Abstract
Objectives
Chronic infections with the opportunistic pathogen Pseudomonas aeruginosa are responsible for the majority of the morbidity and mortality in patients with cystic fibrosis (CF). While P. aeruginosa infections may initially be treated successfully with standard antibiotics, chronic infections typically arise as bacteria transition to a biofilm mode of growth and acquire remarkable antimicrobial resistance. To address the critical need for novel antimicrobial therapeutics that can effectively suppress chronic bacterial infections in challenging physiological environments, such as the CF lung, we have rationally designed a de novo engineered cationic antimicrobial peptide, the 24-residue WLBU2, with broad-spectrum antibacterial activity for pan-drug-resistant P. aeruginosa in liquid culture. In the current study, we tested the hypothesis that WLBU2 also prevents P. aeruginosa biofilm growth.
Methods
Using abiotic and biotic biofilm assays, co-culturing P. aeruginosa with polarized human airway epithelial cells, we examined the ability of WLBU2 to prevent biofilm biogenesis alone and in combination with currently used antibiotics.
Results
We observed a dose-dependent reduction in biofilm growth on an abiotic surface and in association with CF airway epithelial cells. WLBU2 prevented P. aeruginosa biofilm formation when co-cultured with mucus-producing primary human CF airway epithelial cells and using CF clinical isolates of P. aeruginosa, even at low pH and high salt conditions that mimic the CF airway. When used in combination, WLBU2 significantly increases killing by the commonly used antibiotics tobramycin, ciprofloxacin, ceftazidime and meropenem.
Conclusions
While other studies have demonstrated the ability of natural and synthetic antimicrobial peptides to prevent abiotic bacterial biofilm formation, the current studies for the first time demonstrate the effective peptide treatment of a biotic bacterial biofilm in a setting similar to the CF airway, and without negative effects on human airway epithelial cells, thus highlighting the unique potential of this engineered cationic antimicrobial peptide for treatment of human respiratory infections.
Introduction
The genetic lung disease cystic fibrosis (CF) is caused by mutations in the chloride channel, CF conductance transmembrane regulator (CFTR).1 Defects in CFTR-mediated chloride secretion cause dehydration of the airway surface liquid and a reduction in mucociliary clearance, leading to an increase in respiratory infections.2–4 While bacterial infections, such as those caused by Pseudomonas aeruginosa, may initially be treated successfully with standard antibiotics, chronic infections typically display high levels of antimicrobial resistance.5 Chronic pulmonary infections with P. aeruginosa, combined with the ineffective immune response to those infections, are responsible for the majority of the morbidity and mortality in patients with CF.6 Therefore, there is a critical need for novel antimicrobial drugs that can effectively suppress bacterial infections in the challenging environment of the CF lung.
Antimicrobial peptides (AMPs) have been intensely investigated over the last several decades as potential antibiotics against multidrug resistant bacteria.7–12 Most AMPs are cationic peptides with an amphipathic structure as a consensus motif for antimicrobial activities that selectively target the membranes of bacteria via electrostatic forces.13–17 In contrast to standard antibiotics, AMPs are effective against both quiescent and actively growing bacteria as they generally do not require metabolic processes for antimicrobial activity, display rapid (seconds or minutes) killing kinetics and demonstrate a low propensity to invoke selection of bacterial resistance in vitro.18–22 However, AMPs display several limitations that have delayed their successful development for clinical use, including inhibition of activity in the presence of the acidic pH and increased salt seen in a CF lung environment.23 During the last two decades, we have focused on rationally engineering AMP amphipathic structures to overcome these intrinsic limitations.7,18–22,24,25 We previously reported the activity of the engineered cationic AMP (eCAP) WLBU2 (24-mer containing valine, arginine and tryptophan) that has been optimized as an idealized amphipathic helix to maximize antimicrobial properties while minimizing epithelial cell cytotoxicity.18,19 In sharp contrast to the natural AMP LL-37 and the membrane-interactive antibiotic colistin, WLBU2 displays a broader spectrum of activity against extremely resistant clinical isolates of bacteria, including a diverse panel from patients with CF.22 In addition, WLBU2 displays systemic antimicrobial activity against P. aeruginosa in a mouse model of septicaemia.21,22 Based on a previous report of the antibiofilm property of LL-37,26 we hypothesized that WLBU2 could prevent P. aeruginosa biofilm growth.
Hence, the goal of this study was to evaluate if the naturally inspired, rationally engineered synthetic AMP WLBU2 could prevent the biofilm growth of bacteria associated with chronic infections in patients with CF. Our results in abiotic and biotic biofilm models demonstrate that WLBU2 reduces biofilm growth of P. aeruginosa. Moreover, the WLBU2 peptide retains its activity in an environment rich in mucus, low pH and high salt concentrations, all characteristic of the airways of patients with CF. These observations with the WLBU2 peptide antibiotic are to date unmatched among evaluations of numerous natural and synthetic AMPs,22,27 highlighting the unique potential of WLBU2 for treatment of human respiratory infections in patients with CF.
Materials and methods
AMP synthesis
The eCAP WLBU2 (RRWVRRVRRWVRRVVRVVRRWVRR) and the naturally occurring AMP LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) were synthesized by the University of Pittsburgh Peptide Synthesis Facility using the previously described standard Fmoc (9-fluorenylmethoxy carbonyl) synthesis protocols.21 Synthetic peptides were characterized and purified by reversed-phase HPLC on Vydac C18 or C4 columns (The Separations Group, Hesperia, CA, USA) and the identity of each peptide was confirmed by MS (Electrospray Quatro II triple quadrupole mass spectrometer; Micromass, Inc., Manchester, UK). Peptide concentrations were determined by using a quantitative ninhydrin assay, as previously described.21 For comparison purposes, 1 μM WLBU2 represents a peptide concentration of 3.4 mg/L.
Bacterial strains
P. aeruginosa strains PAO1, strain PAO1 with plasmid (pSMC21) to express constitutively the green fluorescent protein (gfp) and CF clinical isolate 1595 used in this study were gifts from George O'Toole (Geisel School of Medicine at Dartmouth).28 Late isolates 31, 33 and 74 were from the University of Washington collection, previously published in Smith et al.29
Reagents
The reagents used in this study were tobramycin (APP Pharmaceuticals, Schaumberg, IL, USA), ceftazidime (Sagent Pharmaceuticals), ciprofloxacin (Fluka), colistimethate (XGen Pharmaceuticals), meropenem (NovaPlus), and LB broth, LB agar and Triton X-100 (Sigma, St Louis, MO, USA).
Abiotic biofilm prevention Crystal Violet assay
Overnight PAO1 cultures were diluted 1:30 in sterile MEM supplemented with 2 mM l-glutamine and varying doses of WLBU2 or LL-37. The dilutions were then plated in a flexible 96-well plate (Corning Life Sciences, Kennebunk, ME, USA) with four replicates per dose and incubated at 37°C for 24–26 h. The bacteria were then removed, wells were washed with H2O, the plate was dried inverted on paper towels and stained with 41% Crystal Violet in 12% ethanol in deionized H2O for 15 min. The wells were again washed with distilled deionized water and allowed to dry. Wells were first imaged on an Epson scanner and then reconstituted with 30% glacial acetic acid, transferred to a new plate and read on a plate reader at 550 nm.
Epithelial cell culture
The cells used in our experiments were human bronchial epithelial cells [CFBE41o–; hereafter called CF airway epithelial cells (AECs)] isolated from a patient with homozygous ΔF508 CF stably transduced with wt-CFTR, which were originally developed by Dr D. Gruenert and colleagues and were a gift from Dr J. P. Clancy (University of Cincinnati, Cincinnati, OH, USA). Cells were maintained in minimal Earle's growth medium supplemented with 10% FBS, 2 mM l-glutamine, 50 U/mL penicillin, 50 mg/L streptomycin and 2 μg/mL puromycin in a 5% CO2/95% air incubator at 37°C. CFBE41o– cells were seeded on to collagen-coated 12 mm transwell semi-permeable membrane inserts with a 0.4 μm pore size (Corning Life Sciences) at 2.5 × 105 cells per filter and grown at an air–liquid interface at 37°C for 8–10 days to allow for cell polarization, as previously published.30 Primary human bronchial epithelial cells were cultured from explanted lungs of patients with CF, under an Institutional Review Board approved protocol at the University of Pittsburgh (PRO11070367 and IRB970946). Cells were enzymatically dissociated, expanded in growth medium and seeded on to Transwell inserts at the air–liquid interface. Cultures were used when well polarized and differentiated.30–32
Transepithelial electrical resistance measurements
Transepithelial electrical resistance was measured hourly for 5 h on air–liquid interface differentiated AECs treated with 10 or 50 μM WLBU2 or LL-37 peptides using a Ag/AgCl electrode (EVOM meter).30
Static co-culture biotic biofilm assays
Biotic biofilm prevention assays on polarized CF AECs were performed with PAO1 P. aeruginosa with a starting moi of 25, as previously published.30,33 CF AECs were inoculated with bacteria in minimal Earle's growth medium for 1 h before removing unattached bacteria and adding the AMPs (10–100 μM) diluted in 0.4% l-arginine in sterile minimal Earle's growth medium for 5 h. After a total of 6 h, the biofilms were disrupted with 0.1% Triton X-100 in minimal Earle's growth medium, serially diluted and enumerated on LB agar plates to determine cfu counts. Antibiotic concentrations were used as follows: 1000 mg/L tobramycin, 0.5 mg/L ciprofloxacin, 350 mg/L ceftazidime, 5 mg/L meropenem and 20 mg/L colistimethate.
Statistics
All data are plotted as mean ± SD. Data were log transformed and tested by one-way ANOVA for statistical significance. If an effect was present, a Tukey post-hoc test was used for individual comparisons using PRISM software (GraphPad, La Jolla, CA, USA).
Results
WLBU2 prevents abiotic P. aeruginosa biofilms
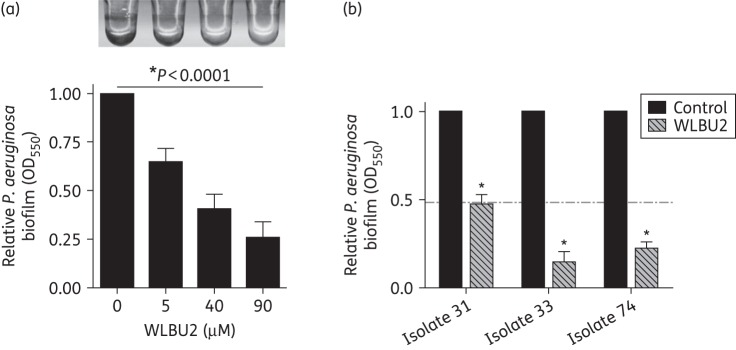
The eCAP WLBU2 has previously been reported to have dose-dependent bactericidal activity for P. aeruginosa in liquid culture. As P. aeruginosa forms bacterial biofilms associated with medical devices and within the host, we examined if WLBU2 could prevent P. aeruginosa biofilm growth. We first determined the dose dependence of abiotic biofilm prevention by WLBU2 in a microtitre dish assay. In the presence of 5–90 μM WLBU2 peptides for 24 h, we observed a dose-dependent decrease in P. aeruginosa biofilm growth (Figure 1a). Using three clinical isolates of P. aeruginosa from patients with CF, we demonstrated that WLBU2 significantly (P < 0.05) decreased P. aeruginosa biofilm growth on an abiotic surface (Figure 1b). Interestingly, at a concentration of 10 μM WLBU2 showed increased efficacy on the clinical isolates, as compared with PAO1, a lab strain of P. aeruginosa (horizontal broken line in Figure 1b).
Figure 1.
WLBU2 prevents abiotic biofilm growth by P. aeruginosa. (a) WLBU2 shows dose-dependent prevention of P. aeruginosa biofilms grown on an abiotic surface. P. aeruginosa strain PAO1 was grown in minimal Earle's growth medium for 24 h in a 96-well microtitre biofilm assay and biofilm growth was assessed by Crystal Violet staining and absorbance measurement at 550 nm. Biofilm values are normalized to the untreated control, set to 1. Representative image is shown above the quantification of results. n = 3, *P < 0.0001 by ANOVA. (b) WLBU2 prevents biofilm growth on an abiotic surface by CF clinical isolates of P. aeruginosa. Clinical isolates were grown in minimal Earle's growth medium for 24 h in a 96-well microtitre biofilm assay in the presence of 10 μM WLBU2 and biofilm growth was assessed by Crystal Violet staining and absorbance measurement at 550 nm. The horizontal broken line represents PAO1 biofilm levels with 10 μM WLBU2, for comparison. Error bars represent standard deviations for replicates of each clinical isolate, *P < 0.01 as compared with isolate control.
WLBU2 prevents P. aeruginosa biofilms grown in association with human CF AECs
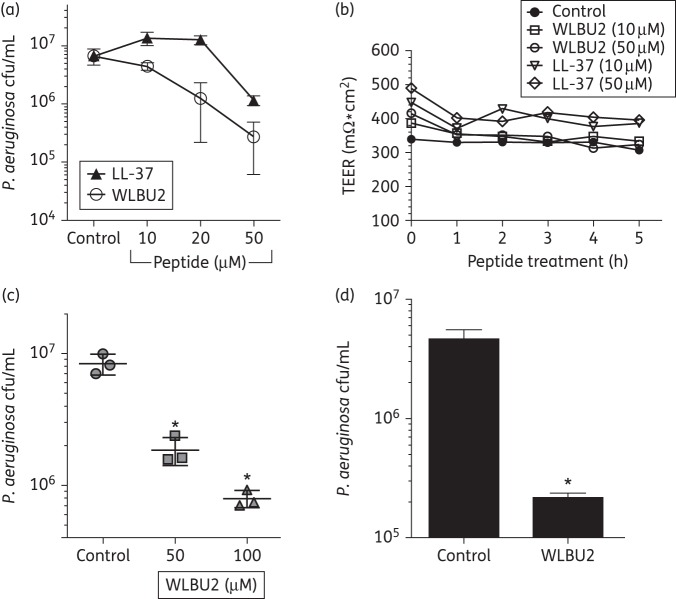
P. aeruginosa biofilms grown in association with the host have remarkable resistance to current antimicrobials used in the clinic.28 We next examined if WLBU2 would prevent P. aeruginosa biofilm growth in the presence of the airway epithelium with negligible cytotoxicity, using a previously published biotic biofilm model.30 In this model, P. aeruginosa biofilms are grown for 6 h on AECs and cfu are enumerated. In this time, P. aeruginosa rapidly forms biofilms resembling those observed in lung samples from transplant and autopsy. Moreover, biofilms grown in association with AECs reach maturity much more rapidly (within 6 h) than on an abiotic surface, as demonstrated in a previous report showing dramatically increased antimicrobial resistance profiles, exopolysaccharide matrix production, induction of quorum sensing and gene expression changes consistent with mature biofilm growth.28 Using increasing doses, we observed that WLBU2 prevented P. aeruginosa biofilms in association with CF AECs in a dose-dependent manner (Figure 2a). In addition, when compared with the naturally occurring lung AMP, cathelicidin (LL-37), WLBU2 was significantly more effective at reducing biotic biofilm growth (i.e. in association with a biotic surface; Figure 2a). For example, WLBU2 at 20 μM reduced bacterial biofilm by 90% while LL-37 failed to reduce bacterial cfu at the same concentration of peptide. To assess if WLBU2 or LL-37 is cytotoxic to the airway epithelium at the doses studied, we examined transepithelial electrical resistance of the polarized CF AECs. Neither WLBU2 nor LL-37 reduced epithelial integrity over the 5 h period examined in these studies (Figure 2b). We also observed significant reductions in P. aeruginosa biofilm growth of 90% in association with mucus-producing, primary AECs cultured from a CF lung (Figure 2c) and even better biofilm reduction on a clinical isolate of P. aeruginosa from a patient with CF (Figure 2d). Thus, while both WLBU2 and LL-37 displayed negligible cytotoxicity, the effect of WLBU2 on the prevention of biofilm formation was significantly greater than that of LL-37 at all test concentrations up to 50 μM.
Figure 2.
WLBU2 prevents P. aeruginosa biofilms on human CF AECs. (a) WLBU2 shows dose-dependent prevention of P. aeruginosa biofilms grown on CF AECs. In a static co-culture biofilm model, CF AECs were inoculated with P. aeruginosa strain PAO1 at moi 25 for 1 h, unattached bacteria were removed and the remaining bacteria were incubated in the presence of 10–50 μM LL-37 (filled triangles) or WLBU2 (open circles) for 5 h. Biofilm growth was assessed by cfu enumeration. Hereafter referred to as the static co-culture biofilm model. n = 3, *P < 0.05 as compared with control. (b) WLBU2 and LL-37 do not alter epithelial integrity. Transepithelial electrical resistance (TEER) was measured hourly for polarized CF AECs treated with 10–50 μM LL-37 or WLBU2. Results are presented of a representative experiment; the experiment was repeated three times. (c) WLBU2 prevents P. aeruginosa biofilms grown on primary CF AECs. The static co-culture biofilm model was used with the substitution of primary CF AECs. P. aeruginosa strain PAO1 bacteria were incubated in the presence of 50–100 μM WLBU2 for 5 h. n = 3, *P < 0.05 as compared with control. (d) WLBU2 prevents biotic biofilm growth of a CF clinical isolate of P. aeruginosa on CF AECs. The static co-culture biofilm model was used with the substitution of a late P. aeruginosa CF clinical isolate and biofilm growth was assessed in the presence of 50 μM WLBU2 for 5 h. n = 3, *P < 0.05.
WLBU2 retains biofilm prevention activity in high salt and low pH environments
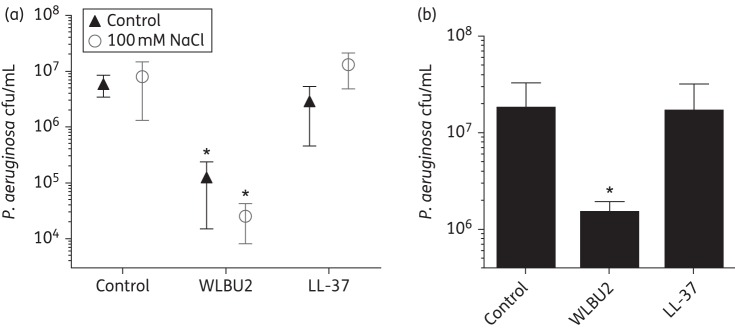
The CF airway thwarts the activity of many naturally occurring AMPs because of the high salt content (∼100 μM) and low pH (∼6.5–7.0) that manifest as a result of the primary defect in CF, altered chloride secretion by the airway epithelium.23,34,35 We next examined if WLBU2 retained its biofilm prevention activity in the presence of NaCl concentrations and pH levels similar to CF airway surface liquid. Interestingly, WLBU2 reduced P. aeruginosa biofilm growth by 2 log on CF AECs in the presence of high NaCl concentrations (100 mM NaCl), similar to those that have been shown to inactivate endogenous AMPs in CF (Figure 3a and Widdicombe34). In addition, WLBU2 retained activity at pH 6.5 (Figure 3b). In marked contrast to WLBU2, LL-37 was completely inhibited in the high salt or lower pH environments (Figure 3a and b). Taken together, these data suggest that in conditions of the CF airway, WLBU2 effectively prevents P. aeruginosa biofilm growth, thus highlighting enhanced activity of the eCAP compared with the reference natural AMP, LL-37 under these conditions.
Figure 3.
WLBU2 prevents biofilm growth in high salt and low pH environments. (a) WLBU2 maintains efficacy in preventing P. aeruginosa biofilms grown on CF AECs in a high salt environment. In a static co-culture biofilm model, CF AECs were inoculated with P. aeruginosa strain PAO1 at an moi of 25 for 1 h, unattached bacteria were removed and the remaining bacteria were incubated in the presence of 100 mM NaCl and 50 μM LL-37 (filled triangles) or WLBU2 (open circles) for 5 h. Biofilm growth was assessed by cfu enumeration. n = 3, *P < 0.001 as compared with control conditions. (b) WLBU2 maintains efficacy in preventing P. aeruginosa biofilms grown on CF AECs in a low pH environment. In the same experimental conditions as in (a), bacteria were incubated at pH 6.5 and treated with 50 μM LL-37 or WLBU2 for 5 h. Biofilm growth was assessed by cfu enumeration. n = 3, *P < 0.05.
WLBU2 improves antibiotic killing of P. aeruginosa biotic biofilms
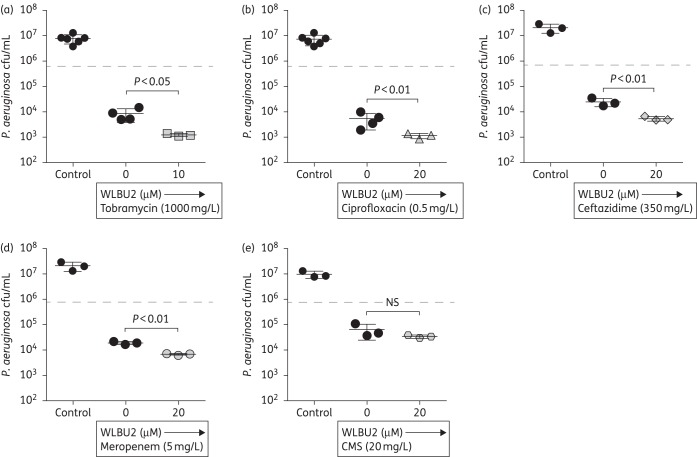
As antimicrobials are often combined in an attempt to control infections involving antimicrobial-resistant bacteria, we next examined if WLBU2 improved the prevention of P. aeruginosa biotic biofilm growth by standard antimicrobials used in treating P. aeruginosa infections. When WLBU2 was applied with subinhibitory doses of antibiotics, WLBU2 significantly increased the observed prevention in biotic biofilm formation by P. aeruginosa for tobramycin (Figure 4a), ciprofloxacin (Figure 4b), ceftazidime (Figure 4c) and meropenem (Figure 4d). The combination of colistimethate and WLBU2 did not have an additional benefit for preventing biotic biofilm growth (Figure 4e), indicating a specificity of the WLBU2-mediated enhancement of the other antibiotics. These results are consistent with the conclusion that WLBU2 is capable of improving the bacterial killing of a number of front-line antimicrobials used to treat chronic P. aeruginosa infections.
Figure 4.
WLBU2 improves killing of P. aeruginosa biotic biofilms by antibiotics. In a static co-culture biofilm model, CF AECs were inoculated with P. aeruginosa strain PAO1 at moi 25 for 1 h, unattached bacteria were removed and the remaining bacteria were incubated in the presence of 20 μM WLBU2 and various currently approved antibiotics for 5 h. (a) Tobramycin (1000 mg/L). (b) Ciprofloxacin (0.5 mg/L). (c) Ceftazadime (350 mg/L). (d) Meropenem (5 mg/L). (e) Colistimethate (CMS; 20 mg/L). Biofilm growth was assessed by cfu enumeration. Horizontal broken line represents killing by WLBU2 alone, for comparison. n = 3. NS, not significant.
Discussion
The development of new antimicrobials to treat chronic bacterial infections in CF lung disease is a critical unmet need. In the current study, we demonstrate the ability of the eCAP, WLBU2, to prevent biofilm formation on abiotic surfaces, as well as on the surface of polarized human CF AECs without cytotoxicity. Importantly, WLBU2 prevented biofilm growth by clinical isolates of P. aeruginosa from patients with CF. WLBU2 also prevented biotic biofilm growth in the presence of mucus (from primary CF AECs), low pH and high salt conditions similar to those of the CF airway. Finally, we demonstrate additional benefit of WLBU2 co-administration with existing antibiotics used to treat P. aeruginosa infections. WLBU2 enhanced the activity of tobramycin, ciprofloxacin, ceftazidime and meropenem, raising the exciting possibility of combining AMP therapy with existing antibiotics to treat chronic P. aeruginosa infections.
Over 2000 AMPs have been identified from diverse species of animals, plants and bacteria, offering the potential for development of novel therapeutics for bacterial infections in humans.36 However, in vitro evaluations of candidate natural AMPs have revealed intrinsic limitations of natural AMPs when tested against diverse bacteria or in different bioenvironments (high salt, low pH, serum, etc.).18 These observations suggest that the remarkable diversity of AMPs in nature reflects a natural evolution of peptide structures to optimize activity against specific pathogens in defined biological environments; changes in target pathogen or environment can then negatively impact peptide activity. For example, due to defective CFTR chloride channel function, the CF airway has an acidic pH, is high in salt and coated in a thick, dehydrated mucus layer.23,35,37 This altered airway environment prevents the action of several natural AMPs with a critical antimicrobial role in host defence of the airway, including lysozyme, lactoferrin, human β-defensin-3 and LL-37.23,35 To address the confounding limitations of natural AMPs as clinical therapeutics, we have engineered a series of naturally inspired, rationally designed cationic peptides that consist of two to three different amino acids arranged in a sequence to achieve an optimized amphipathic helix that maximizes bacterial membrane interaction and killing. WLBU2 is a 24-mer consisting of only valine, arginine and tryptophan.19 We have previously demonstrated the ability of WLBU2 to inactivate a broad spectrum of Gram-negative and Gram-positive bacteria in vitro, including clinical strains that are resistant to standard antibiotics, colistin (drug of last resort) and LL-37 (reference natural AMP produced in the lung).21,22 Our previous studies on the structural determinants of AMP activity have revealed the importance of amino acid composition, peptide length, overall positive charge and amphipathic potential, all factors optimized in the rational design of WLBU2. As such, WLBU2 is highly amphipathic and shows a strong propensity to form helices, which correlates with its antimicrobial activity in high salt environments.19 Thus, we speculated that the structural features of the engineered WLBU2, compared with LL-37, would result in enhanced bacterial membrane interactions that are not effectively suppressed by factors such as serum, salt, pH or divalent cations, which suppress LL-37 activity. In addition to host factors that can limit AMP activity, biofilm lifestyle growth increases antimicrobial resistance and induces exopolysaccharide production that may limit antibiotic penetration, raising concerns that the effectiveness of WLBU2 will be reduced against bacterial biofilms. In the current study, we demonstrate that WLBU2 overcomes the limitations of many natural and synthetic AMPs and shows efficacy in preventing biofilm growth on both abiotic and biotic surfaces.
The lack of efficacy of current antibiotics against infections associated with biofilm has stimulated interest in AMPs as potential antibiofilm agents. Previous studies have demonstrated the ability of diverse natural and synthetic AMPs to prevent bacterial biofilms in an abiotic environment.27 However, it is not clear whether these AMPs would be able to overcome limitations to salt and acidic environments displayed by natural peptides in the context of biotic bacterial biofilms. Since LL-37, despite its antibiofilm properties, is ineffective under conditions associated with chronic respiratory infections (e.g. CF), it was appropriate to compare LL-37 with WLBU2 under high salt and low pH conditions known to occur in CF. The observation of higher effective concentrations of WLBU2 required to prevent P. aeruginosa biofilm growth compared with activity against planktonic bacteria must be appreciated in the context of biofilm–AEC co-culture. As previously demonstrated, the increased effective concentrations of WLBU2 against P. aeruginosa in blood compared with activity in PBS were also associated with reduced cytotoxicity and with systemic efficacy in mice. Hence, effective concentrations should always be considered in the context of environment and mammalian toxicity. While much more progress is still needed, the current study further illustrates our potential to overcome limitations to test conditions proven to dampen activity of most AMPs.
The ability of WLBU2 to prevent abiotic biofilms is similar to that previously published with various natural and synthetic AMPs, with effective peptide concentrations in the micromolar range (extensively reviewed in Batoni et al.38). However, the unique aspect of the current study is the demonstration of WLBU2 efficacy in preventing biotic biofilms associated with AECs, without obvious deleterious effects to the sensitive cell substrate and the retention of WLBU2 activity in a complex biological environment (salt, pH, mucus) that suppresses natural AMP activity. It is important to note that the effective concentration of AMP in biofilm prevention does not necessarily correlate with the MIC values determined against planktonic bacteria;39,40 published effective peptide concentrations for abiotic biofilm prevention can be higher or lower than the respective MIC values for the same peptide–bacteria combination.38 In the case of WLBU2 treatment of abiotic and biotic biofilms, we observed an effective concentration of peptide that is two to four times higher than the MIC determined for planktonic P. aeruginosa.22 It might be expected that the observed effective concentrations of WLBU2 will be highly dependent on the specific experimental conditions of the in vitro assay and not likely to predict the effective concentration of peptide in vivo, particularly in the case of the CF lung environment.
The success of WLBU2 in vitro as an antibacterial and a biofilm prevention agent is complemented by published in vivo studies demonstrating the efficacy of a single dose (intravenous or intraperitoneal) of WLBU2 to clear lung infections in mice inoculated with a lethal dose of P. aeruginosa.18,20,41 The in vivo activity further demonstrates the unique ability of WLBU2 to retain its activity in a complex biological environment that presents numerous potential suppressive factors, including inhibitory factors in serum and mucosal secretions, limitations in biodistribution and toxicity. In conclusion, the eCAP WLBU2 shows promise as a novel antimicrobial therapy to treat chronic P. aeruginosa infections in the challenging setting of the CF airway.
Funding
This work was supported by the National Institutes of Health (P30DK072506 to J. M. P., R. C. M. and J. M. B., R00HL098342 to J. M. B. and T32AI49820 to J. A. M.) and the Cystic Fibrosis Foundation (BOMBER14G0 to J. M. B., Cystic Fibrosis Foundation Research Development Program grant to J. M. P. and MELVIN15F0 to J. A. M.).
Transparency declarations
R. C. M. holds stock in Peptilogics, Inc. and serves on the scientific advisory board for the company. Although a financial conflict of interest was identified based on the author's relationship with Peptilogics, the research findings included in this publication may not necessarily be related to the interests of Peptilogics. All other authors: none to declare.
Acknowledgements
We thank Jane Burns for use of the late CF P. aeruginosa clinical isolates. We also thank Kazi Islam and Ray Yurko of the University of Pittsburgh Peptide and Peptoid Synthesis Facility for their production and characterization of WLBU2.
References
- 1.Choo-Kang LR, Zeitlin PL. Type I, II, III, IV, and V cystic fibrosis transmembrane conductance regulator defects and opportunities for therapy. Curr Opin Pulm Med 2000; 6: 521–9. [DOI] [PubMed] [Google Scholar]
- 2.Manzanares D, Krick S, Baumlin N et al. Airway surface dehydration by transforming growth factor β (TGF-β) in cystic fibrosis is due to decreased function of a voltage-dependent potassium channel and can be rescued by the drug pirfenidone. J Biol Chem 2015; 290: 25710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garland AL, Walton WG, Coakley RD et al. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc Natl Acad Sci USA 2013; 110: 15973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med 2007; 58: 157–70. [DOI] [PubMed] [Google Scholar]
- 5.Vidya P, Smith L, Beaudoin T et al. Chronic infection phenotypes of Pseudomonas aeruginosa are associated with failure of eradication in children with cystic fibrosis. Eur J Clin Microbiol Infect Dis 2016; 35: 67–74. [DOI] [PubMed] [Google Scholar]
- 6.Emerson J, Rosenfeld M, McNamara S et al. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 2002; 34: 91–100. [DOI] [PubMed] [Google Scholar]
- 7.Steckbeck JD, Deslouches B, Montelaro RC. Antimicrobial peptides: new drugs for bad bugs? Expert Opin Biol Ther 2014; 14: 11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipsky BA, Holroyd KJ, Zasloff M. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis 2008; 47: 1537–45. [DOI] [PubMed] [Google Scholar]
- 9.Chen HC, Brown JH, Morell JL et al. Synthetic magainin analogues with improved antimicrobial activity. FEBS Lett 1988; 236: 462–6. [DOI] [PubMed] [Google Scholar]
- 10.Flamm RK, Rhomberg PR, Simpson KM et al. In vitro spectrum of pexiganan activity when tested against pathogens from diabetic foot infections and with selected resistance mechanisms. Antimicrob Agents Chemother 2015; 59: 1751–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamb HM, Wiseman LR. Pexiganan acetate. Drugs 1998; 56: 1047–52; discussion 1053-4. [DOI] [PubMed] [Google Scholar]
- 12.Hancock RE, Patrzykat A. Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr Drug Targets Infect Disord 2002; 2: 79–83. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzaki K, Sugishita K, Miyajima K. Interactions of an antimicrobial peptide, magainin 2, with lipopolysaccharide-containing liposomes as a model for outer membranes of gram-negative bacteria. FEBS Lett 1999; 449: 221–4. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H, Mattila JP, Holopainen JM et al. Comparison of the membrane association of two antimicrobial peptides, magainin 2 and indolicidin. Biophys J 2001; 81: 2979–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Rozek A, Hancock RE. Interaction of cationic antimicrobial peptides with model membranes. J Biol Chem 2001; 276: 35714–22. [DOI] [PubMed] [Google Scholar]
- 16.Hancock RE, Scott MG. The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci USA 2000; 97: 8856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol 2006; 306: 91–110. [DOI] [PubMed] [Google Scholar]
- 18.Deslouches B, Islam K, Craigo JK et al. Activity of the de novo engineered antimicrobial peptide WLBU2 against Pseudomonas aeruginosa in human serum and whole blood: implications for systemic applications. Antimicrob Agents Chemother 2005; 49: 3208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deslouches B, Phadke SM, Lazarevic V et al. De novo generation of cationic antimicrobial peptides: influence of length and tryptophan substitution on antimicrobial activity. Antimicrob Agents Chemother 2005; 49: 316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deslouches B, Gonzalez IA, DeAlmeida D et al. De novo-derived cationic antimicrobial peptide activity in a murine model of Pseudomonas aeruginosa bacteraemia. J Antimicrob Chemother 2007; 60: 669–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deslouches B, Steckbeck JD, Craigo JK et al. Rational design of engineered cationic antimicrobial peptides consisting exclusively of arginine and tryptophan, and their activity against multidrug-resistant pathogens. Antimicrob Agents Chemother 2013; 57: 2511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deslouches B, Steckbeck JD, Craigo JK et al. Engineered cationic antimicrobial peptides to overcome multidrug resistance by ESKAPE pathogens. Antimicrob Agents Chemother 2015; 59: 1329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pezzulo AA, Tang XX, Hoegger MJ et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 2012; 487: 109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phadke SM, Islam K, Deslouches B et al. Selective toxicity of engineered lentivirus lytic peptides in a CF airway cell model. Peptides 2003; 24: 1099–107. [DOI] [PubMed] [Google Scholar]
- 25.Phadke SM, Deslouches B, Hileman SE et al. Antimicrobial peptides in mucosal secretions: the importance of local secretions in mitigating infection. J Nutr 2005; 135: 1289–93. [DOI] [PubMed] [Google Scholar]
- 26.Hell E, Giske CG, Nelson A et al. Human cathelicidin peptide LL37 inhibits both attachment capability and biofilm formation of Staphylococcus epidermidis. Lett Appl Microbiol 2010; 50: 211–5. [DOI] [PubMed] [Google Scholar]
- 27.Batoni G, Maisetta G, Brancatisano FL et al. Use of antimicrobial peptides against microbial biofilms: advantages and limits. Curr Med Chem 2011; 18: 256–79. [DOI] [PubMed] [Google Scholar]
- 28.Moreau-Marquis S, Bomberger JM, Anderson GG et al. The ΔF508-CFTR mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability. Am J Physiol Lung Cell Mol Physiol 2008; 295: L25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith EE, Buckley DG, Wu Z et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA 2006; 103: 8487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zemke AC, Shiva S, Burns JL et al. Nitrite modulates bacterial antibiotic susceptibility and biofilm formation in association with airway epithelial cells. Free Radic Biol Med 2014; 77: 307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasgado-Flores H, Krishna Mandava V, Siman H et al. Effect of apical hyperosmotic sodium challenge and amiloride on sodium transport in human bronchial epithelial cells from cystic fibrosis donors. Am J Physiol Cell Physiol 2013; 305: C1114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myerburg MM, Latoche JD, McKenna EE et al. Hepatocyte growth factor and other fibroblast secretions modulate the phenotype of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2007; 292: L1352–60. [DOI] [PubMed] [Google Scholar]
- 33.Zemke AC, Gladwin MT, Bomberger JM. Sodium nitrite blocks the activity of aminoglycosides against Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 2015; 59: 3329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Widdicombe JH. Altered NaCl concentration of airway surface liquid in cystic fibrosis. News Physiol Sci 1999; 14: 126–7. [DOI] [PubMed] [Google Scholar]
- 35.Abou Alaiwa MH, Reznikov LR, Gansemer ND et al. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc Natl Acad Sci USA 2014; 111: 18703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gogoladze G, Grigolava M, Vishnepolsky B et al. DBAASP: database of antimicrobial activity and structure of peptides. FEMS Microbiol Lett 2014; 357: 63–8. [DOI] [PubMed] [Google Scholar]
- 37.Travis SM, Singh PK, Welsh MJ. Antimicrobial peptides and proteins in the innate defense of the airway surface. Curr Opin Immunol 2001; 13: 89–95. [DOI] [PubMed] [Google Scholar]
- 38.Batoni G, Maisetta G, Esin S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim Biophys Acta 2016; 1858: 1044–60. [DOI] [PubMed] [Google Scholar]
- 39.Reffuveille F, de la Fuente-Nunez C, Mansour S et al. A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob Agents Chemother 2014; 58: 5363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribeiro SM, de la Fuente-Nunez C, Baquir B et al. Antibiofilm peptides increase the susceptibility of carbapenemase-producing Klebsiella pneumoniae clinical isolates to β-lactam antibiotics. Antimicrob Agents Chemother 2015; 59: 3906–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paranjape SM, Lauer TW, Montelaro RC et al. Modulation of proinflammatory activity by the engineered cationic antimicrobial peptide WLBU-2. F1000Res 2013; 2: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]