Abstract
Background
High multivitamin (10-fold AIN-93G, HV) diets fed during pregnancy to Wistar rats increase characteristics of metabolic syndrome in offspring when weaned to the recommended vitamin (RV) diet.
Objective
To determine if the effects of HV gestational diets on obesogenic phenotypes in the offspring arise as a consequence of altered hypothalamic control of feeding behavior and if their increased food intake could be prevented by feeding them HV or high folate (10-fold folate, HFol) diets.
Methods
Male offspring of dams fed HV diet during pregnancy weaned to RV, HV or HFol diets were compared to those born to RV dams and weaned to RV diet for 29 weeks. Food intake over 72 hours and body weight were measured bi-weekly and weekly, respectively. Glucose response to a glucose load was measured at 18 weeks post-weaning. Hypothalamic gene expression of feeding-related neuropeptides including neuropeptide Y, pro-opiomelanocortin (POMC), insulin receptor, leptin receptor, brain-derived neurotrophic factor (BDNF), receptors for dopamine (DopaR1/2/5) and serotonin (SeroR1A/2A/2C), as well as global DNA methylation and brain and plasma folate concentrations were measured at 29 weeks post-weaning.
Results
HV or HFol pup diets increased brain and plasma folate concentrations and prevented the increase in food intake (5%, P=0.03), body weight (8%, P=0.0006) and glucose response to a glucose load (36%, P=0.02) found in those fed the RV diet. Expression of anorexigenic POMC (P=0.004) and BDNF (P=0.02) was higher, and DopaR1 was lower (P=0.06) in pups fed the HV diet. The HFol pup diet partially brought BDNF to the control level (P=0.02) and lowered SeroR2A (P=0.008). Expression of other genes was unaffected. Global DNA methylation was similar among the diet groups.
Conclusion
The obesogenic phenotype in offspring from HV fed dams is prevented by feeding HV or HFol pup diets, possibly due to post-weaning modulation of food intake regulatory mechanisms.
Keywords: Folate, food intake regulation, gestational vitamins, hypothalamus, post-weaning diet
INTRODUCTION
Diets consumed during pregnancy and early postnatal life shape the developmental trajectory of the offspring.1, 2 Although vitamins contribute to all phases of development,3 the effects of multivitamin consumption above requirements (but at non-toxic levels) during pregnancy as determinants of physiologic phenotypes of the offspring have received little attention. We have shown recently that feeding a diet containing 10-fold the recommended multivitamin content (HV) to Wistar rats during pregnancy results in increased food intake, obesity and characteristics of the metabolic syndrome in both adult male4 and female5 offspring weaned to the recommended vitamin (RV) diet. Because the hypothalamus is an important regulator of food intake and appetite control,6 it can be hypothesized that the HV diet alters development of feeding neurocircuitry in the offspring resulting in an obesogenic phenotype. Disordered regulation of feeding pathways can affect growth and metabolism, consequently driving the appearance of metabolic syndrome later in life.7, 8
An extension of the concept that early life factors contribute to chronic diseases is described by the predictive adaptive response (PAR) hypothesis, which suggests that a mismatch between in utero and postnatal environment increases the risk of developing disease in adulthood.9, 10 It has been proposed that the offspring modify their phenotype based on their in utero environment in order to adapt to the predicted postnatal setting. Based on the PAR hypothesis, the high multivitamin gestational diet would lead to developmental adaptation in the offspring consistent with a high vitamin post-weaning environment. If so, it would be predicted that it is this mismatch between the HV diet fed to the dams and the vitamin content of diets fed to the offspring that leads to an obesogenic phenotype. The effect of high vitamin maternal diets on chronic disease in the offspring can be attributed primarily to epigenetic modulation of gene expression leading to programming of regulatory systems.11 Because methyl group vitamins, and specifically folate, have been shown to participate in epigenetic changes through its functions in DNA methylation,12 we also fed offspring a high folate (HFol) diet. The control group was offspring fed the RV diet and born to dams fed the RV diet. Therefore, we hypothesized that the effects of HV gestational diets on obesogenic phenotype in the offspring arise as a consequence of altered hypothalamic control of feeding behavior and these effects could be prevented by feeding them HV or HFol diets. Our primary objective was to compare the effects of RV, HV and HFol pup diets on food intake, body weight, glucose control and hypothalamic gene expression of feeding neuropeptides in offspring born to HV dams with offspring born to RV dams and weaned to RV diet.
MATERIALS AND METHODS
Animals and Diets
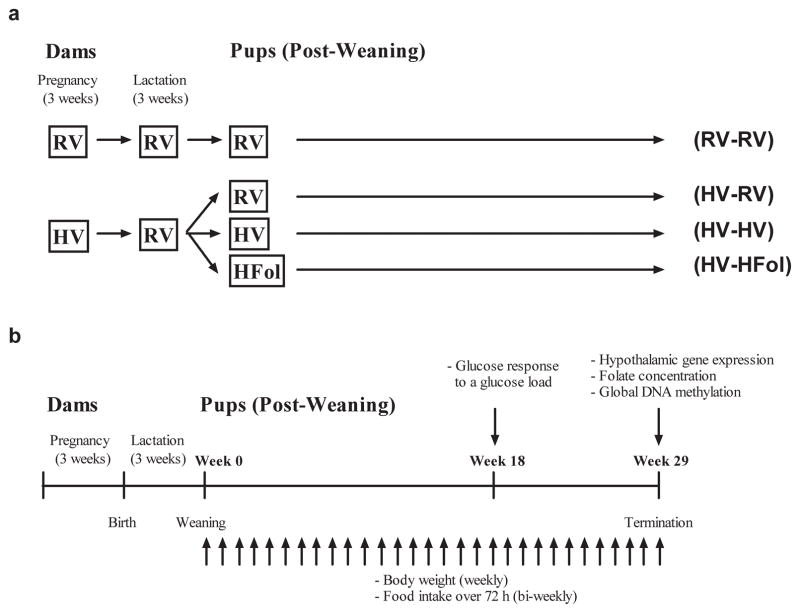
First-time pregnant Wistar rats (day 2–3 of pregnancy) was purchased from Charles River (Montreal, QC, Canada). The dams were housed individually in ventilated plastic transparent cages with bedding in a 12:12 h light-dark cycle (lights on at 0700 at 22 ± 1 °C). Ad libitum access to food and water was provided throughout the study period. The protocol was approved by the University of Toronto Animal Care Committee. From third day of pregnancy to term, dams (n=10 per gestational diet group) were fed the AIN-93G diet13 containing either the RV or HV amount of the multivitamin mix. The composition (in g/kg) of the RV AIN-93G diet is 529.5 cornstarch, 200 casein, 100 sucrose, 70 soybean oil, 50 cellulose, 10 vitamin mixture, 35 mineral mixture, 3 L-cystine, 2.5 choline bitartarate and 0.014 tert-butyl hydroquinone. Because 9.75 g of sucrose is added as a carrier in the 10 g of vitamin mix in the RV diet, we reduced the added sucrose to only 10 g in the HV diet to adjust for the 97.5 g of sucrose from the 100 g of vitamin mix. Figure 1a depicts the study design. Within 24 hours of delivery, each litter was culled to 10 pups to minimize the difference in milk availability. During lactation, all dams were fed the RV diet. At weaning, 10 male offspring (one per dam) per gestational diet group were terminated. All other offspring from dams fed the HV diet were weaned to RV, HV or HFol diets. The RV diet has 2 mg/kg of folate. Thus, to formulate the 10-fold HFol diet, we added 18 mg of folate to make a total of 20 mg/kg. The RV, HV and HFol diets were all of the same energy density (3.8 kcal/g). A control group of offspring from dams fed the RV diet was weaned to the RV pup diet for 29 weeks.
Figure 1.
Schematic sketch of the study (a) design and (b) protocol for male offspring from 0–29 weeks post-weaning. Diet abbreviations: RV, the AIN-93G diet with the recommended vitamins; HV, 10-fold amount of the recommended multivitamin mix; HFol, RV+10-fold the folate content. Diets consumed during pregnancy denoted before the dash line. Diets consumed by the offspring denoted after the dash line.
Food Intake, Weight Gain and Oral Glucose Tolerance Test
Figure 1b outlines the study protocol. Food intake over 72 hours was measured every 2 weeks from 0–29 weeks post-weaning. Body weight was measured at birth and weaning, and weight gain was calculated weekly from 0–29 weeks post-weaning. Glucose response to glucose gavage (0.375 g glucose mL−1, 5 g of glucose per kg body weight) was measured at 18 weeks post-weaning. The rats were fasted overnight for 10 h before glucose gavage. A blood sample was withdrawn from the tail vein and baseline glucose was immediately assayed using a commercial glucometer (MediSense Precision Xtra, Abbott Laboratories, Abbott Park, IL, USA). Upon the glucose gavage, blood glucose concentrations were determined 15 and 60 min later and the incremental area under the curve (iAUC) was calculated.
Tissue Collection
Whole rat brains were removed rapidly after decapitation at 29 weeks post-weaning and immediately frozen on dry ice, and then stored at −80 °C. The brains were thawed on ice and the right and left sides of the hypothalamus were dissected separately using the method described previously.14 The optic chiasma was used as demarcation for the anterior part of the hypothalamus. This passes through the anterior commissure, which was used as a horizontal limit. The line between the posterior hypothalamus and the mammillary bodies was considered a caudal section. The dissected hypothalamic blocks contained preoptic, supraoptic, paraventricular, anterior, suprachiasmatic, dorsomedial, ventromedial, arcuate, mammillary, posterior and lateral complex nuclei. The right side of the hypothalamus was used for relative gene expression and the left side for DNA methylation status. The left side of the remaining brain was used for folate concentration measurement. Trunk blood was collected and centrifuged, and plasma samples were added with 0.5% ascorbic acid to prevent folate oxidation.
Hypothalamic Gene Expression
Each dissected hypothalamus was homogenized using a tissue ruptor homogenizer (Qiagen Tech., Mississauga, ON, Canada). The RNA from the homogenized samples was isolated using Trizol reagent and chloroform extraction by the manufacturer’s protocol (Invitrogen, Grand Island, NY, USA) and quantified by Nanodrop 1000. The cDNA was synthesized using the High Capacity cDNA Archive Kit in the ABI (Applied Biosystems Inc, Foster City, CA, USA) Gene Amp PCR System 2700.
Real-time RT-PCR was performed on the ABI PRISM 7000 Sequence Detection System (SDS) using Taqman assays for the following genes purchased from ABI (Foster City, CA, USA): neuropeptide Y (NPY; Cat# Rn01410146_m1); pro-opiomelanocortin (POMC; Cat# Rn00595020_m1); insulin receptor (InsR; Cat# Rn00567070_m1); leptin receptor (LepR; Cat# Rn01433205_m1); brain-derived neurotrophic factor (BDNF; Cat# Rn02531967_s1); dopamine receptor 1 (DopaR1; Cat# Rn03062203_s1); dopamine receptor 2 (DopaR2; Cat# Rn00561126_m1); dopamine receptor 5 (DopaR5; Cat# Rn00562768_s1); serotonin receptor 1A (SeroR1A; Cat# Rn00561409_s1); serotonin receptor 2A (SeroR2A; Cat# Rn01468302_m1); serotonin receptor 2C (SeroR2C; Cat# Rn00562748_m1). The cycle conditions were 50 °C for 2 minutes, 95 °C for 10 minutes, 40 cycles for 95 °C for 15 seconds, and 60 °C for 1 minute. The relative quantification method was performed using glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Cat# Rn99999916_s1) as an endogenous control, which was selected based on a preliminary screen for its lowest variation. Results were expressed as fold-change by the 2−ΔΔCT method15 and analyzed using ABI DataAssist software version 3.0 (Foster City, CA, USA).
Folate Concentration Analysis
Plasma and brain folate concentrations were determined by the microbiological microtiter plate assay using Lactobacillus casei.16, 17
Hypothalamic Global DNA Methylation Analysis
DNA was isolated using the protocol outlined by the DNeasy Blood and Tissue Kit (Qiagen Tech., Mississauga, ON, Canada). Two spectrophotometric readings of the DNA samples were averaged to determine the concentration, which had a ratio of A260 to A280 between 1.8 and 2.0 and was free of RNA and protein contamination.
Global methylation status of hypothalamic genomic DNA was determined by the in vitro methyl acceptance assay method as described previously.18, 19 This assay involves 3H-methyl-S-adenosylmethionine (New England Nuclear, Boston, MA, USA) as a methyl donor mediated by Sss1 DNA methyltransferase (New England Biolabs, Pickering, ON, Canada). The acceptance capacity of methyl groups in the whole genome is known to be inversely related to the exogenous 3H-methyl incorporation. All samples were run in duplicates and the entire assay was performed twice. The intra- and inter-assay CV were 5%.
Statistical Analyses
Treatment effects on weight gain and 72 h food intake were analyzed using the PROC MIXED model procedure in SAS (Version 9.2, SAS Institute Inc., Carey, NC, USA) with gestational diets, pup diets and time as the main factors. The means at each time point and for hypothalamic gene expression, folate status and glucose response were compared by one-way analysis of variance using the PROC GLM followed by a Tukey’s post-hoc test. Significant differences were reported at P<0.05. All data are expressed as means ± standard error of means (SEM).
RESULTS
Food Intake, Weight Gain and Glucose Response
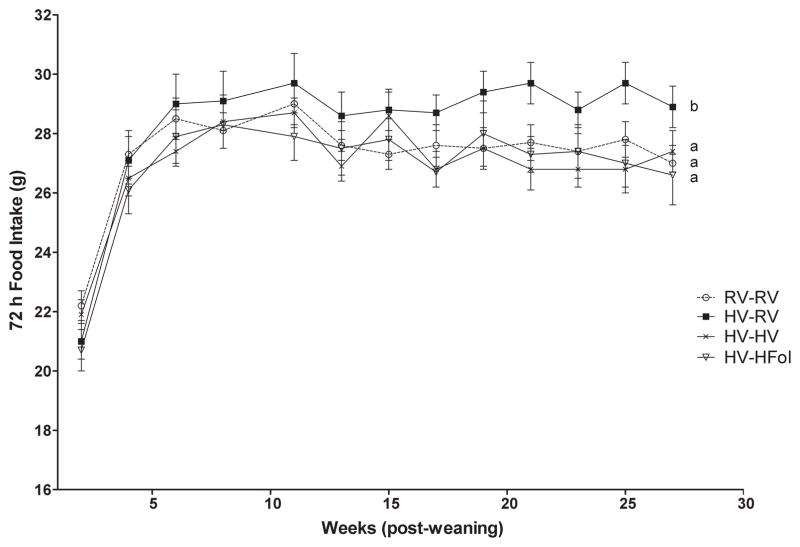
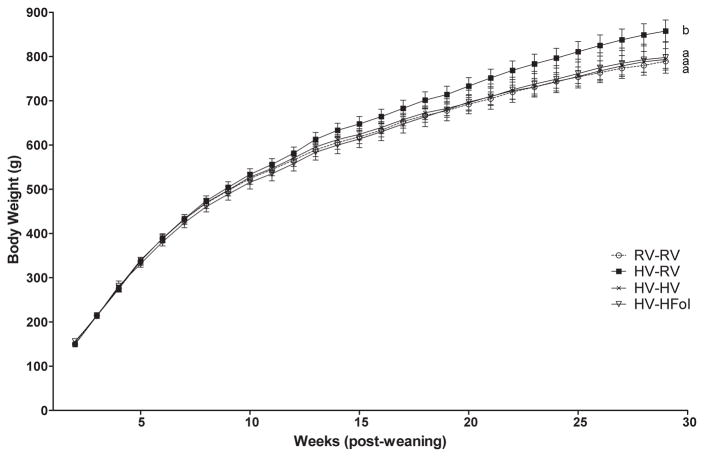
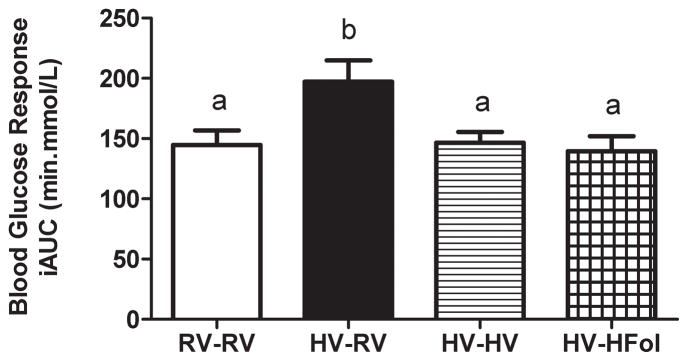
There was no difference in body weight at birth and weaning between the offspring born to dams on the HV diet compared to those born to dams on a RV diet. Male offspring from the HV dams weaned to the RV diet had 5% higher 72 h food intake (Diet: P=0.03, Time: P<0.0001, Diet*Time Interaction: P=0.4), 8% higher weight gain (Diet: P=0.0006, Time: P<0.0001, Diet*Time Interaction: P=0.3) and 36% higher glucose response to glucose gavage (P=0.02) compared to those from the RV dams at 29 weeks post-weaning (Figure 2, 3, 4). In contrast, male offspring from the HV dams weaned to either the HFol or HV pup diet did not differ in food intake, weight gain and glucose response compared to those from the RV dams (Figure 2, 3, 4).
Figure 2.
Food intake over 72 hours of male offspring from 0–29 weeks post-weaning. Diet abbreviations: RV, the AIN-93G diet with the recommended vitamins; HV, 10-fold amount of the recommended multivitamin mix; HFol, RV+10-fold the folate content. Diets consumed during pregnancy denoted before the dash line. Diets consumed by the offspring denoted after the dash line. Diet (P=0.03), Time (P<0.0001), Diet*Time (P=0.4). AB Significantly different by PROC MIXED model repeated measures. Values are mean ± SEM, n=12–14/group.
Figure 3.
Body weight of male offspring from 0–29 weeks post-weaning. Diet abbreviations: RV, the AIN-93G diet with the recommended vitamins; HV, 10-fold amount of the recommended multivitamin mix; HFol, RV+10-fold the folate content. Diets consumed during pregnancy denoted before the dash line. Diets consumed by the offspring denoted after the dash line. Weight Gain: Diet (P=0.0006), Time (P<0.0001), Diet*Time (P=0.3). AB Significantly different by PROC MIXED model repeated measures. Values are mean ± SEM, n=12–14/group.
Figure 4.
Blood glucose response to a glucose load (5 g of glucose per kg of body weight) as incremental area under the curve (iAUC) of male offspring at 18 weeks post-weaning. Diet abbreviations: RV, the AIN-93G diet with the recommended vitamins; HV, 10-fold amount of the recommended multivitamin mix; HFol, RV+10-fold the folate content. Diets consumed during pregnancy denoted before the dash line. Diets consumed by the offspring denoted after the dash line. AB P=0.02 by one-way ANOVA followed by Tukey’s post-hoc test. Values are mean ± SEM, n=8–12/group.
Hypothalamic Gene Expression
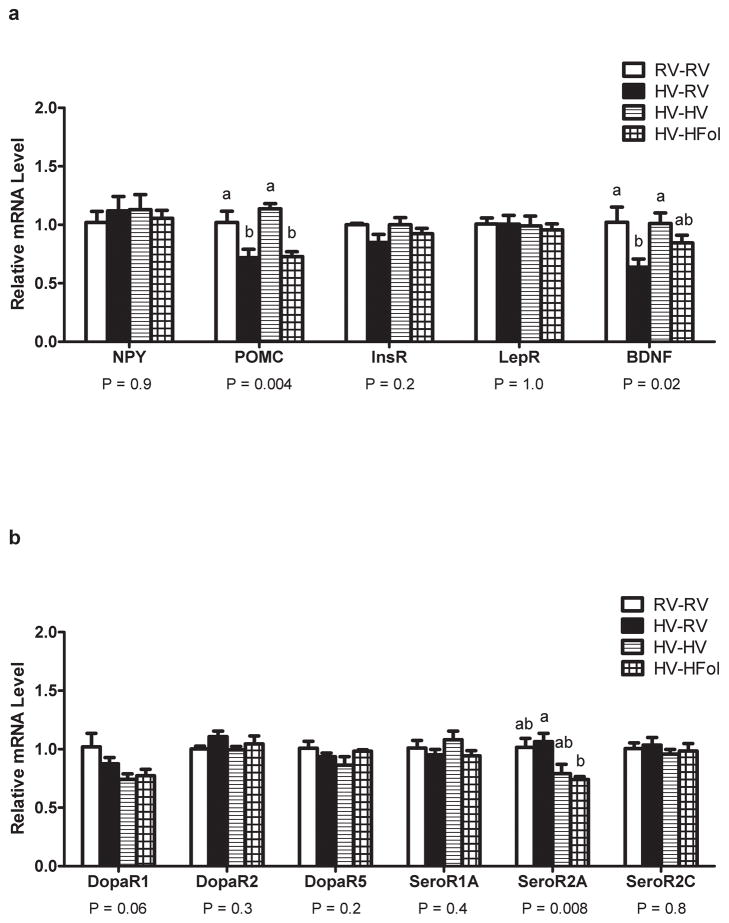
The HV but not HFol pup diet brought POMC expression to control levels in mature offspring from HV dams (P=0.004) (Figure 5a). The HV pup diet brought BDNF expression to control levels but the HFol pup diet only attenuated the decrease in expression caused by the HV gestational diet (P=0.02) (Figure 5a). Only the HFol pup diet lowered SeroR2A expression compared to the HV offspring weaned to a RV pup diet (P=0.008) (Figure 5b). There was a trend for lower DopaR1 expression in the HV offspring weaned to a HV pup diet compared to the RV offspring (P=0.06) (Figure 5b). There was no effect of the gestational or pup diets on hypothalamic expression of other genes.
Figure 5.
Hypothalamic gene expression of (a) neuropeptide Y (NPY), pro-opiomelanocortin (POMC), insulin receptor (InsR), leptin receptor (LepR) and brain-derived neurotrophic factor (BNDF) and (b) dopamine receptors (DopaR1/R2/R5) and serotonin receptors (SeroR1A/2A/2C) in male offspring at 29 weeks post-weaning. Diet abbreviations: RV, the AIN-93G diet with the recommended vitamins; HV, 10-fold amount of the recommended multivitamin mix; HFol, RV+10-fold the folate content. Diets consumed during pregnancy denoted before the dash line. Diets consumed by the offspring denoted after the dash line. AB Significantly different by one-way ANOVA followed by Tukey’s post-hoc test. Values are mean ± SEM, n=4–7/group.
Folate Status
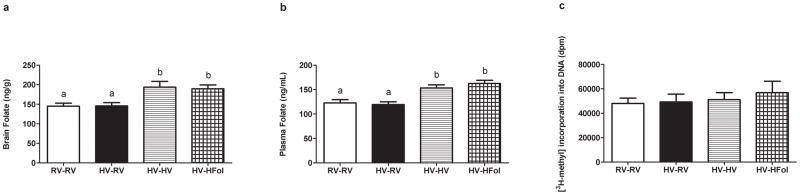
Both brain (Figure 6a) and plasma (Figure 6b) folate levels were unaffected by the diets of the dams but were elevated by the high multivitamin or high folate pup diet.
Figure 6.
Levels of (a) brain folate, (b) plasma folate and (c) global DNA methylation of male offspring at 29 weeks post-weaning. Diet abbreviations: RV, the AIN-93G diet with the recommended vitamins; HV, 10-fold amount of the recommended multivitamin mix; HFol, RV+10-fold the folate content. Diets consumed during pregnancy denoted before the dash line. Diets consumed by the offspring denoted after the dash line. AB P<0.0001 by one-way ANOVA followed by Tukey’s post-hoc test. Values are mean ± SEM, n=10–15/group. Global DNA methylation not significant, n=6–8/group.
Global Methylation Status
Neither the gestational nor post-weaning diet had any effect on the global methylation status in the offspring at 29 weeks post-weaning (Figure 6c).
DISCUSSION
Our findings support the hypothesis that HV or HFol pup diet prevents the increase in food intake, body weight and glucose response to a glucose load in offspring of the HV dams, possibly by post-weaning changes in food intake regulatory systems. Although the role of hypothalamic regulation in determining the phenotype is uncertain, our gene expression data are novel because we show that feeding neurocircuitry undergoes modification both in utero and into maturity.
Two lines of evidence support the PAR hypothesis. First, an unmatched RV post-weaning diet fed the offspring born to HV dams resulted in increased food intake and characteristics of the metabolic syndrome, as observed previously.4 Second, the HV and HFol post-weaning diets prevented these effects. There were significant effect of both diet and time, but no interaction between them on food intake and weight gain, suggesting that vitamin diets strongly alter the phenotype of mature offspring independent of time. Because the negative consequences of the HV gestational diet on food intake, weight gain and glucose control were prevented by feeding either the HV or HFol pup diets, these observations support the PAR hypothesis that offspring fed diets similar to their mothers lead to a more appropriate adaptation to their postnatal environment compared to those fed an unmatched diet. Elevated folate levels in the brain and plasma at maturity confirmed the folate content of the HV and HFol post-weaning diets, and importantly, revealed that the brain responds to the post-weaning diet. Although the HV and HFol post-weaning diets had similar effects on food intake, weight gain and blood glucose response by preventing the negative consequences of the HV gestational diet, they differed in their impact on expression in food intake regulatory genes in the hypothalamus.
Overall the data support the hypothesis that the cause of the phenotype of HV offspring fed RV diets may be accounted for by its effects in utero on development of hypothalamic pathways of intake regulation, leading to excess food intake, and consequently increased body weight and characteristics of the metabolic syndrome.4 Offspring born to dams fed the HV diet during pregnancy had lower expression of POMC and BDNF, which are anorexigenic (appetite-suppressing) genes,20, 21 compared to those born to dams on the RV diet. Lower expression of the anorexigenic genes in the offspring of dams fed the multivamins during pregnancy is consistent with the observed increased food intake. Our results are consistent with other data showing that early nutrition alters the central appetite regulatory network.22 Effects of the maternal diet on the hypothalamic expression of food intake regulatory pathways were found in lambs of ewes fed a diet that exceeded their energy requirement. These lambs were fatter but had higher hypothalamic POMC expression at postnatal day 30 and relative milk intake of these lambs was not different from those born to control-fed mothers after the third postnatal week.23 In contrast, the data from the present study show that higher food intake in adulthood of offspring born to the HV dams and fed the RV diet is consistent with their lower expression of anorexigenic neuropeptides in the hypothalamus. However, there was no gestational HV diet effect on expression of leptin and insulin receptors, which are known to modulate expression of orexigenic and anorexigenic neurons regulating energy intake.24–26 Despite this lack of difference, other neurons downstream regulating food intake such as POMC and BDNF may be affected by the high vitamin diets.
A novel observation of our study is that the hypothalamic feeding pathways respond to the vitamin content of the post-weaning diet. Feeding the HV pup diet restored POMC and BDNF expression to control levels in mature offspring. Because expression of these anorexigenic neuropeptides was normalized, it may be suggested that vitamin content of post-weaning diets contribute to remodeling of hypothalamic functioning in mature as well as developing rats. Furthermore, there was a trend for lower expression of DopaR1 in the HV offspring weaned to a HV diet compared to the control offspring. In the ventromedial hypothalamic nucleus, dopamine release is required to initiate a meal, and is positively associated with meal number and duration.27, 28 By possibly reducing dopamine signaling, the HV pup diet may have provided some protection against excess food intake caused by the HV gestational diet. Although we are the first to show that the brain at maturity responds to vitamins, our results are substantiated by a report of hypothalamic changes in the food intake regulatory genes due to the interaction of maternal and post-weaning diets high in saturated fats in male offspring.29
Surprisingly, a ten-fold addition of folate alone to the RV diet modified some of the effects of the maternal HV diet on the offspring, suggesting a possible primary role for this methyl group vitamin. A significant role for folate in the complete vitamin mixture in determining the effect of the HV diet was supported by the reductions in food intake, body weight and blood glucose. However, folate had fewer effects on the hypothalamus of offspring born to HV dams. Because POMC was not brought to control levels, vitamins other than folate may play a role in correcting POMC. The HFol pup diet reduced SeroR2A expression in mature offspring of the dams fed the HV diet, but not by the HV pup diet (Figure 5b). BDNF was corrected to similar levels by the HV diet, and only partially by the HFol pup diet. Previous studies have shown that BDNF increases serotonin turnover in the hypothalamus,30, 31 suggesting that the effect of gestational and post-weaning diets on BDNF mRNA levels may explain some compensatory response in SeroR2A expression. The fact that we did not observe differences in other subtypes of SeroR may indicate that SeroR2A is more sensitive to BDNF-induced changes by the vitamin diets. Because BDNF may be differentially expressed in specific regions of the hypothalamus, where high levels are present in the ventromedial hypothalamus,32 the relationship between BDNF and SeroR is unknown from whole hypothalamus in our study.
Although folate has been shown to be an active participant in methyltransfer reactions,12 global methylation was not affected by either the gestational or post-weaning diet. However this is not surprising because previous studies have reported that DNA methylation of genes in the absence of a detectable change in global methylation, suggesting that epigenetic changes are site-specific.33 In addition, the RV, HV and HFol diets in our study contain high amounts of choline, which is another factor affecting DNA methylation,34, 35 and it alone may have obscured detection of the effect of folate on methylation. The metabolism of choline and other methyl group vitamins (ie. folate, vitamin B6 and B12) is closely linked.36
A significant weakness of the study arises from the lack of measurement of gene expression at different stages of maturity. Thus, we do not know the stage of maturation when the post-weaning diets altered the hypothalamus. In addition to hypothalamic genes, measurement of genes in regions of the brain involved in reward and motivation may have contributed to a fuller understanding of the overall impact of the diets on regulation of food intake. It has been shown that mice fed post-weaning a high fat diet (60% calories from fat) had lower expression of the μ-opioid receptor gene which influences reward circuitry in the ventral tegmental area, nucleus accumbens and prefrontal cortex.37
In conclusion, the obesogenic phenotype in offspring from HV fed dams may be a consequence of altered hypothalamic control of feeding behavior but can be prevented by feeding them HV or HFol pup diets.
Acknowledgments
SOURCES OF SUPPORT: Canadian Institute of Health Research, Institute of Nutrition, Metabolism and Diabetes (CIHR-INMD), Reference MOP-93624.
We thank the Canadian Institute of Health Research, Institute of Nutrition, Metabolism and Diabetes (CIHR-INMD), Reference MOP-93624 for funding this research. Clara E. Cho is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Postgraduate Scholarship (PGS), Diana Sánchez-Hernández and Sandra A Reza-López by the ConsejoNacional de Ciencia y Tecnologia (Mexico) and Pedro S.P. Huot by the Ontario Graduate Scholarship (OGS).
Footnotes
DISCLOSURE: The authors declare no conflict of interest.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341(8850):938–41. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 2.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 3.Fall CH, Yajnik CS, Rao S, Davies AA, Brown N, Farrant HJ. Micronutrients and fetal growth. J Nutr. 2003;133(5 Suppl 2):1747S–1756S. doi: 10.1093/jn/133.5.1747S. [DOI] [PubMed] [Google Scholar]
- 4.Szeto IM, Aziz A, Das PJ, Taha AY, Okubo N, Reza-Lopez S, et al. High multivitamin intake by Wistar rats during pregnancy results in increased food intake and components of the metabolic syndrome in male offspring. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R575–82. doi: 10.1152/ajpregu.90354.2008. [DOI] [PubMed] [Google Scholar]
- 5.Szeto IM, Das PJ, Aziz A, Anderson GH. Multivitamin supplementation of Wistar rats during pregnancy accelerates the development of obesity in offspring fed an obesogenic diet. Int J Obes (Lond) 2009;33(3):364–72. doi: 10.1038/ijo.2008.281. [DOI] [PubMed] [Google Scholar]
- 6.Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiol Behav. 2004;81(5):781–93. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Cripps RL, Martin-Gronert MS, Ozanne SE. Fetal and perinatal programming of appetite. Clin Sci (Lond) 2005;109(1):1–11. doi: 10.1042/CS20040367. [DOI] [PubMed] [Google Scholar]
- 8.McMillen IC, Adam CL, Muhlhausler BS. Early origins of obesity: programming the appetite regulatory system. J Physiol. 2005;565(Pt 1):9–17. doi: 10.1113/jphysiol.2004.081992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–6. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 10.Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20(10):527–33. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Dominguez-Salas P, Cox SE, Prentice AM, Hennig BJ, Moore SE. Maternal nutritional status, C(1) metabolism and offspring DNA methylation: a review of current evidence in human subjects. Proc Nutr Soc. 2012;71(1):154–65. doi: 10.1017/S0029665111003338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YI. Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutr. 2005;135(11):2703–9. doi: 10.1093/jn/135.11.2703. [DOI] [PubMed] [Google Scholar]
- 13.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127(5 Suppl):838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 14.Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966;13(8):655–69. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods (San Diego, Calif. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43–53. doi: 10.1016/s0076-6879(97)81007-5. [DOI] [PubMed] [Google Scholar]
- 17.Sie KK, Chen J, Sohn KJ, Croxford R, Thompson LU, Kim YI. Folic acid supplementation provided in utero and during lactation reduces the number of terminal end buds of the developing mammary glands in the offspring. Cancer Lett. 2009;280(1):72–7. doi: 10.1016/j.canlet.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Kotsopoulos J, Medline A, Renlund R, Sohn KJ, Martin R, Hwang SW, et al. Effects of dietary folate on the development and progression of mammary tumors in rats. Carcinogenesis. 2005;26(9):1603–12. doi: 10.1093/carcin/bgi117. [DOI] [PubMed] [Google Scholar]
- 19.Kotsopoulos J, Sohn KJ, Martin R, Choi M, Renlund R, McKerlie C, et al. Dietary folate deficiency suppresses N-methyl-N-nitrosourea-induced mammary tumorigenesis in rats. Carcinogenesis. 2003;24(5):937–44. doi: 10.1093/carcin/bgg036. [DOI] [PubMed] [Google Scholar]
- 20.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385(6612):165–8. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 21.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6(7):736–42. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muhlhausler BS, Adam CL, McMillen IC. Maternal nutrition and the programming of obesity: The brain. Organogenesis. 2008;4(3):144–52. doi: 10.4161/org.4.3.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muhlhausler BS, Adam CL, Findlay PA, Duffield JA, McMillen IC. Increased maternal nutrition alters development of the appetite-regulating network in the brain. FASEB J. 2006;20(8):1257–9. doi: 10.1096/fj.05-5241fje. [DOI] [PubMed] [Google Scholar]
- 24.Baskin DG, Wilcox BJ, Figlewicz DP, Dorsa DM. Insulin and insulin-like growth factors in the CNS. Trends Neurosci. 1988;11(3):107–11. doi: 10.1016/0166-2236(88)90155-5. [DOI] [PubMed] [Google Scholar]
- 25.Baskin DG, Breininger JF, Schwartz MW. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes. 1999;48(4):828–33. doi: 10.2337/diabetes.48.4.828. [DOI] [PubMed] [Google Scholar]
- 26.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138(10):4489–92. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 27.Meguid MM, Yang ZJ, Laviano A. Meal size and number: relationship to dopamine levels in the ventromedial hypothalamic nucleus. Am J Physiol. 1997;272(6 Pt 2):R1925–30. doi: 10.1152/ajpregu.1997.272.6.R1925. [DOI] [PubMed] [Google Scholar]
- 28.Meguid MM, Yang ZJ, Koseki M. Eating induced rise in LHA-dopamine correlates with meal size in normal and bulbectomized rats. Brain Res Bull. 1995;36(5):487–90. doi: 10.1016/0361-9230(95)92128-3. [DOI] [PubMed] [Google Scholar]
- 29.Page KC, Malik RE, Ripple JA, Anday EK. Maternal and postweaning diet interaction alters hypothalamic gene expression and modulates response to a high-fat diet in male offspring. Am J Physiol Regul Integr Comp Physiol. 2009;297(4):R1049–57. doi: 10.1152/ajpregu.90585.2008. [DOI] [PubMed] [Google Scholar]
- 30.Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131(2):229–38. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 31.Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96(26):15239–44. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19(6):1290–300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143(7):1084–96. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehedint MG, Niculescu MD, Craciunescu CN, Zeisel SH. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J. 2010;24(1):184–95. doi: 10.1096/fj.09-140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20(1):43–9. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Wijk N, Watkins CJ, Bohlke M, Maher TJ, Hageman RJ, Kamphuis PJ, et al. Plasma choline concentration varies with different dietary levels of vitamins B6, B12 and folic acid in rats maintained on choline-adequate diets. Br J Nutr. 2012;107(10):1408–12. doi: 10.1017/S0007114511004570. [DOI] [PubMed] [Google Scholar]
- 37.Vucetic Z, Kimmel J, Reyes TM. Chronic high-fat diet drives postnatal epigenetic regulation of mu-opioid receptor in the brain. Neuropsychopharmacology. 2011;36(6):1199–206. doi: 10.1038/npp.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]