Summary
The objective of this study is to investigate whether the avidity of proteinase‐3‐anti‐neutrophil cytoplasmic antibody (PR3‐ANCA) changes during follow‐up in different subgroups of patients with granulomatosis with polyangiitis (GPA). We selected 10 patients with renal relapsing GPA, 10 patients with renal non‐relapsing GPA and 10 patients with non‐renal relapsing GPA. In all patients, an ANCA rise occurred during remission. The avidity was measured using a chaotropic approach at the time of an ANCA rise and at the time of a relapse in relapsing patients or time‐matched during remission in non‐relapsing patients. No difference was observed in the avidity at the ANCA rise between renal relapsing patients [26·2% (15·5–47·5)], renal patients without a relapse [39·6% (21·2–63·4)] and non‐renal relapsing patients [34·2% (21·6–59·5)]. In renal relapsing patients, the avidity increased significantly from the moment of the ANCA rise to the relapse [difference 6·4% (0·0–17·1), P = 0·0273]. The avidity did not increase after an ANCA rise in renal non‐relapsing patients [difference 3·5 (−6·0 to 10·1), P = 0·6250] or in non‐renal relapsing patients [difference −3·1% (−8·0 to 5·0), P = 0·5703]. The avidity of PR3‐ANCA increases after an ANCA rise during follow‐up in renal relapsing patients, but not after an ANCA rise in renal patients who remain in remission or in non‐renal relapsing patients.
Keywords: ANCA‐associated vasculitis, avidity, follow‐up, relapse, granulomatosis with polyangiitis, PR3‐ANCA, proteinase 3, relative avidity index
Introduction
Granulomatosis with polyangiitis (GPA; formerly Wegener's), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA; Churg–Strauss syndrome) are inflammatory disease entities affecting small to medium vessels. They are characterized by the presence of anti‐neutrophil cytoplasmic antibodies (ANCA) against myeloperoxidase (MPO) or proteinase‐3 (PR3), and are frequently grouped together under the term ANCA‐associated vasculitis (AAV) 1.
Since the 1980s it has been advocated that ANCA rises predict disease reactivation 2. However, the relation between ANCA rise and relapse of the disease is far from absolute, as many ANCA rises are not followed by a relapse and relapses may occur without a preceding ANCA rise 3, 4. Recently, we have demonstrated that longitudinal ANCA measurements are highly predictive for disease activity in patients with renal involvement, but not in patients with non‐renal disease 5. At present, it is clear that not every ANCA rise is pathogenic, as only a subset of patients who have an ANCA rise will experience disease reactivation within a period of 12 months. Our hypothesis is that the pathogenicity of an ANCA rise is determined by the quality of the autoantibodies, such as the avidity, glycosylation profile or epitope specificity.
The clinical relevance of the avidity of an autoantibody has been demonstrated in patients with anti‐phospholipid syndrome (APS), in which patients with high‐avidity anti‐β2‐glycoprotein I antibodies (anti‐β2‐GPI) have a higher risk of thrombosis compared to patients with low or heterogeneous avidity anti‐β2‐GPI 6. In systemic lupus erythematosus (SLE), high‐avidity anti‐dsDNA antibodies are associated more closely with renal involvement and/or disease activity than low‐ or intermediate‐avidity anti‐dsDNA antibodies 7, 8, 9, 10. Lastly, determination of the avidity of anti‐viral antibodies is useful to differentiate a primary infection from reactivation 11, 12, 13.
Several published studies have investigated the avidity of MPO‐ANCA 14, 15, 16, 17, 18, 19. The avidity from natural autoantibodies against MPO in healthy controls is lower compared to the avidity of MPO‐ANCA in patients with primary AAV 14. The avidity of MPO‐ANCA antibodies reduces during remission in patients with vasculitis induced by propylthiouracil (PTU), but remains constant in primary ANCA‐associated vasculitis 15, 16, 17. Patients with high‐avidity MPO‐ANCA generally suffer from severe vasculitis disease activity, while patients with low avidity suffer more often from mild vasculitis activity 18. To our knowledge, no studies have currently been published on the avidity of PR3‐ANCA.
The primary objective of this study is to evaluate whether the avidity of PR3‐ANCA changes during follow‐up in patients with GPA. The secondary objective is to determine whether the avidity of PR3‐ANCA differs in patients with renal involvement compared to patients without renal involvement during follow‐up.
Materials and methods
Patient inclusion
In our cohort of patients with GPA 5, we defined three different subgroups and selected 10 patients from each subgroup: 10 patients with renal involvement with a relapse during follow‐up (i.e. ‘renal relapsing’), 10 patients without renal involvement with a relapse during follow‐up (i.e. ‘non‐renal relapsing’) and 10 patients with renal involvement without a relapse during follow‐up (i.e. ‘renal non‐relapsing’).
All selected patients fulfilled the following criteria: (1) patients were diagnosed with biopsy‐proven GPA according to the European Medicines Agency (EMA) classification system 20, (2) patients were positive for PR3‐ANCA 21, (3) remission was induced after the initiation of immunosuppressive induction therapy 22, (4) an ANCA rise occurred during follow‐up 5 and (5) serum was available as described below. Serum was obtained for clinical purpose and patients did not object to the (anonymous) use of surplus serum for the purpose of research. Therefore the requirement of ethical approval was waived according to the Dutch law.
Classification of patients
Renal involvement was determined by a renal biopsy showing pauci‐immune necrotizing glomerulonephritis 23. Disease activity was scored using the Birmingham Vasculitis Activity Score (BVAS) version 3 24. Patients were treated according to the European League against Rheumatism (EULAR) guidelines, as described previously 22, 23. Remission was defined as absence of disease activity attributable to active disease during maintenance immunosuppressive therapy of a prednisone dosage of 7·5 mg or lower 25.
Follow‐up
Patients were screened routinely for potential symptoms of a relapse, and blood was drawn 5. Antigen‐specific solid‐phase ANCA tests were performed for the detection and quantification of PR3‐ANCA. An ANCA rise was defined as described previously 5. A relapse was defined as re‐occurrence or new onset of disease attributable to active disease combined with an increase or addition of immunosuppressive treatment 3, 5, 25, 26, 27.
Serum selection
In all patients, a serum sample (T1) was selected at the time of an ANCA rise 5. In renal relapsing and non‐renal relapsing patients, a second serum sample (T2rel) was selected prior to the start of the immunosuppressive induction therapy at the time of the relapse. In renal non‐relapsing patients, a second serum sample (T2rem) was selected after the ANCA rise, whereas the time since the ANCA rise was matched with the time between the ANCA rise and the relapse of the renal relapsing patients.
If available, we also included a diagnostic sample prior to the start of the immunosuppressive induction therapy at diagnosis.
Determination of the avidity of PR3‐ANCA
Sera were tested using the PR3‐hn‐hr enzyme‐linked immunosorbent assay (elisa) (Euroimmun AG, Luebeck, Germany) 28. Briefly, 100 µl of serum diluted 1 : 101 in sample buffer was added to each of three wells coated with PR3 antigen. After incubation for 30 min at room temperature (+18°C to +25°C) and washing, wells were exposed to 200 µl of either a 5 M urea solution, a 3 M urea solution or phosphate‐buffered saline (PBS) for 10 min. After washing three times, wells were incubated with 100 µl peroxidase‐labelled anti‐human IgG for 30 min at room temperature, followed again by three washing cycles. One hundred µl of a chromogenic substrate solution was added, and the reactions were stopped after 15 min by the addition of 100 µl stop solution per well. The reactions were read immediately at a wavelength of 450 nm using a reference wavelength of 650 nm. A highly positive index patient serum was used to generate a standard curve consisting of three calibrators (2, 20 and 200 relative units (RU)/ml). RU were calculated by this standard curve 28. A relative avidity index (RAI) was calculated for each specimen and was expressed as the percentage of reactivity remaining in the urea‐treated wells 13. The inter‐ and intra‐assay coefficients of variability of the PR3‐hn‐hr elisa were 3·4% and 2·1% when using PBS, 4·4% and 2·9% when using 3 M urea and 4·2% and 4·3% when using 5 M urea.
Statistics
Numerical variables were expressed as median [interquartile range (IQR)] and categorical variables as numbers (percentages). Continuous variables were correlated with the Spearman test. Three unpaired columns were compared with the Kruskal–Wallis H‐test and post‐hoc with the Mann–Whitney U‐test. Two paired columns were compared with the Wilcoxon matched‐pairs signed‐rank test. For the main research question regarding the change of avidity over time, we also applied a sensitivity analysis using a linear mixed model with time (1 = ANCA rise, 2 = relapse or time‐matched during remission), group (1 = renal relapsing, 2 = renal non‐relapsing and 3 = non‐renal relapsing) and time × group as fixed factor and an unstructured covariance structure for the repeated measurements.
All statistical analyses were performed using GraphPad Prism version 6·04 for Windows (GraphPad Software, La Jolla, CA, USA) and spss statistics for Windows, version 23·0 (IBM, Armonk, NY, USA). A P‐value < 0·05 was considered significant.
Results
Description of the cohort
Thirty patients were included: 10 renal relapsing patients, 10 renal non‐relapsing patients and 10 non‐renal relapsing patients (see Table 1).
Table 1.
Patient characteristics
| Renal relapse | Renal remission | Non‐renal relapse | |
|---|---|---|---|
| Characteristics at time of previous disease activity | |||
| Patients | 10 | 10 | 10 |
| Included at diagnosis | 7 | 9 | 9 |
| Age in years | 58 (52–72) | 50 (31–54) | 56 (49–63) |
| Women | 0 | 4 | 5 |
| BVAS | 17 (13–21) | 21 (16–24) | 7 (5–11) |
| C‐reactive protein | 68 (15–157) | 87 (16–120) | 121 (24–162) |
| Serum creatinine | 388 (127–489) | 114 (79–274) | 87 (78–102) |
| Organ involvement at previous disease activity | |||
| Arthralgia | 8 | 6 | 7 |
| Cutaneous | 3 | 2 | 1 |
| Eyes | 2 | 3 | 2 |
| Ear, nose, throat | 9 | 8 | 8 |
| Lung | 8 | 9 | 8 |
| Cardiovascular | 1 | 0 | 0 |
| Renal | 10 | 10 | 0 |
| Central nervous system | 1 | 0 | 0 |
| Peripheral nervous system | 1 | 2 | 0 |
| Induction treatment resulting in remission | |||
| Cyclophosphamide + GC | 7 | 7 | 5 |
| Rituximab + GC | 1 | 2 | 0 |
| Methothrexate + GC | 0 | 0 | 3 |
| Mofetil mycophenolate + GC | 0 | 1 | 0 |
| Gusperimus + GC | 2 | 0 | 1 |
| GC monotherapy | 0 | 0 | 1 |
| Characteristics during follow‐up | |||
| Time between previous disease activity and ANCA rise in months | 12·0 (8·5–24·5) | 19·6 (13·1–44·2) | 12·4 (8·6–14·4) |
| Time between ANCA rise and relapse/remission in months | 7·2 (1·9–9·9) | 7·4 (2·8–12·9) | 14·5 (5·6–17·5) |
| Persistently ANCA‐positive | 3 | 3 | 3 |
| Major relapse | 8 | 0 | 3 |
| Minor relapse | 2 | 0 | 7 |
Values are expressed as median (interquartile range, IQR) or as the total. GC = glucocorticoids; BVAS = Birmingham Vasculitis Activity Score; ANCA= anti‐neutrophil cytoplasmic antibodies.
Avidity as measured with different urea concentrations
The median PR3‐ANCA level as measured with PBS was 142 (82–247) RU/ml. One sample in a non‐renal relapsing patient at the time of a minor relapse tested negative for PR3‐ANCA, and this sample was excluded for the evaluation of avidity. The RAI as measured with 3 M and 5 M urea was 59·3% (45·8–73·3) and 35·6% (24·5–49·9), respectively. A strong correlation between the relative avidity index as measured with 3 M and the relative avidity index as measured with 5 M existed (R = 0·951, P < 0·001, see Fig. 1a). In addition, the PR3‐ANCA level as measured with PBS correlated with the relative avidity index as measured with 3 M and 5 M (R = 0.432, P < 0·001 and R = 0·296, P < 0·001, respectively, see Fig. 1b,c). The relative avidity index as measured with 5M urea was used in all further analyses.
Figure 1.
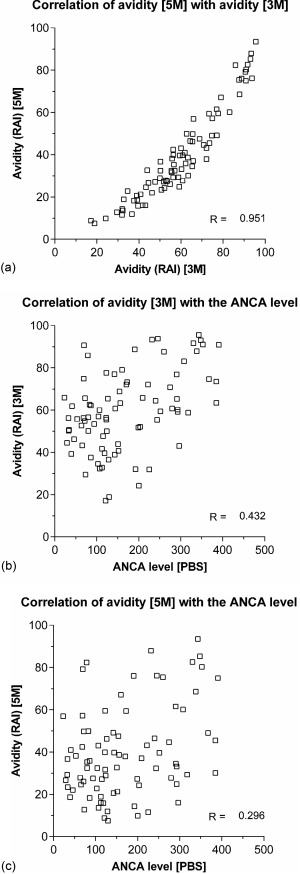
(a) The correlation of the relative avidity index as measured with a urea concentration of 5 M and 3 M. (b) The correlation of the anti‐neutrophil cytoplasmic antibodies (ANCA) level as measured in phosphate‐buffered saline (PBS) with the relative avidity index as measured with a urea concentration of 3 M. (c) The correlation of the ANCA level as measured in PBS with the relative avidity index as measured with a urea concentration of 5 M.
Avidity at the time of the ANCA rise
No difference was observed in the avidity of PR3‐ANCA if obtained at the time of an ANCA rise between renal relapsing patients [26·2% (15·5–47·5) Kruskal–Wallis P = 0·4756], renal patients without a relapse [39·6% (21·2–63·4] and non‐renal relapsing patients [34·2% (21·6–59·5); see Fig. 2].
Figure 2.
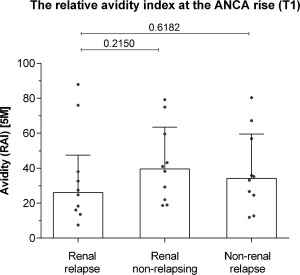
The relative avidity index in renal relapsing patients, renal non‐relapsing patients and non‐renal relapsing patients at the anti‐neutrophil cytoplasmic antibodies (ANCA) rise (T1). Post‐hoc P‐values from the Mann–Whitney U‐test are shown.
Changes in avidity during follow‐up
In renal relapsing patients, the avidity increased significantly from the moment of the ANCA rise to the relapse [difference 6·4% (0·0–17·1), P = 0·0273]. This was not the case in renal non‐relapsing patients, in whom the avidity was similar at the ANCA rise and time‐matched during remission [difference 3·5 (−6·0 to 10·1), P = 0·6250] or in non‐renal relapsing patients, in whom the avidity was similar at the ANCA rise and at the relapse [difference −3·1% (−8·0 to 5·0), P = 0·5703; see Fig. 3]. The sensitivity analysis using a linear mixed model gave similar results as the Wilcoxon matched‐pairs signed‐rank test (P = 0·012, P = 0·662 and P = 0·453 for renal relapsing, renal non‐relapsing and non‐renal relapsing patients). Concomitantly, in renal relapsing patients the ANCA level increased significantly from the moment of the ANCA rise [140 (102–201)] to the relapse [222 (123–340), P = 0·0488], while ANCA levels did not increase in renal non‐relapsing patients and in non‐renal relapsing patients (data not shown).
Figure 3.
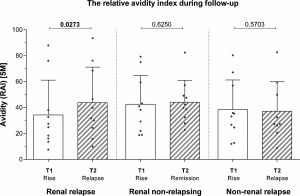
The relative avidity index during follow‐up in renal relapsing patients in renal patients who remain in remission and in non‐renal relapsing patients. P‐values of the Wilcoxon matched‐pairs signed‐rank test are shown.
No significant differences were observed at the relapse or time‐matched in remission in the avidity of PR3‐ANCA between renal relapsing patients [35·8% (22·2–70·5), Kruskal–Wallis P = 0·5912], renal patients without a relapse [41·1% (31·3–51·2), post‐hoc P = 0·6706 versus renal relapsing patients] and/or non‐renal relapsing patients [27·2% (23·3–54·6), post‐hoc P = 0·6447 versus renal relapsing patients].
Avidity at the time of diagnosis
Finally, we examined the avidity at the time of diagnosis to investigate whether there are differences in avidity in the early stages of disease process. A diagnostic sample was available in seven renal relapsing patients, nine renal non‐relapsing patients and seven non‐renal relapsing patients.
No significant differences were observed in the avidity at the diagnosis between renal relapsing patients [49·1% (29·3–60·2), Kruskal–Wallis P = 0·1653], renal patients without a relapse [34·5% (15·2–43·9), post‐hoc P = 0·0712 versus renal relapsing patients] and non‐renal relapsing patients [37·1% (28·9–61·6), post‐hoc P = 0·6894 versus renal relapsing patients].
Discussion
In this study, we report the results of avidity measurement of PR3‐ANCA in patients with GPA. Most importantly, the avidity increases in patients with renal involvement who relapse during follow‐up, while it remains constant in renal patients who do not relapse and in non‐renal patients who relapse during follow‐up. We did not find a difference in the avidity between patients with renal involvement compared to patients with non‐renal disease at the time of the ANCA rise and/or the time of diagnosis.
The affinity of an antibody is defined as the binding energy of a monovalent antibody with a single epitope of the target antigen, while the avidity of an antibody represents the binding energy of the antibody with all available epitopes 29. The avidity of an antibody can be determined using two distinct approaches. The chaotropic method is based on the chemical dissociation of the antigen‐antibody complex by a chaotropic agent, such as urea 12, 13, 30. Another approach is based on competitive inhibition of the binding site of the antibody 14, 18, 19, 31, 32. The results of the distinct approaches are similar, although assays employing a chaotropic agent may detect antibodies more often with inappropriately low avidity 33. The applied method to determine the avidity in this study has been validated extensively with large panels of characterized samples in the field of infectious serology 12, 13.
Interestingly, the avidity increases in patients with renal involvement who relapse during follow‐up, while it remains constant in renal patients who do not relapse and non‐renal patients who relapse during follow‐up. Similar results were found with an additional sensitivity analysis, thereby strengthening the observation. These changes in the avidity of PR3‐ANCA may be linked to the fluctuating presence of the antigen source, as has been suggested in patients with MPO‐ANCA 15, 19, 34. In addition, the immune system in these patients may be skewed to a proinflammatory state, e.g. due to a chronic upper respiratory infection, which may contribute towards avidity maturation.
This study was designed as a pilot study to gain insight into the avidity of PR3‐ANCA during follow‐up in patients with renal involvement and patients with non‐renal disease. Importantly, we did not find a difference in the avidity level at the ANCA rise of patients with renal involvement compared to patients with non‐renal disease. Therefore, we conclude that the avidity of PR3‐ANCA does not explain why ANCA rises are associated highly with a relapse in patients with renal involvement but not in patients with non‐renal disease 5.
The avidity index may be correlated with the ANCA level due to several reasons. First, low ANCA levels and low avidity may represent natural autoantibodies, while high‐avidity PR3‐ANCA production may result in high ANCA levels that are pathogenic 14. Alternatively, the observed correlation between the avidity index and the ANCA level may be related to the method of avidity testing. Avidity assays have been shown to be less sensitive to changes in avidity in samples with a high concentration of antibodies 29. Moreover, using a different assay but utilizing a similar test principle, Dangel et al. have shown that the avidity index varied between various dilutions of the same sample 35.
Our study suffers from several limitations. First, we tested only a small group of highly selected patients. The strength of our study is that patients are well characterized and the avidity of PR3‐ANCA was determined at several moments during follow‐up that are clinically relevant 5. Moreover, the avidity was determined using a method that has been validated extensively 12, 13.
In conclusion, the avidity of PR3‐ANCA increases during follow‐up in renal relapsing patients but not in renal patients who remain in remission or in non‐renal relapsing patients. Whether the avidity of PR3‐ANCA is associated with more severe vasculitic disease activity at diagnosis should be studied further, as has been demonstrated for MPO‐ANCA.
Acknowledgements
The elisa assays were provided by Euroimmun. W. S. is a board member and stakeholder of Euroimmun AG; C. D. is an employee of Euroimmun AG; and M. J. K. has received the Kolff Student Researcher grant by the Nierstichting. We would like to thank Dr B. Winkens (Maastricht University) for his consultation regarding the statistical analysis. The other authors declare that they have no financial or other conflicts of interest.
References
- 1. Wilde B, van Paassen P, Witzke O et al New pathophysiological insights and treatment of ANCA‐associated vasculitis. Kidney Int 2011; 79:599–612. [DOI] [PubMed] [Google Scholar]
- 2. Cohen Tervaert JW, van der Woude F, Fauci A et al Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Intern Med 1989; 159:2461–5. [DOI] [PubMed] [Google Scholar]
- 3. Boomsma MM, Stegeman CA, Van Der Leij MJ et al Prediction of relapses in Wegener's granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: a prospective study. Arthritis Rheum 2000; 43:2025–33. [DOI] [PubMed] [Google Scholar]
- 4. Tomasson G, Grayson PC, Mahr AD et al Value of ANCA measurements during remission to predict a relapse of ANCA‐associated vasculitis – a meta‐analysis. Rheumatology 2012; 51:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kemna MJ, Damoiseaux JGMC, Austen J et al ANCA as a predictor of relapse: useful in patients with renal involvement but not in patients with nonrenal disease. J Am Soc Nephrol 2015; 26:537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Čučnik S, Kveder T, Artenjak A et al Avidity of anti‐β2‐glycoprotein I antibodies in patients with antiphospholipid syndrome. Lupus 2012; 21:764–5. [DOI] [PubMed] [Google Scholar]
- 7. Winfield JB, Faiferman I, Koffler D. Avidity of anti‐DNA antibodies in serum and IgG glomerular eluates from patients with systemic lupus erythematosus. Association of high avidity antinative DNA antibody with glomerulonephritis. J Clin Invest 1977; 59:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ter Borg EJ, Horst G, Hummel EJ et al Measurement of increases in anti‐double‐stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long‐term, prospective study. Arthritis Rheum 1990; 33:634–43. [DOI] [PubMed] [Google Scholar]
- 9. Villalta D, Romelli PB, Savina C et al Anti‐dsDNA antibody avidity determination by a simple reliable ELISA method for SLE diagnosis and monitoring. Lupus 2003; 12:31–6. [DOI] [PubMed] [Google Scholar]
- 10. Suh‐Lailam BB, Chiaro TR, Davis KW et al Evaluation of a high avidity anti‐dsDNA IgG enzyme‐linked immunosorbent assay for the diagnosis of systemic lupus erythematosus. Int J Clin Exp Pathol 2011; 4:748–54. [PMC free article] [PubMed] [Google Scholar]
- 11. Prince HE, Lape‐Nixon M. Role of cytomegalovirus (CMV) IgG avidity testing in diagnosing primary CMV infection during pregnancy. Clin Vaccine Immunol 2014; 21:1377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guisasola ME, Ramos B, Sanz JC et al Comparison of IgG avidity assays in the confirmation of the diagnosis of cytomegalovirus primary infection. APMIS 2010; 118:991–3. [DOI] [PubMed] [Google Scholar]
- 13. Mubareka S, Richards H, Gray M et al Evaluation of commercial rubella immunoglobulin G avidity assays. J Clin Microbiol 2007; 45:231–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu P‐C, Cui Z, Chen M et al Comparison of characteristics of natural autoantibodies against myeloperoxidase and anti‐myeloperoxidase autoantibodies from patients with microscopic polyangiitis. Rheumatology 2011; 50:1236–43. [DOI] [PubMed] [Google Scholar]
- 15. Gao Y, Chen M, Ye H et al Follow‐up of avidity and titre of anti‐myeloperoxidase antibodies in sera from patients with propylthiouracil‐induced vasculitis. Clinical Endocrinol 2007; 66:543–7. [DOI] [PubMed] [Google Scholar]
- 16. Lin W, Chen M, Zhao M‐H. Follow‐up of avidity and titer of anti‐myeloperoxidase antibodies in sera from patients with primary ANCA‐associated vasculitis. Autoimmunity 2009; 42:198–202. [DOI] [PubMed] [Google Scholar]
- 17. Liu L‐J, Chen MIN, Yu F et al IgG subclass distribution, affinity of anti‐myeloperoxidase antibodies in sera from patients with Wegener's granulomatosis and microscopic polyangiitis. Nephrology 2008; 13:629–35. [DOI] [PubMed] [Google Scholar]
- 18. Yoshida M, Sasaki M, Nakabayashi I et al Two types of myeloperoxidase‐antineutrophil cytoplasmic autoantibodies with a high affinity and a low affinity in small vessel vasculitis. Clin Exp Rheumatol 2009; 27:S28–32. [PubMed] [Google Scholar]
- 19. Kokolina E, Noel L‐H, Nusbaum P et al Isotype and affinity of anti‐myeloperoxidase autoantibodies in systemic vasculitis. Kidney Int 1994; 46:177–84. [DOI] [PubMed] [Google Scholar]
- 20. Watts R, Lane S, Hanslik T et al Development and validation of a consensus methodology for the classification of the ANCA‐associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007; 66:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Damoiseaux JGMC, Slot MC, Vaessen M et al Evaluation of a new fluorescent‐enzyme immuno‐assay for diagnosis and follow‐up of ANCA‐associated vasculitis. J Clin Immunol 2005; 25:202–8. [DOI] [PubMed] [Google Scholar]
- 22. Mukhtyar C, Guillevin L, Cid MC et al EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis 2009; 68:310–7. [DOI] [PubMed] [Google Scholar]
- 23. Hilhorst M, Wilde B, van Breda Vriesman P et al Estimating renal survival using the ANCA‐associated GN classification. J Am Soc Nephrol 2013; 24:1371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mukhtyar C, Lee R, Brown D et al Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 2009; 68:1827–32. [DOI] [PubMed] [Google Scholar]
- 25. Hellmich B, Flossmann O, Gross WL et al EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti‐neutrophil cytoplasm antibody‐associated vasculitis. Ann Rheum Dis 2007; 66:605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Slot MC, Cohen Tervaert JW, Boomsma MM et al Positive classic antineutrophil cytoplasmic antibody (C‐ANCA) titer at switch to azathioprine therapy associated with relapse in proteinase 3‐related vasculitis. Arthritis Rheum 2004; 51:269–73. [DOI] [PubMed] [Google Scholar]
- 27. Cohen Tervaert JW, Huitema MG, Sluiter WJ et al Prevention of relapses in Wegener's granulomatosis by treatment based on antineutrophil cytoplasmic antibody titre. Lancet 1990; 336:709–11. [DOI] [PubMed] [Google Scholar]
- 28. Damoiseaux JGMC, Dähnrich C, Rosemann A et al A novel enzyme‐linked immunosorbent assay using a mixture of human native and recombinant proteinase‐3 significantly improves the diagnostic potential for antineutrophil cytoplasmic antibody‐associated vasculitis. Ann Rheum Dis 2009; 68:228–33. [DOI] [PubMed] [Google Scholar]
- 29. Dimitrov JD, Lacroix‐Desmazes S, Kaveri SV. Important parameters for evaluation of antibody avidity by immunosorbent assay. Anal Biochem 2011; 418:149–51. [DOI] [PubMed] [Google Scholar]
- 30. Pullen GR, Fitzgerald MG, Hosking CS. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods 1986; 86:83–7. [DOI] [PubMed] [Google Scholar]
- 31. Friguet B, Chaffotte AF, Djavadi‐Ohaniance L et al Measurements of the true affinity constant in solution of antigen‐antibody complexes by enzyme‐linked immunosorbent assay. J Immunol Methods 1985; 77:305–19. [DOI] [PubMed] [Google Scholar]
- 32. Xu P‐C, Chen M, Cui Z et al Influence of myeloperoxidase by anti‐myeloperoxidase antibodies and its association with the disease activity in microscopic polyangiitis. Rheumatology 2010; 49:2068–75. [DOI] [PubMed] [Google Scholar]
- 33. Berth M, Grangeot‐Keros L, Heskia F et al Analytical issues possibly affecting the performance of commercial human cytomegalovirus IgG avidity assays. Eur J Clin Microbiol Infect Dis 2014; 33:1579–84. [DOI] [PubMed] [Google Scholar]
- 34. Dolman KM, Stegeman CA, van de Wiel BA et al Relevance of classic anti‐neutrophil cytoplasmic autoantibody (C‐ANCA)‐mediated inhibition of proteinase 3‐alpha 1‐antitrypsin complexation to disease activity in Wegener's granulomatosis. Clin Exp Immunol 1993; 93:405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dangel V, Bader U, Enders G. Improvement of cytomegalovirus avidity testing by adjusting the concentration of CMV‐specific IgG in test samples. J Clin Virol 2006; 35:303–9. [DOI] [PubMed] [Google Scholar]


