Summary
A high number of Leishmania‐responder T cells is found in cutaneous leishmaniasis lesions, suggesting that important immunological events occur at the site of infection. Although activated, cytotoxic and regulatory T cells infiltrating into lesions may influence disease pathogenesis, the role of the T cell differentiation pattern of lymphocytes in lesions is unknown. Our aim was to investigate whether the phase of lesion development (early or late) is influenced by the functional status of cells present in inflammatory infiltrate. Activation, cytotoxity and T cell differentiation molecules were evaluated in lesion mononuclear cells by flow cytometry. The frequency of T cells was correlated with the lesion area (r = 0·68; P = 0·020). CD4+CD25+ T cells predominated over CD4+CD69+ T cells in early lesions (less than 30 days), whereas late lesions (more than 60 days) exhibited more CD4+CD69+ T cells than CD4+CD25+ T cells. The duration of illness was correlated positively with CD4+CD69+ (r = 0·68; P = 0·005) and negatively with CD4+CD25+ T cells (r = −0·45; P = 0·046). Most CD8+ T cells expressed cytotoxic‐associated molecules (CD244+), and the percentages were correlated with the lesion area (r = 0·52; P = 0·04). Both CD4+ and CD8+ effector memory T cells (TEM‐CD45RO+CCR7–) predominated in CL lesions and were significantly higher than central memory (TCM‐CD45RO+CCR7+) or naive T cells (CD45RO–CCR7+). An enrichment of TEM cells and contraction of naive T cells were observed in lesions in comparison to blood (P = 0·006) for both CD4+ and CD8+ T cells. Lesion chronicity is associated with a shift in activation phenotype. The enrichment of TEM and activated cytotoxic cells can contribute to immune‐mediated tissue damage.
Keywords: cutaneous leishmaniasis lesions, flow cytometry, memory T cell subsets
Introduction
American tegumentary leishmaniasis (ATL) in Brazil is caused largely by Leishmania (Viannia) braziliensis. The most common clinical form is cutaneous leishmaniasis (CL), which is characterized by single or multiple skin ulcers that may heal spontaneously or after specific treatment. However, the histopathological pattern is quite variable and is composed of granulomas and necrotic areas. Although parasite forms are rare in the lesion infiltrate 1, lymphocytes, macrophages and plasma cells are present in CL skin infiltrate 2.
In‐vivo and in‐vitro studies have shown the key role of the cellular immune response in the pathogenesis of CL 3, 4, 5, 6, 7, 8. As expected, T cell subtypes present in skin ulcers do not reflect the cell sets circulating in blood 9, 10, and the lower percentage of CD8+ T cells in blood in comparison with lesions, along with an increase in Leishmania‐specific T cells in lesions, is probably related to a migratory process between these compartments 5, 9. Together, these results indicate that the most important events may occur in inflammatory sites, possibly playing more relevant roles in disease immunopathogenesis.
Memory CD45RO molecules are present in the cellular inflammatory infiltrate of CL lesions 11, 12, 13. Different functional subtypes of these memory T cells are now recognized based on the expression of CD45RO in association with homing molecules such as CD62L and CCR7 14, 15, 16. CCR7 is a constitutive chemokine receptor that controls migration to secondary lymphoid organs and is considered an adequate marker for memory T cell subtypes, because CD62L expression is heterogeneous in these subtypes. According to this model, effector memory T cells (TEM) express CD45RO but lack molecules associated with homing to lymph nodes (e.g. CCR7); this subtype is ready to respond to a stimulus secreting cytokines and/or cytotoxic mediators. In contrast, central memory T cells (TCM) express both CD45RO and CCR7, as well as CD62L, and consequently recirculate in secondary lymphoid organs. Naive T cells fail to express the CD45RO molecule but exhibit CCR7 and CD62L receptors, trafficking through secondary lymphoid organs until encountering a specific stimulus 14, 17.
Cells expressing different activation molecules are present in the inflammatory infiltrate 9, 11, 12, 13, 18. In chronic CL lesions, activated cells express CD69 molecule 18. Activated CD25+ cells are higher in number in mucosal lesions than in skin lesions 9, suggesting that hyper‐reactivity of cells contributes to a poor disease prognosis. Moreover, regulatory T cells (Treg) CD4+CD25+forkhead box protein 3 (FoxP3+) migrate to leishmaniasis lesions 19 and exert a suppressor role in the inflammatory infiltrate of CL lesions 20. Greater gene expression of FoxP3 in patients with chronic and refractory treatment CL patients suggests that Treg cells in the lesions are functionally suppressive and can influence the pathogenesis of disease 20, 21.
In this work, our aim was to characterize T cells infiltrating CL lesions in terms of activation and memory subsets and to evaluate the relationship of these subtypes within the context of disease immunopathology. Our goal was to investigate whether the phase of lesion development (early or late) is influenced by the functional status of the cells present in inflammatory infiltrate. For these purposes, skin lesions and blood compartments were evaluated.
Materials and methods
Patients
Twenty‐three CL patients participated in the study. CL diagnosis was confirmed by clinical, parasitological and immunological criteria, as described elsewhere 22, 23. The patients were treated with a pentavalent antimonial (N‐methyl‐glucamine) according to the guidelines of the Brazilian Ministry of Health; follow‐up occurred thereafter. Blood was drawn after informed consent was obtained from each subject prior to therapy. All the procedures were approved by the ethical committees for human research (Instituto Nacional de Infectologia Evandro Chagas/FIOCRUZ and Hospital Universitário Edgar Santos/UFBA).
Collection of mononuclear cells from blood and cutaneous leishmaniasis lesions
Peripheral blood mononuclear cells (PBMCs) were separated by centrifugation over a gradient of Ficoll‐Hypaque (Histopaque 1077; Sigma Chemical Company, St Louis, MO, USA). The cells were resuspended in RPMI‐1640 supplemented with 10 mM HEPES, 1·5 μM l‐glutamine, 0·04 mM 2‐mercaptoethanol, (RPMI‐supplemented; Sigma). PBMCs were adjusted to 3 × 106 cells/ml and processed for the phenotypical analysis of surface molecules.
An incisional biopsy from the skin lesion border was performed for diagnostic purposes, and part of the tissue fragment was separated for the extraction of cells. Leishmaniasis lesion mononuclear cells (LMC) were obtained as described elsewhere 9. In brief, the skin specimen, stripped of subcutaneous fat, was placed in a tissue sieve fitted with a 64‐μm mesh filter and containing supplemented RPMI, and the cells were separated mechanically using a stick. The single‐cell suspension obtained was washed once, and the mononuclear cells were separated by centrifugation over a Ficoll–Hypaque gradient (Sigma). The LMCs (106/ml) were resuspended in cold phosphate‐buffered saline (PBS) containing 0·01% sodium azide (NaN3; Sigma) and 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA) (PBSAz/FBS) and processed for phenotypical analysis.
Phenotypical characterization of T cell subsets and surface molecules related to migration
Ex‐vivo PBMCs and LMCs (106 cells in 200 µl of PBSAz/FBS) were incubated for 30 min at 4°C in the presence of 5 μl of fluorescein isothiocyanate (FITC)‐, phycoerythrin (PE)‐, tandem conjugate phycoerythrin‐cyanin 5 (PE‐Cy5)‐ and tandem conjugate phycoerythrin‐cyanin 7 (PE‐Cy7)‐labelled monoclonal antibodies. After incubation, the cells were washed in PBSAz/FBS and resuspended in a fixation solution containing 1% paraformaldehyde in PBS prior to the analysis. Three‐ or four‐colour cytofluorimetry protocols were created for each sample: CD3‐PC5/CD4‐FITC/CD8‐PE, CD4‐PECy7/CD8‐PECy5/CD69‐PE/CD25‐FITC, CD4‐PECy7/CD8‐PECy5/CCR7‐PE/CD45RO‐FITC and CD3‐PECy5/CD8‐FITC/CD244‐PE (Immunotech, Beckman Coulter Corporation, Marseille, France).
For the flow cytometry analysis, 30 000 events in the total lymphocyte gate (R1) per sample were acquired using a fluorescence‐activated cell sorter (CyAn ADP analyzer; Beckman Coulter, Fullerton, CA, USA). Surface molecules were analysed for total lymphocytes or in gates defined electronically in T CD4+ and in T CD8+ cell populations using Summit 4·3 software (DakoCytomation, Fort Collins, CO, USA). The total lymphocyte gate (R1) was considered based on size [forward‐scatter (FSC)] and granularity [side‐scatter (SSC)]. Positive cells were defined (or gated) based on the control sample with isotype antibodies. T cell subtypes (CD4+ or CD8+) were defined in the lymphocyte‐gated cells. The frequency of positive CD25, CD69, CD244 and memory subtypes was determined in the positive T cell subpopulation (CD4+ or CD8+) or in the total lymphocyte gate. For memory subtype characterization using CD45RO and CCR7, after electronic gate selection in CD4+ or in CD8+ cells, a dot‐plot graphic was created with CD45RO+ as the y‐axis and CCR7+ as the x‐axis. The R3, R4 and R6 quadrants correspond to memory effector cells (TEM‐CD45RO+CCR7–), central memory T cell (TCM‐CD45RO+CCR7+) and naive cells (CD45RO–CCR7+), respectively. The results are expressed as the percentage of positive cells and the mean of fluorescent intensity.
Statistical analysis
Mann–Whitney (two‐tailed) and Kruskal–Wallis tests were used to compare quantitative variables expressed as the median and interquartile range. The Fisher exact test was used to compare proportions, and correlations were performed by the Spearman test. The statistical analysis was performed using the software GraphPad Prism version 4·00 for Windows (GraphPad Software, San Diego, CA, USA). The results are expressed as median (interquartil range).
Results
The demographic and clinical features of the 23 CL participants of the study are shown in Table 1. The mean age was 36 ± 13 years, and 16 (69.5%) were male. The number of lesions ranged from one to four and the illness duration from 20 to 180 days. The lesion sizes were also variable, ranging from 1·3 cm2 to 12·3 cm2. The Montenegro skin test was positive in all patients in whom it was performed. The patients had acquired the disease in endemic areas for L. braziliensis infection in Rio de Janeiro State (11 cases) and Corte de Pedra, Bahia State (12 cases).
Table 1.
Clinical characteristics of cutaneous leishmaniasis patients
| Patient number | Age (years) | Gender | Number of lesions | Duration of illness (days) | Lesion size (cm2) | MST (mm) | Origin |
|---|---|---|---|---|---|---|---|
| 1 | 47 | M | 3 | n.d. | n.d. | n.d. | RJ |
| 2 | 38 | M | 3 | 60 | 4·71 | 34 | RJ |
| 3 | 19 | F | 1 | 90 | 3·93 | n.d. | RJ |
| 4 | 34 | M | 1 | 90 | 2·07 | 15 | RJ |
| 5 | 71 | M | 1 | 30 | 0·38 | 45 | RJ |
| 6 | 37 | M | 1 | 120 | n.d. | 52 | RJ |
| 7 | 52 | M | 2 | 60 | n.d. | 07 | RJ |
| 8 | 41 | M | 4 | 60 | 2·12 | n.d. | RJ |
| 9 | 19 | F | 1 | 180 | n.d. | 10 | RJ |
| 10 | 27 | M | 3 | 60 | n.d. | n.d. | RJ |
| 11 | 43 | M | 4 | 45 | n.d. | 10 | RJ |
| 12 | 39 | M | 1 | 30 | 1·43 | 16 | BA |
| 13 | 23 | F | 1 | 30 | 1·30 | 21 | BA |
| 14 | 24 | M | 2 | 30 | 1·51 | 14 | BA |
| 15 | 28 | M | 2 | 40 | 2·09 | 27 | BA |
| 16 | 34 | F | 1 | 21 | 23·5 | 20 | BA |
| 17 | 49 | M | 1 | 20 | 2·36 | 30 | BA |
| 18 | 28 | F | 1 | 30 | 1·57 | 21 | BA |
| 19 | 27 | M | 1 | 30 | 0·33 | 20 | BA |
| 20 | 15 | M | 1 | 120 | 12·37 | 12 | BA |
| 21 | 14 | F | 1 | 15 | 4·7 | 15 | BA |
| 22 | 18 | F | 2 | 30 | 10·6 | 11 | BA |
| 23 | 29 | M | 4 | 30 | 3·92 | 11 | BA |
M = male; F = female; n.d. = not determined; RJ = Rio de Janeiro; BA = Bahia, Corte de Pedra; MST = Montenegro skin test.
CD4+ T cells predominate in early cutaneous leishmaniasis lesions
The frequency of ex‐vivo CD3+ T cells in the CL lesions was 49·5% (71·6–42·1%) in the lymphocyte gate. The frequency of CD3+ T cells present in the lesions was correlated positively with the lesion area (r = 0·68; P = 0·020) (Fig. 1a). The percentage of CD4+CD3+ T lymphocytes was 43·4% (62·8–38·5%); 39·6% (49·9–30·8%) were CD8+CD3+ T lymphocytes. CD3+CD4+ T cells were predominant in recent lesions compared to CD3+CD8+ (P = 0.020) and against CD3+CD4+ in late lesions (P = 0·029) (Table 2). Late lesions showed a similar frequency of CD3+CD4+ and CD3+CD8+ T cells.
Figure 1.
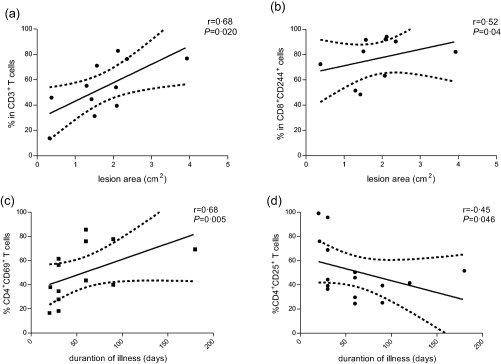
Cutaneous leishmaniasis in‐situ immune response is correlated with clinical parameters. Correlation between frequency of CD3+(a) or CD8 + CD244+(b) cytotoxic T cells and lesion area. Correlation between the duration of illness and CD4+CD69+(c) and CD4+CD25+(d) T cells. The graphs show the best fitted lines with 95% confidence intervals. P = significance level (Spearman's test); r = correlation coefficient.
Table 2.
Phenotypical characteristics of T cell subsets in cellular infiltrate of cutaneous leishmaniasis patients according to illness duration
| T cell markers | Early lesions (less than 30 days) % | Late Lesions (more than 60 days) % |
|---|---|---|
| CD3+ | 46·4 (13·7–76·3) (n = 8) | 54·0 (41·4–84·1) (n = 11) |
| CD3+CD4+ (*) | 51·7 (42·7–70·1) (n = 7) | 39·4 (20·1–74·6) (n = 11) |
| CD3+CD8+ | 41·5 (20·1–50·6) (n = 8) | 35·5 (19·4–66·1) (n = 11) |
| CD4+CD25+(*) | 68·9 (10·0–99·1) (n = 7) | 41·5 (24·4–87·3) (n = 6) |
| CD4+CD69+ (**) | 31·2 (16·4–61·4) (n = 7) | 69·1 (39·9–85·5) (n = 6) |
| CD8+CD25+ | 47·4 (14·1–99·9) (n = 7) | 35·7 (10·8–93·6) (n = 6) |
| CD8+CD69+ | 69·1 (11·0–96·8) (n = 7) | 46·3 (17·9–95·1) (n = 6) |
| CD8+CD244+ | 86·4 (48·4–92·0) (n = 6) | 82·3 (63·2–98·2) (n = 11) |
Comparison between recent and late lesions (*)P < 0·05; (**)P < 0.01. Results are expressed as the median; interquartile range.
CD4+ T cell activation in cutaneous leishmaniasis may depend upon illness duration
The analysis of activated T cells showed that half the T cell subtypes express CD25 or CD69 on their membrane surface [CD4+CD69+: 53·3% (75·9–34·5%); CD4+CD25+: 45·1% (70·6–34·7%)]. However, the percentage of CD4+ T cells presenting activation molecules was heterogeneous.
We assessed the contribution of the CD25+ and CD69+ activated T cell population to the overall inflammatory infiltrate of early lesions (fewer than 30 days of illness) and late lesions (more than 60 days of illness). In early lesions, CD4+CD25+ T cells [68·9% (95·6–36·4%)] predominated over CD4+CD69+ T cells [31·2% (43·8–17·7%)]. In contrast, a significant enrichment of CD4+CD69+ [69·1% (80·9–48·3%)] in comparison to CD4+CD25+ T cells [41·5% (51·4–29·6%)]) was observed in late lesions (Fig. 2, Table 2). These results were reinforced by negative and positive correlations to illness duration: CD4+CD25+ T cells (r = −0·45; P = 0·046) and CD4+CD69+ T cells (r = 0·68, P = 0·005) (Fig. 1c,d, respectively).
Figure 2.
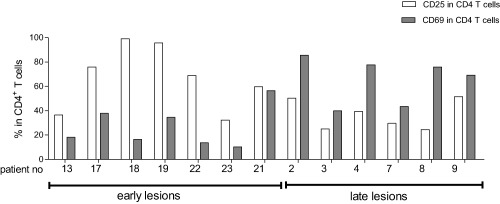
Activation‐associated CD25 and CD69 molecules in CD4+ T lymphocytes for early (duration of illness of less than 30 days; n = 7) or late (duration of illness of more than 60 days n = 6) cutaneous leishmaniasis lesions. The white bars represent the percentage of CD4+CD25+ T cells and the grey bars represent the percentage of CD4+CD69+ T cells. Each evaluated patient is represented by a pair of bars.
The percentages of activated CD8+ T cells expressing CD25+ [41·5% (80·0–15·9%)] or CD69+ molecules [46·3% (93·8–32·4%)] were variable, and no correlation with illness duration or lesion area was detected. However, the majority of CD8+ T cells presented phenotypes associated with cytotoxicity [CD244: 82·4% (93·0–74·4%)]. Lesion area was correlated positively to the presence of CD8+CD244+ T cells (r = 0·52; P = 0·04) in the infiltrate (Fig. 1b).
Enrichment of T effector memory cells in cutaneous leishmaniasis lesions
CD45RO and CCR7 were used to distinguish T lymphocytes into TCM (CD45RO+ CCR7+) or TEM (CD45RO+CCR7–) cells 14, 24, and TEM cells were found to predominate in both CD4+ [90·2% (91·6–79·8%); n = 4; P = 0·028] or CD8+ [68·5% (87·5–47·6%); n = 4; P = 0·028] T cell sets in CL lesions (Fig. 3a,b, respectively). Indeed, these TEM cells were significantly more abundant than TCM cells and naive T cells. The percentages of TCM cells were significantly high in the CD4+ T cell set [7·3% (11·3–5·0%)] when compared to naive T cells [1·1% (2·78–0·2%); P = 0·028], although this was not observed for CD8+ T cells [TCM: 3·4% (4·7–2·3%) and naive: 1·2% (4·9–0·1%)] (Fig. 3).
Figure 3.
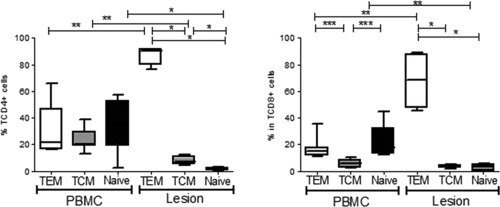
Cellular differentiation of TCD4+ and TCD8+ in effector memory T cells (TEM ‐ CD45RO+ CCR7−), central memory T cells (TCM ‐ CD45RO+CCR7+) and naive T cells (CD45RO−CCR7+) were evaluated in lesions inflammatory cell infiltrates (n = 4) and blood (n = 7) of cutaneous leishmaniasis patients. The results are expressed as median and interquartile interval. PBMC ‐ peripheral blood mononuclear cells *P < 0.05; **P < 0.005; ***P < 0.0005; one‐way analysis of variance (ANOVA).
In contrast, CD4+ blood memory T subtypes (TCM and TEM) and naive T cells had similar levels [TCM: 20·1% (29·9–18·9%); TEM: 21·6% (46·5–16·4%); naive: 31·5% (52·4–18·6%); n = 7] (Fig. 3a). In the CD8+ T cell set, the TCM levels were lower [5·9% (8·4–2·6%)] when compared with TEM [14·8% (17·9–11·8%); P = 0·006] or naive T cells [17·9% (32·5–13·4%); P = 0·006] (Fig. 3b). An enrichment of TEM and a contraction of naive T cells were observed in lesions in comparison with blood (P = 0·006), as observed for both CD4+ and CD8+ T cells (Fig. 3).
Discussion
Cellular immunity plays a central role in leishmaniasis immunopathogenesis. In this work, we showed that TEM cells predominate in CL lesions. In blood, TCM cells were in similar levels than other differentiated T cell subtypes. Both CD4+ and CD8+ compartments of the CL infiltrate exhibited the activation‐associated molecules CD25 and CD69. However, the presence of these molecules on activated CD4+ T cells was dependent upon the duration of illness. In addition, the frequency of CD8+CD244+ T cells was also correlated to the lesion area. Together, our results corroborate the hypothesis that activated effector T cells contribute to the chronicity of lesions, possibility by inducing tissue damage.
Initial studies using histopathology demonstrated an enrichment of T lymphocytes in cutaneous inflammatory infiltrate, relating these cells with the pathogenesis of the disease 1, 2. Corroborating this, immunohistochemistry 9, 10, 11, 13, 25, 26, 27 and flow cytometry analysis 9, 28 have also demonstrated that lymphocytes comprise half the cells present in lesions. Here, we demonstrate that the frequency of CD3+ T cells in infiltrate is correlated to the lesion area, reinforcing the suggestion that lesion extension, and thus tissue damage, can be generated by T lymphocytes 5, 29. In this sense, these features could explain why skin lymphocytes from larger cutaneous lesion areas tend to show the highest index of proliferation 30.
In our study, a great variability was observed in the percentage of infiltrating CD4+ and CD8+ T cells among patients, corroborating previous results 9, 31. This wide variability in the percentage of T cell subtypes occurs regardless of the technique employed (immunohistochemistry or flow cytometry) (data not shown).
In approximately half the CL lesions, T lymphocytes presented an activation phenotype (CD25 and CD69) in both the CD4+ and CD8+ populations. Surprisingly, when the patients were grouped by the duration of illness, the early‐lesion group showed a higher frequency of CD4+CD25+ T cells than CD4+CD69+ T cells. In contrast, CD4+CD69+ T cells predominated in late‐lesion group, represented by chronic lesions, as was also observed in Venezuela 18. These patterns were corroborated by correlation analysis. Together, these results suggest that activated T cells can contribute to tissue damage.
In CL lesions, the majority CD4+CD25+ T cells express regulatory associated molecules [FoxP3, cytotoxic T lymphocyte antigen (CTLA)−4, glucocorticoid‐induced tumour necrosis factor family receptor (GITR)], produce interleukin (IL)−10 and transforming growth factor (TGF)‐β regulatory cytokines and exhibit a functional regulatory characteristic 19. The higher FoxP3 expression in early compared to chronic CL lesions due to L. guyanensis 20 prompted us to hypothesize that the CD4+CD25+ T cells observed in early CL lesions in our study could exhibit a regulatory (Treg) function. The CL chronicity process should be accompanied by a functional switch of the lesion's cellular infiltrate. As a limitation of the present study, the functional phenotype of these two subtypes of activated cells was not evaluated.
The memory T cell phenotype (CD45RO) within CL lesions has been described 12, 13, 32. Notably, compared to CD4+ T cells, the low frequency of CD8+CD45RO+ memory T cells in CL lesions could be a consequence of an increased effector capacity. These highly differentiated CD8+ T cells suffer a loss of CD45RO expression, followed by the re‐expression of CD45RA 14, 16, 24. Another explanation could be the death of CD8+ effector T cells due to apoptosis 33.
Our results showed a higher percentage of the TEM compartment in CL lesions than in blood for both CD4+ and CD8 + T cells. This phenomenon was expected, because TEM is essential in inflammatory sites to maintain tissues homeostasis 34, produces cytokines or cytotoxic mediators 35, 36 and can express peripheral tissue homing molecules 36. In CL lesions, an effector function was suggested by the demonstration of cytokine and/or cytotoxic mediators produced by CD4+ or CD8+ cells 19, 37, 38, 39, 40 and cutaneous leucocyte antigen (CLA)‐positive cells 28. Conversely, a high percentage of TCM cells was observed in blood compared to lesions. As TCM cells home to lymph nodes and have a high proliferating capacity after a secondary stimulus, they constitute a reservoir of TEM‐specific cells 14, 41 in cured patients, potentially renewing effector memory T cells 42, 43. In experimental leishmaniasis, TCM cells protect mice against a second infection with L. major and display a plastic phenotype, potentially differentiating into T helper type 1 (Th1) or Th2 effector cells 41, 44. Beside this, it was recently shown that double negative T cells and NKT are the major cell population of the cytotoxic‐cell pool [45].
In conclusion, our results show that effector memory T, activated and cytotoxic cells predominate in CL lesions. We also show that a shift in the activation phenotype of skin inflammatory cells is associated with disease progression. Effector and activated cells should contribute to the pathogenesis of the disease.
Disclosure
There is no disclosures.
Acknowledgements
The authors thank Dr. Bertho from Plataforma de Citometria de Fluxo from Instituto Oswaldo Cruz and Alessandro M. Santos for sample flow cytometry acquisition. We also thank Dr Adriano Gomes‐Silva for helpful discussion and manuscript revision and Mr Lago and Ms Neuza from Posto de Saúde de Corte de Pedra for their support. This work was supported by IOC/FIOCRUZ internal funds, FAPERJ (E‐26/102.457/2010) and by the National Institutes of Health (NIH AI 30639). C. O. M.‐A. is a post‐doctoral fellow sponsored by FAPERJ/CAPES, Brazil. R. G.‐V. is a doctoral student sponsored by CNPq, Brazil. A. M. D.‐C. is a CNPq and FAPERJ (CNE) researcher fellow. E. M. C. is a senior researcher from CNPq.
References
- 1. Ridley DS. A histological classification of cutaneous leishmaniasis and its geographical expression. Trans R Soc Trop Med Hyg 1980; 74:515–21. [DOI] [PubMed] [Google Scholar]
- 2. Magalhães AV, Moraes MA, Raick AN et al Histopathology of cutaneous leishmaniasis by Leishmania braziliensis braziliensis. 1. Rev Inst Med Trop São Paulo 1986; 28:253–62. [DOI] [PubMed] [Google Scholar]
- 3. Castés M, Tapia FJ. Immunopathology of American tegumentary leishmaniasis. Acta Cient Venez 1998; 49:42–56. [PubMed] [Google Scholar]
- 4. Castés M, Agnelli A, Verde O, Rondon AJ. Characterization of the cellular immune response in American cutaneous leishmaniasis. Clin Immunol Immunopathol 1983; 27:176–86. [DOI] [PubMed] [Google Scholar]
- 5. Conceição‐Silva F, Dorea RC, Pirmez C, Schubach A, Coutinho SG. Quantitative study of Leishmania braziliensis braziliensis reactive T cells in peripheral blood and in the lesions of patients with American mucocutaneous leishmaniasis. Clin Exp Immunol 1990; 79:221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carvalho EM, Johnson WD, Barreto E et al Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol 1985; 135:4144–8. [PubMed] [Google Scholar]
- 7. Mendonça SC, Coutinho SG, Amendoeira RR, Marzochi MC, Pirmez C. Human American cutaneous leishmaniasis (Leishmania b. braziliensis) in Brazil: lymphoproliferative responses and influence of therapy. Clin Exp Immunol 1986; 64:269–76. [PMC free article] [PubMed] [Google Scholar]
- 8. Da‐Cruz AM, Bittar R, Mattos M et al T‐cell‐mediated immune responses in patients with cutaneous or mucosal leishmaniasis: long‐term evaluation after therapy. Clin Diagn Lab Immunol 2002; 9:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Da‐Cruz AM, Bertho AL, Oliveira‐Neto MP, Coutinho SG. Flow cytometric analysis of cellular infiltrate from American tegumentary leishmaniasis lesions. Br J Dermatol 2005; 153:537–43. [DOI] [PubMed] [Google Scholar]
- 10. Modlin RL, Tapia FJ, Bloom BR et al In‐situ characterization of the cellular immune response in American cutaneous leishmaniasis. Clin Exp Immunol 1985; 60:241–8. [PMC free article] [PubMed] [Google Scholar]
- 11. Pirmez C, Cooper C, Paes‐Oliveira M, Schubach A, Torigian VK, Modlin RL. Immunologic responsiveness in American cutaneous leishmaniasis lesions. J Immunol 1990; 145:3100–4. [PubMed] [Google Scholar]
- 12. Martínez‐Arends A, Tapia FJ, Caceres‐Dittmar G, Mosca W, Valecillos L, Convit J. Immunocytochemical characterization of immune cells in lesions of American cutaneous leishmaniasis using novel T cell markers. Acta Trop 1991; 49:271–80. [DOI] [PubMed] [Google Scholar]
- 13. Esterre P, Dedet JP, Frenay C, Chevallier M, Grimaud JA. Cell populations in the lesion of human cutaneous leishmaniasis: a light microscopical, immunohistochemical and ultrastructural study. Virchows Arch A Pathol Anat Histopathol 1992; 421:239–47. [DOI] [PubMed] [Google Scholar]
- 14. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Ann Rev Immunol 2004; 22:745–63. [DOI] [PubMed] [Google Scholar]
- 15. Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol 2003; 4:835–42. [DOI] [PubMed] [Google Scholar]
- 16. Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol 2012; 12:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Badovinac VP, Harty JT. Memory lanes. Nat Immunol 2003; 4:212 [DOI] [PubMed] [Google Scholar]
- 18. Diaz NL, Zerpa O, Ponce LV, Convit J, Rondon AJ, Tapia FJ. Intermediate or chronic cutaneous leishmaniasis: leukocyte immunophenotypes and cytokine characterisation of the lesion. Exp Dermatol 2002; 11:34–41. [DOI] [PubMed] [Google Scholar]
- 19. Campanelli AP, Roselino AM, Cavassani KA et al CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis 2006; 193:1313–22. [DOI] [PubMed] [Google Scholar]
- 20. Bourreau E, Ronet C, Darcissac E et al Intralesional regulatory T‐cell suppressive function during human acute and chronic cutaneous leishmaniasis due to Leishmania guyanensis . Infect Immun 2009; 77:1465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Costa DL, Cardoso TM, Queiroz A et al Tr‐1‐like CD4+CD25‐CD127‐/lowFOXP3‐ cells are the main source of interleukin 10 in patients with cutaneous leishmaniasis due to Leishmania braziliensis . J Infect Dis 2015; 211:708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vieira‐Gonçalves R, Pirmez C, Jorge ME et al Clinical features of cutaneous and disseminated cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis in Paraty, Rio de Janeiro. Int J Dermatol 2008; 47:926–32. [DOI] [PubMed] [Google Scholar]
- 23. Jirmanus L, Glesby MJ, Guimarães LH et al Epidemiological and clinical changes in American tegumentary leishmaniasis in an area of Leishmania (Viannia) braziliensis transmission over a 20‐year period. Am. J Trop Med Hyg 2012; 86:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401:708–12. [DOI] [PubMed] [Google Scholar]
- 25. Barral A, Jesus AR, Almeida RP et al Evaluation of T‐cell subsets in the lesion infiltrates of human cutaneous and mucocutaneous leishmaniasis. Parasite Immunol 1987; 9:487–97. [DOI] [PubMed] [Google Scholar]
- 26. Nilsen R, Mshana RN. In situ characterization of the cutaneous immune response in Ethiopian cutaneous leishmaniasis. Scand J Immunol 1987; 26:503–12. [DOI] [PubMed] [Google Scholar]
- 27. Morgado FN, Schubach A, Rosalino CM et al Is the in situ inflammatory reaction an important tool to understand the cellular immune response in American tegumentary leishmaniasis? Br J Dermatol 2008; 158:50–8. [DOI] [PubMed] [Google Scholar]
- 28. Mendes‐Aguiar CO, Gomes‐Silva A, Nunes E Jr et al The skin homing receptor cutaneous leucocyte‐associated antigen (CLA) is up‐regulated by Leishmania antigens in T lymphocytes during active cutaneous leishmaniasis. Clin Exp Immunol 2009; 157:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett 2005; 101:226–30. [DOI] [PubMed] [Google Scholar]
- 30. Da‐Cruz AM, Oliveira‐Neto MP, Bertho AL, Mendes‐Aguiar CO, Coutinho SG. T cells specific to Leishmania and other nonrelated microbial antigens can migrate to human leishmaniasis skin lesions. J Invest Dermatol 2010; 130:1329–36. [DOI] [PubMed] [Google Scholar]
- 31. Palma GIS, Saravia NG. In situ characterization of the human host response to Leishmania panamensis . Am J Dermatopathol 1997; 19:5. [DOI] [PubMed] [Google Scholar]
- 32. Pirmez C. Immunopathology of American cutaneous leishmaniasis. Mem Inst Oswaldo Cruz 1992; 87(Suppl.5):105–9. [DOI] [PubMed] [Google Scholar]
- 33. Bertho AL, Santiago MA, Da‐Cruz AM, Coutinho SG. Detection of early apoptosis and cell death in T CD4+ and CD8+ cells from lesions of patients with localized cutaneous leishmaniasis. Braz J Med Biol Res 2000; 33:317–25. [DOI] [PubMed] [Google Scholar]
- 34. Wherry EJ, Teichgraber V, Becker TC et al Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol 2003; 4:225–34. [DOI] [PubMed] [Google Scholar]
- 35. Sallusto F. The role of chemokines and chemokine receptors in T cell priming and Th1/Th2‐mediated responses. Haematologica 1999; 84 (Suppl.EHA‐4):28–31. [PubMed] [Google Scholar]
- 36. Mora JR, von Andrian UH. T‐cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol 2006; 27:235–43. [DOI] [PubMed] [Google Scholar]
- 37. Faria DR, Gollob KJ, Barbosa J Jr et al Decreased in situ expression of interleukin‐10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun 2005; 73:7853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Faria DR, Souza PE, Duraes FV et al Recruitment of CD8(+) T cells expressing granzyme A is associated with lesion progression in human cutaneous leishmaniasis. Parasite Immunol 2009; 31:432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bacellar O, Faria D, Nascimento M et al Interleukin 17 production among patients with American cutaneous leishmaniasis. J Infect Dis 2009; 200:75–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Santos CS, Boaventura V, Ribeiro Cardoso C et al CD8(+) granzyme B(+)‐mediated tissue injury vs. CD4(+)IFN gamma(+)‐mediated parasite killing in human cutaneous leishmaniasis. J Invest Dermatol 2013; 133:1533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pakpour N, Zaph C, Scott P. The central memory CD4+ T cell population generated during Leishmania major infection requires IL‐12 to produce IFN‐gamma. J Immunol 2008; 180:8299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keshavarz Valian H, Nateghi Rostami M, Tasbihi M et al CCR7+ central and CCR7‐ effector memory CD4+ T cells in human cutaneous leishmaniasis. J Clin Immunol 2013; 33:220–34. [DOI] [PubMed] [Google Scholar]
- 43. Pereira‐Carvalho R, Mendes‐Aguiar CO, Oliveira‐Neto MP et al Leishmania braziliensis‐reactive T cells are down‐regulated in long‐term cured cutaneous leishmaniasis, but the renewal capacity of T effector memory compartments is preserved. PloS One 2013; 8:e81529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long‐term immunity to Leishmania major in the absence of persistent parasites. Nat Med 2004; 10:1104. [DOI] [PubMed] [Google Scholar]
- 45. R Ferraz, CF Cunha, A Gomes‐Silva, AO Schubach, MI Pimentel, MR Lyra, SC Mendonca, CM Valete‐Rosalino, AM Da‐Cruz, AL Bertho. Apoptosis and frequency of total and effector CD8+ T lymphocytes from cutaneous leishmaniasis patients during antimonial therapy. BMC Infectious Diseases 2015; 15:74. [DOI] [PMC free article] [PubMed] [Google Scholar]


