Summary
The complement receptor 2 (CR2, CD21) is part of a complex (CD21/CD19/CD81) acting as a co‐receptor to the B cell receptor (BCR). Simultaneous triggering of the BCR and CD21 lowers the threshold for B cell activation. Although CD21 is important, B cells that express low amounts or lack surface CD21 (CD21–/low) are increased in conditions with chronic inflammation, e.g. autoimmune diseases. However, little is known about the CD21–/low B cell subset in peripheral blood from healthy donors. Here, we show that CD21–/low cells represent approximately 5% of B cells in peripheral blood from adults but are barely detectable in cord blood, after excluding transitional B cells. The CD21–/low subset can be divided into CD38–24+ and CD38–24low cells, where most of the CD38–24+ are CD27+immunoglobulin (Ig)M+IgD+ and the CD38–24low are switched CD27–. Expression levels of additional markers, e.g. CD95 and CD62L, are similar to those on classical memory B cells. In contrast to naive cells, the majority of CD21–/low cells lack expression of the ABCB1 transporter. Stimulation with a combination of BCR, Toll‐like receptor (TLR)−7/8 and interleukin (IL)−2 induces proliferation and differentiation of the CD21–/low B cells comparable to CD21+CD27+ memory B cells. The response excluding BCR agonist is not on par with that of classical memory B cells, although clearly above that of naive B cells. This is ascribed to a weaker response by the CD38–24low subset, implying that some memory B cells require not only TLR but also BCR triggering. We conclude that the CD21–/low cells in healthy donors are memory B cells.
Keywords: B cells, CD21, memory, peripheral blood, TLR
Introduction
B lymphocytes represent a cell type involved in both adaptive and innate immunity. Under both conditions B cells can differentiate into plasma cells secreting antibodies that bind to invading pathogens and allow their clearance by different pathways. Upon encounter with cognate antigen, mature naive B cells undergo clonal expansion and can thereafter also differentiate into memory B cells, which play an important role upon re‐encounter of the same pathogen. B cells are central in many autoimmune diseases because of their capacity to produce autoantibodies and cytokines and present antigen, and are therefore a subject of extensive research and a target for therapy. For instance, in rheumatoid arthritis (RA) B cell depleting therapy (rituximab) has been successful, and in systemic lupus erythematosus (SLE) a drug that inhibits B cell survival (belimumab) is the first drug to be approved for SLE in more than 50 years 1, 2.
The complement receptor type 2 (CR2, CD21) binds to fragments of complement C3 (C3d, C3dg and iC3b), and together with CD19 and CD81 forms a co‐receptor to the B cell receptor (BCR). The binding of complement‐tagged antigens to the BCR and CD21 results in co‐ligation, which amplifies signals transduced through the BCR and thus lowers the B cell activation threshold 3, 4. In healthy donors, naive and memory B cells express CD21, whereas its expression is low on early transitional B cells and plasmablasts/plasma cells 5.
Despite the apparent importance of CD21, a subset of mature B cells that lacks (or expresses low levels of) this marker, CD21–/low, has been described in both mice and man 6, 7. In mice, a CD21–/low B cell subset accumulates with age and has been referred to as age‐associated B cells (ABCs) 8, 9. A similar subset has been found enriched in young lupus‐prone mice 9 and in mice lacking the surrogate light chain (SLC–/–) 10, 11. In humans, a CD21–/low B cell subset has been described in tonsils 12, an environment that is exposed constantly to various antigens, and hence cells are activated continuously. The CD21–/low B cells, defined by their expression of the inhibitory Fc‐receptor‐like protein 4 (FcRL4), were described as memory B cells although they lacked the conventional memory B cell marker, CD27. A CD21–/low B cell subset is also enriched in peripheral blood in a number of conditions with chronic immune stimulation, e.g. certain pathogenic infections (viral, parasitic) and autoimmune diseases, e.g. RA, SLE, Sjögren's syndrome and common variable immunodeficiency (CVID) type Ia 13, 14, 15, 16, 17, 18, 19. Depending on disease and study design, the CD21–/low B cells have been described as CD27+ or CD27–, expressing immunoglobulin (Ig)M or switched BCRs with or without somatic hypermutation (SHM). Most of these studies concluded that under conditions of chronic immune stimulation the CD21–/low cells are memory B cells. However, little is known about this subset in peripheral blood from healthy donors. Here, we investigate this, and demonstrate that the CD21–/low B cell subset is almost absent in cord blood but present in peripheral blood from healthy adults. As in situations of chronic immune stimulation, this subset is composed mainly of memory B cells. Moreover, it can be subdivided further into CD27+CD38–24+, most of which are IgM+IgD+ and CD27–CD38–24low that are mainly switched cells.
Materials and methods
Study subjects
Peripheral blood from 18 healthy women, aged 24–30 (n = 9) and 55–64 years (n = 9), were collected into lithium heparin tubes (Greiner Bio‐One, Stonehouse, UK). Written consent was not required for these samples, and approval by the Human Research Ethics Committee was not needed as no personal information or identity was recorded (Swedish law 2003: 460, paragraphs 4 and 13). All mothers were given oral and written information for cord blood samples, and gave oral consent to participate in the study. Ethical approval (Ö342–00) was obtained through the Human Research Ethics Committee of the Medical Faculty, University of Gothenburg, Sweden. The cord blood samples were collected from healthy newborn infants (n = 6) born at term (≥ 38 gestational weeks) at the Sahlgrenska University Hospital. Tonsils from six healthy children, aged 1–9 years, undergoing tonsillotomy were collected. As no personal information or identity was recorded, no written consent or approval by the Human Research Ethics Committee was needed (Swedish law 2003: 460, paragraphs 4 and 13).
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were obtained from plasma‐free blood after separation on Ficoll (GE Healthcare, Little Chalfont, UK), according to the manufacturer's protocol. Cells from tonsils were prepared according to standard methods for lymphoid organs. Cells were filtered using a 40 µm filter (BD Biosciences, San Diego, CA, UK), and thereafter stained directly or frozen to be stained later. Cells were stained at a concentration of 10 × 106 cells/ml. Mouse, rat and rabbit serum were used to inhibit unspecific binding to Fc receptors. Cells were stained in 100 μl using antibodies and dilutions as shown in Supporting information, Table S1. Biotinylated antibodies were detected using streptavidin‐V500 (BD Biosciences). The cells were acquired on a FACSCanto 2 or FACSVerse (BD Biosciences), and data were analysed using FlowJo software (TreeStar Inc., Ashland, OR, USA).
ABCB1 transporter expression assay
PBMCs and tonsillar lymphocytes were resuspended in complete medium and allowed to equilibrate at 37°C in a water bath for 30 min with cyclosporin A (CsA; 25 µM), which exposes the epitope of the ABCB1 (CD243)‐activated conformation, hence accessible to antibody binding. The antibody was then added and the tubes were incubated at 37°C for another 30 min. Cells were then washed and stained by regular surface staining at 4°C.
Cell sorting
B cells were purified from PBMCs using immunomagnetic beads (Dynal® B cell Negative Isolation Kit; Invitrogen, Carlsbad, CA, USA), with a purity of 99% (Supporting information, Fig. S1). These cells were stained with antibodies recognizing CD19, CD21 and CD27, and sorted as CD21–/low, CD21+CD27– and CD21+CD27+ CD19+ B cell subsets on a Synergy cell sorter (Sony Biotech, San Jose, CA, USA). Sort purities were > 95% (Supporting information, Fig. S1).
CD69 up‐regulation
PBMCs or total B cells, purified as above, were cultured in 96‐well plates at 1 × 106 cells/well in complete RPMI‐1640 supplemented with L‐glutamine, non‐essential amino acids, sodium pyruvate, penicillin, streptomycin, β‐mercaptoethanol and 10% fetal bovine serum, and activated with the following stimuli: 2·5 μg/ml Toll‐like receptor (TLR)−7/8 agonist R848 (Alexis Biochemicals), 2·5 μg/ml TLR‐9 agonist cytosine–phosphate–guanine (CpG) oligodeoxynucleotide 2006 (5′‐TCGTCGTTTTGTCGTTTTGTCGTTGGGGG–3′) (InvivoGen, San Diego, CA, USA), 2·5 μg/ml goat anti‐human IgA/IgG/IgM F(ab′)2 fragments (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and 20 ng/ml human recombinant interleukin (IL)−2 (R&D Systems, Minneapolis, MN, USA). CD69 up‐regulation was assessed after 3 h by flow cytometry on a FACSVerse (BDBiosciences) and data analysed as above.
Proliferation and differentiation
Total B cells or subsets were purified as above, labelled with CellTraceTM Violet Cell Proliferation Kit (Life Technologies, Paisley, UK) and cultured in 96‐well plates at 1 × 104 cells/well in RPMI‐1640 supplemented as above. The cells were stimulated with different combinations of the stimuli used for CD69 up‐regulation, and at the same concentrations. Cell proliferation and plasmablast differentiation were assessed at day 5 by flow cytometry on a FACSVerse (BD Biosciences) and data analysed as above.
Statistics
The median and the interquartile range give distribution of a cell subset's frequency. Differences between these, as well as age groups, were analysed for statistical significance with the unpaired Mann–Whitney test (Figs 1, 2, 3). Figure 6 was analysed by one‐way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test, using GraphPad Prism version 6 (La Jolla, CA, USA).
Figure 1.
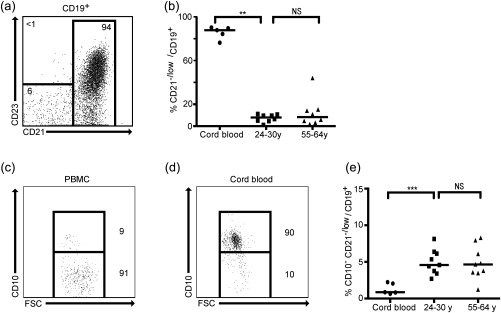
A CD21–/low B cell subset is present in peripheral blood from healthy donors. Peripheral blood mononuclear cells (PBMCs) and cord blood from healthy donors were analysed by flow cytometry. (a) Representative fluorescence activated cell sorter (FACS) plot shows the CD21–/low B cell subset after gating on CD19+ B cells. (b) Graph shows frequency of CD21–/low B cells in three age groups of healthy individuals. Representative FACS plots show CD10+ frequency in (c) PBMCs and (d) cord blood. (e) Graph shows the frequency of CD21–/low B cells (median 4·6%; P25–P75 3·5–6·2%) after excluding CD10+ (transitional) cells; n.s. = not significant; *P < 0·05; **P < 0·01; ***P < 0·001.
Figure 2.
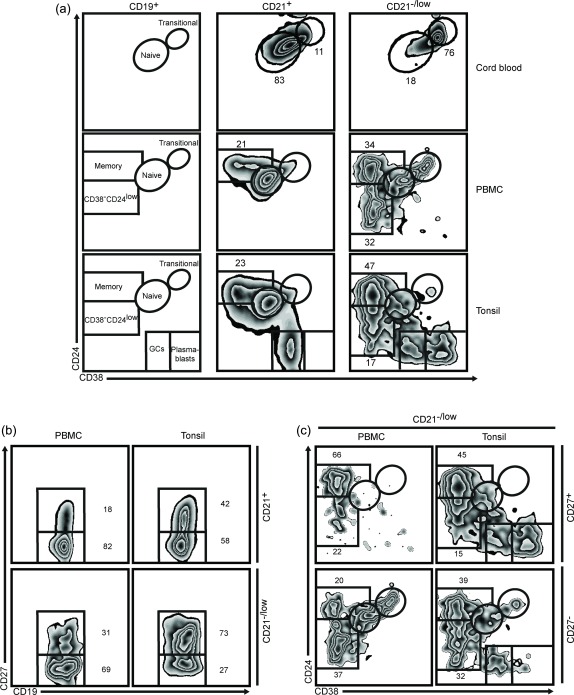
The CD21–/low B cells can be divided into two subsets. Cord blood, peripheral blood mononuclear cells (PBMCs) and tonsils from healthy donors were analysed by flow cytometry. (a) After gating on CD19+ B cells different subsets can be distinguished based on the CD38 and CD24 markers. This is shown schematically (left) and with representative fluorescence activated cell sorter (FACS) plots after gating on CD21+ (middle) and CD21–/low (right) B cells. (b) Representative FACS plots of CD27 expression in CD21+ (upper) and CD21–/low (lower) B cells. (c) CD21–/low B cells were gated as CD27+ (upper) and CD27– (lower) cells and projected into subsets according to the CD38 and CD24 markers. In PBMCs, the frequency of CD27+ cells among CD21+ cells was 25% (P25–P75 21–43%) and among CD21–/low cells was 49% (P25–P75 24–57%).
Figure 3.
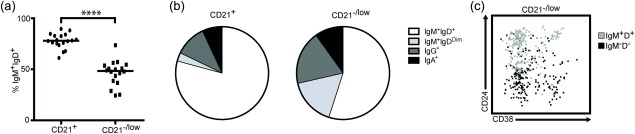
The CD21–/low B cell subset contains both immunoglobulin (Ig)M+ and switched cells. Peripheral blood mononuclear cells (PBMCs) from healthy donors were analysed by flow cytometry. (a) Graph shows percentage of IgM+IgD+ cells in the CD21+ and CD21– B cell subsets. (b) Representative pie charts show frequencies of Ig isotypes expressed by the CD21+ and CD21–/low B cell subsets. CD21+ versus CD21–/low (%): IgM+IgD+, 80 (P25–P75 75–90%) versus 50 (P25–P75 38–54%); IgM+IgDDim, 3 (P25–P75 2–6%) versus 15 (P25–P75 11–18%); IgG+, 11 (P25–P75 5–16%) versus 17 (P25–P75 12–30%); IgA+, 7 (P25–P75 4–12%) versus 9 (P25–P75 8–17%). (c) Representative fluorescence activated cell sorter (FACS) plot shows the distribution of IgM+IgD+ and IgM–IgD– cells in the CD21–/low B cell subset.
Figure 6.
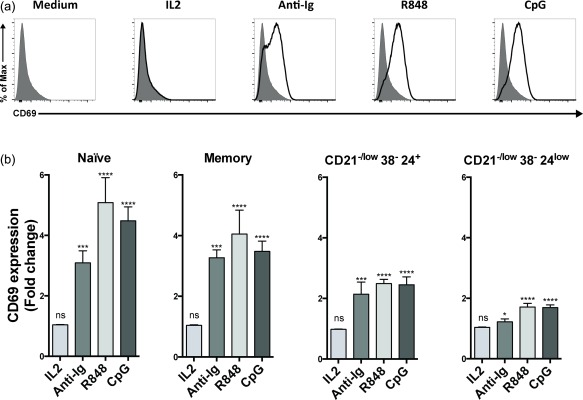
Up‐regulation of CD69 upon activation. Peripheral blood mononuclear cells (PBMCs) from healthy donors were stimulated in vitro followed by flow cytometry analysis. (a) After 3 h with different stimuli, as indicated, the geometric mean fluorescence intensity of CD69 was determined. Representative histograms show overlays of stimulated (bold line) and unstimulated total B cells (grey, filled). (b) As in (a), except that B cells were gated as CD21+ naive (CD38+CD24+) and memory (CD38–CD24+), and CD21–/lowCD38–CD24+ and CD21–/lowCD38–CD24low cells. Shown is CD69 fold change relative to medium controls; n.s. = not significant; *P < 0·05; ***P < 0·001; ****P < 0·0001.
Results
A CD21–/low B cell subset is present in peripheral blood from healthy donors
To assess the presence of a CD21–/low B cell subset, PBMCs from healthy female donors (aged 24–30) were analysed by flow cytometry. In line with our studies in mice 10, 11, we included the CD23 marker in our analysis. A CD21–/lowCD23– subset was clearly present (Fig. 1a), and as all CD21–/low were CD23– we will refer to this subset hereafter as CD21–/low. To determine whether the frequency of the CD21–/low B cell subset changed with age we analysed PBMCs from 55–64‐year‐old healthy female donors. We found that the proportion was similar in this age group, approximately 5% of the total CD19+ B cell population (Fig. 1b). We also investigated whether a CD21–/low B cell subset could be detected at a very young age. Therefore, we analysed B cells from cord blood where we found that approximately 90% appeared to consist of CD21–/low B cells. Because early transitional B cells in peripheral blood are known to be CD21–/low 5, we included the CD10 marker. This showed that fewer than 10% of the CD21–/low cells in adults, but the vast majority in cord blood were CD10+ transitional B cells (Fig. 1c,d). In adults, exclusion or inclusion of CD10+ cells did not change the proportion of CD21–/low B cells significantly, whereas a very different picture emerged when excluding these cells in cord blood; the CD10–CD21–/low B cell subset was now significantly smaller in cord blood than in adults (Fig. 1e). The proportion of early transitional B cells in the CD21–/low B cell subset in adults was not significantly different in the two age groups, and hence the percentage of CD10–CD21–/low B cells was also similar (Fig. 1e). The data from the 24–30‐ and 55–64‐year‐olds were therefore pooled in subsequent analyses. We conclude that a CD10–CD21–/low B cell subset is clearly distinguishable in peripheral blood in healthy donors, a subset that is evident in adults but not newborns.
The CD21–/low B cells can be divided into two subsets based on CD24
Transitional, naive and memory B cells, as well as plasmablasts, can be distinguished by combining the CD38 and CD24 markers 20, 21, 22, 23. Using these we sought to determine whether the CD21–/low B cells were mainly naive and/or memory B cells (Fig. 2a). We first analysed cord blood cells, and could confirm that a majority of the CD21–/low B cells were transitional (CD38hiCD24hi) and that the vast majority of the CD21+ cells were naive (CD38+CD24+) B cells, consistent with a low frequency of memory B cells during the first 2 years of life 24. Thereafter, we analysed PBMCs from adults where the CD21+ cells showed a typical pattern with transitional, naive and classical memory (CD38–CD24+) B cells (Fig. 2a), as expected. Gating on the CD21–/low B cells showed a pattern that was markedly different: consistent with the above data using the CD10 marker, approximately 10% of the CD21–/low cells were found in the transitional gate and 5% in the naive gate. The remaining CD21–/low B cells were CD38–, hence typical of memory B cells. However, based on the CD24 expression levels they could be divided into two subsets, CD38–CD24+ and CD38–CD24low. A CD21–/low B cell subset has also been described in tonsils, based on FcRL4 expression 12, and for comparison we also analysed cells from this tissue. Cells in tonsils are activated continuously, and hence germinal centre (GC) B cells are also distinguishable 25. Nevertheless, excluding GC B cells and plasmablasts (CD38++CD24–) showed that a majority of the CD21–/low subset lacked CD38 and could also be divided into CD38–CD24+ and CD38–CD24low, although the ratio was different from that in PBMCs (Fig. 2a). Another marker often used to characterise human memory B cells is CD27, although it is not an absolute marker for such cells 26, 27. Gating on the CD21+ cells showed that approximately 20% were CD27+ in PBMCs, whereas a higher (approximately 40%) proportion was observed in tonsils (Fig. 2b). In the CD21–/low subset the proportion of CD27+ cells was approximately 30% in PBMCs, whereas a higher (approximately 75%) proportion was observed in tonsils. In PBMCs the CD21–/lowCD38–24+ cells were enriched in the CD27+ and the CD21–/lowCD38–CD24low were enriched in the CD27– cells. This was also observed in tonsils, although with different proportions, due in part to the GC B cells and plasmablasts (Fig. 2c). Taken together, these data demonstrate that in both PBMCs and tonsils the CD21–/lowCD38– cells can be divided into two subsets based on the CD24 levels.
The CD21–/low B cell subset contains both IgM+ and switched cells
To determine the Ig isotype expressed by the CD21–/low B cells in PBMCs from adults, we first analysed the expression of IgM and IgD compared with the CD21+ subset. There was no significant difference including or excluding the CD10 marker (not shown), and this marker was therefore excluded. The proportion of IgM+IgD+ in the CD21+ B cells was approximately 80%, whereas it was approximately 50% in the CD21–/low B cells, and 15% were IgM+IgDDim in CD21–/low B cells, but only approximately 3% of the CD21+ (Fig. 3a,b). Staining for switched isotypes showed that approximately 15% of the CD21–/low B cells were IgG+ and approximately half as many were IgA+, with similar proportions among the CD21+ cells. We also found that most of the CD21–/lowCD38–CD24+ cells were IgM+IgD+ whereas a majority of the CD21–/lowCD38–CD24low subset was IgM–IgD– (switched), supporting that the latter were also memory B cells (Fig. 3c). Taken together, this suggests that after excluding transitional and plasmablasts the vast majority of the CD21–/low cells are memory B cells.
The CD21–/low B cells express markers indicating previous activation
To further investigate the CD21–/low B cells, we determined the expression levels of additional markers. Both CD40 and CD86 were expressed at similar levels on CD21+ naive (CD38+CD24+) and classical memory (CD38–CD24+) as well as on the CD21–/low B cells (Fig. 4). Compared to naive B cells, the levels of CD80 were slightly higher on classical memory and on the CD21–/low subset. The levels of CD95 were also elevated on the two latter subsets, where it showed a bi‐modal expression pattern that was most evident in the CD21–/low subset. The levels of CD62L, a marker that is down‐regulated upon activation 28, followed a bi‐modal expression pattern on the naive, classical memory and CD21–/low B cell subsets, although the proportion of CD62L– cells was higher among the latter. However, the bi‐modal patterns of the CD95 and CD62L markers within the CD21–/low subset could not be correlated with the levels of CD24 (not shown). Thus, the CD21–/low B cells display markers indicating previous activation, analogous to classical memory B cells.
Figure 4.
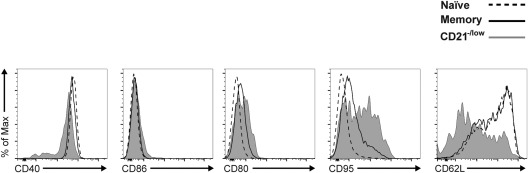
The CD21–/low B cells express markers indicating previous activation. Peripheral blood mononuclear cells (PBMCs) from healthy donors were analysed by flow cytometry. Representative histograms show expression of indicated markers after gating B cells as CD21+CD38+CD24+ naive (dashed line), CD21+CD38–CD24+ memory (bold line) and CD21–/low (grey, filled).
The CD21–/low B cells lack expression of the ABCB1 transporter
Previous work has shown that expression of the active form of the ABCB1 transporter can distinguish between naive and memory B cells 29. Therefore, we stained PBMCs and tonsils for expression of this marker. In both, the CD21+ cells were divided into positive and negative cells, whereas the vast majority of the CD21–/low cells lacked expression of the ABCB1 transporter (Fig. 5a). Gating on CD21+ naive and classical memory B cells in PBMCs demonstrated that the naive cells were positive and the memory cells negative (Fig. 5b), as expected, and that the vast majority of the CD21–/low cells were negative. Thus, this supports that the CD21–/low cells consist mainly of memory B cells.
Figure 5.
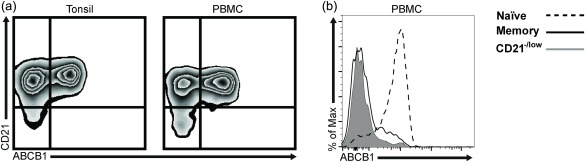
The CD21–/low B cells do not express ABCB1, which is restricted to naive B cells. (a) ABCB1 expression was analysed by flow cytometry in CD21+ and CD21–/low B cells in tonsils (left) and peripheral blood mononuclear cells (PBMCs) (right) from healthy donors. (b) Representative histograms show ABCB1 expression on naive B cells (dashed line, CD21+CD24+CD38+), memory (bold line, CD21+CD24+CD38–) and CD21–/low B cells (grey, filled).
The CD21–/lowCD38–CD24low subset responds poorly to single stimuli
A decrease in surface CD21 levels could lead potentially to unresponsiveness, as it is a part of the B cell co‐receptor and considered important for B cell activation. Therefore, we asked whether the CD21–/low B cells could respond to various single stimuli, e.g. BCR cross‐linking. To this end, we determined the change in CD69 expression before and 3 h after activation, as this is one of the earliest inducible leucocyte markers 30. Consistent with this, total B cells showed elevated expression levels of CD69 in response to anti‐Ig (BCR stimulation), R848 (TLR‐7/8 agonist) and CpG (TLR‐9 agonist), but not to IL‐2 (Fig. 6a). Analysing CD21+ naive and memory B cells showed that both subsets responded to these stimuli, and to approximately the same extent (Fig. 6b). However, the CD21–/low subset did not respond to the same extent to any of these stimuli (not shown), which was due mainly to a lower response by the CD38–CD24low subset (Fig. 6b). Thus, the CD21+ naive and memory B cells and the CD21–/lowCD38–CD24+ subset responded to all single stimuli, whereas the response of the CD21–/lowCD38–CD24low subset was modest.
The CD21–/low B cells proliferate and differentiate into plasmablasts
Although the response of the CD21–/low B cells to single stimuli did not reach that of the CD21+ B cells, the response to a combination of stimuli could be different. To investigate this, we first activated total B cells, which showed substantial proliferation after 5 days in response to R848, which was enhanced by the addition of IL‐2 (Fig. 7a). The response to CpG was not as pronounced, whether in the presence or absence of IL‐2. The non‐proliferating cells in these cultures were most probably the naive B cells 31. Including anti‐Ig showed that this, together with R848 and IL‐2, induced the highest level of proliferation.
Figure 7.
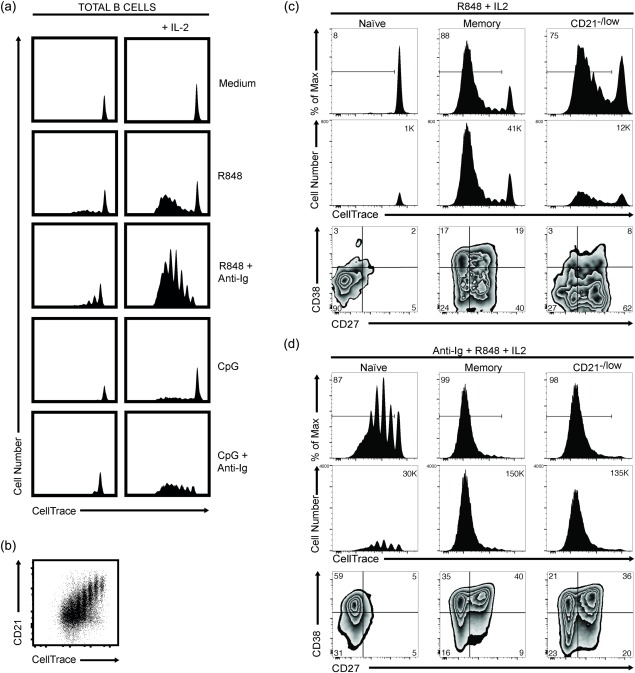
The CD21–/low B cells proliferate and differentiate into plasmablasts. Representative data of peripheral blood mononuclear cells (PBMCs) isolated from healthy donors. Cells were labelled with CellTrace and stimulated in vitro, and analysed by flow cytometry after 5 days. (a) Total B cells were stimulated with R848 and cytosine–phosphate–guanine (CpG) in the presence or absence of F(ab′)2‐fragments of goat anti‐human immunoglobulin (anti‐Ig), with or without interleukin (IL)−2, as indicated. The dilution of CellTrace indicates cell cycles and proportions. (b) Dot‐plot shows CD21 expression versus CellTrace dilution on day 5 after stimulation with anti‐Ig, R848 and IL‐2. (c, d) CD21+ naive (CD27–) and memory (CD27+) B cells, and CD21–/low B cells were stimulated with R848 and IL‐2 without (c) and with (d) anti‐Ig. Proliferation as % of max (upper) and cell number (middle) was determined as in (a), and plasmablast differentiation (lower) as cells being CD27hiCD38hi. Numbers in histograms show percentages and cell numbers, respectively.
To study the response of the CD21–/low B cells, we had to isolate these by cell sorting, as B cell activation results in down‐regulation of CD21 (Fig. 7b) 32, and hence we would not be able to determine the response of the CD21–/low cells by relying on CD21 levels. For comparison, we used CD21+ memory (CD27+) and naive (CD27–) B cells and the response to R848 in combination with IL‐2 was investigated, as this induced a high level of proliferation and seemingly distinguished between naive and memory B cells. Indeed, this combination induced proliferation of the memory but not naive B cells (Fig. 7c). Notably, however, not all memory cells proliferated. The same combination of stimuli induced proliferation in a proportion of CD21–/low B cells similar (% of max) to that of the memory B cells. Although cell numbers were lower in the CD21–/low compared to the memory B cell cultures, they were much higher than those of naive B cells that did not proliferate at all. This indicated that some of the CD21–/low B cells responded poorly to R848, most probably the CD38–CD24low subset (Fig. 6b). We determined, therefore, whether addition of anti‐Ig to the R848 and IL‐2 stimuli enhanced the response. This combination induced proliferation in all cultures, i.e. of naive, memory and CD21–/low B cells (Fig. 7d). However, the response of the naive B cells was not of the same magnitude as that of the memory B cells, whereas that of the CD21–/low cells was. In fact, the response of the CD21–/low cells was very similar to that of the memory B cells in terms of both cell numbers and cycles.
In parallel with the proliferation assay, we investigated the ability of the cells to differentiate into plasmablasts (CD38hiCD27hi). In the presence of R848 and IL‐2, with or without anti‐Ig, the naive B cells gave rise to a very low (< 5%) proportion of plasmablasts (Fig. 7c,d). By contrast, the memory B cells differentiated into plasmablasts in both the absence and presence of anti‐Ig, although not to the same extent: 20 and 40%, respectively. In the presence of R848 and IL‐2, the CD21–/low B cell cultures gave rise to 10% plasmablasts. However, this proportion was increased to approximately 40% when anti‐Ig was also added to these cultures, and hence the response was now very similar to that of the memory B cells. Taken together, these results strongly support that the CD21–/low cells are memory B cells, although they differ in some regard from classical CD21+ memory B cells.
Discussion
Inherent difficulties come with the definition of memory B cells as there is no absolute memory marker, although CD27, CD24 and CD38, in combination with surface Ig isotype, are instructive. Using these in combination with additional markers and assays, our results suggest that the vast majority of the CD21–/low B cells in healthy donors, excluding transitional and plasmablasts, belong to the pool of antigen‐experienced cells, hence we conclude that they are memory B cells. This is also consistent with our observation in cord blood, where a CD21–/low subset is almost absent, after excluding transitional cells and where the frequency of memory B cells is low 24. Moreover, it is consistent with our results from tonsils. A small proportion of the CD21–/low cells might be naive B cells, as proposed previously among the CD27– cells 16. Nevertheless, the CD21–/low B cells could be divided further into two subsets. One of these was CD38–CD24+ and, as these also expressed CD27, they were typical of memory B cells, although most were IgM+. The other subset, also being CD38–, expressed lower levels of CD24 and lacked CD27 but most were switched, and therefore also regarded as memory B cells. Additional memory B cell markers, e.g. CD80, CD95 and CD62L, also showed a bi‐modal pattern, but we were unable to correlate these with the CD24 levels, which would also be consistent with a bi‐modal pattern of these markers among CD21+ classical memory B cells that show more homogeneous CD24 levels.
The presence of SHM in the BCR is often used to define memory B cells, although the absence of SHM does not exclude that the cells are memory cells 33, 34. Another mark of memory cells is their lack of an active ABCB1 transporter, which is expressed uniquely in naive B cells 29. The vast majority of CD21–/low B cells lacked expression of ABCB1, similar to CD21+ classical memory B cells, which also supports that the CD21–/low are memory cells.
Stimulation of memory B cells with TLR agonists can drive their proliferation and differentiation without the need for BCR stimulation, which is in contrast to naive B cells where BCR co‐stimulation is required 35. In vitro, the response of the CD21–/low B cells to TLR‐7/8 agonists and IL‐2 was more similar to that of memory than naive B cells. That the response was not on a par with classical memory B cells is due most probably to a poor response from the CD38–CD24low subset, based on their modest response to single stimuli, in contrast to both naive and memory B cells. Nevertheless, the response to a combination of BCR and TLR‐7/8 agonists was on a par with classical memory B cells, and very different from that of the naive B cells. This indicates that certain memory B cells require BCR engagement in addition to TLR agonists for activation.
Various CD21–/low B cell subsets have also been described in mice, although it is currently unknown whether these are the counterparts of the CD21–/low B cell subsets described in humans. Nevertheless, the CD21–/low subset described in aged mice (ABC) was detected in peripheral lymphoid organs, e.g. spleen as well as in peripheral blood 8. The cells were IgM+IgD+, and it was proposed that this subset was the result of extensive proliferation of mature follicular B cells. Another ABC subset has also been described, although whether the two ABC subsets are the same is unclear, as the former did not express CD11b and the latter was defined as CD21–CD11c+CD11b+. Nonetheless, the development of the CD21–CD11c+CD11b+ subset is dependent upon TLR‐7 signalling, as in the absence of TLR‐7 it failed to accumulate in old female mice 9. The CD21–/low cells in lupus‐prone mice express CD11c, whereas it has not been investigated in SLC–/– mice. In both models, however, these cells secrete autoantibodies upon in‐vitro activation 9, 10.
The CD21–/low B cells in humans have been described in several disorders associated with chronic inflammation, e.g. human immunodeficiency virus (HIV) infection, malaria, CVID, RA and Sjögren's syndrome 13, 14, 15, 16, 17, 18, 19, and in most disorders the cells express CD11c, by analogy to the ABCs in aged and lupus‐prone mice 6, 7. Moreover, in disease the CD21–/low B cells seemingly belong to the memory B cell pool based on their expression of either CD27, mutated and/or switched BCRs. Whether the CD27–CD21–/low B cells in RA and CVID patients are memory or anergic B cells has been discussed 16, 36, 37. Nonetheless, the CD21–/low B cells in many of these disorders are reminiscent of the CD21–/low B cell subset in tonsils from healthy donors 12, in that they also express FcRL4 7. However, the vast majority of CD21–/low B cells in healthy individuals do not express elevated levels of, for example, CD69 or CD86, indicating that in this regard they are different from those in tonsils and disease. The CD21–/low B cells from the patients with the aforementioned disorders were found to respond poorly to BCR stimulation alone, but responded to combinations of stimuli, e.g. TLRs, CD40L and cytokines. This is analogous to the response by the CD21–/low B cells in healthy donors that we attributed to the CD38–CD24low subset. Although the CD24 levels on the CD21–/low B cells in most of these disorders is unknown, those in malaria and CVID type Ia expressed low CD24 levels in addition to being CD27–/low, and thus similar to the CD38–CD24low subset described herein.
The increased frequency of CD21–/low B cells in HIV, hepatitis C virus (HCV) and malaria infections as well as in various autoimmune diseases could be due to an expansion of this B cell subset in response to chronic activation by the infective agent or autoantigen, respectively. This is supported, for example, by results showing that the CD21–/low B cells in these disorders produce antibodies that recognize the infecting virus/parasite, although also CD21+ memory B cells do. It is currently unclear whether the antibody repertoire of the CD21–/low B cells differs from that of conventional memory B cells 38, 39. The presence of CD21–/low memory B cells in healthy individuals could potentially be explained by subclinical chronic infections such as Epstein–Barr virus (EBV), cytomegalovirus (CMV) or Helicobacter pylori. They may also be the result of a normal immune response, as they produce antibodies against the immunized pathogen, as discussed recently 6. Such studies have been performed in mice, whereas it would be more difficult to verify a connection between an infective agent and the frequency of CD21–/low B cells in healthy individuals, as the infective agent would be unknown.
In conclusion, as in disease and tonsils of healthy individuals, the CD21–/low B cell subset in peripheral blood from healthy female adults is composed mainly of memory B cells, although heterogeneous both in expression of markers and in responses to different stimuli.
Disclosure
The authors have no conflicting financial or commercial interests.
Author contribution
I. L. M. and I. G. designed the study; K. T., A. C. and N. C. performed the experiments; I. L. M., I. G., K. T., A, C., N. C., O. G. and L. J. wrote the paper; I. L. M., I. G. and L. J. provided financial support.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Antibodies used in the study
Fig. S1. Strategies to isolate different B cell populations from human peripheral blood. Total B cells were isolated by depletion of non‐B cells using magnetic beads (upper panel). Total B cells were isolated as above, followed by sorting of naïve (CD19+21+27−), memory (CD19+21+27+) and CD21−/low B cells on a cell sorter (lower panel).
Acknowledgements
We would like to thank Linda Bergqvist, Karolina Törn and Prabhanshu Tripathi for cell sorting, donors of blood and tonsils, and funding from: Swedish Science Research Council, Swedish Cancer Foundation, Torsten and Ragnar Söderbergs Stiftelser, AFA, ALF (regional agreement on medical training and clinical research), King Gustav V Stiftelse, IngaBritt och Arne Lundbergs Stiftelse, Swedish Medical Society, Reumatikerförbundet, Lundgrens Stiftelse, Amlövs Stiftelse, Adlerbertska stiftelsen, The Royal Society of Arts and Sciences in Gothenburg, Sigurd och Elsa Golje's minne and the Sahlgrenska Academy.
References
- 1. Lopez‐Olivo MA, Amezaga Urruela M, McGahan L, Pollono EN, Suarez‐Almazor ME. Rituximab for rheumatoid arthritis. Cochrane Database Syst Rev 2015; 1:CD007356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leone A, Sciascia S, Kamal A, Khamashta M. Biologicals for the treatment of systemic lupus erythematosus: current status and emerging therapies. Expert Rev Clin Immunol 2015; 11:109–16. [DOI] [PubMed] [Google Scholar]
- 3. Carter RH, Fearon DT. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science 1992; 256:105–7. [DOI] [PubMed] [Google Scholar]
- 4. Matsumoto AK, Kopicky‐Burd J, Carter RH, Tuveson DA, Tedder TF, Fearon DT. Intersection of the complement and immune systems: a signal transduction complex of the B lymphocyte‐containing complement receptor type 2 and CD19. J Exp Med 1991; 173:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suryani S, Fulcher DA, Santner‐Nanan B et al Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells. Blood 2010; 115:519–29. [DOI] [PubMed] [Google Scholar]
- 6. Rubtsova K, Rubtsov AV, Cancro MP, Marrack P. Age‐associated B cells: a T‐bet‐dependent effector with roles in protective and pathogenic immunity. J Immunol 2015; 195:1933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thorarinsdottir K, Camponeschi A, Gjertsson I, Martensson IL. CD21(–/low) B cells: a snapshot of a unique B cell subset in health and disease. Scand J Immunol 2015; 82:254–61. [DOI] [PubMed] [Google Scholar]
- 8. Hao Y, O'Neill P, Naradikian MS, Scholz JL, Cancro MP. A B cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood 2011; 118:1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubtsov AV, Rubtsova K, Fischer A et al Toll‐like receptor 7 (TLR7)‐driven accumulation of a novel CD11c(+) B cell population is important for the development of autoimmunity. Blood 2011; 118:1305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keenan RA, De Riva A, Corleis B et al Censoring of autoreactive B cell development by the pre‐B cell receptor. Science 2008; 321:696–9. [DOI] [PubMed] [Google Scholar]
- 11. Grimsholm O, Ren W, Bernardi AI et al Absence of surrogate light chain results in spontaneous autoreactive germinal centres expanding V(H)81X‐expressing B cells. Nat Commun 2015; 6:7077. [DOI] [PubMed] [Google Scholar]
- 12. Ehrhardt GR, Hsu JT, Gartland L et al Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue‐based population of memory B cells. J Exp Med 2005; 202:783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moir S, Ho J, Malaspina A et al Evidence for HIV‐associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV‐infected viremic individuals. J Exp Med 2008; 205:1797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Charles ED, Brunetti C, Marukian S et al Clonal B cells in patients with hepatitis C virus‐associated mixed cryoglobulinemia contain an expanded anergic CD21low B cell subset. Blood 2011; 117:5425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Terrier B, Joly F, Vazquez T et al Expansion of functionally anergic CD21–/low marginal zone‐like B cell clones in hepatitis C virus infection‐related autoimmunity. J Immunol 2011; 187:6550–63. [DOI] [PubMed] [Google Scholar]
- 16. Isnardi I, Ng YS, Menard L et al Complement receptor 2/CD21– human naive B cells contain mostly autoreactive unresponsive clones. Blood 2010; 115:5026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wehr C, Eibel H, Masilamani M et al A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol 2004; 113:161–71. [DOI] [PubMed] [Google Scholar]
- 18. Saadoun D, Terrier B, Bannock J et al Expansion of autoreactive unresponsive CD21–/low B cells in Sjogren's syndrome‐associated lymphoproliferation. Arthritis Rheum 2013; 65:1085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weiss GE, Crompton PD, Li S et al Atypical memory B cells are greatly expanded in individuals living in a malaria‐endemic area. J Immunol 2009; 183:2176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood 2005; 105:4390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev 2004; 197:179–91. [DOI] [PubMed] [Google Scholar]
- 22. Perez‐Andres M, Paiva B, Nieto WG et al Human peripheral blood B cell compartments: a crossroad in B cell traffic. Cytometry B Clin Cytom 2010; 78 Suppl 1:S47–S60. [DOI] [PubMed] [Google Scholar]
- 23. Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol 2008; 20:67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smet J, Mascart F, Schandene L. Are the reference values of B cell subpopulations used in adults for classification of common variable immunodeficiencies appropriate for children? Clin Immunol 2011; 138:266–73. [DOI] [PubMed] [Google Scholar]
- 25. Weller S, Mamani‐Matsuda M, Picard C et al Somatic diversification in the absence of antigen‐driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J Exp Med 2008; 205:1331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fecteau JF, Cote G, Neron S. A new memory CD27‐IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J Immunol 2006; 177:3728–36. [DOI] [PubMed] [Google Scholar]
- 27. Wei C, Anolik J, Cappione A et al A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol 2007; 178:6624–33. [DOI] [PubMed] [Google Scholar]
- 28. Morrison VL, Barr TA, Brown S, Gray D. TLR‐mediated loss of CD62L focuses B cell traffic to the spleen during Salmonella typhimurium infection. J Immunol 2010; 185:2737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wirths S, Lanzavecchia A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur J Immunol 2005; 35:3433–41. [DOI] [PubMed] [Google Scholar]
- 30. Vazquez BN, Laguna T, Carabana J, Krangel MS, Lauzurica P. CD69 gene is differentially regulated in T and B cells by evolutionarily conserved promoter‐distal elements. J Immunol 2009; 183:6513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pinna D, Corti D, Jarrossay D, Sallusto F, Lanzavecchia A. Clonal dissection of the human memory B cell repertoire following infection and vaccination. Eur J Immunol 2009; 39:1260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zabel MD, Weis JH. Cell‐specific regulation of the CD21 gene. Int Immunopharmacol 2001; 1:483–93. [DOI] [PubMed] [Google Scholar]
- 33. Klein U, Kuppers R, Rajewsky K. Evidence for a large compartment of IgM‐expressing memory B cells in humans. Blood 1997; 89:1288–98. [PubMed] [Google Scholar]
- 34. Toyama H, Okada S, Hatano M et al Memory B cells without somatic hypermutation are generated from Bcl6‐deficient B cells. Immunity 2002; 17:329–39. [DOI] [PubMed] [Google Scholar]
- 35. Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll‐like receptors in acquired immunity: up‐regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood 2003; 101:4500–4. [DOI] [PubMed] [Google Scholar]
- 36. Rakhmanov M, Keller B, Gutenberger S et al Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate‐like B cells. Proc Natl Acad Sci USA 2009; 106:13451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rakhmanov M, Gutenberger S, Keller B, Schlesier M, Peter HH, Warnatz K. CD21low B cells in common variable immunodeficiency do not show defects in receptor editing, but resemble tissue‐like memory B cells. Blood 2010; 116:3682–3. [DOI] [PubMed] [Google Scholar]
- 38. Muellenbeck MF, Ueberheide B, Amulic B et al Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies. J Exp Med 2013; 210:389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zinocker S, Schindler CE, Skinner J et al The V gene repertoires of classical and atypical memory B cells in malaria‐susceptible West African children. J Immunol 2015; 194:929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Antibodies used in the study
Fig. S1. Strategies to isolate different B cell populations from human peripheral blood. Total B cells were isolated by depletion of non‐B cells using magnetic beads (upper panel). Total B cells were isolated as above, followed by sorting of naïve (CD19+21+27−), memory (CD19+21+27+) and CD21−/low B cells on a cell sorter (lower panel).


