Abstract
Type 2 diabetes (T2D) is a disease of pandemic proportions, one defined by a complex aetiological mix of genetic, epigenetic, environmental, and lifestyle risk factors. Whilst the last decade of T2D genetic research has identified more than 100 loci showing strong statistical association with disease susceptibility, our inability to capitalise upon these signals reflects, in part, a lack of appropriate human cell models for study. This review discusses the impact of two complementary, state-of-the-art technologies on T2D genetic research: the generation of stem cell-derived, endocrine pancreas-lineage cells and the editing of their genomes. Such models facilitate investigation of diabetes-associated genomic perturbations in a physiologically representative cell context and allow the role of both developmental and adult islet dysfunction in T2D pathogenesis to be investigated. Accordingly, we interrogate the role that patient-derived induced pluripotent stem cell models are playing in understanding cellular dysfunction in monogenic diabetes, and how site-specific nucleases such as the clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 system are helping to confirm genes crucial to human endocrine pancreas development. We also highlight the novel biology gleaned in the absence of patient lines, including an ability to model the whole phenotypic spectrum of diabetes phenotypes occurring both in utero and in adult cells, interrogating the non-coding ‘islet regulome’ for disease-causing perturbations, and understanding the role of other islet cell types in aberrant glycaemia. This article aims to reinforce the importance of investigating T2D signals in cell models reflecting appropriate species, genomic context, developmental time point, and tissue type.
Keywords: human endocrine pancreas derivation, Induced pluripotent stem cells, CRISPR-Cas9, maturity-onset diabetes of the young, Wolfram syndrome
Introduction
Main question or problem
Type 2 diabetes (T2D) is a global health burden. Given that more than 415 million individuals are currently affected and that the incidence is predicted to rise faster than the adult population growth rate 1, it could be argued that our current preventative and therapeutic strategies against this disorder are inadequate.
Understanding T2D pathophysiology is inherently difficult because of its complex aetiology; an individual’s disease risk is based on a combination of genetic, epigenetic, environmental, and lifestyle risk factors 2, 3. However, the last decade or so has seen a transformation in our understanding of the genetic basis of this disease; through large-scale international collaborations and DNA samples from hundreds of thousands of individuals, common and rare variant association studies have identified more than 100 genomic loci influencing T2D susceptibility 4, 5. Also, for T2D, and unlike many other complex genetic disorders, we have a good handle on the tissue driving pathogenesis; despite perturbations to both insulin secretion and sensitivity, multiple studies place pancreatic islet dysfunction at centre stage in terms of disease susceptibility and progression 6– 8.
Despite this wealth of information, our ability to go from genetic signal to mechanism (and even therapeutic target) has progressed at a pace far slower than that of the initial discoveries of these disease susceptibility loci. Why?
Specifics about the questions or problem
Multiple factors underlie the difficulties in biological interpretation of genome-wide association study data. Firstly, we need to know which transcript(s) are driving the phenotypic signal. This has formed a huge stumbling block for researchers as (i) extensive regions of linkage-disequilibrium mean that most associated loci harbour many genes and transcripts, (ii) many signals lie within poorly annotated, non-coding regions of the genome (although efforts to map the ‘islet regulome’ are beginning to bear fruit 9– 12), and (iii) the modest effect sizes of disease-associated variants make functional interrogation of risk versus non-risk alleles problematic (odds ratios are usually between 1.1 and 1.4 4, 5).
Secondly, far and away one of the biggest challenges has been the lack of appropriate human islet cell models for study. Until very recently, this was limited to animal models and rodent insulinoma cell lines, which present numerous challenges; there are multiple instances in which human diabetic phenotypes are not recapitulated in the analogous murine model of gene haploinsufficiency 13– 24, and differences in islet architecture, ion channel composition, nutrient sensitivity, and other physiological parameters 25– 31 limit the functional inferences that can be made from rodent-derived data. Human islet isolation programmes and the subsequent availability of this tissue for research purposes have gone some way to alleviate this bottleneck, as has the recent generation of human beta-cell lines from pancreas explants 32, 33, although these latter cells are only just beginning to be characterised 34.
Thirdly, despite increasing access to human islets and cell lines, many technical constraints remain: (i) human islets are heterogeneous in terms of donor genotype and function/viability after surgical extraction, (ii) the restriction of islet isolation programmes to adult donors limits study to mature cells, (iii) human beta-cell lines represent only a single islet cell type, and (iv) low recombination rates and an inability to expand single clones make genomic manipulation via site-specific nucleases challenging.
What is to come in the rest of the review
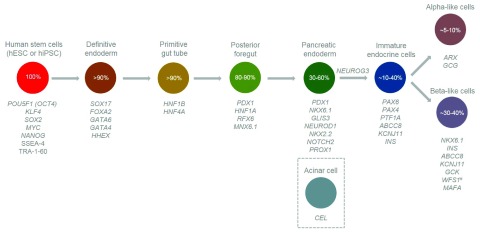
This article will focus on one of the most exciting emerging fields in diabetes research at present: human endocrine pancreas derivation in a dish. The utilisation of state-of-the-art in vitro differentiation techniques to turn human pluripotent stem cells into those of the islet lineage 35– 41 allows researchers to sequentially generate definitive endoderm cells (expressing SOX17 and FOXA2) through to pancreatic progenitors ( PDX1- and NKX6.1-positive), all the way to cells expressing insulin, glucagon, and islet transcription factors regulating mature cell function ( MAFA).
This model system has broad application in many areas of islet biology and diabetes research. Firstly, it can be used as a platform for drug discovery efforts aimed at increasing functional beta-cell mass, and importantly, one which is without many of the ethical, legal, and practical considerations surrounding the routine use of human tissue (both foetal and adult). Induced pluripotent stem cells (iPSCs) specifically bypass the need for embryonic tissue as they can be generated by reprogramming any somatic cell 42, 43. Secondly, the ability to further mature these cells in vivo, and to phenotypically correct diabetes in immunocompromised mice 38– 40, 44– 49, also shows the translational potential of such cells, with analogous clinical trials beginning to take place in humans 50. Both of these areas have been reviewed extensively elsewhere 51– 56. Instead, the rest of this article will focus on the potential of stem cell-derived islet-lineage cells in disease modelling, in particular how they can be manipulated with genome editing tools such as CRISPR-Cas9 57, 58, so as to accurately recapitulate the genomic, developmental, and mature cell perturbations underlying T2D pathogenesis 59 ( Figure 1).
Figure 1. Expression time points for genes important to endocrine pancreas development and diabetes pathology.
Circles represent discrete developmental stages, with derivation efficiency estimates also shown 59. Genes discussed in this article are listed according to the developmental stage at which they are first expressed and any subsequent stages where they perform important biological functions or are crucial for cell identity ( #except WFS1 which is expressed at all stages; however, the diabetes observed in patients with Wolfram syndrome is believed to result from selective beta-cell loss via apoptosis 76). CEL is expressed in acinar cells, which differentiate from multipotent pancreatic progenitor cells and subsequently exocrine progenitor cells (not depicted in the figure). hESC, human embryonic stem cell; hiPSC, human induced pluripotent stem cell.
Diabetes modelling using patient-derived cells
Recent methodological advances in endocrine pancreas differentiation have promoted formation of mono-hormonal cells with function similar to (but not quite yet the same as) that of human islets 38– 41. However, variation in line-to-line differentiation efficiencies 60– 62 coupled with an inability to make fully mature cells 63 has so far limited disease modelling to monogenic diabetes caused by highly penetrant, large-effect mutations.
One of the first proof-of-principle studies 64 generated iPSC lines from individuals with maturity-onset diabetes of the young (MODY) by using a polycistronic lentiviral vector overexpressing the so-called ‘Yamanaka factors’ ( POU5F1 [OCT4], KLF4, SOX2, and MYC), these needed for somatic cell reprogramming to pluripotency 42. This included lines from patients with mutations in endocrine pancreas developmental transcription factors ( HNF1B, HNF4A, and HNF1A), as well as those with perturbed enzymes governing glucose-stimulated insulin secretion (GSIS) in mature cells ( GCK), and even exocrine pancreas function ( CEL). Regardless of mutated gene, all lines were shown to fulfil basic iPSC quality control: expression of pluripotency genes via fluorescence-activated cell sorting (OCT4, SOX2, NANOG, SSEA-4, and TRA-1-60), spontaneous teratoma formation upon transplant into immunocompromised mice (cells capable of generating all three germ layers), and a diploid ‘stable’ karyotype 64. Another study aimed at generating iPSCs from patients with HNF1A-MODY 65 again produced cells passing basic pluripotency QC, and which were able to differentiate from embryoid bodies, into those expressing insulin and glucagon. Of note here is that these hormones were not present at levels comparable to those seen in other studies 39, 40, perhaps reflecting the quite different in vitro differentiation strategies employed. Likewise, the inability of these cells to form teratomas spontaneously in vivo suggests that reprogramming to full pluripotency may not have been achieved.
Other diabetes iPSC models have focussed on characterising cellular dysfunction apparent within mature islets, making endocrine pancreas differentiation essential for phenotyping patient-derived cells. Individuals with heterozygous GCK mutations have a mild phenotype whereby fasting plasma glucose levels are marginally elevated (6 to 8 mmol/L) because of a higher threshold for GSIS, which is governed by altered beta-cell glucose uptake and glycolytic flux 66, 67. Directed differentiation of iPSCs from patients with GCK-MODY down the islet lineage occurred with an efficiency comparable to that of control cells, with the only observable defects mirroring patient phenotype (elevated GSIS set-point), thus validating this as a physiologically representative model for studying monogenic GCK mutations 68.
iPSC models have also been generated for syndromic diabetes disorders, such as Wolfram syndrome. This disorder is caused by mutations in WFS1 69, with patients suffering from multi-organ dysfunction, including diabetes, optic atrophy, and neurodevelopmental defects 70. Such a broad phenotype reflects the multi-tissue expression of WFS1, with the encoded Wolframin protein performing vital roles in endoplasmic reticulum (ER) Ca 2+ homeostasis 71– 73 as well as alleviating ER stress in cells with high translational load 74, such as those with secretory function 75. This is thought to explain the childhood-onset diabetes in these individuals, with post-mortem study of Wolfram syndrome pancreases suggesting selective beta-cell loss via apoptosis 76. Directed differentiation of iPSCs from patients with Wolfram syndrome down the islet lineage showed that these cells had elevated levels of chemically induced ER stress, which resulted in translational stasis and decreased insulin processing and content. Likewise, in vivo maturation of patient cells showed that grafts declined in function much more rapidly than control cells, perhaps reflecting enhanced apoptosis 77.
The need for phenotypic correction of patient stem cells
Importantly, the cellular dysfunction observed in both diabetes iPSC-derived models was corrected via genetic (zinc finger nuclease 68) or chemical (4-phenylbutyric acid 77) means. This phenotypic correction is fundamental in assigning causality to the studied mutation of interest, particularly as large-scale sequencing studies are continuing to identify previously reported ‘disease-causing’ mutations in unaffected individuals within the general population, leading to continued revision and reduction of penetrance estimates 78. Likewise, comparing patient lines to isogenic controls removes any differentiation efficiency or phenotypic effects driven by factors extrinsic to the particular mutation of interest, including reprogramming efficiency and epigenetic or sequence variation (or both) in the donor genome 60– 62.
A methodological advance which has revolutionised the ease at which we can generate isogenic control lines is the expansion of site-directed nuclease, so-called ‘genome editing’ technologies, from zinc finger nucleases 79, 80 to TALENs (transcription activator-like effector nucleases) 81– 85 and more recently to CRISPR-Cas9 57, 58, 86– 100. The most popular of these editing methods, CRISPR-Cas9, exploits a bacterial innate immune system response to pathogens, whereby the Cas9 endonuclease is targeted to invading phage DNA by a sequence-specific guide RNA molecule 101. In recent years, manipulation of this system so that it can target eukaryotic (specifically mammalian) genomes has allowed its full translational potential to be realised 57, 58, 102. The ability to target more or less any sequence in the human genome for gene knockout via non-homologous end-joining 103, nucleotide-level manipulation via homology-directed repair 104, 105, or larger recombination events to generate reporter lines 90, 106 or even bring into close proximity mediators of gene expression (such as activators or repressors tethered to modified Cas9 protein) 86– 88, 92, 93, 107 means that every type of genetic perturbation is theoretically possible. Use of this technology has also extended into simultaneous targeting of multiple genes 57, 87, 88, 98– 100 as well as inducible 89 and epigenome-modifying 91, 108 systems.
Accordingly, CRISPR-Cas9 and other site-specific nucleases are a very attractive tool for the generation or correction (or both) of diabetes-relevant mutations in human stem cell-derived models, stem cells being particularly amenable to this technology because of their clonal nature and highly recombinogenic genome. Both gene knockout via Cas9-induced indels 109 and doxycycline-inducible gain-of-function transgenes (targeted to the AAVS1 safe harbour locus using TALENs 110) have been used to definitively establish the role of NEUROG3 in human pancreas development. Whilst Neurog3 is essential for murine pancreas development and derivation of all islet cell types 111– 113, individuals with homozygous NEUROG3 mutations retain some islet function 114– 116. Complete gene knockout showed that NEUROG3 −/− cells could not mature past pancreatic progenitors into endocrine pancreas; however, with graded perturbation to gene dosage via small hairpin RNA (shRNA), as little as 10% residual NEUROG3 activity still led to some islet hormone-positive cells 109. These data are directionally consistent with analogous experiments whereby inducible NEUROG3 overexpression in human embryonic stem cell (hESC)-derived pancreatic progenitors leads to increased numbers of endocrine pancreas-like cells expressing INS, NKX2.2, NEUROD1, and other relevant islet transcription factors 110. Drastically reduced NEUROG3 levels are therefore sufficient for the development of human islets, an effect not recapitulated in mice.
Although many reports have begun to emerge of mutation introduction or correction via homology-directed repair in both control and patient-derived cell lines, these remain as yet unpublished, perhaps reflecting the low efficiency of this technique and repeated cleavage of repaired sites 117, alongside the additional scrutiny of these experimental techniques in terms of off-target effects 118, 119.
Interrogating diabetes pathology in the absence of patient-derived lines
Patient-derived iPSCs facilitate study of the precise mutational mechanisms underlying an individual’s diabetes risk and progression; however, their use so far has been limited to monogenic disease. Although we may not yet have phenotypic resolution to assay dysfunction underlying more complex disease, the ability to generate cellular models of islet development opens up a whole new avenue of investigation for T2D pathogenesis 59.
T2D pathology may result from dysfunction in both foetal and adult islets
We know from studying monogenic diabetes and pancreatic agenesis that there is substantial overlap between the genes causing these phenotypically severe Mendelian disorders and those harbouring more common and incompletely penetrant variants predisposing to T2D risk 4, 120. It follows that within these cellular pathways, the extent of perturbation dictates when diabetes presents: either in utero/early life if severe or much later as T2D if more subtle.
At the extreme end of this scale is pancreas hypoplasia or even lack of a pancreas completely (agenesis). Haploinsufficiency for GATA6 is the most common cause of pancreatic agenesis in humans 14. Individuals with this haploinsufficiency may also experience cardiac or gastrointestinal abnormalities, reflecting the role of GATA6 in organogenesis for multiple tissues. As phenotypic presentation of GATA6 mutation carriers varies (some individuals experience dysfunction in only a subset of these tissues), a potential redundant role for the related transcription factor GATA4 has been proposed in humans. This hypothesis is well established in mouse development 121, 122 but continues to be the subject of debate in humans, despite the identification of individuals with neonatal diabetes (one with pancreatic agenesis) resulting from heterozygous GATA4 mutations 20.
Biallelic inactivation of RFX6, a key transcription factor in gut- and pancreatic-endoderm specification, causes both neonatal 123– 125 and childhood-onset diabetes 126, with phenotype severity correlating with loss of RFX6 gene dosage, and subsequently islet cell development/hypoplasia 126. An elegant CRISPR-Cas9 hESC knockout study showed that loss of RFX6 alters or delays pancreatic progenitor formation through perturbed PDX1 induction 110, thus implicating RFX6 in the regulation of both foetal and adult islet cell function (in which it helps maintain mature beta-cell identity 127, 128). Heterozygous mutations in HNF1B 129, a gene switched on within cells in the primitive gut tube where it is responsible for regional gut specification and branching morphogenesis as well as later cell fate decisions in multipotent pancreatic progenitors 130, cause MODY 131, 132, pancreatic hypoplasia/agenesis 133– 136, and renal abnormalities 137– 139.
GATA6 and HNF1B map to genomic loci implicated in later-onset diabetes 4, 120; therefore, understanding their role in foetal and adult human islets is crucial for investigating T2D pathogenesis. Because mice haploinsufficient for Gata6, Gata4, and Hnf1b do not have diabetes 15– 17 and with homozygous knockouts causing embryonic lethality 140– 142, dual developmental and adult characterisation would not be possible without human cell models representative of both time points.
Stem cells can be used to model the whole spectrum of diabetes phenotypes
The severity of a diabetes phenotype may be influenced, in part, by the temporal expression pattern of a mutated gene. For example, one of the downstream targets of HNF1B is GLIS3, a zinc finger transcription factor involved in regulating the transient spike in NEUROG3 expression important for endocrine fate commitment 130. Although GLIS3 mutations have been shown to cause neonatal diabetes and T2D in humans, these same individuals do not experience pancreatic agenesis 143, and this fits with the later expression of GLIS3 (versus HNF1B) in the foetal pancreas. This suggests that these individuals are able to make some endocrine pancreas tissue and that disease pathology results from insufficient insulin secretion from a reduced functional beta-cell mass potentially both in utero and in adult life. Analogous observations have been made for individuals with mutations in the foetal pancreatic transcription factors PAX6 144, NEUROD1 145, NKX2.2 146, and MNX1 146, 147.
In a similar vein, heterozygous mutations in other genes important for islet progenitor function can cause the milder phenotype of MODY; this is characterised by onset of non-insulin-dependent diabetes before 25 years of age 148. Mutations in HNF family members HNF4A and HNF1A are the most common cause of MODY in Europeans 149– 153, and these genes also map to genomic regions associated with T2D risk 4, 120. Whilst both disorders could result from defective insulin secretion from mature islets (the two transcription factors regulate genes governing GSIS 24), they also perform distinctive roles in the foetal pancreas, as dictated by discrete spatiotemporal expression patterns for each of the multiple HNF4A and HNF1A transcript isoforms 154– 156. Studying both HNFs in foetal versus adult tissue has also shown big differences in post-translational regulation; in adult islets these two HNF transcription factors regulate expression of each other and themselves 157 whereas only HNF4A mutations have been shown to cause the more severe phenotype of neonatal diabetes, suggesting that this gene has a more dominant role in foetal pancreas development 155. The association of HNF4A variants with macrosomia and hypoglycaemia in neonates 158 also suggests that perturbations to this gene transiently increase foetal insulin secretion, a phenomenon not observable if studying (i) adult islets alone (as HNF4A mutations cause the opposite phenotype of beta-cell dysfunction and hyperglycaemia 153) or (ii) rodent pancreas ( Hnf4a +/− and Hnf1a +/− mice are phenotypically normal 13, 18, 24). Accordingly, understanding the temporal relationship between HNF4A gene dosage and insulin secretion is fundamental to managing pregnancy as well as neonatal and young-onset diabetes and T2D.
Irrespective of a previous implication in Mendelian diabetes, knowing the developmental expression pattern of genes mapping to T2D-associated regions of the genome can also help refine likely effector transcripts at these loci, particularly considering the well-established role of islet dysfunction in the progression of this disease 6– 8. HHEX, NOTCH2, and PROX1 map to T2D loci containing multiple putative effector transcripts and potentially causal variants 4, 120. Although none of these genes harbour mutations implicated in monogenic diabetes, strong candidacy for their role as effector transcript comes from their importance in endocrine pancreas development: HHEX regulates ventral pancreas organogenesis 159, NOTCH2 is involved in fate decisions of pancreatic progenitors 160, and PROX1 marks pancreatic progenitor cells in the endoderm (later becoming specific to NEUROG3-positive cells) 161. Thus, using human models of endocrine pancreas differentiation to understand how subtle perturbations to these genes during development may impact upon risk of diabetes in later life is fundamental to the functional characterisation, and consequent assignment of variant/transcript causality, at these T2D-associated genomic loci 59.
This same principle can be applied to disentangling disease-associated genetic perturbations mapping to non-coding regions of the genome. As many islet enhancers are tissue-specific 162, and with studies in stem cell-derived endocrine pancreas-lineage cells also showing these and other regulatory marks to be developmental stage specific too 163, it follows that characterisation of non-coding regions harbouring disease-associated genetic variations is possible only if developmental pancreas cell models are employed. A good example of this approach comes from a recent study of multiple consanguineous families with recessive pancreatic agenesis of unknown aetiology 164. All affected individuals were absent of coding mutations in previously established pancreatic agenesis genes ( GATA6 14, PTF1A 165, and PDX1 166, 167) and accordingly were subjected to whole genome sequencing. Homozygosity mapping showed that no biallelic coding changes co-segregated with disease. Extended analysis into non-coding regions of the genome showed that multiple affected individuals harboured biallelic mutations in a 400-base pair sequence about 25 kB downstream of PTF1A, a transcription factor mediating early pancreas specification from the foregut 168. ChIP-seq in hESC-derived pancreatic progenitors showed that this region overlapped binding sites for the foetal pancreas transcription factors FOXA2 and PDX1 as well as an H3K4me1 active enhancer site. Enhancer activity was shown to be tissue- and developmental stage-specific and was abolished upon introduction of the agenesis mutations 164. As PTF1A maps to a locus associated with T2D 4, 120, it follows that similar developmental enhancers may also be important in adult-onset disease.
The usefulness of a model capable of recapitulating all islet cell types
Although as diabetes researchers we can put a large emphasis on understanding insulin secretory defects, aberrant glycaemia can also result from dysfunction in other islet cell types.
Because differentiated stem cells make cells positive for all islet hormones 39, 40, one can use the same systems to study aberrant glycaemia resulting from perturbations in non-beta cell types. Diffuse congenital hyperinsulinism in infancy (CHI) is characterised by insulin over-secretion despite hypoglycaemia 169. Mutations in the ATP-sensitive islet potassium channel subunit genes ABCC8 and KCNJ11 are the most common cause of CHI; the unregulated closure of this channel is thought to result in sustained insulin release 170, 171. However, study of pancreas tissue from 10 individuals with KCNJ11-mediated CHI showed that functional beta-cell mass was maintained as constant since, despite increased proliferation, a concomitant elevation in cell type-specific apoptosis was also observed 172. Intriguingly, and consistent with the disorganised islet architecture observed in Kcnj11 knockout mice 21, the human CHI islets had downregulated PAX4 and ARX levels (the latter transcription factor specific to alpha cells 173) as well as elevated NKX2.2 expression (particularly in delta cells, 10% of which also demonstrated nucleomegaly) 172, 174, 175. Consistent with the use of somatostatin analogues in the treatment of some CHI cases 169, these data suggest that alteration of multiple endocrine pancreas cell lineages (not just beta cells) is driving phenotype 172. Despite disorganised islets, Kcnj11 and Abcc8 knockout mice do not exactly recapitulate the phenotype of human CHI 21, 22, making the further investigation of this disorder in stem cell-derived endocrine pancreas models attractive.
Summary
This review highlights the need for human, physiologically relevant cell models which accurately recapitulate both foetal and adult islet function for interrogation of diabetes pathogenesis. Although a lot of our knowledge regarding pancreas development has come from studying the mouse, there are many cases in which murine models fall phenotypically short and so translating genetic signals into disease mechanisms is limited. The huge advances that have been made in differentiating human stem cells (both embryonic and induced pluripotent) into all cell types of the developing endocrine pancreas have transformed how we are able to characterise disease-causing and -associated genetic perturbations. However, although we are now able to make endocrine pancreas-like cells with some islet function, it is important to temper expectations and remember that we are still some way from making the perfect beta cell. Although the most recent studies from leading labs report glucose-responsive insulin secretion and Ca 2+ channel activity 39, 40, this function does not fully recapitulate that of human islets. Accordingly, we as a field must make an effort to standardise phenotyping assays and subject them to the same scrutiny as that used to interrogate primary tissue. Efforts to deposit functional 176 and omics-level 59 data for both primary tissue and stem cell-derived endocrine pancreas-like cells are helping researchers generating their own pancreas-in-a-dish to compare, contrast, and truly evaluate their model systems. Once this methodological standardisation is achieved, we can collectively increase the complexity of our routine phenotyping of parameters such as hormone secretion and ion currents and move towards physiologically relevant doses of mixed nutrient stimuli, amongst other assays.
Regardless of these current functional bottlenecks, coupling stem cell-derived endocrine pancreas-like cells with the excitement of genome editing technologies places diabetes researchers in an extremely powerful position of novel biology discovery and genetic signal validation. Armed with these new experimental tools, one can start probing more complex forms of the disease such as T2D 59 and, with a pluripotent cell type, model the complex multi-organ dysfunction occurring in cells derived from the same patient. The dream of a true ‘personalised medicine’ approach to diabetes is in our midst.
Acknowledgements
Nicola L. Beer is a Naomi Berrie Fellow in Diabetes Research. Anna L. Gloyn is a Wellcome Trust Senior Fellow in Basic Biomedical Research (095101).
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
James Johnson, Department of Cellular and Physiological Sciences, University of British Columbia, Vancouver, BC, Canada
Timo Otonkoski, Research Program for Molecular Neurology and Biomedicum Stem Cell Center, University of Helsinki, Helsinki, Finland
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. International Diabetes Federation: IDF Diabetes Atlas - 7th Edition.2015. Reference Source [PubMed] [Google Scholar]
- 2. Almgren P, Lehtovirta M, Isomaa B, et al. : Heritability and familiality of type 2 diabetes and related quantitative traits in the Botnia Study. Diabetologia. 2011;54(11):2811–9. 10.1007/s00125-011-2267-5 [DOI] [PubMed] [Google Scholar]
- 3. Hu FB: Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–57. 10.2337/dc11-0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morris AP, Voight BF, Teslovich TM, et al. : Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44(9):981–90. 10.1038/ng.2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium, . Asian Genetic Epidemiology Network Type 2 Diabetes (AGEN-T2D) Consortium, . South Asian Type 2 Diabetes (SAT2D) Consortium, et al. : Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. 2014;46(3):234–44. 10.1038/ng.2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dimas AS, Lagou V, Barker A, et al. : Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes. 2014;63(6):2158–71. 10.2337/db13-0949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ingelsson E, Langenberg C, Hivert MF, et al. : Detailed physiologic characterization reveals diverse mechanisms for novel genetic Loci regulating glucose and insulin metabolism in humans. Diabetes. 2010;59(5):1266–75. 10.2337/db09-1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Voight BF, Scott LJ, Steinthorsdottir V, et al. : Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42(7):579–89. 10.1038/ng.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fadista J, Vikman P, Laakso EO, et al. : Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc Natl Acad Sci U S A. 2014;111(38):13924–9. 10.1073/pnas.1402665111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaulton KJ, Ferreira T, Lee Y, et al. : Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat Genet. 2015;47(12):1415–25. 10.1038/ng.3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parker SC, Stitzel ML, Taylor DL, et al. : Chromatin stretch enhancer states drive cell-specific gene regulation and harbor human disease risk variants. Proc Natl Acad Sci U S A. 2013;110(44):17921–6. 10.1073/pnas.1317023110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van de Bunt M, Manning Fox JE, Dai X, et al. : Transcript Expression Data from Human Islets Links Regulatory Signals from Genome-Wide Association Studies for Type 2 Diabetes and Glycemic Traits to Their Downstream Effectors. PLoS Genet. 2015;11(12):e1005694. 10.1371/journal.pgen.1005694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dukes ID, Sreenan S, Roe MW, et al. : Defective pancreatic beta-cell glycolytic signaling in hepatocyte nuclear factor-1alpha-deficient mice. J Biol Chem. 1998;273(38):24457–64. 10.1074/jbc.273.38.24457 [DOI] [PubMed] [Google Scholar]
- 14. Lango Allen H, Flanagan SE, Shaw-Smith C, et al. : GATA6 haploinsufficiency causes pancreatic agenesis in humans. Nat Genet. 2012;44(1):20–2. 10.1038/ng.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuo CT, Morrisey EE, Anandappa R, et al. : GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11(8):1048–60. 10.1101/gad.11.8.1048 [DOI] [PubMed] [Google Scholar]
- 16. Morrisey EE, Tang Z, Sigrist K, et al. : GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12(22):3579–90. 10.1101/gad.12.22.3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molkentin JD, Lin Q, Duncan SA, et al. : Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11(8):1061–72. 10.1101/gad.11.8.1061 [DOI] [PubMed] [Google Scholar]
- 18. Pontoglio M, Prié D, Cheret C, et al. : HNF1alpha controls renal glucose reabsorption in mouse and man. EMBO Rep. 2000;1(4):359–65. 10.1093/embo-reports/kvd071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Servitja JM, Ferrer J: Transcriptional networks controlling pancreatic development and beta cell function. Diabetologia. 2004;47(4):597–613. 10.1007/s00125-004-1368-9 [DOI] [PubMed] [Google Scholar]
- 20. Shaw-Smith C, De Franco E, Lango Allen H, et al. : GATA4 mutations are a cause of neonatal and childhood-onset diabetes. Diabetes. 2014;63(8):2888–94. 10.2337/db14-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seino S, Iwanaga T, Nagashima K, et al. : Diverse roles of K ATP channels learned from Kir6.2 genetically engineered mice. Diabetes. 2000;49(3):311–8. 10.2337/diabetes.49.3.311 [DOI] [PubMed] [Google Scholar]
- 22. Seghers V, Nakazaki M, DeMayo F, et al. : Sur1 knockout mice. A model for K ATP channel-independent regulation of insulin secretion. J Biol Chem. 2000;275(13):9270–7. 10.1074/jbc.275.13.9270 [DOI] [PubMed] [Google Scholar]
- 23. Shiota C, Larsson O, Shelton KD, et al. : Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem. 2002;277(40):37176–83. 10.1074/jbc.M206757200 [DOI] [PubMed] [Google Scholar]
- 24. Stoffel M, Duncan SA: The maturity-onset diabetes of the young (MODY1) transcription factor HNF4alpha regulates expression of genes required for glucose transport and metabolism. Proc Natl Acad Sci USA. 1997;94(24):13209–14. 10.1073/pnas.94.24.13209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Vos A, Heimberg H, Quartier E, et al. : Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest. 1995;96(5):2489–95. 10.1172/JCI118308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hay CW, Docherty K: Comparative analysis of insulin gene promoters: implications for diabetes research. Diabetes. 2006;55(12):3201–13. 10.2337/db06-0788 [DOI] [PubMed] [Google Scholar]
- 27. Fiaschi-Taesch N, Bigatel TA, Sicari B, et al. : Survey of the human pancreatic beta-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human beta-cell replication and function in vivo. Diabetes. 2009;58(4):882–93. 10.2337/db08-0631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCulloch LJ, van de Bunt M, Braun M, et al. : GLUT2 ( SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: implications for understanding genetic association signals at this locus. Mol Genet Metab. 2011;104(4):648–53. 10.1016/j.ymgme.2011.08.026 [DOI] [PubMed] [Google Scholar]
- 29. McDonald TJ, Tu E, Brenner S, et al. : Canine, human, and rat plasma insulin responses to galanin administration: species response differences. Am J Physiol. 1994;266(4 Pt 1):E612–7. [DOI] [PubMed] [Google Scholar]
- 30. Peschke E, Bähr I, Mühlbauer E: Melatonin and pancreatic islets: interrelationships between melatonin, insulin and glucagon. Int J Mol Sci. 2013;14(4):6981–7015. 10.3390/ijms14046981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steiner DJ, Kim A, Miller K, et al. : Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2(3):135–45. 10.4161/isl.2.3.11815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ravassard P, Hazhouz Y, Pechberty S, et al. : A genetically engineered human pancreatic β cell line exhibiting glucose-inducible insulin secretion. J Clin Invest. 2011;121(9):3589–97. 10.1172/JCI58447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scharfmann R, Pechberty S, Hazhouz Y, et al. : Development of a conditionally immortalized human pancreatic β cell line. J Clin Invest. 2014;124(5):2087–98. 10.1172/JCI72674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andersson LE, Valtat B, Bagge A, et al. : Characterization of stimulus-secretion coupling in the human pancreatic EndoC-βH1 beta cell line. PLoS One. 2015;10(3):e0120879. 10.1371/journal.pone.0120879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bruin JE, Erener S, Vela J, et al. : Characterization of polyhormonal insulin-producing cells derived in vitro from human embryonic stem cells. Stem Cell Res. 2014;12(1):194–208. 10.1016/j.scr.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 36. Cho CH, Hannan NR, Docherty FM, et al. : Inhibition of activin/nodal signalling is necessary for pancreatic differentiation of human pluripotent stem cells. Diabetologia. 2012;55(12):3284–95. 10.1007/s00125-012-2687-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. D'Amour KA, Bang AG, Eliazer S, et al. : Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392–401. 10.1038/nbt1259 [DOI] [PubMed] [Google Scholar]
- 38. Nostro MC, Sarangi F, Yang C, et al. : Efficient generation of NKX6–1 + pancreatic progenitors from multiple human pluripotent stem cell lines. Stem Cell Reports. 2015;4(4):591–604. 10.1016/j.stemcr.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pagliuca FW, Millman, JR, Gürtle M, et al. : Generation of functional human pancreatic β cells in vitro. Cell. 2014;159(2):428–39. 10.1016/j.cell.2014.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rezania A, Bruin JE, Arora P, et al. : Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121–33. 10.1038/nbt.3033 [DOI] [PubMed] [Google Scholar]
- 41. Russ HA, Parent AV, Ringler JJ, et al. : Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34(13):1759–72. 10.15252/embj.201591058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takahashi K, Tanabe K, Ohnuki M, et al. : Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- 43. Takahashi K, Yamanaka S: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 44. Bruin JE, Rezania A, Xu J, et al. : Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia. 2013;56(9):1987–98. 10.1007/s00125-013-2955-4 [DOI] [PubMed] [Google Scholar]
- 45. Kelly OG, Chan MY, Martinson LA, et al. : Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29(8):750–6. 10.1038/nbt.1931 [DOI] [PubMed] [Google Scholar]
- 46. Kroon E, Martinson LA, Kadoya K, et al. : Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52. 10.1038/nbt1393 [DOI] [PubMed] [Google Scholar]
- 47. Nostro MC, Sarangi F, Ogawa S, et al. : Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138(5):861–71. 10.1242/dev.055236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rezania A, Bruin JE, Riedel MJ, et al. : Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–29. 10.2337/db11-1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rezania A, Bruin JE, Xu J, et al. : Enrichment of human embryonic stem cell-derived NKX6.1–expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells. 2013;31(11):2432–42. 10.1002/stem.1489 [DOI] [PubMed] [Google Scholar]
- 50. Viacyte: A Safety, Tolerability, and Efficacy Study of VC-01™ Combination Product in Subjects With Type I Diabetes Mellitus. In ClinicalTrials.gov [Internet]. National Library of Medicine (US), Bethesda (MD),2014;2016 Reference Source [Google Scholar]
- 51. Bruin JE, Rezania A, Kieffer TJ: Replacing and safeguarding pancreatic β cells for diabetes. Sci Transl Med. 2015;7(316):316ps23. 10.1126/scitranslmed.aaa9359 [DOI] [PubMed] [Google Scholar]
- 52. Cogger K, Nostro MC: Recent advances in cell replacement therapies for the treatment of type 1 diabetes. Endocrinology. 2015;156(1):8–15. 10.1210/en.2014-1691 [DOI] [PubMed] [Google Scholar]
- 53. Nair G, Hebrok M: Islet formation in mice and men: lessons for the generation of functional insulin-producing β-cells from human pluripotent stem cells. Curr Opin Genet Dev. 2015;32:171–80. 10.1016/j.gde.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pagliuca FW, Melton DA: How to make a functional β-cell. Development. 2013;140(12):2472–83. 10.1242/dev.093187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Quiskamp N, Bruin JE, Kieffer TJ: Differentiation of human pluripotent stem cells into β-cells: Potential and challenges. Best Pract Res Clin Endocrinol Metab. 2015;29(6):833–47. 10.1016/j.beem.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 56. Robinton DA, Daley GQ: The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481(7381):295–305. 10.1038/nature10761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cong L, Ran FA, Cox D, et al. : Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–23. 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mali P, Yang L, Esvelt KM, et al. : RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–6. 10.1126/science.1232033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van de Bunt M, Lako M, Barrett A, et al. : Insights into islet development and biology through characterization of a human iPSC-derived endocrine pancreas model. Islets. 2016;8(3):83–95. 10.1080/19382014.2016.1182276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kajiwara M, Aoi T, Okita K, et al. : Donor-dependent variations in hepatic differentiation from human-induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109(31):12538–43. 10.1073/pnas.1209979109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kyttala A, Moraghebi R, Valensisi C, et al. : Genetic Variability Overrides the Impact of Parental Cell Type and Determines iPSC Differentiation Potential. Stem Cell Reports. 2016;6(2):200–12. 10.1016/j.stemcr.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rouhani F, Kumasaka N, de Brito MC, et al. : Genetic background drives transcriptional variation in human induced pluripotent stem cells. PLoS Genet. 2014;10(6):e1004432. 10.1371/journal.pgen.1004432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hrvatin S, O'Donnell CW, Deng F, et al. : Differentiated human stem cells resemble fetal, not adult, β cells. Proc Natl Acad Sci U S A. 2014;111(8):3038–43. 10.1073/pnas.1400709111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Teo AK, Windmueller R, Johansson BB, et al. : Derivation of human induced pluripotent stem cells from patients with maturity onset diabetes of the young. J Biol Chem. 2013;288(8):5353–6. 10.1074/jbc.C112.428979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stepniewski J, Kachamakova-Trojanowska N, Ogrocki D, et al. : Induced pluripotent stem cells as a model for diabetes investigation. Sci Rep. 2015;5:8597. 10.1038/srep08597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Matschinsky FM: Regulation of pancreatic beta-cell glucokinase: from basics to therapeutics. Diabetes. 2002;51(Suppl 3):S394–404. 10.2337/diabetes.51.2007.S394 [DOI] [PubMed] [Google Scholar]
- 67. Stride A, Vaxillaire M, Tuomi T, et al. : The genetic abnormality in the beta cell determines the response to an oral glucose load. Diabetologia. 2002;45(3):427–35. 10.1007/s00125-001-0770-9 [DOI] [PubMed] [Google Scholar]
- 68. Hua H, Shang L, Martinez H, et al. : iPSC-derived β cells model diabetes due to glucokinase deficiency. J Clin Invest. 2013;123(7):3146–53. 10.1172/JCI67638 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69. Inoue H, Tanizawa Y, Wasson J, et al. : A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat Genet. 1998;20(2):143–8. 10.1038/2441 [DOI] [PubMed] [Google Scholar]
- 70. Barrett TG, Bundey SE: Wolfram (DIDMOAD) syndrome. J Med Genet. 1997;34(10):838–41. 10.1136/jmg.34.10.838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Takeda K, Inoue H, Tanizawa Y, et al. : WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Hum Mol Genet. 2001;10(5):477–84. 10.1093/hmg/10.5.477 [DOI] [PubMed] [Google Scholar]
- 72. Takei D, Ishihara H, Yamaguchi S, et al. : WFS1 protein modulates the free Ca 2+ concentration in the endoplasmic reticulum. FEBS Lett. 2006;580(24):5635–40. 10.1016/j.febslet.2006.09.007 [DOI] [PubMed] [Google Scholar]
- 73. Yurimoto S, Hatano N, Tsuchiya M, et al. : Identification and characterization of wolframin, the product of the wolfram syndrome gene ( WFS1), as a novel calmodulin-binding protein. Biochemistry. 2009;48(18):3946–55. 10.1021/bi900260y [DOI] [PubMed] [Google Scholar]
- 74. Fonseca SG, Ishigaki S, Oslowski CM, et al. : Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Invest. 2010;120(3):744–55. 10.1172/JCI39678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fonseca SG, Fukuma M, Lipson KL, et al. : WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J Biol Chem. 2005;280(47):39609–15. 10.1074/jbc.M507426200 [DOI] [PubMed] [Google Scholar]
- 76. Karasik A, O'Hara C, Srikanta S, et al. : Genetically programmed selective islet beta-cell loss in diabetic subjects with Wolfram's syndrome. Diabetes Care. 1989;12(2):135–8. 10.2337/diacare.12.2.135 [DOI] [PubMed] [Google Scholar]
- 77. Shang L, Hua H, Foo K, et al. : β-cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabetes. 2014;63(3):923–33. 10.2337/db13-0717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Flannick J, Beer NL, Bick AG, et al. : Assessing the phenotypic effects in the general population of rare variants in genes for a dominant Mendelian form of diabetes. Nat Genet. 2013;45(11):1380–5. 10.1038/ng.2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Porteus MH, Carroll D: Gene targeting using zinc finger nucleases. Nat Biotechnol. 2005;23(8):967–73. 10.1038/nbt1125 [DOI] [PubMed] [Google Scholar]
- 80. Urnov FD, Rebar EJ, Holmes MC, et al. : Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11(9):636–46. 10.1038/nrg2842 [DOI] [PubMed] [Google Scholar]
- 81. Cermak T, Doyle EL, Christian M, et al. : Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39(12):e82. 10.1093/nar/gkr218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hockemeyer D, Wang H, Kiani S, et al. : Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. 2011;29(8):731–4. 10.1038/nbt.1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Joung JK, Sander JD: TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol. 2013;14(1):49–55. 10.1038/nrm3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Miller JC, Tan S, Qiao G, et al. : A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29(2):143–8. 10.1038/nbt.1755 [DOI] [PubMed] [Google Scholar]
- 85. Reyon D, Tsai SQ, Khayter C, et al. : FLASH assembly of TALENs for high-throughput genome editing. Nat Biotechnol. 2012;30(5):460–5. 10.1038/nbt.2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Balboa D, Weltner J, Eurola S, et al. : Conditionally Stabilized dCas9 Activator for Controlling Gene Expression in Human Cell Reprogramming and Differentiation. Stem Cell Reports. 2015;5(3):448–59. 10.1016/j.stemcr.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cheng AW, Wang H, Yang H, et al. : Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res. 2013;23(10):1163–71. 10.1038/cr.2013.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dahlman JE, Abudayyeh OO, Joung J, et al. : Orthogonal gene knockout and activation with a catalytically active Cas9 nuclease. Nat Biotechnol. 2015;33(11):1159–61. 10.1038/nbt.3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. González F, Zhu Z, Shi ZD, et al. : An iCRISPR platform for rapid, multiplexable, and inducible genome editing in human pluripotent stem cells. Cell Stem Cell. 2014;15(2):215–26. 10.1016/j.stem.2014.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. He X, Tan C, Wang F, et al. : Knock-in of large reporter genes in human cells via CRISPR/Cas9-induced homology-dependent and independent DNA repair. Nucleic Acids Res. 2016;44(9):e85. 10.1093/nar/gkw064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hilton IB, D'Ippolito AM, Vockley CM, et al. : Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol. 2015;33(5):510–7. 10.1038/nbt.3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kearns NA, Genga RM, Enuameh MS, et al. : Cas9 effector-mediated regulation of transcription and differentiation in human pluripotent stem cells. Development. 2014;141(1):219–23. 10.1242/dev.103341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Konermann S, Brigham MD, Trevino AE, et al. : Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517(7536):583–8. 10.1038/nature14136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mali P, Aach J, Stranges PB, et al. : CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol. 2013;31(9):833–8. 10.1038/nbt.2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pyzocha NK, Ran FA, Hsu PD, et al. : RNA-guided genome editing of mammalian cells. Methods Mol Biol. 2014;1114:269–77. 10.1007/978-1-62703-761-7_17 [DOI] [PubMed] [Google Scholar]
- 96. Ran FA, Hsu PD, Wright J, et al. : Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–308. 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ran FA, Hsu PD, Lin CY, et al. : Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154(6):1380–9. 10.1016/j.cell.2013.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sanjana NE, Shalem O, Zhang F: Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11(8):783–4. 10.1038/nmeth.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shalem O, Sanjana NE, Hartenian E, et al. : Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343(616):84–7. 10.1126/science.1247005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shalem O, Sanjana NE, Zhang F: High-throughput functional genomics using CRISPR-Cas9. Nat Rev Genet. 2015;16(5):299–311. 10.1038/nrg3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Deltcheva E, Chylinski K, Sharma CM, et al. : CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471(7340):602–7. 10.1038/nature09886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hsu PD, Lander ES, Zhang F: Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–78. 10.1016/j.cell.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bibikova M, Golic M, Golic KG, et al. : Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics. 2002;161(3):1169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liang F, Han M, Romanienko PJ, et al. : Homology-directed repair is a major double-strand break repair pathway in mammalian cells. Proc Natl Acad Sci U S A. 1998;95(9):5172–7. 10.1073/pnas.95.9.5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. San FJ, Sung P, Klein H: Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–57. 10.1146/annurev.biochem.77.061306.125255 [DOI] [PubMed] [Google Scholar]
- 106. Liu H, Yang H, Zhu D, et al. : Systematically labeling developmental stage-specific genes for the study of pancreatic beta-cell differentiation from human embryonic stem cells. Cell Res. 2014;24(10):1181–200. 10.1038/cr.2014.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Maeder ML, Linder SJ, Cascio VM, et al. : CRISPR RNA-guided activation of endogenous human genes. Nat Methods. 2013;10(10):977–9. 10.1038/nmeth.2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Konermann S, Brigham MD, Trevino AE, et al. : Optical control of mammalian endogenous transcription and epigenetic states. Nature. 2013;500(7463):472–6. 10.1038/nature12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. McGrath PS, Watson CL, Ingram C, et al. : The Basic Helix-Loop-Helix Transcription Factor NEUROG3 Is Required for Development of the Human Endocrine Pancreas. Diabetes. 2015;64(7):2497–505. 10.2337/db14-1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhu Z, Li QV, Lee K, et al. : Genome Editing of Lineage Determinants in Human Pluripotent Stem Cells Reveals Mechanisms of Pancreatic Development and Diabetes. Cell Stem Cell. 2016;18(6):755–68. 10.1016/j.stem.2016.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gradwohl G, Dierich A, LeMeur M, et al. : neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97(4):1607–11. 10.1073/pnas.97.4.1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lee JC, Smith SB, Watada H, et al. : Regulation of the pancreatic pro-endocrine gene neurogenin3. Diabetes. 2001;50(5):928–36. 10.2337/diabetes.50.5.928 [DOI] [PubMed] [Google Scholar]
- 113. Xu X, D'Hoker J, Stange G, et al. : Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207. 10.1016/j.cell.2007.12.015 [DOI] [PubMed] [Google Scholar]
- 114. Pinney SE, Oliver-Krasinski J, Ernst L, et al. : Neonatal diabetes and congenital malabsorptive diarrhea attributable to a novel mutation in the human neurogenin-3 gene coding sequence. J Clin Endocrinol Metab. 2011;96(7):1960–5. 10.1210/jc.2011-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rubio-Cabezas O, Jensen JN, Hodgson MI, et al. : Permanent Neonatal Diabetes and Enteric Anendocrinosis Associated With Biallelic Mutations in NEUROG3. Diabetes. 2011;60(4):1349–53. 10.2337/db10-1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang J, Cortina G, Wu SV, et al. : Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355(3):270–80. 10.1056/NEJMoa054288 [DOI] [PubMed] [Google Scholar]
- 117. Isalan M: Zinc-finger nucleases: how to play two good hands. Nat Methods. 2012;9(1):32–4. 10.1038/nmeth.1805 [DOI] [PubMed] [Google Scholar]
- 118. Smith C, Gore A, Yan W, et al. : Whole-genome sequencing analysis reveals high specificity of CRISPR/Cas9 and TALEN-based genome editing in human iPSCs. Cell Stem Cell. 2014;15(1):12–3. 10.1016/j.stem.2014.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Veres A, Gosis BS, Ding Q, et al. : Low incidence of off-target mutations in individual CRISPR-Cas9 and TALEN targeted human stem cell clones detected by whole-genome sequencing. Cell Stem Cell. 2014;15(1):27–30. 10.1016/j.stem.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mahajan A, Sim X, Ng HJ, et al. : Identification and functional characterization of G6PC2 coding variants influencing glycemic traits define an effector transcript at the G6PC2-ABCB11 locus. PLoS Genet. 2015;11(1):e1004876. 10.1371/journal.pgen.1004876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Carrasco M, Delgado I, Soria B, et al. : GATA4 and GATA6 control mouse pancreas organogenesis. J Clin Invest. 2012;122(10):3504–15. 10.1172/JCI63240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Xuan S, Borok MJ, Decker KJ, et al. : Pancreas-specific deletion of mouse Gata4 and Gata6 causes pancreatic agenesis. J Clin Invest. 2012;122(10):3516–28. 10.1172/JCI63352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Concepcion JP, Reh CS, Daniels M, et al. : Neonatal diabetes, gallbladder agenesis, duodenal atresia, and intestinal malrotation caused by a novel homozygous mutation in RFX6. Pediatr Diabetes. 2014;15(1):67–72. 10.1111/pedi.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Smith SB, Qu HQ, Taleb N, et al. : Rfx6 directs islet formation and insulin production in mice and humans. Nature. 2010;463(7282):775–80. 10.1038/nature08748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Spiegel R, Dobbie A, Hartman C, et al. : Clinical characterization of a newly described neonatal diabetes syndrome caused by RFX6 mutations. Am J Med Genet A. 2011;155A(11):2821–5. 10.1002/ajmg.a.34251 [DOI] [PubMed] [Google Scholar]
- 126. Sansbury FH, Kirel B, Caswell R, et al. : Biallelic RFX6 mutations can cause childhood as well as neonatal onset diabetes mellitus. Eur J Hum Genet. 2015;23(12):1744–8. 10.1038/ejhg.2015.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chandra V, Albagli-Curiel O, Hastoy B, et al. : RFX6 regulates insulin secretion by modulating Ca 2+ homeostasis in human β cells. Cell Rep. 2014;9(6):2206–18. 10.1016/j.celrep.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 128. Piccand J, Strasser P, Hodson DJ, et al. : Rfx6 maintains the functional identity of adult pancreatic β cells. Cell Rep. 2014;9(6):2219–32. 10.1016/j.celrep.2014.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bellanne-Chantelot C, Chauveau D, Gautier JF, et al. : Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med. 2004;140(7):510–7. 10.7326/0003-4819-140-7-200404060-00009 [DOI] [PubMed] [Google Scholar]
- 130. De Vas MG, Kopp JL, Heliot C, et al. : Hnf1b controls pancreas morphogenesis and the generation of Ngn3 + endocrine progenitors. Development. 2015;142(5):871–82. 10.1242/dev.110759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Bellanné-Chantelot C, Clauin S, Chauveau D, et al. : Large genomic rearrangements in the hepatocyte nuclear factor-1beta ( TCF2) gene are the most frequent cause of maturity-onset diabetes of the young type 5. Diabetes. 2005;54(11):3126–32. 10.2337/diabetes.54.11.3126 [DOI] [PubMed] [Google Scholar]
- 132. Horikawa Y, Iwasaki N, Hara M, et al. : Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nat Genet. 1997;17(4):384–5. 10.1038/ng1297-384 [DOI] [PubMed] [Google Scholar]
- 133. Body-Bechou D, Loget P, D'Herve D, et al. : TCF2/HNF-1beta mutations: 3 cases of fetal severe pancreatic agenesis or hypoplasia and multicystic renal dysplasia. Prenat Diagn. 2014;34(1):90–3. 10.1002/pd.4264 [DOI] [PubMed] [Google Scholar]
- 134. Edghill EL, Bingham C, Slingerland AS, et al. : Hepatocyte nuclear factor-1 beta mutations cause neonatal diabetes and intrauterine growth retardation: support for a critical role of HNF-1beta in human pancreatic development. Diabet Med. 2006;23(12):1301–6. 10.1111/j.1464-5491.2006.01999.x [DOI] [PubMed] [Google Scholar]
- 135. Haumaitre C, Fabre M, Cormier S, et al. : Severe pancreas hypoplasia and multicystic renal dysplasia in two human fetuses carrying novel HNF1beta/MODY5 mutations. Hum Mol Genet. 2006;15(15):2363–75. 10.1093/hmg/ddl161 [DOI] [PubMed] [Google Scholar]
- 136. Yorifuji T, Kurokawa K, Mamada M, et al. : Neonatal diabetes mellitus and neonatal polycystic, dysplastic kidneys: Phenotypically discordant recurrence of a mutation in the hepatocyte nuclear factor-1beta gene due to germline mosaicism. J Clin Endocrinol Metab. 2004;89(6):2905–8. 10.1210/jc.2003-031828 [DOI] [PubMed] [Google Scholar]
- 137. Bingham C, Bulman MP, Ellard S, et al. : Mutations in the hepatocyte nuclear factor-1beta gene are associated with familial hypoplastic glomerulocystic kidney disease. Am J Hum Genet. 2001;68(1):219–24. 10.1086/316945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Carbone I, Cotellessa M, Barella C, et al. : A novel hepatocyte nuclear factor-1beta (MODY-5) gene mutation in an Italian family with renal dysfunctions and early-onset diabetes. Diabetologia. 2002;45(1):153–4. 10.1007/s125-002-8258-8 [DOI] [PubMed] [Google Scholar]
- 139. Nishigori H, Yamada S, Kohama T, et al. : Frameshift mutation, A263fsinsGG, in the hepatocyte nuclear factor-1beta gene associated with diabetes and renal dysfunction. Diabetes. 1998;47(8):1354–5. 10.2337/diab.47.8.1354 [DOI] [PubMed] [Google Scholar]
- 140. Barbacci E, Reber M, Ott MO, et al. : Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development. 1999;126(21):4795–805. [DOI] [PubMed] [Google Scholar]
- 141. Coffinier C, Thepot D, Babinet C, et al. : Essential role for the homeoprotein vHNF1/HNF1beta in visceral endoderm differentiation. Development. 1999;126(21):4785–94. [DOI] [PubMed] [Google Scholar]
- 142. Haumaitre C, Barbacci E, Jenny M, et al. : Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci U S A. 2005;102(5):1490–5. 10.1073/pnas.0405776102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Senee V, Chelala C, Duchatelet S, et al. : Mutations in GLIS3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat Genet. 2006;38(6):682–7. 10.1038/ng1802 [DOI] [PubMed] [Google Scholar]
- 144. Solomon BD, Pineda-Alvarez DE, Balog JZ, et al. : Compound heterozygosity for mutations in PAX6 in a patient with complex brain anomaly, neonatal diabetes mellitus, and microophthalmia. Am J Med Genet A. 2009;149A(11):2543–6. 10.1002/ajmg.a.33081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Rubio-Cabezas O, Minton JA, Kantor I, et al. : Homozygous mutations in NEUROD1 are responsible for a novel syndrome of permanent neonatal diabetes and neurological abnormalities. Diabetes. 2010;59(9):2326–31. 10.2337/db10-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Flanagan SE, De Franco E, Lango AH, et al. : Analysis of transcription factors key for mouse pancreatic development establishes NKX2-2 and MNX1 mutations as causes of neonatal diabetes in man. Cell Metab. 2014;19(1):146–54. 10.1016/j.cmet.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bonnefond A, Vaillant E, Philippe J, et al. : Transcription factor gene MNX1 is a novel cause of permanent neonatal diabetes in a consanguineous family. Diabetes Metab. 2013;39(3):276–80. 10.1016/j.diabet.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 148. Tattersall R: Maturity-onset diabetes of the young: a clinical history. Diabet Med. 1998;15(1):11–4. [DOI] [PubMed] [Google Scholar]
- 149. Frayling TM, Bulamn MP, Ellard S, et al. : Mutations in the hepatocyte nuclear factor-1alpha gene are a common cause of maturity-onset diabetes of the young in the U.K. Diabetes. 1997;46(4):720–5. 10.2337/diab.46.4.720 [DOI] [PubMed] [Google Scholar]
- 150. Kropff J, Selwood MP, McCarthy MI, et al. : Prevalence of monogenic diabetes in young adults: a community-based, cross-sectional study in Oxfordshire, UK. Diabetologia. 2011;54(5):1261–3. 10.1007/s00125-011-2090-z [DOI] [PubMed] [Google Scholar]
- 151. Shields BM, Hicks S, Shepherd MH, et al. : Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53(12):2504–8. 10.1007/s00125-010-1799-4 [DOI] [PubMed] [Google Scholar]
- 152. Yamagata K, Oda N, Kaisaki PJ, et al. : Mutations in the hepatocyte nuclear factor-1alpha gene in maturity-onset diabetes of the young (MODY3). Nature. 1996;384(6608):455–8. 10.1038/384455a0 [DOI] [PubMed] [Google Scholar]
- 153. Yamagata K, Furuta H, Oda N, et al. : Mutations in the hepatocyte nuclear factor-4alpha gene in maturity-onset diabetes of the young (MODY1). Nature. 1996;384(6608):458–60. 10.1038/384458a0 [DOI] [PubMed] [Google Scholar]
- 154. Harries LW, Ellard S, Stride A, et al. : Isomers of the TCF1 gene encoding hepatocyte nuclear factor-1 alpha show differential expression in the pancreas and define the relationship between mutation position and clinical phenotype in monogenic diabetes. Hum Mol Genet. 2006;15(14):2216–24. 10.1093/hmg/ddl147 [DOI] [PubMed] [Google Scholar]
- 155. Harries LW, Locke JM, Shields B, et al. : The diabetic phenotype in HNF4A mutation carriers is moderated by the expression of HNF4A isoforms from the P1 promoter during fetal development. Diabetes. 2008;57(6):1745–52. 10.2337/db07-1742 [DOI] [PubMed] [Google Scholar]
- 156. Harries LW, Brown JE, Gloyn AL: Species-specific differences in the expression of the HNF1A, HNF1B and HNF4A genes. PLoS One. 2009;4(11):e7855. 10.1371/journal.pone.0007855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Ferrer J: A genetic switch in pancreatic beta-cells: implications for differentiation and haploinsufficiency. Diabetes. 2002;51(8):2355–62. 10.2337/diabetes.51.8.2355 [DOI] [PubMed] [Google Scholar]
- 158. Pearson ER, Boj SF, Steele AM, et al. : Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med. 2007;4(4):e118. 10.1371/journal.pmed.0040118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bort R, Martinez-Barbera JP, Beddington RS, et al. : Hex homeobox gene-dependent tissue positioning is required for organogenesis of the ventral pancreas. Development. 2004;131(4):797–806. 10.1242/dev.00965 [DOI] [PubMed] [Google Scholar]
- 160. Cras-Meneur C, Li L, Kopan R, et al. : Presenilins, Notch dose control the fate of pancreatic endocrine progenitors during a narrow developmental window. Genes Dev. 2009;23(17):2088–101. 10.1101/gad.1800209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wang J, Kilic G, Aydin M, et al. : Prox1 activity controls pancreas morphogenesis and participates in the production of "secondary transition" pancreatic endocrine cells. Dev Biol. 2005;286(1):182–94. 10.1016/j.ydbio.2005.07.021 [DOI] [PubMed] [Google Scholar]
- 162. Pasquali L, Gaulton KJ, Rodriguez-Segui SA, et al. : Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat Genet. 2014;46(2):136–43. 10.1038/ng.2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Wang A, Yue F, Li Y, et al. : Epigenetic priming of enhancers predicts developmental competence of hESC-derived endodermal lineage intermediates. Cell Stem Cell. 2015;16(4):386–99. 10.1016/j.stem.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Weedon MN, Cebola I, Patch AM, et al. : Recessive mutations in a distal PTF1A enhancer cause isolated pancreatic agenesis. Nat Genet. 2014;46(1):61–4. 10.1038/ng.2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Sellick GS, Barker KT, Stolte-Dijkstra I, et al. : Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet. 2004;36(12):1301–5. 10.1038/ng1475 [DOI] [PubMed] [Google Scholar]
- 166. Schwitzgebel VM, Mamin A, Brun T, et al. : Agenesis of human pancreas due to decreased half-life of insulin promoter factor 1. J Clin Endocrinol Metab. 2003;88(9):4398–406. 10.1210/jc.2003-030046 [DOI] [PubMed] [Google Scholar]
- 167. Stoffers DA, Zinkin NT, Stanojevic V, et al. : Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15(1):106–10. 10.1038/ng0197-106 [DOI] [PubMed] [Google Scholar]
- 168. Burlison JS, Long Q, Fujitani Y, et al. : Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev Biol. 2008;316(1):74–86. 10.1016/j.ydbio.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Dunne MJ, Cosgrove KE, Shepherd RM, et al. : Hyperinsulinism in infancy: from basic science to clinical disease. Physiol Rev. 2004;84(1):239–75. 10.1152/physrev.00022.2003 [DOI] [PubMed] [Google Scholar]
- 170. Kapoor RR, Flanagan SE, Arya VB, et al. : Clinical and molecular characterisation of 300 patients with congenital hyperinsulinism. Eur J Endocrinol. 2013;168(4):557–64. 10.1530/EJE-12-0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Snider KE, Becker S, Boyajian L, et al. : Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J Clin Endocrinol Metab. 2013;98(2):E355–63. 10.1210/jc.2012-2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Salisbury RJ, Han B, Jennings RE, et al. : Altered Phenotype of β-Cells and Other Pancreatic Cell Lineages in Patients With Diffuse Congenital Hyperinsulinism in Infancy Caused by Mutations in the ATP-Sensitive K-Channel. Diabetes. 2015;64(9):3182–8. 10.2337/db14-1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Collombat P, Mansouri A, Hecksher-Sorensen J, et al. : Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17(20):2591–603. 10.1101/gad.269003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Papizan JB, Singer RA, Tschen SI, et al. : Nkx2.2 repressor complex regulates islet β-cell specification and prevents β-to-α-cell reprogramming. Genes Dev. 2011;25(21):2291–305. 10.1101/gad.173039.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Rahier J, Fält K, Müntefering H, et al. : The basic structural lesion of persistent neonatal hypoglycaemia with hyperinsulinism: deficiency of pancreatic D cells or hyperactivity of B cells? Diabetologia. 1984;26(4):282–9. 10.1007/BF00283651 [DOI] [PubMed] [Google Scholar]
- 176. Wills QF, Boothe T, Asadi A, et al. : Statistical approaches and software for clustering islet cell functional heterogeneity. Islets. 2016;8(2):48–56. 10.1080/19382014.2016.1150664 [DOI] [PMC free article] [PubMed] [Google Scholar]