized to control R1 ESCs. Error bars s.d. (n = 3). (d) Micrographs show suspension-reprogrammed primary fibroblasts differentiated in vitro and stained for ACTN1 (mesoderm), TUBB3 (ectoderm) and FOXA2 (endoderm). (e) FACS analysis of the indicated factor expression in MEF-derived and TTF-derived primary reprogrammed SiPS cells. Significant differences are depicted with horizontal brackets (Nanog: ANOVA P = 0.023 and Tukey post-hoc P < 0.022.). Error bars s.d. (n = 3). (f) Kinetics of SSEA-1 activation in secondary fibroblasts under different conditions (left, n = 2) and in primary fibroblasts transduced with different virus doses (right, n = 2).
We compared the kinetics of fibroblast reprogramming under adherent and suspension conditions. SSEA-1-positive cell populations exhibited comparable profiles over the reprogramming process, with moderately increased SSEA-1 induction kinetics for suspension conditions (Fig. 4f). Primary cells depended on the optimal delivery of the reprogramming factors and displayed slower reprogramming compared to secondary MEFs. We tested the effect of two different virus doses on reprogramming kinetics. For the high virus does (eight times more virus than lower dose), reprogramming was significantly faster over the first seven days (low MOI: slope = 4.64 ± 0.68, P = 0.002; high MOI: slope 6.86 ± 0.56, P = 0.001), suggesting that more of the fibroblasts were transduced with the factors and started initiating reprogramming (Fig. 4f). Later in the process, however, the two curves converged, indicating that rapidly dividing cells undergoing reprogramming out-compete non-reprogramming cells regardless of the initial virus dose.
Integrated suspension reprogramming and differentiation
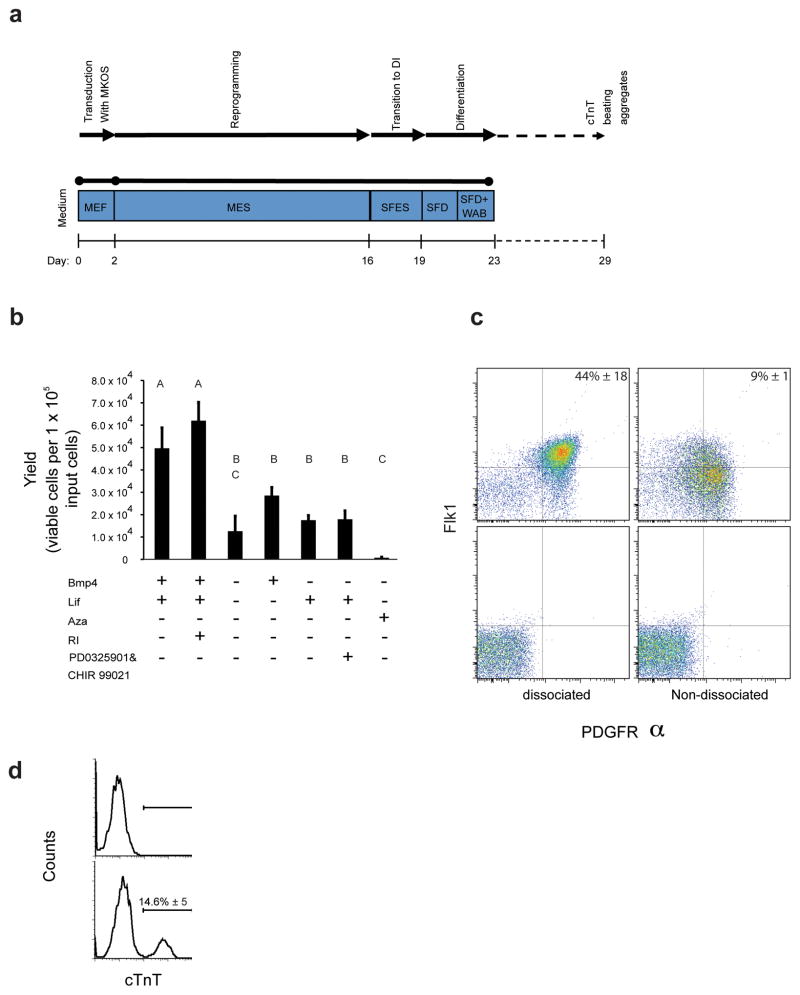
We next subjected primary fibroblasts to reprogramming, expansion and differentiation in an integrated stirred suspension system (Fig. 5a). We observed that the transition to doxycycline independence and differentiation induction led to decreased growth rate and increased cell death; this phenomenon was also observed in adhesion cultures although it was masked by the efficiency of re-adhesion upon passaging. We tested different conditions to optimize this transition step and measured cell survival after three days of culture in the absence of doxycycline (Fig. 5b). We observed the best cell survival was a transition to SFES medium ± ROCK inhibitor (RI). We shifted cells to doxycycline-free SFES medium from day 16 to day 19. The cultures began to form compact SiPSC aggregates during this transition period. Cell yields in culture three days after doxycycline removal were on the order of 0.5–0.6 per input cell suggesting that further selection occurs during reprogramming during the transition to factor independence (Fig. 5b).
Figure 5.
Integrated derivation, expansion and differentiation of SiPSCs towards cardiac progenitors. (a) Schematic of the suspension-based reprogramming/differentiation process. (b) Cell yields after transitioning SiPSCs from doxycycline-containing mESC medium to the indicated doxycycline-free media at day 19 of culture. Conditions that do not share a letter are significantly different from each other (ANOVA P = 0.001 and Tukey post-hoc with P = 0.0042). Error bars s.d. (n = 3). (c) FACS analysis for FLK1 and PDGFRα double-positive cardiac progenitor cells at day 23 after virus transduction.] After suspension reprogramming for 16 days, doxycycline was removed and cultures were maintained for three days before cells were induced to differentiate towards cardiac progenitor cells18, 19 either without dissociation (right, top) or after dissociation to single cells (left, top). Corresponding isotype controls are shown (bottom). Values are expressed as means ± s.d. (n ≥ 3) (d) FACS analysis of cTNT expression at day 29 after the initiation of reprogramming. Values are expressed as mean ± s.d. (n = 3).
We next transitioned the cells (either dissociated or not) into serum-free media for cardiac differentiation18. We obtained on the order of 40% FLK1/PDGFRα double positive cells at the end of the process (Fig. 5c), comparable with differentiation results previously obtained for optimized conditions19. Dissociation of the aggregates prior to differentiation led to considerably higher frequencies of double positive cell fractions but reduced overall cell yields (Fig. 5c). We further differentiated the cardiac progenitor cells in suspension along the cardiac lineage. At Day 29 after the induction of fibroblast reprogramming about 14% of the cells stained positive for the definitive cardiac specific marker Tnnt2 (cTnT) (Fig. 5d) and formed aggregates that exhibited spontaneous beating (supplementary Movie 1).
Discussion
Our study demonstrates the successful derivation of iPSCs from somatic mouse cells in suspension cultures in the absence of serum, tissue culture substrates or supporting feeder-cell layers. Global gene expression profiles, expression of pluripotency markers and functional characteristics of suspension-reprogrammed cells were comparable to control cultures reprogrammed under conventional adherence-dependent conditions and to other published reports11, 17. The subset of genes that were differentially expressed between suspension and adherent reprogramming were mainly associated with transcripts involved in cell adhesion and extracellular matrix interactions, suggesting that these processes may not be critical to transitioning cells towards pluripotency. Suspension based reprogramming allows for the continuous analysis of reprogramming in the absence of repeated cell passaging (adhesive selection) steps. Moreover, stringent control of environmental parameters in suspension systems might allow for the reduction of confounding factors introduced by static culture systems. Finally, compared to reprogramming under adherent conditions, suspension reprogramming simplifies the process, reduces overall costs and should ultimately enable iPSC culture under conditions amenable to bioprocess engineering strategies (O2 control, media perfusion, etc).
Mouse and human embryonic stem cells have been previously shown to be amenable to expansion in microcarrier-free suspension cultures if appropriate conditions and signaling cues are provided13, 16, 20, 21. We demonstrate successful SiPSC derivation under scalable culture conditions, both in serum-containing and in defined serum-free medium. Reprogramming kinetics in adherent and suspension culture conditions were comparable and reprogrammed cells became independent of exogenous factor expression at the end of the processes. Commonly, suspension culture protocols for pluripotent stem cells involve repeated dissociation and re-aggregation steps14, 15. However, fibroblasts transduced with reprogramming factors were amenable to reprogramming and expansion without repeated dissociation, in the presence of doxycycline. This allowed for improved scalability of the process and enabled us to continuously produce cardiac progenitors under stirred suspension conditions, demonstrating the feasibility of generating desired target cells from mixed input populations.
We note that suspension reprogramming using primary somatic cells leads to non-uniform populations of SiPSCs. The absence of clonal selection can yield cultures with variegated transgene expression levels due to positional effects. The presence of partially reprogrammed cells or incomplete transgene repression would be expected to impact the differentiation potential of such populations2, 22–24. For both primary and secondary systems, we focused in this study on transgene-cassettes driven by doxycycline responsive promoters25. The advantage of inducible configurations is the efficient repression of the exogenous reprogramming factors upon removal of doxycycline. Cells that are incompletely reprogrammed undergo apoptosis and differentiation upon doxycyline withdrawal, increasing the frequency of generation of bona fide iPSC compared to non-inducible configurations23. With this set-up, cells that survive and proliferate upon doxycycline removal, exhibited typical embryonic stem cell-like properties.
In conclusion, we demonstrate that adult and embryonic mouse somatic cells can be reprogrammed, expanded and differentiated in suspension in a feeder free system. Translating this technology to human cells, as well as implementing high density perfusion-based cell production systems, would overcome several of the key bottlenecks in current pluripotent cell-mediated cell production4, thus accelerating the development of iPSC biology and technology.
Methods
Cell Maintenance and reprogramming
Mouse embryonic fibroblasts (MEFs) (inducible 6C, 1B lines)11 and rtTA-MEFs (Rosa26 rtTA-IRES-GFP knock-in)26 were maintained in DMEM medium supplemented with 10% (v/v) FBS (Gibco), 1 mM sodium pyruvate (Gibco), 2 mM Glutamax™ (Invitrogen) and 1% (v/v) Penicillin and Streptomycin (Invitrogen). Mouse fibroblasts were reprogrammed either in serum containing mouse (m) ESC medium consisting of DMEM supplemented with 15% (v/v) FBS (Wisent), 0.1 mM β-mercapto-ethanol (BME, Sigma), 1 mM sodium pyruvate, 0.1 mM MEM non-essential amino acid (NEAA, Gibco), 2 mM Glutamax™, 1% (v/v) Penicillin-Streptomycin and 1000 U ml−1 of LIF (Millipore) or in serum-free ESC medium (SFEM) consisting of DMEM/F12 (Gibco)/Neurobasal (Gibco) based medium supplemented with N2 (Gibco), B27 (Gibco), 0.05% (w/v) BSA, 2mM Glutamax, 1% (v/v) Penicillin and Streptomycin, 1.5 × 10−4 M monothioglycerol, 1000 U ml−1 LIF, and 10 ng ml−1 BMP418. Mouse ES lines (R1 and rtTA-GFP) were maintained in mES medium. Reprogramming in adherent conditions: secondary inducible 6C MEFs were plated in gelatin coated 6-well plates (Sarstedt) and induced with 1 μg ml−1 doxycycline (Sigma) the day after. Medium exchanges were carried out every 48 hours during the entire reprogramming process. Primary mouse fibroblasts were transduced with respective viral preparations 24 hours and 36 hours after seeding. Culture medium was supplemented with doxycycline 24 hours after the last viral transduction and cells were reprogrammed following the same protocols as for secondary fibroblasts. Reprogramming in suspension conditions: secondary inducible 6C MEFs were trypsinized 8 hours after doxycycline induction and seeded either into Sigmacote™ (Sigma) treated spinner flasks (Integra biosciences) at 0.5–1 × 105 cells ml−1 or in low cell binding plates (Nunc). Primary mouse fibroblasts were transduced with viral preparations 24 hours and 36 hours after seeding. Cultures were supplemented with doxycycline 24 hours after the last viral transduction. 8–12 hours after induction (doxycycline addition), cells were trypsinized and seeded into spinner flasks at 2 × 105 cells ml−1. Culture volumes were between 30–50 ml with a constant stirring speed of 65 rpm. One third of the culture medium was replaced every day. Spinner flasks were replaced every 6 days to prevent sticking of cells to vessel walls. To remove large aggregates from high-density cultures, cells were passed through 100 μm cell strainers (BD Biosciences). All adherent cultures, spinner flasks and low cell binding plates were incubated in a humidified 5% (v/v) CO2 air environment at 37°C.
Suspension reprogramming of secondary chimera derived spleen cells and T-cell progenitors
The spleen of secondary chimeric adult 1B mice were isolated, minced and cultured in αMEM medium (Gibco) containing 16.7% (v/v) FBS (HyClone), 1% (v/v) Penicillin and Streptomycin, 10 ng ml−1 human IL-2 and 5 ng ml−1 mouse granulocyte macrophage colony-stimulating factor (GM-CSF) (R&D Systems) to promote survival. The cells were seeded at 1 × 106 cells ml−1 density in low adhesion plates (Nunc) in the presence of doxycycline for 8 hours. After 8 hours cells were seeded at 1 × 105 cells ml−1 density in spinner flasks in αMEM media with cytokines and doxycycline as above or at a density of 5 × 104 cells per 6-well on irradiated feeder MEFs. After 2 days, the medium was replaced with fresh αMEM media supplemented with 40% mESC media. Once the cells started forming aggregates after 4 days, stirring was initiated in the spinner flasks at 65 rpm and the medium was replaced to 100% ESC medium containing IL-2, GM-CSF and doxycycline. Blood cytokines were completely removed after 8 days. Doxcycline was removed after 15 days for expansion of spleen-derived SiPSCs.
For isolation of double negative (DN1) progenitor T cells, the thymus of secondary chimeric adult 1B mice were isolated, minced and stained for CD4−CD8−CD25−CD44+ surface marker expression. The FACS sorted DN1-T cells were cultured in low adhesion plates (Nunc) at 0.1 M ml−1 density in αMEM medium (Gibco) containing 16.7% (v/v) FBS (HyClone), 1% (v/v) Penicillin and Streptomycin, 50 ng ml−1 mouse stem cell factor (SCF), 10 ng ml−1 human FLT3L and 10 ng ml−1 mouse IL-7 (R&D Systems) to promote survival and doxcycline (1 mg ml−1) to initiate reprogramming. After 2 days, the medium was replaced with fresh αMEM media supplemented with 40% (v/v) mES medium. After 4 days, the medium was exchanged with 100% mES medium containing SCF, FLT3L, IL-7 and doxycycline. After 6 days, reprogramming DN1-T cells started forming aggregates and stirring was initiated. ESC medium was replaced with half the concentration of blood cytokines which were completely removed after 8 days. Doxcycline was removed after 15 days for expansion of DN1-derived SiPSCs.
Integrated production of cardiac progenitors
RtTA-MEF cells were transduced in T75 flasks at day 0 and day 1. Cells were induced with doxycycline at day 2 and 8 hours after induction shifted to suspension conditions in spinner flasks. After day 10 cells were diluted to concentrations below 1 106 cells ml−1 to avoid medium depletion. At day 16 cells were shifted from mES medium with doxycycline to SFES medium without doxycycline and re-incubated in spinner flasks or in low adherence 10 cm petri-dishes (Fisher) placed on an orbital shaker (65–75 rpm). Day 19 cultures were passed trough a 40 μm strainer (BD Biosciences) and filter retentates were used for cardiac induction as described in18. Input populations were either non-dissociated aggregates or single cells after trypsin dissociation. Differentiation to cTnT cells was performed by dissociating day 21 cultures and re-aggregating single cells as 100 cell aggregates in 400 μm microwell inserts27 in SFD medium18 supplemented with BMP4 (R&D) 10 ng ml−1, Activin A (R&D) 10 ng ml−1 and VEGF (Sigma) 5 ng ml−1 for 3 days. On day 24, aggregates were transferred from microwells to 6 cm plates containing Stempro® media (Invitrogen) supplemented with DKK1 (R&D) 150 ng ml−1, VEGF 5 ng ml−1, bFGF (Peprotech) (10 ng ml−1), FGF10 (10 ng ml−1) and cultured for an additional 5 days and subsequently analyzed by FACS.
Small molecules used were: 5-azacytidine (3 μM, Sigma), Rock inhibitor (Y27632, 10 μM, Sigma), PD0325901 (1 μM, Stemgent), CHIR 99021 (3 μM, Stemgent). 2i medium consists of SFES medium without BMP4, supplemented with PD0325901 and CHIR99021.
Plasmid generation and virus production
pDFTET-Myc and pDFTET-Sox were generated as follows: The cMyc and Sox2 open reading frames were PCR amplified from retroviral backbones (Addgene) using primers cMyc_fwd/cMyc_rev and Sox2_fwd/Sox2_rev (all primers used are listed in supplementary Table S2). The amplified cMyc and Sox2 fragments were subcloned by ligating the NheI/NotI restricted inserts into the corresponding sites of pCEP4 (Invitrogen). Fragments were excised from these intermediates by NheI/XhoI restriction and subsequently ligated into the corresponding sites of pMF35128 generating PDFCMV-cMyc and PDFCMV-Sox2. In a second step the Tet promoter (PTET) was amplified by PCR from PB-TET-Myc11 using primers PTET_fwd/PTET_rev, restricted using AscI/NheI and subsequently ligated into the AscI/NheI restricted pDFCMV-Myc and pDFCMV-Sox2 backbones.
PDFTET-KOS was generated by PCR amplifying KOS from PB-TET-MKOS11 using primers Klf4_fwd/Sox2_rev. The KOS containing fragment was restricted using NheI/NotI and ligated into the corresponding sites of PDFTET-Sox2.
Viral particles were produced by transfection of HEK293-T cells with calcium phosphate-DNA precipitates. Medium was shifted to Advanced DMEM (Gibco) supplemented with 1% (v/v) Penicillin and Streptomycin, 1% (v/v) Glutamax, 2% (v/v) FBS (Gibco) and 0.01 mM cholesterol 16 hours after transfection. Supernatants were harvested twice, 36 and 60 hours after transfection, filtered through a 0.45 μm filter, aliquoted and stored at −80 °C. For standard transductions 8 ml of MEF culture medium was mixed with 400 μl of each viral supernatant and added to MEF cells grown in T75 tissue culture flasks in the presence of 8 μg ml−1 Polybrene (Sigma).
Microarray analysis
Whole genome expression analysis of cells reprogramming under adherent and suspension conditions was carried out by isolating total RNA at indicated timepoints using RNAeasy RNA isolation kits (Qiagen). Hybridizations were carried out on Affimetrix mouse Gene 1.0 ST arrays (one array per timepoint and condition) and normalized using RMA (Affimetrix expression console). Fold changes and Pearson correlation coefficients were calculated in Excel. Hierarchical clustering was performed using Cluster 3.0 (similarity metric: correlation (centered), clustering method: average linkage) and visualized by JavaTree software. GO term enrichment analysis was performed using the DAVID bioinformatics resource 29.
Quantitative PCR analysis
Total RNA was extracted from cells using Qiagen RNAeasy miniprep columns according to the manufacturers protocol. Total RNA was used to generate cDNA using Superscript-III reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. Generated cDNA was mixed with respective primers and SYBR green mix (Roche, Sigma) and run on an Applied Biosystems 7900 HAT Real time PCR machine. Relative expression levels of described genes were determined by delta-delta Ct method with the expression of Gapdh as an internal reference. Primer sequences are listed in supplementary Table S2.
Differentiation protocols
Mesoderm/Endoderm Differentiation
Differentiation was carried out by dissociating iPSC and seeding in low adhesion plates at a density of 1 × 106 cells per 10 ml in DMEM containing 15% (v/v) FBS, 1% (v/v) Penicillin and Streptomycin, 2 mM Glutamax, 0.1 mM BME, and 0.1 mM NEAA. Cells were cultured for 4 days on an orbital shaker (65 rpm) with medium exchange at day 2. After 4 days, suspension aggregates were seeded on gelatin coated tissue culture plates and cultured for another 5 days before staining with corresponding antibodies.
Ectoderm: differentiation was performed according to30. Briefly, iPS cells were trypsinized and plated at 5 × 105 cells per 10ml in SFEB medium (GMEM supplemented with 5% (v/v) knock-out serum replacement, 0.1 mM NEAA, 1 mM sodium pyruvate, 1% (v/v) Penicillin and Streptomycin and 0.1 mM BME). Cells were cultured for 3 days in low adherence plates. At day 3 cells were re-fed by replacing 70% of the medium and cultured for another 2 days. Spheres were transferred intact to matrigel coated 6-well plates and incubated for 5 days in N2B27 media (DMEM/F12/Neurobasal media supplemented with B27, N2 supplements, 0.005% (w/v) BSA, 1 mM sodium pyruvate) before staining for TUBB3 expression.
Protein extraction and Western Blot analysis
Cell pellets were washed in chilled phosphate-buffer saline (PBS) and incubated in ice cold lysis buffer containing freshly added protease inhibitors (Sigma). Lysates were cleared by centrifugation at 4 °C for 15 min at 12,000 g and protein concentrations determined using a bicinchoninic acid assay (Thermo Scientific). For Western-Blot analysis 20 μg of total protein was size fractionated by SDS-PAGE on a TGX™AnykD™ precast gel (BioRad), transferred to a Hybond nitrocellulose membrane (GE Healthcare) in transfer buffer containing 10% methanol. The membrane was probed with specific primary antibodies (Supplementary Table S2) and secondary horseradish-peroxidase-conjugated antibodies (anti-mouse IgG-HRP, HAF007; anti-rabbit IgG, HAF008, R&D Systems). Antibody-protein complexes were detected using ECL-plus (GE Healthcare) on a GelDoc™ 2000 (BioRad).
Immunocytochemistry, flow cytometry, cell staining
Utilized antibodies for flow cytometry and immunostainings are listed in supplementary Table S3. All surface stainings for flow cytometry were performed in the presence of 7AAD (Molecular Probes) and populations were gated on live cells. For cell sorting, cells isolated from the thymus were first blocked for 10 minutes on ice with mouse CD16/CD32 Fc block (clone 2.4G2, BD 553142). The cells were subsequently labeled with the conjugated antibodies above for 20 minutes on ice to sort for CD4−CD8−CD25−CD44+ expressing DN1 T cells using a FACSAria flow cytometer (BD Biosciences). For intracellular stainings cells were fixed with PBS containing 4% formaldehyde and then permeabilized with methanol. Analysis was performed on FACSDiva (BD Biosciences) as well as FlowJo (Tree Star). Immunocytochemistry stainings were performed by fixing cells in PBS containing 4% (v/v) formaldehyde. Cells were permeabilized in PBS containing 0.1% (v/v) Triton X-100 and subsequently blocked in PBS containing 10% (v/v) donkey serum. Samples were incubated with primary and secondary antibodies in PBS containing 1% (w/v) BSA and imaged using a confocal microscope (FV1000 laser scanning confocal; Olympus) with 5 μm optical sections. Images represent the z-stack projection of five to ten confocal optical sections. Annexin V (Invitrogen) staining was carried out according to the manufacturers protocol. EdU cell proliferation assays were performed according to the manufacturers protocol (Invitrogen). Calcein-AM and Ethidium homodimer I staining (Sigma) was performed as indicated in the manufacturers protocol.
Generation of chimeras
Doxycycline-independent suspension iPS cells were plated and collected as cell clumps of 8–15 cells from gelatinized dishes by gentle trypsinization. For diploid chimeras, 2.5 d.p.c. Hsd:ICR(CD-1) embryos were aggregated with iPS cell clumps, and cultured overnight at 37 °C in 5% (v/v) CO2 in KSOM medium (Millipore). Embryos were transferred into pseudopregnant recipient ICR females 24 hours later. For LacZ detection, pregnant dams were treated with doxycycline (1.5 mg ml−1 doxycycline; 5% (w/v) sucrose in water) 20 hours before dissection. LacZ staining and sectioning were performed as described in11. The mice were housed in a pathogen free environment and the care of the animals was in accordance with institutional guidelines (Toronto Centre for Phenogenomics, Toronto, Ontario, Canada).
Statistical Analysis
Results are expressed as mean ± s.d. from at least three independent replicates if not otherwise stated. Data were checked for normalcy using the Kolmogorov-Smirnov test with P = 0.05. Multiple comparisons were performed using ANOVA with Tukey post-hoc comparisons to determine significant differences between groups (P < 0.05, two-tailed). Results were confirmed using non-parametric Mann-Whitney U tests. Calculations were performed using Minitab 16.2.1 (Minitab, Inc.).
Supplementary Material
Supplementary Figure 1. FACS analysis of Nanog expression during suspension reprogramming in the presence of dox.
Supplementary Figure 2. Example micrographs of suspension reprogrammed iPSC aggregate cultures after dox removal.
Supplementary Figure 3. Sustained SSEA-1 and NANOG expression in suspension reprogrammed-expanded secondary inducible MEF cells.
Supplementary Figure 4. Western Blot analysis of pluripotency factor expression.
Supplementary Figure 5. Q-PCR analysis of pluripotency factor expression in spleen derived SiPSCs and non induced parental spleen cells from the same chimera.
Supplementary Figure 6. Suspension reprogrammed iPSCs from purified DN1-progenitor T cells from the thymus.
Supplementary Figure 7. Schematic representation of vector constructs used to reprogram primary mouse fibroblasts.
Supplementary Figure 8. FACS analysis of SSEA-1 expression in adult tail tip derived fibroblasts reprogramming in suspension.
Supplementary Table 1A. Gene list of transcripts grouped under the GO term “extracellular matrix” exhibiting > two-fold expression differences between adherent and suspension conditions at day two after induction.
Supplementary Table 1B. Gene list of transcripts grouped under the GO term “cell adhesion” exhibiting > two-fold expression differences between adherent and suspension conditions at day two after induction.
Supplementary Table 2. Sequences of oligonucleotides used in this study.
Supplementary Table 3. Antibodies used in this study.
Supplementary Video. Spontaneously beating aggregates generated from primary fibroblast cells reprogrammed and differentiated towards the cardiac lineage in an integrated suspension process.
Acknowledgments
We are grateful to the Toronto Centre for Phenogenomics for chimera generation. We thank K. Woltjen for providing 6C secondary fibroblasts as well as providing PB-TET-cMyc and PB-TET-MKOS plasmids. PMF351 was kindly provided by M. Fussenegger. This work was funded by NSERC, the Canadian Institute of Health Research (MOP-57885) and an Ontario Research Fund – Global Leadership Round in Genomics & Liefe Sciences award to A.N. D.A.F was supported by a McLean Award to P.W.Z. P.W.Z. is the Canada Research Chair in Stem Cell Bioengineering.
Footnotes
Author Contributions
D.A.F. designed and performed most of the experiments. P.D.T. performed chimera experiments, part of the Q-PCR experiments and contributed to draft the manuscript. H.S. performed cTnT differentiation experiments and did confocal imaging. R. P. B. performed western blot analysis and did part of the secondary MEF, spleen and adult fibroblast reprogramming experiments. N.S. did part of the secondary MEF and spleen reprogramming experiments. S.S. performed secondary DN1 blood reprogramming experiments. G.C. performed statistical analysis. D.A.F. and P.W.Z. designed the project and wrote the manuscript.
Competing Financial Interests
The authors declare no competing financial interest
References
- 1.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 4.Kirouac DC, Zandstra PW. The systematic production of cells for cell therapies. Cell stem cell. 2008;3:369–381. doi: 10.1016/j.stem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 6.Taylor CJ, et al. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366:2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- 7.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou H, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell stem cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren L, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell stem cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woltjen K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell stem cell. 2008;3:595–605. doi: 10.1016/j.stem.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Steiner D, et al. Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension. Nat Biotechnol. 2010;28:361–364. doi: 10.1038/nbt.1616. [DOI] [PubMed] [Google Scholar]
- 14.Fok EY, Zandstra PW. Shear-controlled single-step mouse embryonic stem cell expansion and embryoid body-based differentiation. Stem Cells. 2005;23:1333–1342. doi: 10.1634/stemcells.2005-0112. [DOI] [PubMed] [Google Scholar]
- 15.Andang M, Moliner A, Doege CA, Ibanez CF, Ernfors P. Optimized mouse ES cell culture system by suspension growth in a fully defined medium. Nat Protoc. 2008;3:1013–1017. doi: 10.1038/nprot.2008.65. [DOI] [PubMed] [Google Scholar]
- 16.Zweigerdt R, Olmer R, Singh H, Haverich A, Martin U. Scalable expansion of human pluripotent stem cells in suspension culture. Nat Protoc. 2011;6:689–700. doi: 10.1038/nprot.2011.318. [DOI] [PubMed] [Google Scholar]
- 17.Samavarchi-Tehrani P, et al. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell stem cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Song H, et al. Interrogating functional integration between injected pluripotent stem cell-derived cells and surrogate cardiac tissue. Proc Natl Acad Sci U S A. 2010;107:3329–3334. doi: 10.1073/pnas.0905729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kattman SJ, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell stem cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Dang SM, Gerecht-Nir S, Chen J, Itskovitz-Eldor J, Zandstra PW. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells. 2004;22:275–282. doi: 10.1634/stemcells.22-3-275. [DOI] [PubMed] [Google Scholar]
- 21.Amit M, et al. Dynamic suspension culture for scalable expansion of undifferentiated human pluripotent stem cells. Nat Protoc. 2011;6:572–579. doi: 10.1038/nprot.2011.325. [DOI] [PubMed] [Google Scholar]
- 22.Brambrink T, et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell stem cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell stem cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sommer CA, et al. Excision of reprogramming transgenes improves the differentiation potential of iPS cells generated with a single excisable vector. Stem Cells. 2010;28:64–74. doi: 10.1002/stem.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maherali N, et al. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell stem cell. 2008;3:340–345. doi: 10.1016/j.stem.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belteki G, et al. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res. 2005;33:e51. doi: 10.1093/nar/gni051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ungrin MD, Joshi C, Nica A, Bauwens C, Zandstra PW. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS One. 2008;3:e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitta B, et al. Advanced modular self-inactivating lentiviral expression vectors for multigene interventions in mammalian cells and in vivo transduction. Nucleic Acids Res. 2002;30:e113. doi: 10.1093/nar/gnf112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe K, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. FACS analysis of Nanog expression during suspension reprogramming in the presence of dox.
Supplementary Figure 2. Example micrographs of suspension reprogrammed iPSC aggregate cultures after dox removal.
Supplementary Figure 3. Sustained SSEA-1 and NANOG expression in suspension reprogrammed-expanded secondary inducible MEF cells.
Supplementary Figure 4. Western Blot analysis of pluripotency factor expression.
Supplementary Figure 5. Q-PCR analysis of pluripotency factor expression in spleen derived SiPSCs and non induced parental spleen cells from the same chimera.
Supplementary Figure 6. Suspension reprogrammed iPSCs from purified DN1-progenitor T cells from the thymus.
Supplementary Figure 7. Schematic representation of vector constructs used to reprogram primary mouse fibroblasts.
Supplementary Figure 8. FACS analysis of SSEA-1 expression in adult tail tip derived fibroblasts reprogramming in suspension.
Supplementary Table 1A. Gene list of transcripts grouped under the GO term “extracellular matrix” exhibiting > two-fold expression differences between adherent and suspension conditions at day two after induction.
Supplementary Table 1B. Gene list of transcripts grouped under the GO term “cell adhesion” exhibiting > two-fold expression differences between adherent and suspension conditions at day two after induction.
Supplementary Table 2. Sequences of oligonucleotides used in this study.
Supplementary Table 3. Antibodies used in this study.
Supplementary Video. Spontaneously beating aggregates generated from primary fibroblast cells reprogrammed and differentiated towards the cardiac lineage in an integrated suspension process.