Summary
During the past 10 years, pneumococcal conjugate vaccine (PCV) has become part of the standard childhood vaccination programme. This may impact upon the diagnosis of polysaccharide antibody deficiency by measurement of anti‐polysaccharide immunoglobulin (Ig)G after immunization with unconjugated pneumococcal polysaccharide vaccine (PPV). Indeed, contrary to PPV, PCV induces a T‐dependent, more pronounced memory response. The antibody response to PPV was studied retrospectively in patients referred for suspected humoral immunodeficiency. The study population was divided into four subgroups based on age (2–5 years versus ≥ 10 years) and time tested (1998–2005 versus 2010–12). Only 2–5‐year‐old children tested in 2010–12 had been vaccinated with PCV prior to PPV. The PCV primed group showed higher antibody responses for PCV–PPV shared serotypes 4 and 18C than the unprimed groups. To a lesser extent, this was also found for non‐PCV serotype 9N, but not for non‐PCV serotypes 19A and 8. Furthermore, PCV‐priming elicited a higher IgG2 response. In conclusion, previous PCV vaccination affects antibody response to PPV for shared serotypes, but can also influence antibody response to some non‐PCV serotypes (9N). With increasing number of serotypes included in PCV, the diagnostic assessment for polysaccharide antibody deficiency requires careful selection of serotypes that are not influenced by prior PCV (e.g. serotype 8). Further research is needed to identify more serotypes that are not influenced.
Keywords: pneumococcal conjugate vaccine, polysaccharide antibody deficiency, specific antibody deficiency, unconjugated pneumococcal polysaccharide vaccine
Introduction
Patients with a specific polysaccharide antibody deficiency (SAD) suffer from recurrent respiratory tract infections (sinusitis, otitis, bronchitis or pneumonia) with encapsulated bacteria (e.g. Streptococcus pneumoniae). They have normal serum immunoglobulin levels and mount normal antibody responses to protein antigens (e.g. tetanus toxoid), but have a deficient antibody response to capsular polysaccharides 1, 2. Antibody deficiency to pneumococcal polysaccharides can also be found as a trait of broader primary and secondary immunodeficiencies, such as common variable immunodeficiency, Wiskott–Aldrich syndrome and acquired immunodeficiency syndrome or post‐splenectomy 3.
Measurement of the antibody response to pneumococcal polysaccharide vaccine (PPV) Pneumovax 23®, a 23‐valent pneumococcal polysaccharide vaccine, is the current method to identify a deficiency in anti‐polysaccharide antibody production. Anti‐pneumococcal polysaccharide antibodies are assessed before and 2–4 weeks after immunization with PPV 4, 5, 6, 7, 8. The interpretation of the antibody response to PPV is complex and integrates several criteria: (i) post‐vaccination antibody concentration, (ii) fold‐increase in antibody concentration and (iii) percentage of serotypes to which there is a good response. An adequate response to PPV is currently considered as a post‐vaccination serotype‐specific antibody concentration > 1·3 mg/l and a twofold or higher increase in antibody level over the prevaccination concentration for more than 70% of serotypes tested (50% for children aged 2–5 years) 4, 7.
Pneumococcal conjugate vaccine (PCV) (Prevnar7®, Prevnar13®, Synflorix®) has become part of the standard vaccination programe for children. Responses to PCV (protein‐coupled polysaccharides) are T cell‐dependent, whereas responses to PPV (pure polysaccharides) are T cell‐independent. To establish the diagnosis of specific polysaccharide antibody deficiency, it is important to evaluate the antibody response to T cell‐independent antigens. Serotype‐specific immunoglobulin (Ig)G to PPV or after exposure to S. pneumoniae are almost exclusively of the IgG2 subclass, whereas IgG responses after PCV are of the IgG1 subclass 9, 10. PCV is more immunogenic than PPV, and induces long‐term protection by inducing a memory IgG response 11, 12. An influence of previous vaccination with PCV on the magnitude and nature of the antibody response to PPV can be suspected. In 33 children with SAD, previous PCV vaccination led to a higher and faster antibody response to PPV–PCV shared serotypes 4, 6B, 9V, 14, 18C, 19F and 23F, but not to serotype 5, not contained in PCV 12. In contrast, in HIV‐infected adults polysaccharide responsiveness was not biased by prior PCV vaccination 13. The extent to which previous vaccination with a conjugated vaccine affects serotype‐specific responses to PPV remains uncertain 14.
The aim of this study was to evaluate the effect of previous PCV vaccination on serotype‐specific antibody response to subsequent vaccination with PPV. The findings will be important to adapt practice guidelines for SAD diagnosis in patients who have been vaccinated with PCV prior to diagnostic PPV vaccination. The antibody response to PPV was studied retrospectively in a large cohort of patients who underwent diagnostic pneumococcal antibody testing in the context of suspected immunodeficiency. A group aged 2–5 years, tested between 2010 and 2012 and vaccinated with PCV‐7 as part of the standard vaccination programme, was compared to a group with the same age, tested between 1998 and 2005, and thus not vaccinated with PCV‐7. Patients of 10 years and older, tested for PPV antibody response during these same time‐periods, but not vaccinated with PCV‐7, served as control populations to exclude confounding factors.
Patients and methods
Patients
All consecutive patients, aged 2–5 years or 10 years and older, tested for antibody response to PPV at the University Hospitals Leuven clinical laboratory from January 1998 to December 2005 and from January 2010 to December 2012, were identified retrospectively. All patients in whom paired pre‐ and post‐vaccination‐specific IgG for serotypes 3, 4 and 9N had been determined were included into the study, independent of clinical status or immunocompetence. The study population was divided into four groups, based on age (2–5 versus ≥ 10 years) and time of pneumococcal antibody response testing (1998–2005 versus 2010–12) (Table 1). The 7‐valent PCV (Prevenar®) vaccination was introduced in Belgium in 2004. As a consequence, children aged 2–5 years and tested in 2010–12 (n = 240) had received PCV‐7 before anti‐PPV antibody response testing. Vaccination with PCV‐7 was performed at 2, 4 and 12 months of age at governmentally organized health‐care visits. For infants aged 18–24 months, vaccination coverage of PCV‐7 was 89·1% and 96·5% in 2008 and 2012, respectively [http://www.zorg-en-gezondheid.be/vaccinatiegraad/].
Table 1.
Median post‐vaccination immunoglobulin (Ig)G and median fold increase of antibody level post‐ over prevaccination for serotypes 3, 4 and 9N with level of statistical significance for the difference between groups [Kruskal–Wallis one‐way analysis of variance (anova) and Dunn's post‐hoc test].
| 2–5 years | ≥ 10 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1998–2005 (n = 159) | 2010–12 (n = 240) | 1998–2005 (n = 112) | 2010–12 (n = 58) | 1998–2005 versus 2010–12 | 2–5 versus ≥ 10 years | |||||
| Median age (Q1–Q3) | Serotype | PCV‐7 | 3 years (3–4) | 3 years (3–4) | 31 years (15–47) | 30 years (16–47) | 2–5 years | ≥ 10 years | 1998–2005 | 2010–12 |
| Median IgG post‐vaccination (Q1–Q3) (mg/l) | 3 | – | 2·7 (0·9–5·0) | 4·2 (2·4–7·7) | 2·1 (0·9–4·3) | 2·4 (1·2–4·3) | *** | n.s. | n.s. | *** |
| 9N | – | 3·8 (1·6–7·1) | 9·6 (4·7–19·2) | 5·4 (2·4–9·7) | 3·6 (1·8–11·6) | *** | n.s. | n.s. | *** | |
| 4 | + | 3·3 (1·5–4·8) | 14·6 (7·9–21·0) | 4·0 (1·7–5·6) | 1·7 (1·1–5·5) | *** | n.s. | n.s. | *** | |
| Median fold increase IgG (Q1–Q3) | 3 | – | 8·9 (1·5–34·5) | 5·0 (1·7–11·5) | 2·2 (1·3–4·7) | 2·1 (1·4–2·9) | * | n.s. | *** | *** |
| 9N | – | 7·8 (2·9–21) | 16·7 (6·9–34·2) | 2·1 (1·5–5·3) | 6·0 (1·7–14·8) | *** | * | *** | *** | |
| 4 | + | 10·6 (5·0–21·8) | 18·5 (10·2–30·1) | 2·5 (1·4–6·5) | 3·4 (1·5–8·2) | *** | n.s. | *** | *** | |
Only 2–5‐year‐old children tested in 2010–12 were vaccinated with PCV‐7 (grey column). PCV = pneumococcal conjugate vaccine; n = the number of included subjects; Q1 and Q3 = first and third quartiles; n.s. = not significant. *P‐value between 0·01 and 0·05; **P‐value between 0·001 and 0·01; *** P‐value < 0·001.
Because PCV was not marketed in Belgium before 2004, children aged 2–5 years tested for anti‐PPV antibody response in 1998–2005 (n = 159) were not vaccinated with PCV‐7 and none of the patients aged ≥ 10 years received PCV‐7 [neither when tested in 1998–2005 (n = 112) nor when tested in 2010–12 (n = 58)].
Ethical approval was granted by the local research ethics committee (University Hospitals Leuven).
Pneumococcal antibody response
IgG antibodies specific to pneumococcal polysaccharide serotypes were measured by the World Health Organization (WHO)‐recommended third‐generation enzyme‐linked immunosorbent assay (ELISA), incorporating absorption of samples with cell wall polysaccharides (CPS) and capsular polysaccharide 22F 5. Pooled sera with high antibody concentrations were used as a secondary standard and quality control. Serum lot 89‐SF [Food and Drug Administration (FDA), Silver Spring, MD, USA] was used as the standard and to assign antibody concentrations to the secondary standard. The same ELISA protocol was used during both included time‐periods and for the additional testing on historical samples. For analysis of IgM and IgG2 levels, horseradish peroxidase‐conjugated monoclonal antibodies to human IgM (Nordic Immunology Laboratories, Tilburg, the Netherlands) and horseradish peroxidase‐conjugated monoclonal antibodies to human IgG2 (Invitrogen, Carlsbad, CA, USA) were used, respectively. IgG2 levels are expressed in arbitrary units (U/l) compared to a secondary standard, as no quantifications of serotype‐specific IgG2 have been assigned to standard serum 89‐SF. When antibody concentration was above the upper limit of detection of the ELISA, the upper limit of detection was taken as antibody level for further calculations. When antibody level was below the lower detection limit of the ELISA, half the lower detection limit was used as absolute value for further calculations.
Additional testing on stored samples
To assess the effect of previous PCV‐7 vaccination on (i) specific IgG response to three additional pneumococcal serotypes (8, 18C and 19A) and (ii) specific IgG2 and IgM response to pneumococcal polysaccharide serotype 4 (contained in PCV‐7), the WHO‐recommended ELISA was performed on stored (−20°C) historical samples from patients included in the study. Eighteen subjects from each group were selected randomly (Table 2). Patients older than 18 years were excluded before random selection to limit heterogeneity in the smaller groups. A correction of the randomized groups was applied in order to ensure that the number of patients with SAD was comparable within each age group and in accordance with the prevalence of SAD in a similar patient population 3. The diagnosis of SAD was based on the results of the three historically tested serotypes, using the definition of SAD as described above.
Table 2.
Median post‐vaccination antibody level and median fold increase of antibody level post‐ over prevaccination for serotypes 8, 19A, 18C and 4 [immunoglobulin (Ig)M and IgG2] with level of statistical significance for the difference between groups [Kruskal–Wallis one‐way one‐way analysis of variance (anova) and Dunn's post‐hoc test].
| 2–5 years | 10–18 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1998–2005 (n = 18) | 2010–2012 (n = 18) | 1998–2005 (n = 18) | 2010–12 (n = 18) | 1998–2005 versus 2010–12 | 2–5 versus 10–18 years | |||||
| Median age (Q1–Q3) | Serotype | PCV–7 | 3·5 years (2–4) | 3 years (2–4) | 13 years (12–16) | 16 years (14–17) | 2–5 years | 10–18 years | 1998–2005 | 2010–12 |
| Median antibody level post‐vaccination (Q1–Q3) (mg/l) | 8 | – | 8·4 (4·3–12·7) | 6·9 (3·7–13·1) | 3·5 (1·4–6·7) | 9·5 (3·6–13·2) | n.s. | n.s. | n.s. | n.s. |
| 19A | – | 1·6 (0·9–2·3) | 1·6 (0·7–4·9) | 2·9 (1·6–5·6) | 3·3 (1·0–7·9) | n.s. | n.s. | n.s. | n.s. | |
| 18C | + | 1·6 (1·3–3·9) | 15·5 (5·9–30·6) | 1·8 (0·9–5·5) | 5·0 (1·7–18·6) | *** | n.s. | n.s. | n.s. | |
| 4 IgM | + | 1·1 (0·6–1·6) | 1·4 (1·2–2·2) | 1·5 (0·8–1·9) | 1·5 (0·6–2·7) | n.s. | n.s. | n.s. | n.s. | |
| 4 IgG2 | + | 28 (12–63) † | 718 (146–1003) † | 41 (13–523) † | 96 (19–391) † | *** | n.s. | n.s. | n.s. | |
| Median fold increase antibody level (Q1–Q3) | 8 | – | 12·1 (5·6–19·5) | 16·0 (9·2–34·2) | 6·8 (1·0–11·8) | 10·4 (2·0–25·5) | n.s. | n.s. | n.s. | n.s. |
| 19A | – | 1·0 (0·8–1·7) | 1·2 (1·0–1·6) | 2·1 (1·3–4·3) | 1·4 (1·2–3·5) | n.s. | n.s. | ** | n.s. | |
| 18C | + | 3·2 (1·0–7·5) | 15·8 (9·0–29·4) | 3·2 (1·2–5·9) | 5·5 (2·5–11·5) | *** | n.s. | n.s. | * | |
| 4 IgM | + | 3·4 (2·2–4·5) | 4·6 (3·7–5·7) | 3·2 (1·8–6·7) | 3·8 (1·7–7·1) | n.s. | n.s. | n.s. | n.s. | |
| 4 IgG2 | + | 2·7 (1·0–7·2) | 24·2 (10·6–54·9) | 2·5 (1·1–17·6) | 4·0 (1·5–17·4) | *** | n.s. | n.s. | * | |
Only 2–5‐year‐old children tested in 2010–12 were vaccinated with PCV‐7 (grey column). PCV = pneumococcal conjugate vaccine; n = the number of included subjects; Q1 and Q3 = first and third quartiles; n.s. = not significant; *P‐value between 0·01 and 0·05; **P‐value between 0·001 and 0·01; ***P‐value < 0·001; †arbitrary units/litre.
Reproducibility of the ELISA for quantification of serotype‐specific antibodies
A pooled control sample was included in each run. The calculated coefficients of variance (CV) for serotypes 3, 4 and 9N were 12·8, 11·4 and 7·1% at concentrations of 1·4, 1·6 and 2·1 mg/l, respectively, during the time‐period 2010–2012 (n = 137 runs per serotype). For the time‐period 1998–2005, CV calculations were not available. For serotypes 8, 18C and 19A, CVs were 5·1, 3·6 and 11·4% at concentrations of 6·1, 3·9 and 4·3 mg/l, respectively (n = 26 runs). For serotype 4‐specific IgM and IgG2, CVs were 7·4 and 6·1% at a concentration of 0·8 mg/l and 89·8 units/litre (n = 26 runs), respectively.
Statistical analysis
Data were analysed using Graphpad Prism software version 5·02 for Windows. Because most data were not distributed normally, non‐parametric tests were used. Differences between groups were compared by Kruskal–Wallis one‐way analysis of variance (anova) with Dunn's post‐hoc multiple comparison test. The threshold of significance was set at a P‐value < 0·05.
Results
Retrospective analysis of pneumococcal antibody response to serotypes 3, 4 and 9N
Results are shown in Fig. 1 and Table 1.
Figure 1.
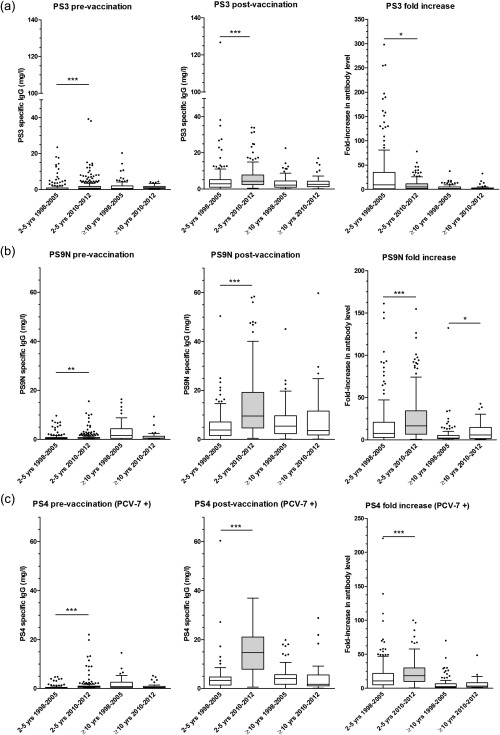
Box‐plot showing pre‐ [left] and post‐vaccination [middle] pneumococcal antibody concentrations as well as fold‐increase of antibody level (post‐ over prevaccination) (right) for four groups based on age and time of pneumococcal antibody testing. Only 2–5‐year‐old children tested in 2010–12 were vaccinated with PCV‐7 (grey box‐plot). The results are shown for serotype 3 (a) (not included in PCV‐7), serotype 9N (b) (not included in PCV‐7) and serotype 4 (c) (included in PCV‐7). *P‐value between 0·01 and 0·05; **P‐value between 0·001 and 0·01; ***P‐value < 0·001. Median (horizontal line) and quartiles (box) are shown.
For serotype 3 (present in PPV, not in PCV‐7), the pre‐ and post‐vaccination antibody levels in the age group ≥ 10 years were similar in both time‐periods. In the 2–5‐year age group, the pre‐ and post‐vaccination antibody levels were significantly higher in the 2010–12 cohort compared to the 1998–2005 cohort. Conversely, however, fold‐increase was significantly lower in the 2010–12 cohort.
Serotype 9N is also contained in PPV but not in PCV‐7. In 2–5‐year‐old children, significantly higher post‐vaccination antibody levels and fold‐increase were found in the PCV‐7‐primed group (2010–12 cohort) compared to the unprimed group (1998–2005 cohort). However, fold‐increase was also slightly higher in patients aged ≥ 10 years tested in 2010–12 than in patients aged ≥ 10 years tested in 1998–2005, although they had not been primed with PCV‐7.
For serotype 4 (present in PPV and in PCV‐7), post‐vaccination antibody levels were markedly and significantly higher in children aged 2–5 years who had been primed with PCV‐7 (cohort 2010–12) than in unprimed children aged 2–5 years (cohort 1998–2005). Also in 2–5‐year‐old children, antibody levels increased almost twice as much in the cohort tested between 2010 and 2012 than in the cohort tested between 1998 and 2005. There were no significant differences between the two time‐periods in post‐vaccination antibody level and fold‐increase in patients aged ≥ 10 years.
To summarize, a strong increase in antibody response to capsular serotype 4 (included in PCV‐7) was seen in children who had been primed with PCV‐7 before boost with PPV. A more subtle increase in antibody response to serotype 9N, although not included in PCV‐7, was also observed. For serotype 3, also not included in PCV‐7, the post‐vaccination antibody level was higher in the PCV‐7 primed group than in the unprimed group. However, the fold‐increase of antibody level upon vaccination was lower in the primed group than in the unprimed group. To determine further whether the enhancement of antibody response was related to previous vaccination with PCV‐7, antibody responses to three additional serotypes were tested using stored sera of a small subpopulation, selected in a semi‐randomized manner.
Additional testing of pneumococcal antibody response to serotypes 8, 18C and 19A
Results are shown in Fig. 2 and Table 2.
Figure 2.
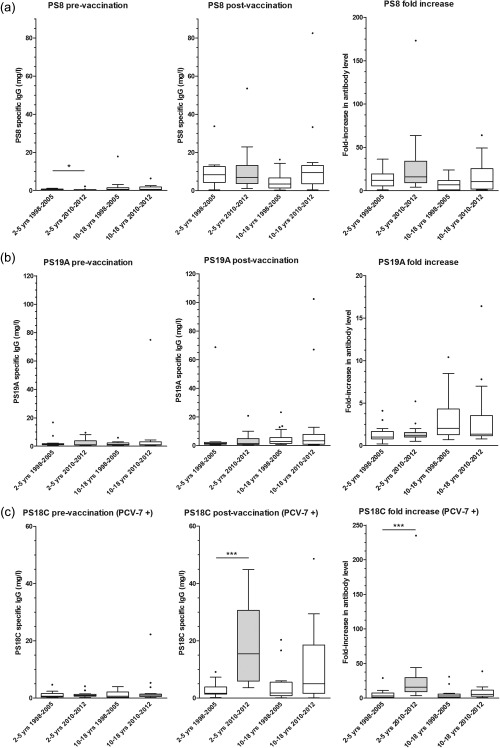
Box‐plot showing pre‐ and post‐vaccination serotype‐specific immunoglobulin (Ig)G levels and fold‐increase for non‐PCV‐7 serotype 8 (a), non‐PCV‐7 serotype 19A (b) and PCV‐7 serotype 18C (c). Only 2–5‐year‐old children tested in 2010–12 were vaccinated with PCV‐7 (grey box‐plot). *P‐value between 0·01 and 0·05; **P‐value between 0·001 and 0·01; ***P‐value < 0·001. Median (horizontal line) and quartiles (box) are shown.
Capsular polysaccharides serotypes 8 and 19A are contained in PPV but not in PCV‐7. For these two serotypes, no significant differences in IgG post‐vaccination antibody level or fold‐increase were found between primed children (aged 2–5 years tested in 2010–12) and unprimed children (aged 2–5 years tested in 1998–2005). Also, no differences were found between the two time‐periods for the antibody responses in children aged 10–18 years. For capsular polysaccharide serotype 18C (contained in PVC‐7), specific IgG post‐PPV vaccination was significantly higher in the PCV‐7‐primed 2–5‐year‐old children compared to the non‐primed 2–5‐year‐old children, similar to serotype 4 responses. No significant difference was found between the two time periods for non‐PCV‐7‐primed 10–18‐year‐old children.
IgM and IgG2 response
To study the effect of previous PCV‐7 vaccination on the IgM and IgG2 subclass antibody response induced by PPV, specific IgM and IgG2 to serotype 4 (present in PCV‐7 and in PPV) were measured in the same subgroup of semi‐randomly selected patients as described above. Results are shown in Fig. 3 and Table 2. No significant differences in post‐vaccination serotype 4‐specific IgM levels were found between the different groups. Polysaccharide 4‐specific IgG2 post‐vaccination antibody levels and fold‐increase of antibody level were significantly higher in PCV‐7‐primed than in non‐primed 2–5‐year‐old children. This difference was not found in 10–18‐year‐old children. This demonstrates that the PPV‐induced increase in serotype 4‐specific IgG after previous PCV‐7 vaccination can be explained at least partially by an increased IgG2 response.
Figure 3.
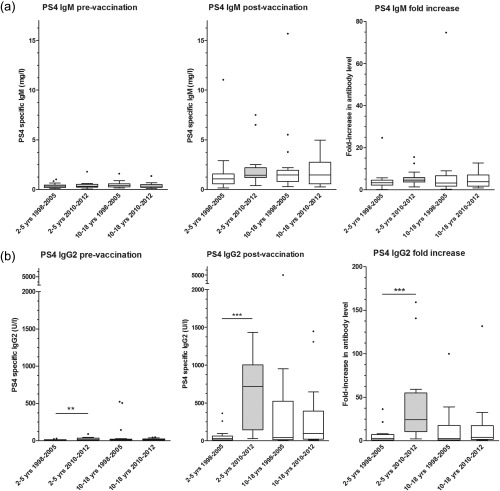
Box‐plot showing pre‐ and post‐vaccination serotype 4‐specific IgM (a) and immunoglobulin (Ig)G2 levels (bB) as well as fold increase. Only 2–5‐year‐old children tested in 2010–12 were vaccinated with PCV‐7 (grey box‐plot). *P‐value between 0·01 and 0·05; **P‐value between 0·001 and 0·01; ***P‐value < 0·001. Median (horizontal line) and quartiles (box) are shown.
Discussion
Our retrospective study investigated the effect of previous PCV priming in a large cohort of children in a current clinical setting, in which children are vaccinated with PCV through the standard vaccination programme and PPV is administered at variable intervals from PCV immunization for SAD diagnosis. Our results demonstrate higher antibody responses to PCV–PPV shared serotypes (4 and 18C) after PPV immunization in children who have been primed previously with PCV‐7 than in children who have not been primed with PCV‐7 (median post‐vaccination concentration 4.4‐fold higher for polysaccharide 4 and 9.7‐fold higher for polysaccharide 18C). Clearly, a pronounced memory response to PCV serotypes is elicited in children who have been primed with PCV before boost with PPV. Some studies have shown that priming with PCV enhances the magnitude of a subsequent antibody response to PPV for serotypes that are shared between the two vaccines 12, 15, 16. These studies were conducted in specific risk populations: patients with proven SAD, adults aged ≥ 70 years and HIV‐infected patients, respectively. However, this effect was not shown in other studies 17, 18, 19, 20. Bernth‐Jensen et al. concluded that polysaccharide responsiveness was not biased by prior pneumococcal conjugate vaccination. They found that in 47 HIV‐infected adults, all vaccinated with both PCV and PPV, the propensity to trigger a sufficient antibody response for PCV‐PPV shared serotypes was not different from pure PPV serotypes 13.
Given the observation that PCV vaccination can overcome the impaired immune response to plain pneumococcal polysaccharides in patients with low response to PPV 12, 21, 22, we suspected that non‐PCV serotypes were more suitable to investigate the polysaccharide antibody response in patients with suspected SAD. Therefore, we also studied the immune response to 4 PPV‐specific serotypes: 9N, 19A, 3 and 8. Serotypes 9N and 19A belong to the same serogroup as, respectively, serotypes 9V and 19F, both included in PCV‐7. Serotypes 3 and 8 do not belong to a serogroup included in PCV‐7. The antibody response to serotypes 8 and 19A was not influenced by previous PCV priming. Unexpectedly, priming with PCV‐7 did influence the antibody response to non‐PCV serotype 9N (median post‐vaccination concentration 2.5‐fold higher). For serotype 3, results were inconclusive: higher post‐vaccination antibody levels were found in the PCV‐primed group but a higher fold‐increase of antibody level was found in the unprimed group. Previous PCV vaccination might affect response to non‐PCV serotypes through in‐vivo and/or in‐vitro cross‐reactivity. Rose et al. also found a higher percentage of responders to non‐PCV serotype 5 in a PCV‐primed group than in an unprimed group, although post‐vaccination antibody levels to serotype 5 did not differ between a primed and an unprimed group 12. To our knowledge, cross‐reactivity of polysaccharide‐specific antibodies with serotype 9N or 3 has not been described. However, a conjugate vaccine can increase antibody avidity, thereby increasing cross‐reactivity to closely related pneumococcal serotypes 23, 24, 25. Genetic similarity of the encoding regions for different capsular polysaccharides does not predict cross‐reactivity between serotypes. For example, serotypes 9N and 9V were classified historically as one serogroup by their pattern of seroreactivity, but they differ significantly at the genetic level 26. Serotypes 19A and 19F are genetically very similar but, although higher 19A antibodies may be elicited after PCV‐7 vaccination (some formulations), cross‐reactive antibodies induced by serotype 19F provide limited protection against 19A disease 27, 28.
As expected, post‐vaccination serotype 4‐specific IgM levels did not differ significantly between primed and unprimed groups, as there is no memory response for IgM production. Serotype‐specific antibodies to the bacterium or to pure polysaccharide antigens are reported to be largely IgG2 10, in contrast to antibodies against protein antigens which are IgG1 subclass. We show that despite previous PCV, there is still a robust IgG2 antibody response to polysaccharide 4 after subsequent PPV vaccination. Serotype 4‐specific IgG2 after PPV vaccination was much higher in PCV‐primed than in unprimed children. This contrasts the earlier finding of an absent IgG2 response in children vaccinated with PCV and PPV 9, but confirms other reports that PPV elicits serotype‐specific IgG2 after priming with PCV 29, 30.
As shown previously, we found that age has a significant influence on polysaccharide responsiveness, and that there is a serotype‐dependent variation 5, 31, 32, 33.
We were able to investigate the effect of PCV introduction in the standard vaccination scheme on the response to PPV in a large cohort of samples for six different serotypes in the target population for SAD diagnosis. A control population within this cohort (older age, not primed with PCV‐7), examined during the same time‐period, allowed us to exclude confounding factors such as possible changes in epidemiology or changes in assay performance over time. Limitations of the study are its retrospective design and the smaller number of samples tested for the additional serotypes. It would have been interesting to assess antibody functionality by opsonophagocytosis assay, to evaluate whether PCV priming increases the functionality of polysaccharide‐specific antibodies to PPV‐specific serotypes. Previous studies have consistently shown good correlation of pneumococcal opsonophagocytosis and antibody concentration 12, 34, 35.
A limitation of our study is that we studied the immune response to only a limited number of serotypes that are PPV‐specific (thus not included in PCV). The following serotypes are contained in PPV but not in PCV‐13: 2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F and 33F. Therefore, additional studies need to address whether the antibody response to these serotypes is influenced by previous PCV‐13 vaccination. It should be noted that if serotype 22F is used for pre‐absorption, then the antibody response to this serotype cannot be evaluated.
In summary, we show that PCV vaccination enhances the antibody response to subsequent PPV vaccination and induces an IgG2 memory response for shared PCV‐PPV serotypes. PCV vaccination can also enhance the antibody response to non‐shared PCV‐PPV serotypes (e.g. 9N), albeit to a lesser extent. With the increasing number of shared serotypes included in PCV, the diagnostic assessment for polysaccharide antibody deficiency requires careful selection of serotypes that are not influenced by prior PCV (e.g. serotype 8). This needs further investigation.
Alternatively, future research should focus upon the diagnostic potential of other methods for measuring polysaccharide antibody responses. A sophisticated way may be to perform serum glycan array – currently, the cost and complexity of the assay limit its use to research questions 36. A less expensive and less complicated assay may consist of determination of antibody responses to Vi capsular polysaccharide from Salmonella typhii. Research is needed to investigate the diagnostic usefulness of these approaches for diagnosing specific polysaccharide antibody deficiency.
Disclosure
There are no disclosures.
Author contributions
H. S. and X. B. designed the study, analysed the data and prepared the manuscript. I. M., M. P., K. D. B. and F. V. took care of the patients, helped with collection of data and reviewed the manuscript. G. W. and D. D. performed the laboratory measurements and reviewed the manuscript. G. F. and L. M. reviewed the manuscript.
Acknowledgements
This study was supported by a research grant from the Research Council of the Catholic University. H. S. is supported by a PhD fellowship grant of the Research Foundation Flanders (FWO). I. M. is supported by a KOF grant of the KU Leuven – University of Leuven and by the Jeffrey Modell Foundation. We thank the laboratory staff from the immunology laboratory of the University Hospitals Leuven for their technical support.
References
- 1. Ambrosino DM, Siber GR, Chilmonczyk BA, Jernberg JB, Finberg RW. An immunodeficiency characterized by impaired antibody responses to polysaccharides. N Engl J Med 1987; 316:790–3. [DOI] [PubMed] [Google Scholar]
- 2. Boyle RJ, Le C, Balloch A, Tang ML‐K. The clinical syndrome of specific antibody deficiency in children. Clin Exp Immunol 2006; 146:486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schaballie H, Vermeulen F, Verbinnen B et al Value of allohemagglutinins in the diagnosis of a polysaccharide antibody deficiency. Clin Exp Immunol 2015; 180:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orange JS, Ballow M, Stiehm ER et al Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol 2012; 130:S1–24. [DOI] [PubMed] [Google Scholar]
- 5. Jeurissen A, Moens L, Raes M et al Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens. Clin Chem 2007; 53:505–10. [DOI] [PubMed] [Google Scholar]
- 6. Borgers H, Moens L, Picard C et al Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clin Immunol 2010; 134:198–205. [DOI] [PubMed] [Google Scholar]
- 7. Ballow M. Vaccines in the assessment of patients for immune deficiency. J Allergy Clin Immunol 2012; 130:283–4.e5. [DOI] [PubMed] [Google Scholar]
- 8. Bonilla FA, Bernstein IL, Khan DA et al Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol 2005; 94:S1–63. [DOI] [PubMed] [Google Scholar]
- 9. Uddin S, Borrow R, Haeney MR et al Total and serotype‐specific pneumococcal antibody titres in children with normal and abnormal humoral immunity. Vaccine 2006; 24:5637–44. [DOI] [PubMed] [Google Scholar]
- 10. Mond JJ, Lees A, Snapper CM. T cell‐independent antigens type 2. Annu Rev Immunol 1995; 13:655–92. [DOI] [PubMed] [Google Scholar]
- 11. Choo S, Seymour L, Morris R et al Immunogenicity and reactogenicity of a pneumococcal conjugate vaccine administered combined with a haemophilus influenzae type B conjugate vaccine in United Kingdom infants. Pediatr Infect Dis J 2000; 19:854–62. [DOI] [PubMed] [Google Scholar]
- 12. Rose MA, Schubert R, Strnad N, Zielen S. Priming of immunological memory by pneumococcal conjugate vaccine in children unresponsive to 23‐valent polysaccharide pneumococcal vaccine. Clin Diagn Lab Immunol 2005; 12:1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bernth‐Jensen JM, Søgaard OS. Polysaccharide responsiveness is not biased by prior pneumococcal‐conjugate vaccination. PLOS ONE 2013; 8:e75944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sorensen RU, Leiva LE. Measurement of pneumococcal polysaccharide antibodies. J Clin Immunol 2014; 34:127–8. [DOI] [PubMed] [Google Scholar]
- 15. Lesprit P, Pédrono G, Molina J‐M et al Immunological efficacy of a prime‐boost pneumococcal vaccination in HIV‐infected adults. AIDS 2007; 21:2425–34. [DOI] [PubMed] [Google Scholar]
- 16. de Roux A, Schmöle‐Thoma B, Siber GR et al Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 2008; 46:1015–23. [DOI] [PubMed] [Google Scholar]
- 17. Peñaranda M, Payeras A, Cambra A, Mila J, Riera M, Majorcan Pneumococcal Study Group. Conjugate and polysaccharide pneumococcal vaccines do not improve initial response of the polysaccharide vaccine in HIV‐infected adults. AIDS 2010; 24:1226–8. [DOI] [PubMed] [Google Scholar]
- 18. Goldblatt D, Southern J, Andrews N et al The immunogenicity of 7‐valent pneumococcal conjugate vaccine versus 23‐valent polysaccharide vaccine in adults aged 50–80 years. Clin Infect Dis 2009; 49:1318–25. [DOI] [PubMed] [Google Scholar]
- 19. Feikin DR, Elie CM, Goetz MB et al Randomized trial of the quantitative and functional antibody responses to a 7‐valent pneumococcal conjugate vaccine and/or 23‐valent polysaccharide vaccine among HIV‐infected adults. Vaccine 2001; 20:545–53. [DOI] [PubMed] [Google Scholar]
- 20. Powers DC, Anderson EL, Lottenbach K, Mink CM. Reactogenicity and immunogenicity of a protein‐conjugated pneumococcal oligosaccharide vaccine in older adults. J Infect Dis 1996; 173:1014–8. [DOI] [PubMed] [Google Scholar]
- 21. Zielen S, Bühring I, Strnad N, Reichenbach J, Hofmann D. Immunogenicity and tolerance of a 7‐valent pneumococcal conjugate vaccine in nonresponders to the 23‐valent pneumococcal vaccine. Infect Immun 2000; 68:1435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sorensen RU, Leiva LE, Giangrosso PA et al Response to a heptavalent conjugate Streptococcus pneumoniae vaccine in children with recurrent infections who are unresponsive to the polysaccharide vaccine. Pediatr Infect Dis J 1998; 17:685–91. [DOI] [PubMed] [Google Scholar]
- 23. Anttila M, Eskola J, Ahman H, Käyhty H. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine 1999; 17:1970–7. [DOI] [PubMed] [Google Scholar]
- 24. Anttila M, Eskola J, Ahman H, Käyhty H. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J Infect Dis 1998; 177:1614–21. [DOI] [PubMed] [Google Scholar]
- 25. Licciardi PV, Balloch A, Russell FM, Mulholland EK, Tang MLK. Antibodies to serotype 9V exhibit novel serogroup cross‐reactivity following infant pneumococcal immunization. Vaccine 2010; 28:3793–800. [DOI] [PubMed] [Google Scholar]
- 26. Bentley SD, Aanensen DM, Mavroidi A et al Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLOS Genet 2006; 2:e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grant LR, O'Brien SE, Burbidge P et al Comparative immunogenicity of 7 and 13‐valent pneumococcal conjugate vaccines and the development of functional antibodies to cross‐reactive serotypes. PLOS ONE 2013; 8:e74906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hausdorff WP, Hoet B, Schuerman L. Do pneumococcal conjugate vaccines provide any cross‐protection against serotype 19A? BMC Pediatr 2010; 10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Breukels MA, Rijkers GT, Voorhorst‐Ogink MM, Zegers BJ, Sanders LA. Pneumococcal conjugate vaccine primes for polysaccharide‐inducible IgG2 antibody response in children with recurrent otitis media acuta. J Infect Dis 1999; 179:1152–6. [DOI] [PubMed] [Google Scholar]
- 30. O'Brien KL, Steinhoff MC, Edwards K, Keyserling H, Thoms ML, Madore D. Immunologic priming of young children by pneumococcal glycoprotein conjugate, but not polysaccharide, vaccines. Pediatr Infect Dis J 1996; 15:425–30. [DOI] [PubMed] [Google Scholar]
- 31. Balloch A, Licciardi PV, Russell FM, Mulholland EK, Tang MLK. Infants aged 12 months can mount adequate serotype‐specific IgG responses to pneumococcal polysaccharide vaccine. J Allergy Clin Immunol 2010; 126:395–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bossuyt X, Borgers H, Moens L, Verbinnen B, Meyts I. Age‐ and serotype‐dependent antibody response to pneumococcal polysaccharides. J Allergy Clin Immunol 2011; 127:1079–80. [DOI] [PubMed] [Google Scholar]
- 33. Sorensen RU, Leiva LE, Javier FC et al Influence of age on the response to Streptococcus pneumoniae vaccine in patients with recurrent infections and normal immunoglobulin concentrations. J Allergy Clin Immunol 1998; 102:215–21. [DOI] [PubMed] [Google Scholar]
- 34. Puumalainen T, Ekström N, Zeta‐Capeding R et al Functional antibodies elicited by an 11‐valent diphtheria–tetanus toxoid‐conjugated pneumococcal vaccine. J Infect Dis 2003; 187:1704–8. [DOI] [PubMed] [Google Scholar]
- 35. Song JY, Moseley MA, Burton RL, Nahm MH. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother 2013; 19:412–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maglione PJ, Simchoni N, Black S et al IRAK‐4 and MyD88 deficiencies impair IgM responses against T‐independent bacterial antigens. Blood 2014; 124:3561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


