Summary
It is well known that a patient in clinical remission of visceral leishmaniasis (VL) remains immune to reinfection, which provides a rationale for the feasibility of a vaccine against this deadly disease. In earlier studies, observation of significant cellular responses in treated Leishmania patients as well as in hamsters against leishmanial antigens from different fractions led to its further proteomic characterization, wherein S‐adenosyl‐L‐homocysteine hydrolase (AdoHcy) was identified as a helper type 1 (Th1) stimulatory protein. The present study includes immunological characterization of this protein, its cellular responses [lymphoproliferation, nitric oxide (NO) production and cytokine responses] in treated Leishmania‐infected hamsters and patients as well as prophylactic efficacy against Leishmania challenge in hamsters and the immune responses generated thereof. Significantly higher cellular responses were noticed against recombinant L. donovani S‐adenosyl‐L‐homocysteine hydrolase (rLdAdoHcy) compared to soluble L. donovani antigen in treated samples. Moreover, stimulation of peripheral blood mononuclear cells with rLdAdoHcy up‐regulated the levels of interferon (IFN)‐γ, interleukin (IL)−12 and down‐regulated IL‐10. Furthermore, vaccination with rLdAdoHcy generated perceptible delayed‐type hypersensitivity response and exerted considerably good prophylactic efficacy (∼70% inhibition) against L. donovani challenge. The efficacy was confirmed by the increased expression levels of inducible NO synthase and Th1‐type cytokines, IFN‐γ and IL‐12 and down‐regulation of IL‐4, IL‐10 and transforming growth factor (TGF)‐β. The results indicate the potentiality of rLdAdoHcy protein as a suitable vaccine candidate against VL.
Keywords: cellular immune response prophylactic efficacy, rLdAdoHcy, treated Leishmania infected hamsters and patients
Introduction
Visceral leishmaniasis (VL) is the most distressing ailment among all types of leishmaniasis and is caused by obligate intracellular protozoan parasites, particularly by the species Leishmania donovani and L. infantum. VL is a noteworthy public health problem in the Indian subcontinent, with more than 90% of the world's cases affecting the poorest population primarily in rural areas. Emergence of resistance against the available anti‐leishmanial drugs in addition to their toxicity, availability and affordability is posing a serious problem in the control of VL. Therefore, the unavailability of satisfactory chemotherapy against VL demands alternate strategies, such as prevention through vaccination for the control of this disease. Generation of a long‐lasting protection against reinfection in recovered Leishmania patients shows that a protective vaccine can be accomplished 1, 2, 3, 4. It has been well documented that the ability to induce a proinflammatory response is essential for control of leishmaniasis, in which the T helper type 1 (Th1) cytokines interleukin (IL)−2, IL‐12 and interferon (IFN)‐γ play a very important role 1, 5 by mediating the effector functions of the macrophages and eliciting a Th1 immune response 6. These outcomes suggest that any intervention that facilitates the shifting of the Th2 immune response towards a Th1 immune response will have a significant impact on the treatment of VL. Hence, a candidate having a Th1 immune response could be used as a vaccine. Based on this, earlier immunoproteomics studies in our laboratory have identified numerous potential protective antigens from L. donovani promastigotes that induced a Th1 immune response in the peripheral blood mononuclear cells (PBMCs) of cured/endemic Leishmania patients 7, 8. Interestingly, S‐adenosyl‐L‐homocysteine hydrolase (AdoHcy), which is responsible for reversible conversion of S‐adenosyl‐L‐homocysteine to adenosine and homocysteine 9, was one of the potential antigens. Because AdoHcy is a product inhibitor of all S‐adenosylmethionine (AdoMet)‐dependent methyltransferases, in eukaryotic cells the catalytic activity of AdoHcy is necessary to maintain the ordinary cellular level of AdoHcy and to promote the various transmethylation reactions needed in typical cell functions to continue 9, 10. In eukaryotic cells, inhibition of AdoHcy hydrolase causes the hindrance of AdoMet‐dependent methyltransferases and also promotes the increment of cellular levels of AdoHcy that result in different pharmacological effects (e.g. anti‐arthritic, anti‐viral, immunosuppressive, anti‐tumour, etc.) 9, 10. Parasites such as Leishmania spp. 11, 12, Trypanasoma spp. 12, 13 and Plasmodium spp. 14, 15 produce their own AdoHcy hydrolase, so this parasitic enzyme is a potential target for developing anti‐parasitic agents. Moreover, earlier studies have shown that rAdoHcy could be a potential antigen candidate for the development of subunit vaccines against Brucella melitensis.To date, there has been no report of rAdoHcy as a vaccine candidate in any form of leishmaniasis. Therefore, the present study was undertaken for the immunological characterization of rLdAdoHcy followed by evaluation of the prophylactic potential against VL. The results from the present study have shown that rLdAdoHcy is a good antigen candidate for recombinant protein vaccine against VL, with adequate immunogenicity and optimum protective efficacy.
Materials and methods
Ethics statement
Experimental protocols on the animals (hamsters) were carried out as per the guidelines of the Institutional Animal Ethics Committee (IAEC) of the Central Drug Research Institute (CDRI) (approval number 154/10/Para/IAEC dated 4.10.10), which is based on the National Guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) under the Ministry of Environment and Forest, Government of India. The protocol and patient study was approved by the Ethics Committee of the Kala‐azar Medical Research Centre, Muzaffarpur (protocol no. EC‐ KAMRC/Vaccine/VL/2007‐01) and written informed consent was obtained from patients before enrolment into this study. All the human subjects underwent clinical examination by a local physician for leishmanial and other possible infections.
Host and parasite
Laboratory‐bred golden hamsters (Mesocricetus auratus) of either sex (male/female), weighing 45–50 g, were procured from the Institute's animal house facility and served as experimental hosts. They were housed in a climatically controlled room and fed with standard rodent food pellets (Lipton India Ltd., Bombay, India) and water ad libitum. The L. donovani strain, isolated from patient at the Kala‐Azar Medical Research Centre, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India was cultured in vitro as described elsewhere 16. Promastigotes were grown in complete RPMI medium [cRPMI; RPMI + 10% fetal bovine serum (FBS) + antibiotics] at 26οC (Sigma‐Aldrich, St Louis, MO, USA) in 75 cm2 culture flasks (Nunc, Roskilde, Denmark) 17. The strain was also maintained in hamsters through serial passages, i.e. from amastigote to amastigote, in order to maintain its virulence 18.
Soluble L. donovani (SLD) antigen from promastigote
SLD was prepared as per the protocol described earlier 7. Briefly, repeatedly washed log phase promastigotes (109) (3–4‐day‐old culture) were resuspended in phosphate‐buffered saline (PBS), pH‐7·4 and sonicated under cool conditions for two periods of 1·5 min (separated by an interval of 3 min) at medium amplitude. The supernatant obtained was finally ultracentrifuged at 40 000 g for 30 min. The resultant antigen, after assessing the protein contents (Bradford method), was distributed in small aliquots and stored at −80°C.
In‐silico prediction of antigenicity of LdAdoHcy
The antigenicity of LdAdoHcy was determined by Kolaskar and Tongaonkar's semi‐empirical method, which is known to predict the antigenic determinants on the basis of the physicochemical characterstics of amino acid residues and the frequencies of their occurrence in known segmental epitopes 19. Prediction of immunodominant T cell antigenic sites from the primary sequence of LdAdoHcy was determined by the ProPred program, which predicts peptide binding to major histocompatibility complex (MHC). ProPred uses robust statistical models for both class I human leucocyte antigen (HLA) alleles (HLA‐A*3302, HLA‐B*3701, HLA‐B*4403, HLA‐A*0205 and HLA‐*3801) and class II HLA alleles (DRB1_0101, DRB1_0102, DRB1_1307, DRB1_0402, DRB1_0802, DRB1_1114, DRB1_1323, DRB1_0813, DRB1_1107, DRB1_1121 20.
Treatment of L. donovani‐infected hamsters and isolation of mononuclear cells from lymph node
Approximately 30 hamsters of either sex were infected with 107 amastigotes intracardially (i.c.) and the infection level was checked as described elsewhere 21. Animals having > 20–30 amastigotes/100 macrophage cell nuclei were then treated with Miltefosine (SynphaBase, Pratteln, Switzerland) given orally with 40 mg/kg body weight for 5 days. Animals were checked for complete cure on day 30 post‐treatment by performing splenic biopsy and the parasite burden assessment was performed in the Giemsa‐stained dab smears. Mononuclear cells were separated from lymph nodes of cured, infected and uninfected hamsters according to the protocol described elsewhere 17. Finally, a suspension of 106cells/ml was made in cRPMI (Sigma‐Aldrich), which was utilized further for lymphoproliferative assay and the estimation of nitric oxide (NO) production.
Isolation of peripheral blood mononuclear cells (PBMCs) from different groups of human patients
The study on human samples was approved by the Ethics Committee of the Kala‐azar Medical Research Centre, Muzaffarpur, Bihar, India (protocol no. EC‐KAMRC/Vaccine/VL/2007‐01). The groups were classified as follows.
Eight treated patients (two males and six females, age range 2–32 years) were from hyperendemic areas of Bihar and had received a complete course of amphotericin B. Samples were collected from 2 months to 1 year after the completion of treatment. Diagnosis was established in all cases by demonstration of parasites in splenic aspirates and found negative at the time of study.
Six endemic household contacts (two males and four females, age ranging from 5 to 45 years) were the relatives of Leishmania patients and neither showed any clinical symptoms nor received any treatment for VL. They were also not positive to Leishmania test.
Six infected patients (three males and three females, age range 5–49 years) were again from hyperendemic areas of Bihar showing clinical symptoms of Kala‐azar.
Six normal healthy donors (four males and two female, age range 25–30 years) were from non‐endemic areas without any history of leishmaniasis and served as negative controls.
Heparinized venous blood (10 ml each) was collected from all study subjects and peripheral blood mononuclear cells (PBMCs) were isolated from blood by Ficoll Hypaque density gradient centrifugation (Histopaque 1077; Sigma, St Louis, MO, USA), as described by Garg et al. 17. A final suspension of 1 × 106 cells/ml was made in cRPMI after determining cell viability by the trypan blue staining method. These were used for various immunological assays.
Assessment of prophylactic efficacy of rLdAdoHcy + BCG in hamsters against L. donovani challenges
Four groups of hamsters (12–15 per group) were used for immunization studies, in which the first three groups served as controls: group 1, unvaccinated and unchallenged (normal control); group 2, unvaccinated and challenged (infected control); and group 3, vaccinated with bacillus Calmette–Guérin (BCG) alone. The animals in group 4 (vaccinated group) were immunized with rLdAdoHcy (50 µg/50 µl per animal) intradermally (i.d.) on the back together with an equal volume of BCG (100 µl per animal) in emulsified form. A booster dose of half the amount of rLdAdoHcy together with BCG was given i.d. 15 days later to all the hamsters in group 4 and BCG only to group 3. All the experimental animals belonging to groups 2–4 were challenged intracardially with 107 metacyclic promastigotes of L. donovani 21 days later. On necropsy at different time intervals, i.e. on days 0, 45, 60, 90 and 120 post‐challenge (p.c.), the body, spleen and liver weight of hamsters of all the experimental groups were measured and the assessment of parasite burden was performed in the Giemsa‐stained dab smears of spleen, liver and bone marrow of vaccinated hamsters as the number of amastigotes/1000 cell nuclei in each organ in comparison to the unvaccinated controls. Peritoneal exudate cells, inguinal lymph nodes and blood were also collected at these time‐points to obtain cells and sera for evaluation of cellular and antibody responses as per the protocols described above. The criterion for prophylactic efficacy in the experimental group was the assessment of percentage inhibition (PI) of Leishmania parasite multiplication in comparison to the unvaccinated control using the following formula 22:
PI = (no. of parasite count from infected control – no. of parasite from vaccinated group/no. of parasite count from infected control) × 100.
Immunological assays
Delayed‐type hypersensitivity (DTH) in hamsters
DTH was performed by injecting 50 µg/50 µl of SLD in PBS intradermally into one footpad and PBS alone into the other footpad of each of the vaccinated and unvaccinated controls. The response was evaluated 48 h later by measuring the difference in footpad swelling between the two with and without SLD for each animal 23.
Lymphocyte proliferation assay through lymphocyte transformation test (LTT) in hamsters and patients
Lymphocyte suspension (1 × 106 cells/ml) of cured/exposed patients as well as normal, infected (30 days p.c.), cured and vaccinated hamsters was cultured in 96‐well flat‐bottomed tissue culture plates (Nunc). This assay was carried out as per the protocol described by Garg et al. 17 with some modifications, where XTT (Roche Diagnostics, Basel, Switzerland) was used instead of [3H] thymidine. Approximately 100 μl of a predetermined concentration of mitogen phytohaemagglutinin (PHA, 10 μg/ml; Sigma) for patients' PBMCs and concanavalin A (ConA) (10 µg/ml; Sigma) for hamster lymphocytes, as well as antigens rLdAdoHcy and SLD (10 µg/ml each), were added to the wells in triplicate. Wells without stimulants served as blank controls. Cultures were incubated at 37°C in a CO2 incubator with 5% CO2 for 3 days in the case of the mitogens and for 5 days in the case of the antigens. Eighteen hours prior to termination of the experiment, 50 µl of XTT was added to 100 µl of supernatants of each well and absorbance measured at 480 nm, with 650 nm as reference wavelength.
Estimation of NO activity in macrophages of hamsters
Isolated mononuclear cells from all three study groups of hamsters, namely normal, infected (30 days postinfected) and cured, were suspended in culture medium and plated at 105 cells/well and stimulated for 3 days in the mitogen [lipopolysaccharide (LPS), 10 μg/ml] and 5 days in the antigens (rLdAdoHcy, SLD) at 10 μg/ml. The presence of NO was assessed using Griess reagent (Sigma) in the culture supernatants of naive hamster peritoneal macrophages 17 after exposure with 100 μl supernatant of stimulated mononuclear cells. The supernatants (100 μl) collected from macrophage cultures 24 h after incubation at 37°C in a CO2 incubator were mixed with an equal volume of Griess reagent (Sigma) and left for 10 min at room temperature. The reaction absorbance was measured at 540 nm in an enzyme‐linked immunosorbent assay (ELISA) reader 24. The nitrite concentration in the macrophage culture supernatant samples was extrapolated from the standard curve plotted with sodium nitrite. The same protocol for measuring NO production was used in the rLdAdoHcy vaccination study.
Assessment of cytokine levels of IFN‐γ/IL‐12/IL‐10/IL‐4 in PBMCs of patients in clinical remission and their uninfected contacts
Further, the levels of T helper type 1 (Th1) and Th2 cytokines were estimated in PBMCs from patients in clinical remission as well as in endemic contacts in response to rLdAdoHcy. PBMC cultures (1 × 106 cells/ml) were plated in 96‐well culture plates, and SLD and rLdAdoHcy were added at a concentration of 10 µg/ml in triplicate wells. The levels of IFN‐γ, IL‐12, IL‐10 and IL‐4 were estimated by using commercial ELISA kits according to the manufacturer's protocol (BD OptEIA kit; BD Pharmingen, San Jose, CA, USA). The results were expressed as picograms of cytokine/ml, based on the standard curves generated using a recombinant cytokine provided in the kit. The lower detection limits for various cytokines were as follows: 4·7pg/ml for IFN‐γ, 7·8 pg/ml for IL‐12, 5·1 pg/ml for IL‐4 and 7·0 pg/ml for IL‐10.
Measurement of immunoglobulin (Ig)G and its serotypes (IgG1 and IgG2) in vaccinated hamsters
Anti‐leishmanial antibodies, IgG and its isotypes IgG1 and IgG2, in serum samples from hamsters of different groups were measured as described earlier 28. The 96‐well ELISA plates were coated with rLdAdoHcy (0·2 µg/100 µl/well in PBS) overnight at 4°C and blocked with 1·5% bovine serum albumin (BSA) at room temperature (RT) for 1 h. The sera were used at a dilution of 1 : 50 for 2 h at RT. Biotin‐conjugated mouse anti‐Armenian and anti‐Syrian hamster IgG, mouse anti‐Armenian hamster IgG1 and mouse anti‐Syrian hamster IgG2 (BD Pharmingen) were added for 1 h and the plates were incubated further for 1 h with horseradish peroxidase (HRP)‐conjugated streptavidin and finally developed using o‐Phenylenediamine dihydrochloride (OPD) (Sigma‐Aldrich) as substrate.
Evaluation of mRNA cytokines and inducible NO synthase (iNOS) in vaccinated hamsters by quantitative real time reverse transcription–polymerase chain reaction (qRT–PCR)
qRT–PCR was performed to assess the expression level of mRNAs for various cytokines and inducible nitric oxide synthase (iNOS) in splenic cells post‐rLdAdoHcy + BCG vaccination. The splenic tissues of hamsters from each experimental group at different time intervals were collected and total RNA was isolated using Tri‐reagent (Sigma‐Aldrich). Following quantification using Gene‐quanta (Bio‐Rad, Hercules, CA, USA), 1 µg of total RNA was employed for the synthesis of cDNA using a first‐strand cDNA synthesis kit (Fermentas, Fremont, CA, USA). The primers for qRT–PCR were designed using Beacon Designer software (Bio‐Rad) on the basis of cytokines and iNOS mRNA sequences, as shown in Supporting information, Table S1 25. Real‐time PCR was performed as described elsewhere 21 using the iQ5 multicolour real‐time PCR system (Bio‐Rad). cDNA from infected hamsters were used as ‘comparator samples’ for quantification of those corresponding to test samples. The housekeeping gene hypoxanthine phosphoribosyltransferase (HGPRT) was used for normalization in all quantifications. A no‐template control cDNA was also included to eliminate contamination or non‐specific reactions. Differences in gene expression were calculated by the comparative CT method 26, which compares test samples to a comparator sample and uses results obtained with a uniformly expressed control gene (HGPRT) to correct for differences in the amounts of RNA present in the two samples being compared to generate a ΔCT value. Results are expressed as the degrees of difference between ΔCT values of test and comparator samples.
Post‐challenge survival of vaccinated hamsters
Survival of hamsters belonging to group 4 was checked until day 180 p.c. in comparison to the normal hamsters (group 1). Animals in all the groups were given proper care and their physical condition was observed for the duration of their survival period. Survival of individual hamsters was recorded and the mean survival period was calculated.
Statistical analysis
Results were expressed as mean ± standard deviation (s.d.). Two sets of experiments were performed for vaccination studies and 15–20 animals were used in each experiment. The results (pooled data of two independent experiments) were analysed by one‐way analysis of variance (anova) test followed by Tukey's test using the GraphPad Prism (version 5·0) software program. The one‐way anova statistical test was used to assess the significance of the differences among various groups and a P‐value of < 0·05 was considered significant.
Results
Reactivity of rLdAdoHcy in serum samples of active VL patients
Using Western blot, rLdAdoHcy showed reactivity against pooled sera of VL patients while it was absent in pooled sera from uninfected healthy controls (Fig. 1a).
Figure 1.
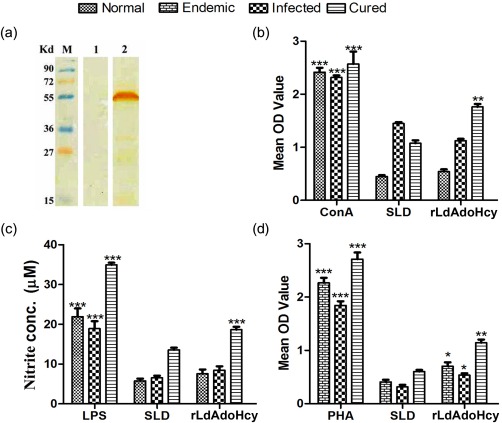
Cellular immune response to recombinant Leishmania donovani S‐adenosyl‐L‐homocysteine hydrolase (rLdAdoHcy) in treated hamsters and visceral leishmaniasis (VL) patients. (a) Reactivity of human sera to rLdAdoHcy: M = molecular weight marker; lane 1 = reactivity of rLdAdoHcy with pooled normal human sera (n = 5); lane 2 = reactivity of rLdAdoHcy with pooled VL patients' sera (n = 5). (b) LTT response of mononuclear cells from normal, L. donovani‐infected and treated hamsters in response to concanavalin (ConA), soluble L. donovani antigen (SLD) and rLdAdoHcy at concentration of 10 µg/ml each. Each bar represents the pooled data [mean ± standard deviation (s.d.) value] of six hamsters and the data represent the means of triplicate wells ± s.d. of each hamster as mean optical density (OD) of stimulated cells and mean OD of unstimulated control cells. (c) Nitric oxide (NO) production (µM): peritoneal macrophages were stimulated with the supernatants of stimulated lymphocytes of normal/infected/cured hamsters in response to rLdAdoHcy, SLD and lipopolysaccharide (LPS), respectively, at 10 µg/ml each. The estimation of NO production was performed using Griess reagent in supernatants collected from macrophage cultures 24 h after incubation, and OD was measured at 540 nm. (d) Lymphocyte transformation test (LTT) response of peripheral blood mononuclear cells (PBMCs) from L. donovani‐infected endemic and patients in clinical remission against phytohaemagglutinin (PHA), SLD and rLdAdoHcy stimulation at a concentration of 10 µg/ml each. Proliferative response was represented as mean OD of stimulated cell – mean OD of unstimulated control. Each bar represents the pooled data (mean ± s.d. value) of stimulated PBMCs of each group. Significance values in figures b,c and d indicate the differences between ConA, LPS, PHA and rLdAdoHcy stimulation against SLD (*P < 0·05, **P < 0·01; ***P < 0·001).
LdAdoHcy reveals MHC‐I‐restricted high antigenicity in silico
The Kolaskar–Tongaonkar semi‐empirical method predicted three antigenic determinants in protein, i.e.YKVKDISL, ASKPLAGAKIAGCLHMTVQTAVLIETLKALGA and RWSSCNI. The average antigenic propensity of the protein was found to be 1·0344, revealing its highly immunogenic nature (Table 1). ProPred analysis for HLA‐DR alleles showed 10 peptide sequences (mainly LVNLGCASG, IELWTNRDS and VKNLYKRLA) to be the highest‐scoring promiscuous binders that could bind to HLA‐DR alleles (Table 2). In ProPred analysis for HLA‐A/B alleles, 13 peptide sequences identified, namely DISLAEWGR, EMPGLMELR and ESLVDGIKR, had affinity to HLA‐A*3302, peptides GDKAGVFFL and RDDAIVCNI had affinity to HLA‐A*3701, while MILDDGGDL, VLIETLKAL and LVIDHHPEL had affinity to HLA‐A*0205. However, all the peptide sequence‐identified HLA‐A/B alleles by ProPred analysis covered the highest peptide score and proved to be the highest promiscuous binder (Table 3).
Table 1.
Prediction of antigenic determinants for Leishmania donovani S‐adenosyl‐L‐homocysteine hydrolase (LdAdoHcy) using the Kolaskar–Tongaonkar semi‐empirical method.
| S no | Antigenic determinant | Position |
|---|---|---|
| 1 | YKVKDISL | 4 |
| 2 | ASKPLAGAKIAGCLHMTVQTAVLIETLKALGA | 38 |
| 3 | RWSSCNI | 72 |
Table 2.
ProPed analysis of human leucocyte antigen D‐related (HLA‐DR) binding Leishmania donovani S‐adenosyl‐L‐homocysteine hydrolase (LdAdoHcy) peptides.
| S. no. | HLA allele | Peptide sequences | Position | Peptide score | Threshold score |
|---|---|---|---|---|---|
| 1 | DRB1_0101 | LVNLGCASG | 342 | 1.7 | 0.14 |
| 2 | DRB1_1307 | YKRLAKGKL | 163 | 2.7 | 0.6 |
| 3 | DRB1_0102 | LVNLGCASG | 342 | 2.7 | 0.7 |
| 4 | DRB1_0102 | LRAFGARVV | 230 | 2.67 | 0.7 |
| 5 | DRB1_0402 | IELWTNRDS | 368 | 6.4 | 1.8 |
| 6 | DRB1_0802 | YKVKDISLA | 3 | 3.3 | 1 |
| 7 | DRB1_0802 | WCIRQTLKG | 108 | 3.3 | 1 |
| 8 | DRB1_1114 | VKNLYKRLA | 159 | 3.5 | 1.3 |
| 9 | DRB1_1323 | VKNLYKRLA | 159 | 3.5 | 1.3 |
| 10 | DRB1_0813 | YKVKDISLA | 3 | 5.1 | 1.9 |
| 11 | DRB1_1107 | LVIDHHPEL | 136 | 5.5 | 2.1 |
| 12 | DRB1_1101 | YKRLAKGKL | 163 | 2.8 | 1.1 |
| 13 | DRB1_1121 | VKNLYKRLA | 159 | 4.5 | 1.8 |
Table 3.
ProPed analysis of human leucocyte antigen (HLA)‐a/B binding Leishmania donovani S‐adenosyl‐L‐homocysteine hydrolase (LdAdoHcy) peptides.
| S. no. | HLA ‐A/B allele | Peptide sequences | Position | Peptide score | Threshold score |
|---|---|---|---|---|---|
| 1 | HLA‐A*3302 | DISLAEWGR | 8 | 3.8067 | −0.105 |
| 2 | HLA‐A*3302 | EMPGLMELR | 25 | 3.8067 | −0.105 |
| 3 | HLA‐A*3302 | ESLVDGIKR | 196 | 3.8067 | −0.105 |
| 4 | HLA‐B*3701 | GDKAGVFFL | 383 | 5.2983 | 0.405 |
| 5 | HLA‐B*4403 | DDIITSAHF | 278 | 4.723 | 0.405 |
| 6 | HLA‐B*3701 | RDDAIVCNI | 290 | 4.0943 | 0.405 |
| 7 | HLA‐A*0205 | MILDDGGDL | 126 | 4.6738 | 0.519 |
| 8 | HLA‐A*0205 | VLIETLKAL | 59 | 4.2683 | 0.519 |
| 9 | HLA‐A*0205 | LVIDHHPEL | 137 | 4.2683 | 0.519 |
| 10 | HLA‐*3801 | HHPELVPKI | 141 | 3.3351 | 0.445 |
| 11 | MHC‐Kb | FSGDGFPNM | 118 | 2.4849 | 0.365 |
| 12 | MHC‐Dd | DGPFKPDHY | 427 | 5.663 | 0.875 |
| 13 | HLA‐B8 | LAKGRLVNL | 338 | 5.7683 | −0.916 |
rLdAdoHcy‐induced LTT and NO responses in cured hamsters
The cellular responses of lymph node cells of cured hamsters were assessed by XTT against the mitogen ConA as well as SLD and rLdAdoHcy at a predetermined concentration of 10 µg/ml. The lymphoproliferative response of lymphocytes from cured hamsters was compared with that of normal as well as L. donovani‐infected lymphocytes, which served as controls. Both the normal and cured Leishmania‐infected groups had shown significantly higher proliferative responses [mean optical density (OD) 2·416 ± 0·188, 2·316 ± 0·088 and 2·571 ± 0·521, respectively] against ConA compared to the L. donovani‐infected group, indicating procedural sensitivity. Stimulation with rLdAdoHcy showed a significantly more proliferative response in cured hamsters (mean OD 1·762 ± 0·120) compared to SLD (mean OD 1·077 ± 0·118). The difference was statistically significant (P < 0·01) (Fig. 1b).
The NO‐mediated macrophage effector mechanism is also a measure of cellular immune response. Therefore, NO production was performed in peritoneal macrophages of cured hamsters after 24 h of incubation in the presence of rLdAdoHcy and SLD. For comparison, NO production in mitogen (LPS)‐stimulated and ‐unstimulated cells served as positive and negative controls, respectively. NO production was recorded to be ∼1–1·5‐fold (P < 0·001) higher against rLdAdoHcy compared to SLD (Fig. 1c).
LTT response and cytokine (IFN‐γ, IL‐12p40 and IL‐10) profile in PBMCs of Leishmania patients in clinical remission in response to rLdAdoHcy
The cellular responses of the hamster data were validated further in PBMCs of Leishmania‐infected patients as well as in patients in clinical remission and endemic contacts. All the individuals in each study group were found to show different responses. Proliferation and cytokine responses of rLdAdoHcy in PBMCs from endemic contacts of patients in clinical remission as well as those with active VL were compared with SLD.
Endemic contacts of Leishmania patients in clinical remission, as well as Leishmania‐infected patients, exhibited higher mean OD values against PHA, i.e. 2·268 ± 0·226, 2·711 ± 0·310 and 1·844 ± 0·190, respectively. PBMCs from all the study groups proliferated in response to rLdAdoHcy with mean OD values of 1·143 ± 0·150, 0·537 ± 0·085 and 0·704 ± 0·177, which were higher than SLD values (mean OD values of 0·604 ± 0·72, 0·316 ± 0·095 and 0·409 ± 0·098), respectively. The difference was found to be significant (P < 0·01) and rLdAdoHcy was observed as a potent T cell antigen, as recognized by the majority of individuals belonging to the different study groups (Fig. 1d).
To validate the Th1/Th2 stimulatory potential of rLdAdoHcy, the estimation of cytokine levels of IFN‐γ, IL‐12p40 and IL‐10 was carried out further in PBMCs from endemic contacts of Leishmania patients in clinical remission as well as Leishmania ‐infected patients. The levels of IFN‐γ and IL‐12p40 cytokines, the signatures of Th1 immune responses, were found to be elevated in the supernatant of patients in clinical remission stimulated with rLdAdoHcy. Following stimulation with rLdAdoHcy, the level of IFN‐γ was found to be higher in patients in clinical remission, ranging from 773 to 2150 pg/ml, and in endemic contacts it ranged from 332 to 1553 pg/ml in comparison to SLD (105–705 and 165–591 pg/ml, respectively, for patients in clinical remission and endemic contacts). Similarly, the IL‐12p40 level was found elevated in patients in clinical remission, ranging from 823 to 1470 pg/ml, and in endemic contacts from 844 to 1262 pg/ml in contrast to SLD (178–541 and 122–488 pg/ml, respectively, for patients in clinical remission and endemic contacts). Conversely, a very low level of IL‐10 cytokines against rLdAdoHcy was detected in patients in clinical remission (7–27 pg/ml) and also in endemic contacts (23–68 pg/ml) compared to SLD (239–436 and 66–291 pg/ml, respectively, for patients in clinical remission and endemic contacts) (Fig. 2a–c). Moreover, no or few detectable amounts of IFN‐γ and IL‐12p40 levels were observed with lymphocytes of healthy individuals as well as L. donovani‐infected patients against the rLdAdoHcy (data not shown). A mixed Th1/Th2 cytokine profile was observed in the PBMCs of patients in clinical remission/endemic contacts against SLD, while high levels of IL‐10 and very low levels of IFN‐γ and IL‐12p40 were observed in response to SLD in patients in clinical remission as well as in endemic contacts.
Figure 2.
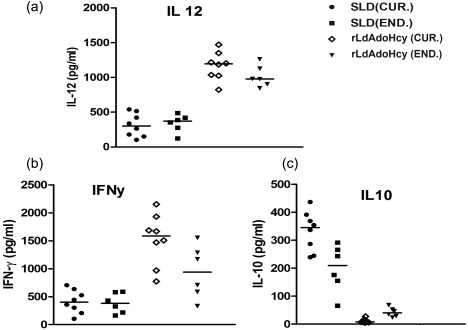
Cytokine production. (a) Interleukin (IL)‐12, (b) interferon (IFN)‐γ and (c) IL‐10 in stimulated peripheral blood mononuclear cells (PBMCs) of cured visceral leishmaniasis (VL) patients (n = 8) and endemic controls (n = 6) in response to recombinant Leishmania donovani S‐adenosyl‐L‐homocysteine hydrolase (rLdAdoHcy) antigens; each data point represents one individual. The x‐axis represents the group of individuals (cured and endemic) and the y‐axis corresponds to the values of respective cytokine concentrations in pg/ml. The mean concentration of cytokine for each group is indicated by the horizontal bars. Cytokine production was tested in triplicate in two independent experiments and the results were comparable. The lower detection limits for various cytokines were as follows: 5·1 pg/ml for IFN‐γ, 30·8 pg/ml for IL‐12 and 6·9 pg/ml for IL‐10.
rLdAdoHcy + BCG vaccination offered optimum level of protection in hamsters against virulent L. donovani challenges
The hamsters vaccinated with rLdAdoHcy + BCG were found to be protected against virulent L. donovani challenge. The vaccinated hamsters gained significant weight in comparison to the hamsters vaccinated with BCG alone and infected animals when observed simultaneously for the same time‐period, i.e. on days 45, 60, 90 and 120 p.c. (Fig. 3a). Furthermore, no hepatosplenomegaly was observed in the vaccinated hamsters compared to the unvaccinated and BCG‐treated challenged hamsters (Fig. 3b,c). A significant (P < 0·001) reduction in parasite load (∼70%) was observed in the hamsters vaccinated with rLdAdoHcy + BCG (Fig. 3d). Conversely, hamsters vaccinated with BCG alone showed no protection, and a progressive increase in parasite load was found in these hamsters as well as in unimmunized ones, and they succumbed to virulent L. donovani challenge within 2–3 months (Fig. 3d). Moreover, parasite loads in liver and bone marrow decreased sharply after day 45 p.c. and parasites were almost absent by day 180 p.c. in the vaccinated group (Fig. 3e,f). All the hamsters vaccinated with rLdAdoHcy + BCG survived longer after the lethal challenge of L. donovani and remained healthy until the completion of the experiment up to 6 months post‐infection (Fig. 7).
Figure 3.
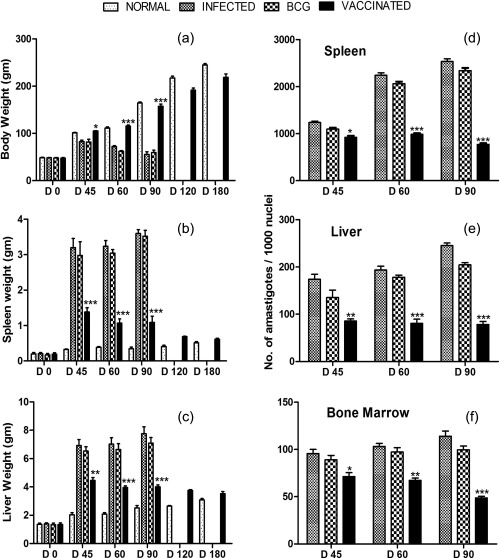
Prophylactic efficacy of recombinant Leishmania donovani S‐adenosyl‐L‐homocysteine hydrolase (rLdAdoHcy) in hamsters against Leishmania donovani challenges: body weight (a), spleen weight (b) and liver weight (c) of hamsters in grams (gm) on days 0, 45, 60, 90, 120 and 180 post‐challenge (p.c.); parasite burden (number of amastigotes per 1000 macrophage nuclei) in the dab smears of spleen (d), liver (e) and bone marrow (f) of hamsters on days 45, 60 and 90 p.c. As the non‐vaccinated challenged (infected control), the bacillus Calmette–Guérin (BCG)‐vaccinated and challenged group died after day 90 (D 90) of the study period, their corresponding bars are not shown in (d,e,f). Significance values indicate the difference between the rLdAdoHcy‐vaccinated groups and infected group (*P < 0·05; **P < 0·01; ***P < 0·001).
Figure 7.
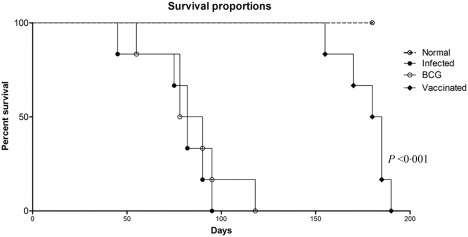
Survival curve analysis of different experimental groups: survival of animals (six hamsters in each experimental group) was observed up to day 180 post‐challenge (p.c.). Significance values indicate the difference between the vaccinated and infected (unvaccinated) control groups.
rLdAdoHcy + BCG vaccination stimulates significant DTH, mitogenic and Leishmania‐specific cellular responses against L. donovani challenge in hamsters
ConA‐induced lymphoproliferation (Fig. 4Ia–c) in vaccinated animals was observed to be similar to normal animals throughout the entire p.c. period (days 45, 60 and 90 p.c.), whereas it was lower in other control groups. A lesser proliferative response was observed in hamsters vaccinated with BCG alone, as well as in unvaccinated infected controls (Fig. 4Ia–c).
Figure 4.
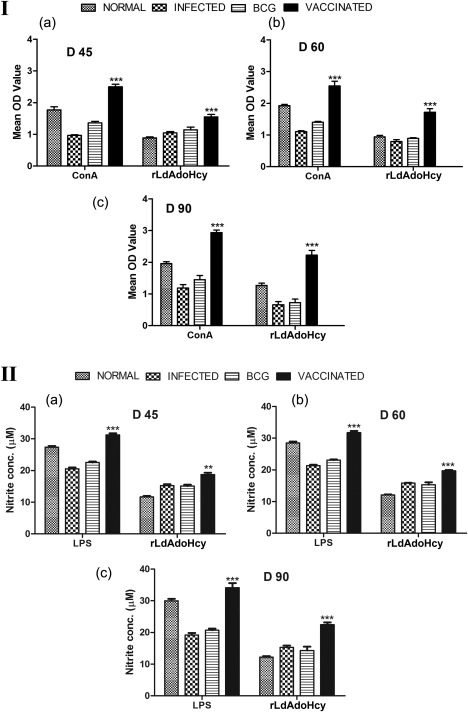
Cellular responses of recombinant Leishmania donovani S‐adenosyl‐L‐homocysteine hydrolase (rLdAdoHcy) + bacillus Calmette–Guérin (BCG)‐vaccinated hamsters. (I) Lymphocyte transformation test (LTT) response [mean optical density (OD) values] to concanavalin A (ConA) and rLdAdoHcy in normal, infected and BCG controls as well as in vaccinated animals on days 45, 60 and 90 post‐challenge (p.c.). (II) Nitric oxide (NO) production (µM) to lipopolysaccharide (LPS) and rLdAdoHcy in the naive macrophages co‐incubated with supernatants of mononuclear cells isolated from normal, infected controls and BCG controls as well as rLdAdoHcy + BCG‐vaccinated hamsters on days 45, 60 and 90 p.c. Significance values indicate the difference between the vaccinated and infected groups (*P < 0·05; **P < 0·01; ***P < 0·001).
The mononuclear cell‐mediated activation of macrophages to produce NO is an important factor for controlling for leishmaniasis. Lymphocyte‐mediated activation of macrophages to produce NO for leishmanicidal activities was found to differ between control and experimental groups of hamsters. Therefore, macrophages isolated from naive hamsters, when incubated with stimulated supernatants of lymphocytes from rLdAdoHcy + BCG‐vaccinated hamsters, produced significantly more NO (P < 0·01) than the other control groups on day 45 p.c. (Fig. 4IIa). A further increase in NO level was observed by days 60 and 90 p.c. (P < 0·001) (Fig. 4IIb,c).
rLdAdoHcy + BCG vaccination stimulates significant DTH and serological response (IgG and its isotypes IgG1 and IgG2) in vaccinated hamsters
DTH is a marker of cell‐mediated immunity in vivo and, as an antigen specific in vitro for T cell proliferation assay, revealed the status of cellular responses produced in vaccinated animals. Hence, it was necessary to determine the DTH and proliferative responses elicited by vaccinated and challenged animals. The hamsters vaccinated with rLdAdoHcy + BCG displayed significant DTH responses, which increased progressively up to 90 days p.c., and was higher compared to the infected groups at all time‐points throughout the experiments (P < 0·001) (Fig. 5I).
Figure 5.
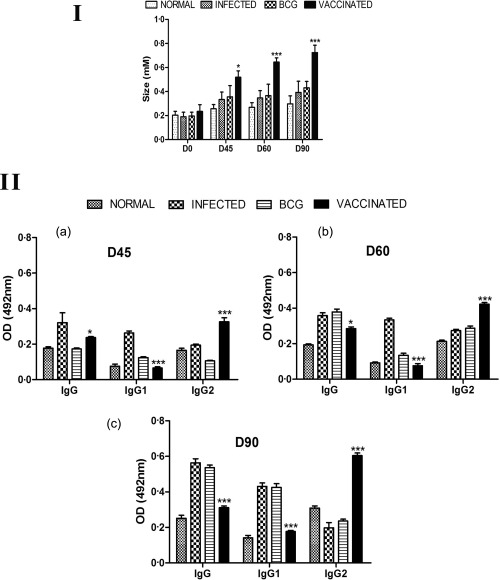
Cellular responses of recombinant Leishmania donovani S‐adenosyl‐L‐homocysteine hydrolase (rLdAdoHcy) + bacillus Calmette–Guérin (BCG)‐vaccinated hamsters. (I) Delayed‐type hypersensitivity (DTH) response (mm) to soluble L. donovani (SLD) antigen as footpad swelling on days 45, 60 and 90 post‐challenge (p.c). Significance values indicate the difference between the vaccinated and infected groups (*P < 0·05; **P < 0·01; ***P < 0·001). (II) Serological response in rLdAdoHcy + BCG‐vaccinated hamsters: immunoglobulin (Ig)G and its isotypes IgG1 and IgG2 response in rLdAdoHcy‐vaccinated hamsters in comparison to the unvaccinated infected control hamsters on days 45, 60 and 90 p.c. Significance values indicate the difference between the vaccinated and unvaccinated infected groups (*P < 0·05; **P < 0·01; ***P <0·001).
Active VL is known to be associated with the production of a high level of Leishmania‐specific antibody, which is observed prior to detection of parasite‐specific T cell responses. In kala‐azar, antibody levels correlate with the intensity of infection harboured by the host. The serum levels of Leishmania‐specific IgG and its isotypes (IgG1 and IgG2) from all the experimental groups were estimated by ELISA. With time, the anti‐Leishmania IgG and IgG1 were elevated progressively to a high level in all the experimental groups except the vaccinated group (Fig. 5II). In contrast, rLdAdoHcy + BCG‐vaccinated animals were the only group that showed a significant increase in the level of IgG2 by two‐ to threefold over the others (P < 0·001) (Fig. 5II). As a measure of cell‐mediated immunity (CMI), the elevation of IgG2 was consistent with the development of effective immune responses.
rLdAdoHcy + BCG‐vaccinated hamster elicited prominent Th1 cytokine responses
It is well known that the cytokine milieu at the commencement of infection is critical in determining disease outcome 27, 28. Therefore, to understand the relationship between the disease‐healing proinflammatory cytokines IFN‐γ and IL‐12 and disease‐associated anti‐inflammatory cytokines IL‐10 and IL‐4, the expression of these cytokines as well as the level of iNOS transcript was investigated by qRT–PCR.
The mRNA expression of Th1 and Th2 cytokines IFN‐γ, TNF‐α, IL‐12, transforming growth factor (TGF)‐β, IL‐4, IL‐10 and iNOS in hamsters of the rLdAdoHcy + BCG‐vaccinated group was evaluated on days 45 and 90 p.c. and compared to animals infected with L. donovani as well as normal control groups. An elevated expression level of Th1 cytokines was observed on days 45 and 90 p.c., and a significantly high level of IFN‐γ (P < 0·001) was observed on day 90 p.c. in comparison to the infected controls (∼2·5‐fold) (Fig. 6). There was a progressive increase in the expression levels of TNF‐α (P < 0·01) and IL‐12 (P < 0·05) mRNA transcripts, but in the case of iNOS it was increased moderately on day 45 p.c.; these cytokines were highly significant on day 90 p.c. (P < 0·001 for iNOS, IL‐12 and P < 0·01 for TNF‐α).
Figure 6.
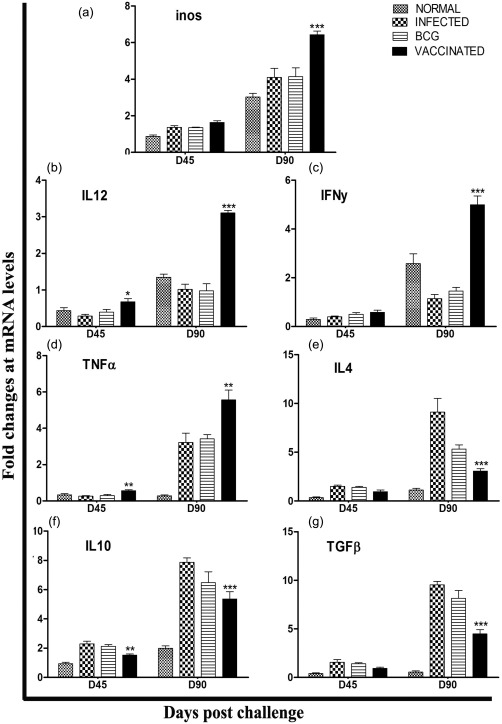
Analysis of mRNA expression profile of recombinant Leishmania donovani S‐adenosyl‐L‐homocysteine hydrolase (rLdAdoHcy) + bacillus Calmette–Guérin‐vaccinated hamsters by quantitative real time reverse transcription–polymerase chain reaction (qRT–PCR). Splenic inducible nitric oxide synthase (iNOS) (a) and cytokines, interleukin (IL)−12 (b), interferon (IFN)‐γ (c), tumour necrosis factor (TNF)‐α (d), IL‐4 (e), IL‐10 (f) and transforming growth factor (TGF)‐β (g), mRNA expression profile was assessed by qRT–PCR in all the experimental groups of hamsters on days 45 and 90 post‐challenge (p.c. Data are presented as means ± standard deviation (s.d.) and are representative of two independent experiments with similar results. Significance values indicate the difference between the vaccinated and unvaccinated infected control groups (*P < 0·05; **P < 0·01; ***P < 0·001).
Conversely, extreme down‐regulation in the expression levels of Th2 cytokines was observed in the vaccinated group compared with the infected groups at both time‐points, i.e. on days 45 and 90 p.c. Among these, the expression of TGF‐β, IL‐10 and IL‐4 was down‐regulated significantly (P < 0·001) at day 90 p.c. (Fig. 6). No clear changes at the level of mRNA transcript were observed at day 60 p.c. (data not shown). The up‐regulation of Th2 cytokines in BCG as well as infected controls are indicative of progressive VL, while these cytokines were highly down‐regulated in vaccinated hamsters, promoting the cure of VL.
Discussion
In VL, Th1 immune responses play an important role in controlling the disease. Thus, T cell stimulatory proteins are thought to be good vaccine targets. Consequently, the search for such antigens in Leishmania parasites, which induce cellular responses in PBMCs/lymphocytes of treated Leishmania infected hamsters and patients, has been demonstrated. Earlier studies have documented that stimulation with SLD, as well as its subfractions, led to the generation of significant T cell responses in treated Leishmania‐infected hamsters and patients 29. Further proteomic characterization of this subfraction revealed a number of immunogenic proteins 30, including AdoHcy. This was identified as a drug target in other organisms 31, but in this study AdoHcy was developed as recombinant protein and was eventually reassessed for immunogenicity using lymphocytes/PBMCs of treated Leishmania‐infected hamsters and patients.
It is well documented that recovery from VL depends mainly on the induction of Th1 of immune responses characterized by the production of IL‐12, IFN‐γ and TNF‐α, and require a balance between immunoregulatory mechanisms of proinflammatory IFNγ/TNF‐α and regulatory IL‐10 cytokines 32. We therefore characterized the cellular immune response of the rLdAdoHcy using lymphocytes of treated Leishmania‐infected hamsters, and we then further validated the observations in amphotericin B‐treated VL patients. 17, 33. The rLdAdoHcy induced significant lymphoproliferative responses which were supported further by the high levels of IFN‐γ, IL‐12 production. Interestingly, the cellular response of rLdAdoHcy was stronger than SLD, particularly the higher IL‐12 level. This can be supported by the observation that IL‐12, which is produced mainly by phagocytic cells in response to infection with intracellular pathogens or in response to other microbial products, can promote the development of Th1 cells 34, 35 and augments cytotoxicity 34, 36, 37. Once secreted, IL‐12 is a strong stimulator of IFN‐γ production by T and natural killer (NK) cells. Conversely, the level of IL‐10, a Th2 cytokine which promotes intracellular infection 36, 37, was found to be suppressed against recombinant protein in treated patients as well as in endemic contacts. These results indicate strongly that rLdAdoHcy possessed a dominant Th1 stimulatory property and can thus be developed as a potential vaccine candidate. This view is well supported by in‐silico analysis, which demonstrated the highly antigenic nature of protein. Further, we have predicted antigenic determinants for the LdAdoHcy using the Kolaskar–Tongaonkar semi‐empirical method and found three major antigenic determinants showing a more than 1·0 antigen propensity that could up‐regulate B cell proliferation (Table 1). On ProPred analysis with HLA‐A/B and HLA‐DR alleles the identified peptide sequences showed that the nature of protein is restricted with both classes MHC‐I and MHC‐II, which is also supported by our in‐vitro (T cell proliferation and antibody response) studies. Further, the treated individuals with reduced parasite load either following treatment or due to adequate immunity, as in endemic contacts, demonstrate good T cell reactivity to the Leishmania antigen. Hence, we further assessed the prophylactic potential of rLdAdoHcy along with BCG in naive hamsters against L. donovani challenges. BCG was selected due to its well‐known adjuvant property 38, 39, 40 via activating macrophages, resulting in induced NO release 41, 42 and long‐lasting cellular and humoral immune responses 43. Vaccination with rLdAdoHcy + BCG offered a considerably good prophylactic efficacy of ∼70%. The parasite load in the vaccinated hamsters decreased progressively, reaching a negligible level by day 120 p.c., and the vaccinated animals survived and remained healthy until the termination of the experiment at day 180 p.c., whereas all non‐immunized hamsters succumbed to the lethal L. donovani challenge within 3–4 months p.c. Thus, the longer survival of the vaccinated hamsters suggest its superiority as a potential vaccine candidate against VL. We further characterized the cellular and humoral immune response in the vaccinated hamsters. T cell proliferation, NO production and DTH responses 29, which are impaired during active VL 44, were evaluated on days 45, 60 and 90 p.c., as by this time the hamsters of most of the experimental groups survived. There was a significant lymphoproliferative (∼twofold) response on days 45, 60 and 90 p.c compared to the other control groups. Moreover, in addition to LTT response, a ∼two‐ to threefold increase in NO level was observed in the lymphocytes of vaccinated hamsters, which also confirms the up‐regulation of iNOS by Th1 cell‐associated cytokines which, in turn, activates the macrophages to kill the intracellular parasites 45. Further, it is well known that successful vaccination in humans and animals is related mainly to antigen‐induced DTH responses in vivo 46. In the present study, the vaccinated hamsters showed the strongest DTH response among all the experimental groups, which can be correlated between CMI responses and immunity to infection in this model. The generation of humoral immune response indicated by high levels of the Leishmania‐specific antibody is associated with active VL, which is reflected by the increase in the titres of IgG and IgG1 antibodies 46. The lower level of these antibodies and the higher level of IgG2, which is a measure of CMI response, is thus consistent with the decreasing parasite loads seen in the vaccinated group, indicating the development of effective immune responses 46.
Moreover, although LTT is known to be the main effector mechanism of immunity, other factors such as IFN‐γ and Leishmania‐specific T cells are also responsible for activating macrophages for the killing of intracellular parasites 47. Similarly, the antigen‐stimulated cells from the rLdAdoHcy‐vaccinated hamsters produced a remarkable level of NO. This supports the view involving the up‐regulation of iNOS with the aid of Th1 cell‐associated cytokines, and confirms that the NO‐mediated macrophage effector mechanism is important to control parasite growth in the animal model 40. The related existence of Th1 and Th2 clones producing both IFN‐γ and IL‐4 obtained from treated VL patients led us to assess whether or not the protecting response, which was elicited mainly by vaccination in hamsters, can exhibit this characteristic of clinical findings 48, 49, 50. Interestingly, hamsters vaccinated with rLdAdoHcy + BCG exhibited a significant elevation of Th1 type cytokines compared to infected controls. IFN‐γ, which is a marker cytokine of the Th1 immune response and has a dominant effect on macrophages in the production of microbicidal responses, as well as effector killing mechanisms, was found to be expressed considerably 51, along with low levels of TNF‐α production in infected hamsters. The transcripts of IFN‐γ and TNF‐α, often reported to act in concert to activate iNOS for the production of NO 52, showed a substantial increase in all the immunized groups of hamsters. A higher concentration of TNF‐α might be required to mount a NO generating signal, as observed during a protective response in vaccinated hamsters. In infected animals, the expression of mRNA for IL‐12 and IFN‐γ was found to be down‐regulated. Moreover, in the present study the low level of IL‐12 was observed in L. donovani‐infected hamsters, while the highest expression of IL‐12 of mRNA transcripts was found in vaccinated hamsters. The synergism of IL‐12 with IFN‐γ might have an additional paramount effect on leishmanicidal activities of L. donovani in this tissue 53.
Conversely, IL‐10, a Th2 cytokine, is reported to have a strong relation with an acute phase of VL during which a progressive increase of IL‐10 transcripts in tissues was generated, but IL‐10 mRNA was not detectable after successful chemotherapy 25, 54. Similar results were observed in the IL‐4 cytokine, which was down‐regulated in vaccinated hamsters compared to infected control hamsters 55. TGF‐β, a cytokine which has various functions and is also known to inhibit the activities of immune cells, was found to be down‐regulated in vaccinated hamsters compared with the infected controls 25, 51, 53.
In summary, our results indicate that rLdAdoHcy could be of great interest as a potential vaccine candidate against visceral leishmaniasis. As this protein is highly conserved among various Leishmania spp., this could be also evaluated for its cross‐protective properties. Furthermore, antigenic determinant from the protein could be identified which would generate a specific T cell response and could be further taken up for the development of a next‐generation vaccine.
Disclosures
The authors declare that they have no disclosures.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Sequences of forward and reverse primers of hamster cytokines used for quantitative real‐time reverse transcription–polymerase chain reaction (qRT–PCR).
Acknowledgements
Grateful acknowledgement is due to the Director, Central Drug Research Institute (CDRI), India, for providing facilities for the experiments. We are thankful to Mr Sumit Joshi, Senior Research Fellow for his valuable inputs in the revision of the manuscript.
References
- 1. Bacellar O, Brodskyn C, Guerreiro J et al Interleukin‐12 restores interferon‐gamma production and cytotoxic responses in visceral leishmaniasis. J Infect Dis 1996; 173:1515–8. [DOI] [PubMed] [Google Scholar]
- 2. Modabber F. Development of vaccines against leishmaniasis. Scand J Infect Dis Suppl 1990; 76:72–8. [PubMed] [Google Scholar]
- 3. Hailu A, Menon JN, Berhe N et al Distinct immunity in patients with visceral leishmaniasis from that in subclinically infected and drug‐cured people: implications for the mechanism underlying drug cure. J Infect Dis 2001; 184:112–5. [DOI] [PubMed] [Google Scholar]
- 4. Kubar J, Fragaki K. Recombinant DNA‐derived Leishmania proteins: from the laboratory to the field. Lancet Infect Dis 2005; 5:107–14. [DOI] [PubMed] [Google Scholar]
- 5. Bacellar O, D'Oliveira A Jr, Jeronimo S, Carvalho EM. IL‐10 and IL‐12 are the main regulatory cytokines in visceral leishmaniasis. Cytokine 2000; 12:1228–31. [DOI] [PubMed] [Google Scholar]
- 6. Kushawaha PK, Gupta R, Sundar S, Sahasrabuddhe AA, Dube A. Elongation factor‐2, a Th1 stimulatory protein of Leishmania donovani, generates strong IFN‐gamma and IL‐12 response in cured Leishmania‐infected patients/hamsters and protects hamsters against Leishmania challenge. J Immunol 2011; 187:6417–27. [DOI] [PubMed] [Google Scholar]
- 7. Gupta SK, Sisodia BS, Sinha S et al Proteomic approach for identification and characterization of novel immunostimulatory proteins from soluble antigens of Leishmania donovani promastigotes. Proteomics 2007; 7:816–23. [DOI] [PubMed] [Google Scholar]
- 8. Kumari S, Samant M, Khare P, Sundar S, Sinha S, Dube A. Induction of Th1‐type cellular responses in cured/exposed Leishmania‐infected patients and hamsters against polyproteins of soluble Leishmania donovani promastigotes ranging from 89.9 to 97 kDa. Vaccine 2008; 26:4813–8. [DOI] [PubMed] [Google Scholar]
- 9. Yin H, Norris DE, Lanzaro GC. Sibling species in the Llutzomyia longipalpis complex differ in levels of mRNA expression for the salivary peptide, maxadilan. Insect Mol Biol 2000; 9:309–14. [DOI] [PubMed] [Google Scholar]
- 10. Chiang SC, Yu JC, Lee ST. Transkinetoplastidy in arsenite‐resistant Leishmania major . Mol Biochem Parasitol 1996; 82:121–4. [DOI] [PubMed] [Google Scholar]
- 11. Henderson DM, Hanson S, Allen T et al Cloning of the gene encoding Leishmania donovani S‐adenosylhomocysteine hydrolase, a potential target for antiparasitic chemotherapy. Mol Biochem Parasitol 1992; 53:169–83. [DOI] [PubMed] [Google Scholar]
- 12. Avila JL, Avila A, Polegre MA, Marquez VE. Specific inhibitory effect of 3‐deazaneplanocin A against several Leishmania mexicana and L. braziliensis strains. Am J Trop Med Hyg 1997; 57:407–12. [DOI] [PubMed] [Google Scholar]
- 13. Seley KL, Schneller SW, Rattendi D, Lane S, Bacchi CJ. Synthesis and anti‐trypanosomal activity of various 8‐aza‐7‐deaza‐5'noraristeromycin derivatives. J Med Chem 1997; 40:625–9. [DOI] [PubMed] [Google Scholar]
- 14. Creedon KA, Rathod PK, Wellems TE. Plasmodium falciparum S‐adenosylhomocysteine hydrolase. cDNA identification, predicted protein sequence, and expression in Escherichia coli . J Biol Chem 1994; 269:16364–70. [PubMed] [Google Scholar]
- 15. Whaun JM, Miura GA, Brown ND, Gordon RK, Chiang PK. Antimalarial activity of neplanocin A with perturbations in the metabolism of purines, polyamines and S‐adenosylmethionine. J Pharmacol Exp Ther 1986; 236:277–83. [PubMed] [Google Scholar]
- 16. Khare P, Jaiswal AK, Tripathi CD, Joshi S, Sundar S, Dube A. Efficacy of Leishmania donovani trypanothione reductase, identified as a potent Th1 stimulatory protein, for its immunogenicity and prophylactic potential against experimental visceral leishmaniasis. Parasitol Res 2013; 113:851–62. [DOI] [PubMed] [Google Scholar]
- 17. Garg R, Gupta SK, Tripathi P, Naik S, Sundar S, Dube A. Immunostimulatory cellular responses of cured Leishmania‐infected patients and hamsters against the integral membrane proteins and non‐membranous soluble proteins of a recent clinical isolate of Leishmania donovani . Clin Exp Immunol 2005; 140:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dube A, Singh N, Sundar S. Refractoriness to the treatment of sodium stibogluconate in Indian kala‐azar field isolates persist in in vitro and in vivo experimental models. Parasitol Res 2005; 96:216–23. [DOI] [PubMed] [Google Scholar]
- 19. Kolaskar AS, Tongaonkar PC. A semi‐empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett 1990; 276:172–4. [DOI] [PubMed] [Google Scholar]
- 20. Mustafa AS, Shaban FA. ProPred analysis and experimental evaluation of promiscuous T‐cell epitopes of three major secreted antigens of Mycobacterium tuberculosis . Tuberculosis (Edinb) 2006; 86:115–24. [DOI] [PubMed] [Google Scholar]
- 21. Kushawaha PK, Gupta R, Tripathi CD et al Leishmania donovani triose phosphate isomerase: a potential vaccine target against visceral leishmaniasis. PLOS ONE 2012; 7:e45766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garg R, Gupta SK, Tripathi P et al Leishmania donovani: identification of stimulatory soluble antigenic proteins using cured human and hamster lymphocytes for their prophylactic potential against visceral leishmaniasis. Vaccine 2006; 24:2900–9. [DOI] [PubMed] [Google Scholar]
- 23. Bhowmick S, Ravindran R, Ali N. Leishmanial antigens in liposomes promote protective immunity and provide immunotherapy against visceral leishmaniasis via polarized Th1 response. Vaccine 2007; 25:6544–56. [DOI] [PubMed] [Google Scholar]
- 24. Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol 1988; 141:2407–12. [PubMed] [Google Scholar]
- 25. Melby PC, Tryon VV, Chandrasekar B, Freeman GL. Cloning of Syrian hamster (Mesocricetus auratus) cytokine cDNAs and analysis of cytokine mRNA expression in experimental visceral leishmaniasis. Infect Immun 1998; 66:2135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Samant M, Gupta R, Kumari S et al Immunization with the DNA‐encoding N‐terminal domain of proteophosphoglycan of Leishmania donovani generates Th1‐type immunoprotective response against experimental visceral leishmaniasis. J Immunol 2009; 183:470–9. [DOI] [PubMed] [Google Scholar]
- 27. Reiner SL, Zheng S, Wang ZE, Stowring L, Locksley RM. Leishmania promastigotes evade interleukin 12 (IL‐12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med 1994; 179:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA 1993; 90:10188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gupta R, Kumar V, Kushawaha PK et al Characterization of glycolytic enzymes–rAldolase and rEnolase of Leishmania donovani, identified as Th1 stimulatory proteins, for their immunogenicity and immunoprophylactic efficacies against experimental visceral leishmaniasis. PLoS One 2014; 9:e86073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kumari S, Samant M, Misra P et al Th1‐stimulatory polyproteins of soluble Leishmania donovani promastigotes ranging from 89.9 to 97.1 kDa offers long‐lasting protection against experimental visceral leishmaniasis. Vaccine 2008; 26:5700–11. [DOI] [PubMed] [Google Scholar]
- 31. Khare P, Gupta AK, Gajula PK et al Identification of novel S‐adenosyl‐L‐homocysteine hydrolase inhibitors through homology‐model‐based virtual screening, synthesis, and biological evaluation. J Chem Inf Model 2012; 52:777–91. [DOI] [PubMed] [Google Scholar]
- 32. Coler RN, Duthie MS, Hofmeyer KA et al From mouse to man: safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH‐F3+GLA‐SE. Clin Transl Immunology 2015; 4:e35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tripathi P, Ray S, Sunder S, Dube A, Naik S. Identification of Leishmania donovani antigens stimulating cellular immune responses in exposed immune individuals. Clin Exp Immunol 2006; 143:380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heinzel FP, Rerko RM, Hatam F, Locksley RM. IL‐2 is necessary for the progression of leishmaniasis in susceptible murine hosts. J Immunol 1993; 150:3924–31. [PubMed] [Google Scholar]
- 35. Sypek JP, Chung CL, Mayor SE et al Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med 1993; 177:1797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bloom S. Parasite genome similarities offer hope for new drugs and vaccines. J Clin Invest 2005; 115:2300–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ghalib HW, Whittle JA, Kubin M et al IL‐12 enhances Th1‐type responses in human Leishmania donovani infections. J Immunol 1995; 154:4623. [PubMed] [Google Scholar]
- 38. Khalil EA, Elhassan AM, Zijlstra EE et al Safety and immunogenicity of an autoclaved Leishmania major vaccine. East Afr Med J 2000; 77:468–70. [PubMed] [Google Scholar]
- 39. Misra A, Dube A, Srivastava B et al Successful vaccination against Leishmania donovani infection in Indian langur using alum‐precipitated autoclaved Leishmania major with BCG. Vaccine 2001; 19:3485–92. [DOI] [PubMed] [Google Scholar]
- 40. Armijos RX, Weigel MM, Calvopina M, Hidalgo A, Cevallos W, Correa J. Safety, immunogenecity, and efficacy of an autoclaved Leishmania amazonensis vaccine plus BCG adjuvant against New World cutaneous leishmaniasis. Vaccine 2004; 22:1320–6. [DOI] [PubMed] [Google Scholar]
- 41. MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol 1997; 15:323–50. [DOI] [PubMed] [Google Scholar]
- 42. Nozaki Y, Hasegawa Y, Ichiyama S, Nakashima I, Shimokata K. Mechanism of nitric oxide‐dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect Immun 1997; 65:3644–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Warren HS, Vogel FR, Chedid LA. Current status of immunological adjuvants. Annu Rev Immunol 1986; 4:369–88. [DOI] [PubMed] [Google Scholar]
- 44. Liew FY, Millott S, Parkinson C, Palmer RM, Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L‐arginine. J Immunol 1990; 144:4794–7. [PubMed] [Google Scholar]
- 45. Scott P, Pearce E, Heath S, Sher A. Identification of T‐cell‐reactive antigens that protect BALB/c mice against Leishmania major . Ann Inst Pasteur Immunol 1987; 138:771–4. [DOI] [PubMed] [Google Scholar]
- 46. Kedzierski L, Zhu Y, Handman E. Leishmania vaccines: progress and problems. Parasitology 2006; 133 (Suppl):S87–112. [DOI] [PubMed] [Google Scholar]
- 47. Bretscher PA, Ogunremi O, Menon JN. Distinct immunological states in murine cutaneous leishmaniasis by immunising with different amounts of antigen: the generation of beneficial, potentially harmful, harmful and potentially extremely harmful states. Behring Inst Mitt 1997; 98:153–9. [PubMed] [Google Scholar]
- 48. Kemp K. Cytokine‐producing T cell subsets in human leishmaniasis. Arch Immunol Ther Exp (Warsz) 2000; 48:173–6. [PubMed] [Google Scholar]
- 49. Kemp K, Kemp M, Kharazmi A et al Leishmania‐specific T cells expressing interferon‐gamma (IFN‐gamma) and IL‐10 upon activation are expanded in individuals cured of visceral leishmaniasis. Clin Exp Immunol 1999; 116:500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kemp M, Kurtzhals JA, Bendtzen K et al Leishmania donovani‐reactive Th1‐ and Th2‐like T‐cell clones from individuals who have recovered from visceral leishmaniasis. Infect Immun 1993; 61:1069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Melby PC, Tabares A, Restrepo BI, Cardona AE, McGuff HS, Teale JM. Leishmania donovani: evolution and architecture of the splenic cellular immune response related to control of infection. Exp Parasitol 2001; 99:17–25. [DOI] [PubMed] [Google Scholar]
- 52. Liew FY, Li Y, Millott S. Tumour necrosis factor (TNF‐alpha) in leishmaniasis. II. TNF‐alpha‐induced macrophage leishmanicidal activity is mediated by nitric oxide from L‐arginine. Immunology 1990; 71:556–9. [PMC free article] [PubMed] [Google Scholar]
- 53. Basu MK, Ray M. Macrophage and Leishmania: an unacceptable coexistence. Crit Rev Microbiol 2005; 31:145–54. [DOI] [PubMed] [Google Scholar]
- 54. Ghalib HW, Piuvezam MR, Skeiky YA et al Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J Clin Invest 1993; 92:324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Noben‐Trauth N, Lira R, Nagase H, Paul WE, Sacks DL. The relative contribution of IL‐4 receptor signaling and IL‐10 to susceptibility to Leishmania major . J Immunol 2003; 170:5152–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Sequences of forward and reverse primers of hamster cytokines used for quantitative real‐time reverse transcription–polymerase chain reaction (qRT–PCR).


