Abstract
Drought stress caused by unpredictable precipitation poses a major threat to food production worldwide, and its impact is only expected to increase with the further onset of climate change. Understanding the effect of drought stress on crops and plants' response is critical for developing improved varieties with stable high yield to fill a growing food gap from an increasing population depending on decreasing land and water resources. When a plant encounters drought stress, it may use multiple response types, depending on environmental conditions, drought stress intensity and duration, and the physiological stage of the plant. Drought stress responses can be divided into four broad types: drought escape, drought avoidance, drought tolerance, and drought recovery, each characterized by interacting mechanisms, which may together be referred to as drought resistance mechanisms. The complex nature of drought resistance requires a multi-pronged approach to breed new varieties with stable and enhanced yield under drought stress conditions. High throughput genomics and phenomics allow marker-assisted selection (MAS) and genomic selection (GS), which offer rapid and targeted improvement of populations and identification of parents for rapid genetic gains and improved drought-resistant varieties. Using these approaches together with appropriate genetic diversity, databases, analytical tools, and well-characterized drought stress scenarios, weather and soil data, new varieties with improved drought resistance corresponding to grower preferences can be introduced into target regions rapidly.
Keywords: Drought stress, Genomics, Phenomics, Breeding, Marker-assisted selection, Physiological processes
1. DROUGHT STRESS: CURRENT AND FUTURE GLOBAL CHALLENGES FOR FOOD PRODUCTION
Drought is a major threat to food production worldwide and is caused by insufficient rainfall and/or erratic rainfall patterns [1]. One third of the world’s land area is arid or semi-arid and inhabited by some of the poorest human populations [2] whereas rain-fed agricultural land accounts for 80% of food production and grows about 60% of the world’s staple food [3]. Climate change and population increase further increase yield losses stemming from drought stress and their impact. Drought stress is when a plant’s water demand is not met by water supply, causing plant-water deficit that is high enough to induce injury to the plant [4]. With the undernourishment rate of about 830 million and population growth estimated to reach 9 billion by 2050, it is predicted that food production needs to be increased by 70%-100% within the same period, from decreased available land, with reduced water and soil quality [5, 6]. Also, the middle class is expected to expand, particularly in developing countries, adding to food demands [5]. The requirements for food production are significantly threatened by climate change, a phenomenon that has become ever more important over the last two decades, with associated temperature rise due to global warming. The global temperature has risen by an estimated 1.2°C over the past century and is expected to rise by another 3°C by 2100 [7]. There could be temperature shocks as high as 6°C, unpredictable precipitation, and decrease in water and soil quality as well. Elevated temperature has consequences on crop production, as it increases evapotranspiration rates, thereby disrupting physiological processes in crop plants [8]. According to Trenberth [9], global warming also has a direct influence on precipitation, through increased soil evaporation and expedited surface drying, increasing the intensity and duration of droughts. This makes development of crop plants with improved performance under drought stress a major breeding objective. However, abiotic stresses are unpredictable, constantly increasing or decreasing on average, with frequent and sporadic spikes, and can also lead to pest and disease outbreaks. Furthermore, no one stress comes alone, when there are droughts, temperatures are usually high and vice versa. Similarly, drought and heat stress can increase salt levels in the upper soil surface, and high salinity can have a similar impact on plant response as drought stress. These factors make breeding for performance under drought stress a complicated activity [10]. In addition to non-ideal environmental conditions, developing countries often experience great yield gaps between yield potential and actual yield in farmers' fields due to low input and poor management practices, and the use of unimproved germplasm not adapted to the current environment. Minimizing this gap by developing adapted germplasm, and improving crop and resource management practices, will ultimately enable the meeting of food needs for the future [11].
2. PLANTS’ RESPONSES TO DROUGHT STRESS
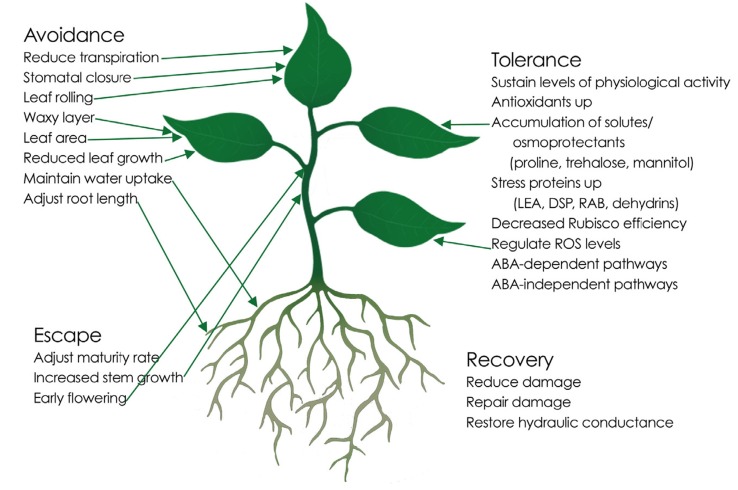
As 80-95% of the mass of growing plant tissues, water has a critical role in most major biological functions of plants and their structural stability, as well as in dissolved nutrient uptake via xylem vessels from roots to leaves. Plants respond quickly to fluctuations in quantity and quality of water, and drought, which is caused by insufficient rainfall or altered precipitation patterns, is the most devastating abiotic stress affecting crop plants [1]. Drought stress can occur at any developmental stage and affects a plant's response and crop productivity to various degrees depending on the time of onset, duration and intensity. For example, shortage of water at emergence or planting could lead to no emergence at all and at the reproductive stage can reduce the yield by up to 50% [12], while stress close to the end of the growing cycle can lead to low quality harvestable products. Overall, plant response to drought is complicated, and plants under drought stress integrate diverse responses and adaptive mechanisms at the morphological, physiological and molecular levels, with large genotypic variations in each (Fig. 1).
Fig. (1).
Plant responses to drought stress is complex can be categorized into four broad types: drought escape, drought avoidance, drought tolerance, and drought recovery, each characterized by interacting mechanisms. Mechanisms more closely linked to a category are shown, but are often part of other categories at the same time.
2.1. Mechanisms of Drought Response in Plants
When faced with drought stress, the plant species, environment, and the timing and intensity of the drought determine the type of response. Plants use four major categories of mechanisms in response to drought stress, namely drought escape, drought avoidance, drought tolerance, and drought recovery (Fig. 1). These have been reviewed in detail [13]. Drought escape involves adjustment of rate of maturity, rapid phenological development, developmental plasticity and remobilization of assimilates in order to escape dry seasons [14]. This enables plants to complete their life cycle before the onset of severe drought stress [10]. In a drought avoidance strategy, a plant maintains fundamental normal physiological processes under mild or moderate drought stress by adjusting morphological structures or growth rates to reduce transpiration or sustain water uptake to keep water levels high within the plant [14, 15]. Such adjustments may include reduction in water losses such as through stomatal closure, leaf rolling and increased wax accumulation on the leaf surface [16]. Drought can also be avoided through enhanced water uptake by root modification or through increasing/decreasing the rate of development from vegetative to reproductive stages [17]. In some cases, plants use a drought tolerance mechanism, which implies maintaining plant health and productivity despite low internal water potential, involving the regulation of hundreds of genes and series of metabolic pathways in order to reduce and/or repair the damage resulting from drought stress, which in turn enables a plant to sustain a certain level of physiological activities under severe drought stress [18, 19]. Finally, some plants can recover after exposure to severe drought stress thereby resuming growth and gaining yield, a concept referred to as drought recovery [19]. Drought avoidance and drought tolerance mechanisms can be referred to together as drought resistance, as the ability of a plant to live, grow and reproduce satisfactorily with limited and irregular water supply or under periodic water deficit conditions [4, 20, 21]. In the current review, we use the term ‘drought resistance’ to refer to both drought avoidance and drought tolerance mechanisms of plant adaptation to drought stress. It should not be confused with ‘resistance’ in the physiological sense, as it is practically impossible for a plant to resist the effects of severe drought stress.
2.2. Physiological Responses to Drought Stress in Plants
Strategies to overcome drought stress can be observed at different levels (Fig. 1). At the physiological level, plants can adjust their rates of photosynthesis by modifying photosystem II, stomatal closure, and low electron transport, carbohydrate and nitrogen metabolism, nucleic acid and protein activity, and growth as a whole [13]. Among the key strategies to sustain normal metabolism is the regulation of osmotic potential and turgor pressure through leaf traits, such as smaller leaves with thick cuticles [22], fine-tuning production of osmolytes such as proline [23], trehalose [24], mannitol, glycine betaine, or myo-inositol, and regulating cell membrane features [25, 26]. The osmoprotective function of proline, an amino acid with exceptional conformational rigidity, has been reported during various stresses including drought [27], high salinity [28], high light and UV irradiation [29], heavy metals [30], oxidative stress [31] as well as other abiotic stresses.
The control of cell expansion plays an important role in maintaining normal plant growth under drought stress. Osmotic regulation of turgor pressure maintains cell growth using substances such as potassium ions (K+), sugars and amino acids [32], and is in turn regulated by drought stress-induced molecular events regulated by abscisic acid (ABA), a phytohormone that is known to accumulate under stress conditions and initiate stress-related signaling cascades [33]. Anion channel activation causes depolarization of guard cell plasma membranes, leading to eventual loss of turgor pressure and stomatal closure [34]. Dehydrins also respond to ABA signals, and are a distinct biochemical group of late embryogenesis abundant (LEA) proteins that can be induced by drought and other abiotic stresses [35]. They are thought to be involved in cellular protection, allowing the cell to maintain normal function or recover it after normal water conditions return [36]. The equilibrium between production and removal of reactive oxygen species (ROS) such as 1O2 H2O2, O2.- and OH., normal by-products of aerobic metabolism, is normally disrupted under drought stress, leading to cell injury and sometimes cell death [37]. The accumulation of ROS can act as a signal to and from the ABA pathway, but at the same time is managed by ascorbic acid and glutathione production, both chemical antioxidants involved in reducing the toxicity of reactive oxygen species such as superoxide dismutase and glutathione S-transferase [38, 39].
As the primary conduit for water uptake, roots are critical in the plant's response to drought. Roots are the first to sense reduction in moisture levels, and activate the signaling pathways leading to production of osmoprotectants, stomatal closure, and other drought resistance mechanisms [40]. Besides the activation of resistance to drought, roots are one of the main components of drought avoidance, as at the whole-plant level, root length, angle, spread, and density, or collectively root architecture, can greatly influence the amount of water the plant is able to access and take up [41-43]. Roots can respond to drought stress directly by, for example, growing longer to reach new potential sources of water [44].
2.3. The Genetic Basis of Drought Resistance
The genetic basis of the physiological adaptations to drought stress has to be understood for designing effective improvement strategies. Numerous candidate genes, most with minor contributions, have been identified using both forward and reverse genetics, and characterized for their functions in drought response, many for roles related to ABA, whether its metabolism, signaling, or localization (reviewed by 45). For example, the ABA signal transduction network regulates stomatal closure, a key drought stress response. Calcium-dependent protein kinases (CDPKs) mediate stomatal movement under drought stress via the ABA and Ca2+ signaling pathways in Arabidopsis and rice [46]. Overexpression of 9-cis-epoxycarotenoid dioxygenase 3 (NCED3), which drought stress rapidly induces, enhances drought tolerance by catalyzing a key step in ABA biosynthesis [47, 48]. The correct localization of ABA is also important to its function, and thus ABA transport and import genes such as membrane-localized transporters of the ABC family ABCG25 and ABCG40 and nitrate transporter family AIT1/NRT1.2/NPF4.6 also regulate water stress responses [49, 50].
Transcription factors are well known to play a critical role in downstream gene expression and to regulate stress response pathways [45]. ABA-responsive elements (ABRE), C-repeat/drought responsive/low-temperature-responsive elements (CRT/DRE/LTRE), Myeloblastosis (MYB) and Myelocytomatosis (MYC) are regulatory elements in the promoter region of genes encoding stress-inducible dehydrins. Several transcription factors bind to these regulatory elements, including ABRE binding factors (ABFs or AREBs), CBF4/DREB1D, which binds to CRT/DRE/ LTRE, MYBFs and MYCFs which bind to MYB and MYC, respectively via the ABA-dependent signaling pathway, and DREB2A and DREB2B, which bind to CRT/DRE/LTRE via the ABA-independent signaling pathway [51-53]. The drought-inducible nuclear transcription factor (NFYA5) and stress-responsive NAC1 (SNAC1) are also both involved in stomatal regulation [54, 55].
Cytosolic ascorbate peroxidase encoding genes (APXs) are induced by overexpression of zinc-finger transcription factors ZAT10 and ZAT12, and act as ROS scavengers for chloroplast proteins under drought stress [56, 57]. Controlling ROS metabolism and regulating ROS homeostasis are essential for normal functioning under stress and many genes are involved. These include manganese superoxide dismutase (MnSOD), which is controlled by an oxidative stress inducible promoter SWPA2 in rice [58], a gene encoding glutathione peroxidase in Arabidopsis (ATGPX3) [59], and OsSKIPa and OsSROC1c genes in rice [60], among others.
For the manufacture of osmoprotectants, 1-pyrroline-5-carboxylate synthetase (P5CS) controls the rate-limiting step in glutamate-derived biosynthesis of proline, an amino acid. In Arabidopsis, P5CS is encoded by two differentially regulated genes, AtP5CS1 and AtP5CS2, which are regulated by ABA1, ABI1, and AXR2 [61]. Alternatively, proline can be synthesized from ornithine-delta-aminotransferase (δ-OAT), producing glutamate-semialdehyde (GSA) and pyrroline-5-carboxylate (P5C), which is then converted to proline [62, 63]. Other osmoprotectants include trehalose, which is synthesized via trehalose-6-phosphate synthase (TPS1) [64], and betaine, biosynthesis under the control of betaine aldehyde dehydrogenase (BADH) [65].
Several other retrograde signaling pathways related to drought stress have been identified. SAL1/ALX8/FRY1, which regulates the retrograde 3’-phosphoadenosine 5’-phosphate (PAP) pathway, has the capacity to alter nuclear gene expression during drought stress [66, 67]. Methylerythritol cyclodiphosphate (MEcPP), a precursor of isoprenoids generated by the methylerythritol phosphate (MEP) pathway, is another retrograde pathway that can induce expression of nuclear encoded stress responsive genes [68]. The full scope of the genetics behind plants’ responses to drought stress is too broad to fully cover here, as different drought stress scenarios also trigger unique pathways and genes, and each response is complex in itself.
3. PHENOTYPING FOR DROUGHT RESISTANCE
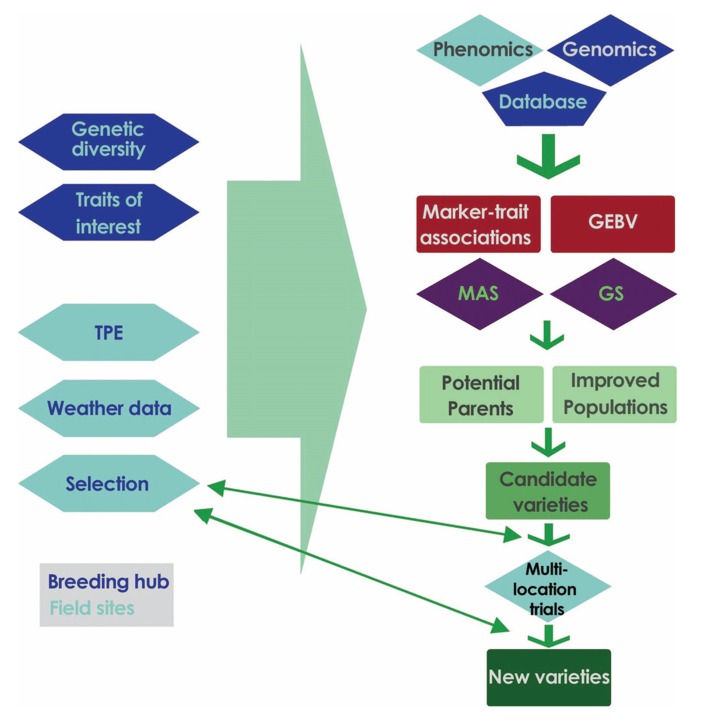
Genetic variation can be found in crop species for various types of drought stress responses. These differences provide plant breeders with the opportunity to generate combinations of traits in elite genetic backgrounds, and develop cultivars with wider adaptability to various drought stress scenarios and stable yield under favorable and unfavorable environmental conditions. As mentioned earlier, many genes are responsible for a number of traits that can lead to small incremental increases in overall yield and yield stability under a particular drought stress level at a specific physiological stage of the plant. Therefore, a well-defined breeding strategy needs thorough characterization of the stress environment, good trait evaluation, and a well-defined screening methodology, coupled with new tools for selection of superior genotypes (Fig. 2), in order to make significant improvement towards developing drought adapted cultivars [69, 70, 10]. As a complex trait, drought resistance requires examination of thousands of genotypes grown in replications across multiple environments to assess differential expression of multiple genes. This in turn poses a challenge in obtaining adequate and relevant phenotypic data from observable physical characteristics on a plot- or plant- basis [71].
Fig. (2).
The complex nature of drought tolerance needs a multi-pronged approach to develop new varieties with stable and enhanced yield under stress conditions. High throughput genomics and phenomics offer rapid and targeted drought-tolerant varieties and genetic gains via Marker-assisted selection (MAS) and genomic selection (GS). The breeding program should be split between a breeding hub (shapes in dark blue) for centralized activities such as genotyping, definition of traits of interest, and maintenance of genetic diversity, databases, and analytical tools, and field sites (shapes in light blue) with well-characterized stress scenarios, weather data, and soil analysis for targeted-site specific phenotyping and stress trials. In this figure, hexagons stand for data required as an input, diamonds are steps to be performed, and rectangles are outputs.
3.1. The Elusive Precise Phenotyping For Drought Resistance
To be able to accurately estimate drought resistance per se implies the absence of other abiotic and biotic stresses that influence plant growth and function. This is hardly the case, since under field conditions, the primary growing environment, drought stress rarely occurs in isolation, but rather in combination with other stresses such as high temperatures, high irradiance, salinity, and nutrient deficiencies, among others [69, 10]. Mittler [72] provides a comprehensive review on abiotic stress combinations under field conditions. As a consequence, some biochemical mechanisms may have opposing effects under different stresses. Increased stomatal conductance is an adaptive strategy under heat stress while it is undesirable under drought stress when a plant uses drought tolerance mechanisms rather than drought avoidance. Similarly, proline, an osmoprotectant under drought conditions, may have toxic effects under heat stress [73, 10]. This has consequences at the molecular level where gene networks controlling several traits overlap. For instance, about 40% of drought or high salinity inducible genes were also shown to be induced by cold stress in rice [74]. The difficulty of obtaining reproducible and precise phenotypic data on complex quantitative traits like drought resistance within a larger germplasm collection remains a challenge and could in part explain the disparity between the results reported so far on molecular control of drought resistance and their practical application in breeding [75, 76].
This complexity therefore calls for proper characterization of the environment, including timing, frequency and intensity of drought as critical to breeding for drought resistance. Soil pH, salinity levels, and nutrient availability all have strong interaction with drought stress and ultimately impact plant performance and yield (Fig. 2). Different structural types of soil have different moisture holding capacity, and plants might need different traits to access water [77]. Therefore, it is essential to know field capacity, soil characteristics, and moisture content from the beginning of a field trial and throughout the growth cycle via soil analytics and soil moisture sensors.
The water table and its relationship to deep and vigorous root architecture should also be well understood. For example, in a low water table, breeding for longer roots might come at a significant cost to the plant. Weather patterns, including rainfall, temperature fluctuations, radiation intensity, relative humidity and day length are all key to predicting yield and quality of crop products, and should be similarly monitored with weather stations installed for the duration of a trial. Environmental characterization can be achieved by the use of geographic information system (GIS) for crop monitoring and water balance models [78, 79], such as the GIS system, CIMMYT was able to use to identify six mega environments for maize [80]. These are a form of target population of environments (TPEs), which allow targeted breeding strategies to each mega environment rather than relying on broad and often contradictory conditions. Defining a TPE instead of a single target environment allows screening for the expected real-life range of growing conditions and potential stresses, and better predicts potential performance of the variety [81]. Within such TPEs, it is important to select testing environments where drought stress intensity, frequency and timing can be reliably managed in order to expose genetic variation for traits from season to season [81]. In this regard, Salekdeh et al. [73] proposed the creation of minimum information about drought experiment (MIADE), which describes the target drought scenario, including the conditions of the micro-environment and agronomic practices, together with their possible interaction with genetic background [82].
3.2. High Through-put Phenomics For Drought Resistance
Good phenotyping involves the use of appropriate genetic materials and precise and accurate measurements, together with relevant experimental conditions that are representative of the TPE [69]. Phenotyping with controlled experiments has the advantage of precise control of the main environmental parameters that are greatly variable under field conditions. Such controlled conditions can include greenhouses, growth chambers, rainout shelters, among others, involving hydroponic systems, aeroponic systems, use of gel- or soil filled chambers [83], soil sacs, paper pouches, paper rolls, and pot experiments, among others [84, 69]. Following standard protocols in controlled experiments can give insight into different mechanisms that a plant could be using when encountering a specific stress situation. Although these experiments might not be fully representative of field conditions, they are excellent for looking at stresses individually, controlling other factors that could influence the performance, and in dissecting the genetic basis of target traits. As there are large numbers of drought stress scenarios interacting and operating in different agro-ecologies, and multiple combinations of traits and strategies that a plant can use to tackle them, controlled growth facility experiments cannot simulate all these scenarios and replicate target environments, particularly for quantitative traits like drought resistance [77, 85-87]. One way to get around this is to move the trait evaluations and experiments to the target regions. In Australia, for example, multi-site managed experimental facilities at three locations, Merredin, Narrabri, and Yanco, have been established for wheat abiotic stress evaluations. Managed environmental facilities (MEFs) represent the selection environments and can be used for trait evaluations for estimating G x E interactions, gene/marker discovery, and direct population improvement using genomic selection [87]. MEFs should be strategically located to represent important target crop production areas and equipped with basic field facilities, including weather stations, access to soil analysis, and some low technology field phenotyping equipment [87, 80]. An established network of MEFs could evaluate large advanced breeding populations with standardized trait evaluation protocols and best practices for crop and field management over several growing seasons. In order to achieve results that are truly indicative of performance in the field, standard crop management practices should be used with uniform and repeatable stress levels (Fig. 2). Such an approach will be representative of the real situation in the field in the target region, resulting in accelerating the development of useful drought resistant and wider adapted cultivars.
Different high-throughput phenotyping methods have been proposed for evaluating traits under field conditions. These include proximal remote sensing and imaging, high throughput metabolomics analysis, near-infrared reflectance spectroscopy (NIRS), and magnetic resonance imaging (MRI) [85]. These methods generate a huge volume of data, which should be deposited to a central repository and later can be used to identify marker-trait associations, G X E analysis and to develop genomic selection models using a central genotyping platform. Once markers linked to traits are identified and genomic selection models developed, they can then be incorporated into breeding programs. These strategically located experimental field sites will identify parents for future crosses, as well as traits that are important in a specific stress scenario and in the production system.
3.3. Phenotyping Target Traits For Drought Tolerance
Drought resistance can have a different meaning to a plant physiologist, to a geneticist, or a breeder, and thus to define the traits of importance is not straightforward. From farmers' and breeders' perspectives, yield stability between normal/optimal growing conditions and moisture stress condition is drought resistance. Even so, stable yield has little value if the yields under normal conditions are not reliably high as well [88, 89]. However, for a plant physiologist, stability in terms of a physiological process e.g., WUE might be drought resistance [4]. Yield itself is a complex trait to which several traits contribute individually and in combinations [10]. Indirect selection for component traits can enhance the yield overall, and this can be very effective, particularly as directly selecting for yield under stress conditions might not reflect the underlying contribution of individual traits, and may lead to unexpected results in a different environment. Selecting traits of focus and prioritizing them should be based on their value and contribution toward increasing the yield and yield stability. Some traits might have very little contribution and investment towards improving them might not be cost-effective, while traits like early vigor and early maturity might have great impact on yield under early drought stress but are not directly related to drought resistance. Beside traits directly measuring yield, traits that lead to drought resistance and ultimately contribute to stable and enhanced yield under drought stress measure overall health, photosynthetic activity, or water status of the plant [90, 69]. Several target traits that might have a role in any of the plant response types should be evaluated to monitor and quantify plant's response to drought stress and to identify their role in yield stability under drought. Root architectural traits such as weight, length, volume and density could be studied non-destructively using digital imaging [91]. It is important to capture root growth and architecture in real time during development under stress as the root is the main organ that is in direct contact with moisture in the ground and responds quickly to changes in it. Because roots grow underground, most sampling of roots for characterizing and evaluating traits is destructive, with the plants being dug out of the ground. However, this digging out has several drawbacks, including damage to the root system while pulling out the plant, being laborious to evaluate hundreds of genotypes, and inability to characterize the development of the same plant over time [92]. Non-contact underground sensing methods like EMI and ground penetrating radar (GPR) sensors have been proposed to use for direct and indirect measurements of RSA development [93]. Plant roots change the electric conductivity (EC) of the surrounding soil, so EMI sensors that are designed to measure EC of the top soil layer can be used to measure root architectural traits. So far, GPR has only been used for coarse root analysis e.g., for trees, as it has not been easy to separate roots from background. However, research on increasing the precision and accuracy of the GPR signal can improve greater accuracy and imaging capabilities [94], but for full application, development of better hardware, and improved analytical tools is needed. MRI is another tool that could be applied to resolving root structure and separating root systems under drought stress [95].
Canopy traits such as canopy cover, leaf temperature, biomass traits, and specific leaf area are used for indirect measurement of plant health and indicate plant response to drought stress. Normalized difference vegetation index (NDVI), which is also dependent on leaf area index and fractional vegetation cover, could be derived from satellite images and analyzed [96]. NIRS have also been applied to study canopy traits such as plant water status and plant transpiration to detect early drought stress [97]. Canopy temperature refers to the difference between the canopy surface and the surrounding air which can be measured by infrared thermography to identify genotypes with cooler canopies under drought stress [69]. Photosynthetic pigment traits can be measured quickly using SPAD leaf meters [98], such as SPAD-502, which can be used to calculate the ratio of absorbance at 650 nm and at 940 nm to indirectly estimate the chlorophyll content of the leaves. Alternatively, NIRS could also be applied to assess chlorophyll content [99]. Stomatal conductance, which plays an important role in regulating plant water balance and WUE, can be measured using carbon isotope discrimination ∆13C or oxygen isotope composition d18O [69], while osmotic adjustment involving the increase in concentration of organic and inorganic substances such as sugars, polyols, amino acids, among others in the cytochylema during drought stress can be measured using laboratory analyses of these compounds [69]. WUE refers to the amount of dry matter produced per unit of water lost through evapotranspiration [100]. However, according to Blum [89], effective use of water may be more important than WUE for yield stability i.e. maintaining high stomatal transpiration for high productivity, while maximizing soil moisture capture by the plant and minimizing any other kind of water loss such as non-stomatal transpiration or soil evaporation.
3.4. Challenges of Data Analysis in High-throughput Phenotyping
Considering the number of genotypes required for field evaluation experiments, particularly with the added factor of a complex trait like drought tolerance, high-throughput phenotyping techniques need to be rapid, flexible, reliable and repeatable. These large and complex datasets call for high-throughput analytic capabilities and software systems as well as management protocols that maximize reliability and efficiency of the phenotype [71]. For maximum use, data should be geo-referenced with positional environmental information to combine it with the phenotype, requiring development and availability of geo-referencing tools. Most high-throughput phenotyping techniques rely on plant imaging to measure physiological, growth, development, and other phenotypic characteristics of plants using automated processes. However, these generally are only able to capture above-ground traits, which is often not fully indicative of the plant’s response to drought stress. Also, many of these platforms must be employed under controlled conditions not representative of real field growing conditions, and remain prohibitively expensive for many researchers. Extracting functional data from the images also poses a significant challenge. In recent years, several plant image analysis databases have been established, although they remain few compared with DNA sequence databases, reviewed by Fahlgren and colleagues [101], for example, HTPheno [102], Plant Image Analysis database [103] and Integrated Analysis Platform [104]. Three-dimensional reconstructions of the plant canopy with multispectral images can resolve phenotypic differences at the genotypic level for a large number of plants. However, this requires an enormous volume of high-resolution data, which is computationally intensive and requires special skills and software for data analysis. Image analysis from complex, large data sets from field experiments remains a bottleneck in high-throughput phenomics that will need further development for implementation to proceed more broadly.
4. GENOMICS-ASSISTED BREEDING FOR DROUGHT RESISTANCE
Crop species have great genetic variability for the traits that directly or indirectly contribute to drought resistance, and which thus offer the potential to be improved through selection in breeding. Genomics-assisted breeding can be approached in two main ways (Fig. 2): marker-assisted selection (MAS) and genomic selection (GS). For MAS, the first step is the identification of molecular markers that are strongly associated with traits of interest, which can then be implemented for selection in breeding programs. Meanwhile, GS relies on development of selection models based on dense genetic markers distributed across the whole genome and phenotyping of a training population to select individuals with high genome-estimated breeding values (GEBVs) in the breeding population [105]. While MAS has been in use since a few decades, and has become a key part of breeding programs for many crops, GS is relatively new, has only recently started to be applied to crops, and has as yet untested promise.
4.1. Marker-assisted Selection
MAS involves the use of molecular markers that map close to specific genes or quantitative trait loci (QTLs), whose association with the target trait has been established and can be used to select individuals with favorable alleles [105]. Reliable, accurate and high-throughput trait evaluation and dense molecular markers across the genome can either be used to identify marker-trait associations via QTL mapping or genome-wide association mapping approaches. Based on these methods, QTLs for traits associated with drought resistance have been identified in important crops such as maize [106], rice [107], wheat [108], soybean [109], sorghum [110], foxtail millet [111], pearl millet [112], among other crops. However, many drought-related QTLs identified are not stable in different environments. A QTL can have positive or negative additive effects, depending on the drought condition due to strong genotype-by-environment interaction (G x E), [113]. Thus, there is real need to first define the target drought scenario for drought resistance QTL identification. In chick pea, precise phenotyping coupled with dense marker data has enabled the identification of several stable and robust main effect QTLs for 13 out of 20 drought tolerance related traits, which explain between 10-58% of the observed variation [114]. Once identified, these QTLs could be fine-mapped to identify drought responsive genes. In maize, 22 differentially expressed genes were identified in a microarray experiment on four susceptible and tolerant recombinant inbred lines (RILs), co-located on the genetic map with QTLs for drought tolerance [115]. Eventually these QTLs could then be introgressed and multiple QTLs can even be pyramided into elite breeding material through marker-assisted backcrossing (MABC; main effect QTL) or through increasing the frequency of several beneficial alleles with small individual effects and additive effect through marker-assisted recurrent selection (MARS). ‘QTL-hotspot’, a genomic region found to harbor 12 out of 25 main effect QTLs for 12 traits related to drought tolerance, has been successfully introgressed into the genetic backgrounds of elite varieties in chick pea using MABC [114]. MABC has also been successfully deployed in rice breeding with a notable example of the popular variety IR64 [116]. IR64 is a high-yielding but drought-susceptible cultivar that is popular in many countries of Asia [117]. Several drought QTLs such as qDTY2.2 and qDTY4.1 were introgressed in IR64 background, resulting in higher grain yield under reproductive stage drought stress [118, 119]. Similarly, MARS has been successfully deployed in crops like maize, soybean, sunflower, wheat, sorghum, and rice, mainly by the private sector [70]. Monsanto developed genotyping systems and information tools that allowed molecular marker-assisted methodologies to increase mean performance of elite breeding populations in maize [120]. In sorghum, four stay-green (Stg) QTLs that regulate canopy size through multiple pathways, resulting in developmental plasticity, have been mapped to a number of key chromosomal regions. These QTLs are currently being introgressed into different backgrounds to develop better adapted sorghum varieties [121, 122].
To identify genes responsible for physiological processes involved in drought resistance, comparison of gene expression profiles after exposing plants to a specific level of drought stress can be complementary to other marker identification approaches. Several studies have identified genes from drought resistance mechanisms and validated them with quantitative PCR, for instance, Schafleitner et al. [123] developed a gene index for hexaploid sweetpotato under drought stress. New high-throughput methods have been developed to look at RNA sequencing (RNA-seq) profiles in time series experiments that are robust, more accurate, and cost effective. RNA-seq experiments should be conducted in controlled conditions, and then identified genes and alleles validated in larger populations. It is becoming the technology of choice to study differential expression of genes in both model and non-model plants such as lodgepole pine [124], chestnut [125], sweetpotato [126], chickpea [127], and cassava [128], among others. This can be a powerful tool for identifying genes related to drought resistance, which can be validated through qPCR, QTL analysis, or GWAS studies, and finally to develop molecular markers for use in MAS.
Prediction of performance in the field under drought stress conditions can also be modeled in silico, by using mathematical models that combine environmental and genetic data, and crop and genetic models [129]. It helps to test physiological hypotheses with a combination of multiple alleles of varied effect in different environmental scenarios and physiological stages by analyzing the QTL for each parameter of the model. Using this approach, the response of leaf growth to temperature and water deficits in maize was broken down into traits such as intrinsic elongation rate [130].
4.2. Potential For Genomic Selection For Drought Resistance
Drought tolerance is complicated by the number of physiological traits, underlying genes and pathways, and the small effects of individual genes to yield, so identification of individual effects and introgressing them into elite cultivars is challenging. Therefore, complementary or improved methods of selection have to be explored. There have been enormous developments in genome sequencing technologies over the last few decades, from Sanger to 3rd generation sequencing [131]. Throughput and sequence read lengths have increased tremendously, while the error rate and costs have been reduced. This has not only increased options for de novo sequencing of new genomes, but also offered opportunities for re-sequencing genotypes within a species. New kinds of marker systems have been developed based on genome sequencing. Hybridization-based marker systems with thousands of markers such as Infinium SNP assays, or sequence-based genotyping methods are in increasing use. Methods such as genotyping-by-sequencing (GBS) and DArTseq (Diversity Array Technology sequencing) are based on the principle of genome complexity reduction through the right choice of methylation-sensitive restriction enzymes [132, 133]. Restriction enzymes are chosen to chop off high copy number repetitive genome sequences, attaching barcoded adapters for identification of genotypes in multiplexed sequencing lanes to sequence only the low copy number genome. Afterwards, bioinformatics tools are used to identify polymorphisms in the sequence reads. These marker systems promise hundreds and thousands of SNP markers in a short time with very low cost and can be used in marker-trait association identification across whole genome, and crop improvement programs based on GEBVs [105].
GS uses all available markers for a population as predictors of GEBVs, based on formulae estimated from a training population with both genotypic and phenotypic data, to bypass marker-trait association identification. GS models are then used in selecting elite individuals in the breeding population without further phenotyping [134]. Unlike MAS, GS does not require prior knowledge of a few large effect genes or QTLs [105]. Instead, GS requires higher density marker data than MAS, and its application has only been made possible by the availability of high throughput, low cost, genome-wide marker coverage genotyping technologies. GS was originally applied in animal breeding programs [135] and since few years ago, it has started gaining interest in crop breeding, as it promises rapid and accelerated genetic gains for complex quantitative traits. The prediction accuracy of GS models depends on several factors, including the relationship between the training population and the breeding population, the number of generations that separate the training and the breeding populations, the type and number of markers used, the accuracy of the phenotyping, and the heritability of the trait [134]. GS is currently being applied for drought resistance breeding in maize by the international maize and wheat improvement center, CIMMYT [136]. Efforts towards this approach are on course for other crops as well, including sugarcane [137], chick pea, pigeon pea and ground nut [138], white spruce [139], cassava [140, 141], apple [142], wheat [143], among others. The results from these GS breeding programs, their accuracy of performance prediction and the genetic gains they achieve will not be known for a few more years.
4.3. Complementary Approaches
Overcoming the current yield gaps by ensuring stable yield under moisture stress requires a broader and deeper look on multiple fronts for sources of tolerance. Staying within existing well-known genepools is not enough. Our crop species have enormous genetic diversity that gives them the ability to adapt to changing environments, including pests, diseases, and climate change. Much of this genetic diversity for important crops and their wild relatives is preserved in genebanks and represents large potential as a source of stress tolerance [144]. For instance, the International Potato Center (CIP) gene bank holds about 80% of the world’s native potato and sweetpotato germplasm and about 80% of wild species [personal communication, CIP genebank]. CIMMYT also maintains a large collection of maize and wheat germplasm [145], as do other CGIAR centers for different crops. Germplasm of crops has been screened for various abiotic stresses, and has shown great variation for response to many stresses due to the presence of novel allelic variations e.g. rice [146] and wheat [147]. This variation has potential as a new source of drought resistance and plays a critical role in developing improved cultivars, either through genomic assisted breeding, or through new biotechnology approaches to rapidly incorporate desirable genes into advanced and consumer-preferred backgrounds. Seeds of Discovery, a program funded by the Government of Mexico, is providing advanced tools for identification of stress tolerance alleles in the maize germplasm [80]. A large number of rice accessions from the genebank of the International Rice Research Institute (IRRI) were screened to identify drought tolerance donors to use in breeding and several accessions with high grain yields in both well-watered and drought stress conditions were recommended for use in drought resistance breeding programs [144]. Potato production worldwide is also strongly affected by drought stress, and improving drought resistance has become a priority for potato breeders. At CIP, 918 accessions, including improved varieties, genetic stocks and landraces from world potato collection were evaluated under different irrigation regimes. Significant differences were found for tuber traits, and Andean landraces were identified as potential sources of drought tolerance [148]. Another study screened 550 maize inbred lines from global breeding programs for drought resistance. Results identified maize lines developed for temperate regions with strong drought resistance when tested under tropical conditions, indicating that they could be used in drought resistance breeding for both temperate and tropical environments [149].
Transgenic refers to an individual or a cell with a DNA sequence that has been integrated into the native DNA by techniques of genetic engineering [150], and offers another option for introgressing new sources of drought resistance. Transgenic research has also been applied in studies aiming to understand drought resistance in plants [151]. For example, Saijo et al. [152] used transgenic rice plants to study the physiological function of OsCDPK7 gene, and Iuchi et al. [47] used gene manipulation of the 9-cis-epoxycarotenoid dioxygenase to study the regulation of drought resistance in Arabidopsis. However, success from transgenic research for drought resistance has been limited because transfer is generally limited to single genes, and thus may only be practical for genes with major effects [13]. A multi-gene transformation strategy combining several major functional or regulatory genes may offer a better option for drought resistant transgenics. Efforts by Monsanto in collaboration with CIMMYT and other institutions in sub-Saharan Africa have used transgenic technology to develop drought tolerant maize in a project referred to as water efficient maize for Africa, WEMA [80].
Another approach is to use gene editing, where a guided RNA is used to create targeted mutations in candidate genes of key pathways to identify their effects and create new variation in a relatively short time [153]. Clustered regularly interspersed short palindromic repeats/CAS [CRISPR-CAS] systems are adaptive immunity systems that are present in many archea and bacteria, encoded by operons with diverse architecture, a high rate of evolution for the CAS genes, and unique spacer content [154]. Gene editing can be achieved through the use of these CRISPR-CAS systems where short RNAs direct CAS nucleases to induce precise cleavage at endogenous loci with nicking enzymes for facilitated homology-directed repair with minimal mutagenic activity. The multiple guided sequences are then encoded into a single CRISPR array for simultaneous editing of several sites [153].
5. CONCLUSIONS
Drought is a major threat to food production worldwide and its impact is only expected to increase with the further onset of climate change. Understanding the effect of drought on plants is critical for developing improved varieties with stable high yield. However, plant responses to drought stress are complex, depending on environmental conditions, frequency and duration of the stress, the species and variety of the plant, and the physiological stage of the plant at the time of the stress. There are myriads of physiological processes, signaling pathways, and genes involved, and untangling the responses for practical application requires a multi-pronged approach. There needs to be precise and accurate phenotyping and high-throughput genotyping, characterization of the target environments and stress scenarios, and good analytical tools to integrate the different components. For a successful breeding program, enough genetic diversity must be present in the starting population to find the right allelic combinations to enhance the resistance level through MAS or GS. New developments in biotechnology can create new sources of resistance and with potential to rapidly introgress in elite backgrounds. Using these approaches, new varieties with improved drought tolerance corresponding to grower preferences can be rapidly introduced into target regions.
ACKNOWLEDGEMENTS
Authors would like to thank Dr. Merideth Bonierbale for reading the article and giving useful feedback.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Mir R.R., Zaman-Allah M., Sreenivasulu N., Trethowan R., Varshney R.K. Integrated genomics, physiology and breeding approaches for improving drought tolerance in crops. Theor. Appl. Genet. 2012;125(4):625–645. doi: 10.1007/s00122-012-1904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malagnoux M., Sene E.H., Atzmon N. Forests, Trees and Water in Arid Lands: A delicate Balance. In: Perlis A., editor. Forests and Water. FAO. 58(4). 2007. pp. 24–29. [Google Scholar]
- 3.Food and Agriculture Organization of the United Nations . Food Outlook: Global Market Analysis. Rome: FAO; 2008. [Google Scholar]
- 4.Blum A. Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Crop Pasture Sci. 2005;56(11):1159–1168. [Google Scholar]
- 5.Bruinsma J. The resource outlook to 2050In Expert Meeting on How to Feed the World in 2050. http://gfs.wur.nl/docs/Bruinsma_pres.pdf. 2009.
- 6.FAO . IFAD.; WFP. The State of Food Insecurity in the World 2013: The Multiple Dimensions of Food Security. Rome: FAO; 2013. [Google Scholar]
- 7.Schneider S.H., Semenov S., Patwardhan A., Burton I., Magadza C.H., Oppenheimer M., Pittock A.B., Rahman A., Smith J.B., Suarez A., Yamin F. In: Assessing key vulnerabilities and the risk from climate change. Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Parry, M.L.; Canziani, O.F.; Palutikof, J.P.; van der Linden, P.J. Hanson C.E., editor. Cambridge, UK: Cambridge University Press; 2007. pp. 779–810. [Google Scholar]
- 8.Garruna-Hernandez R., Orellana R., Larque-Saavedra A., Canto A. Canto A. Understanding the physiological responses of a tropical crop (Capsicum Chinense Jacq.) at high temperature. PLoS one. 2014 doi: 10.1371/journal.pone.0111402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trenberth K.E. Changes in precipitation with climate change. Clim. Res. 2011;47(1):123. [Google Scholar]
- 10.Fleury D., Jefferies S., Kuchel H., Langridge P. Genetic and genomic tools to improve drought tolerance in wheat. J. Exp. Bot. 2010;61(12):3211–3222. doi: 10.1093/jxb/erq152. [DOI] [PubMed] [Google Scholar]
- 11.Cooper P.J., Dimes J., Rao K.P., Shapiro B., Shiferaw B., Twomlow S. Coping better with current climatic variability in the rain-Fed farming systems of sub-Saharan Africa: An essential first step in adapting to future climate change? Agr. Eco. Env. 2008;126(1):24–35. [Google Scholar]
- 12.Venuprasad R., Lafitte H.R., Atlin G.N. Response to direct selection for grain yield under drought stress in rice. Crop Sci. 2007;47(1):285–293. [Google Scholar]
- 13.Fang Y., Xiong L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015;72(4):673–689. doi: 10.1007/s00018-014-1767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner N.C. Drought Resistance and Adaptation to Water Deficits in Crop Plants. In: Mussel H., Staples R.C., editors. Stress Physiology in Crop Plants. New York: Wiley; 1979. pp. 344–372. [Google Scholar]
- 15.Levitt J. Responses of Plants to Environmental Stresses, Vol II. Water, Radiation, Salt, and Other Stresses. London: Academic Press; 1980. [Google Scholar]
- 16.Zhang J-Y., Broeckling C.D., Blancaflor E.B., Sledge M.K., Sumner L.W., Wang Z-Y. Overexpression of WXP1, a putative medicago truncatula AP2 domain-containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago Sativa). Plant J. 2005;42(5):689–707. doi: 10.1111/j.1365-313X.2005.02405.x. [DOI] [PubMed] [Google Scholar]
- 17.Tardieu F. Plant response to environmental conditions: Assessing potential production, water demand, and negative effects of water deficit. Front. Physiol. 2013;4:17. doi: 10.3389/fphys.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blum A. Towards standard assays of drought resistance in crop plants. Production in Water-Limited Environments; 2000. p. 29. [Google Scholar]
- 19.Luo L.J. Breeding for water-saving and drought-resistance rice (WDR) in China. J. Exp. Bot. 2010:erq185. doi: 10.1093/jxb/erq185. [DOI] [PubMed] [Google Scholar]
- 20.May L., Milthorpe F. Drought resistance of crop plants. Proc. Field. Crop. Abstr. 1962;15:171–179. [Google Scholar]
- 21.Levitt J. Responses of plants to environmental stresses. New York: Academic Press; 1972. [Google Scholar]
- 22.Pantin F., Simonneau T., Muller B. Coming of leaf age: Control of growth by hydraulics and metabolics during Leaf ontogeny. New Phytol. 2012;196(2):349–366. doi: 10.1111/j.1469-8137.2012.04273.x. [DOI] [PubMed] [Google Scholar]
- 23.Szabados L., Savouré A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010;15(2):89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Crowe J.H., Crowe L.M., Chapman D. Preservation of membranes in anhydrobiotic organisms: The role of trehalose. Science. 1984;223(4637):701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- 25.Bianchi G., Gamba A., Limiroli R., Pozzi N., Elster R., Salamini F., Bartels D. The unusual sugar composition in leaves of the resurrection plant Myrothamnus flabellifolia. Physiol. Plant. 1993;87(2):223–226. [Google Scholar]
- 26.Ranieri A., Bernardi R., Lanese P., Soldatini G.F. Changes in free amino acid content and protein pattern of maize seedlings under water stress. Environ. Exp. Bot. 1989;29(3):351–357. [Google Scholar]
- 27.Yoshiba Y., Kiyosue T., Katagiri T., Ueda H., Mizoguchi T., Yamaguchi-Shinozaki K., Wada K., Harada Y., Shinozaki K. Correlation between the Induction of a gene for Δ1-Pyrroline-5-Carboxylate Synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. Plant J. 1995;7(5):751–760. doi: 10.1046/j.1365-313x.1995.07050751.x. [DOI] [PubMed] [Google Scholar]
- 28.Nagata S., Wang Y., Zhang H., Sasaki H., Oshima A., Ishida A. Effect of moderate salinity stress treatment on the stimulation of proline uptake and growth in escherichia coli CSH4 and its mutants under high salinity. J. Biosci. Bioeng. 2009;108(3):205–210. doi: 10.1016/j.jbiosc.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Saradhi P. AliaArora, P.S.; Prasad, K.V.S.K. Proline accumulates in plants exposed to UV radiation and protects them against UV-induced peroxidation. Biochem. Biophys. Res. Commun. 1995;209(1):1–5. doi: 10.1006/bbrc.1995.1461. [DOI] [PubMed] [Google Scholar]
- 30.Schat H., Sharma S.S., Vooijs R. Heavy metal-induced accumulation of free proline in a metal-tolerant and a nontolerant ecotype of Silene vulgaris. Physiol. Plant. 1997;101(3):477–482. [Google Scholar]
- 31.Zhang L., Alfano J.R., Becker D.F. Proline metabolism increases katG expression and oxidative stress resistance in Escherichia coli. J. Bacteriol. 2015;197(3):431–440. doi: 10.1128/JB.02282-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebaudy A., Véry A-A., Sentenac H.K. + channel activity in plants: Genes, regulations and functions. FEBS Lett. 2007;581(12):2357–2366. doi: 10.1016/j.febslet.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 33.Tuteja N. Abscisic acid and abiotic stress signaling. Plant Signal. Behav. 2007;2(3):135–138. doi: 10.4161/psb.2.3.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osakabe Y., Arinaga N., Umezawa T., Katsura S., Nagamachi K., Tanaka H., Ohiraki H., Yamada K., Seo S.U., Abo M., Yoshimura E., Shinozaki K., Yamaguchi-Shinozaki K. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell. 2013;25(2):609–624. doi: 10.1105/tpc.112.105700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dure L., III, Crouch M., Harada J., Ho T-H., Mundy J., Ralph Quatrano R., Thomas T., Sung Z.R. Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol. Biol. 1989;12(5):475–486. doi: 10.1007/BF00036962. [DOI] [PubMed] [Google Scholar]
- 36.Hanin M., Brini F., Ebel C., Toda Y., Takeda S., Masmoudi K. Plant dehydrins and stress tolerance: Versatile proteins for complex mechanisms. Plant Signal. Behav. 2011;6(10):1503–1509. doi: 10.4161/psb.6.10.17088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cruz de Carvalho M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008;3(3):156–165. doi: 10.4161/psb.3.3.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. 2012.
- 39.Malik B., Pirzadah T.B., Tahir I., Rehman R.U., Hakeem K.R., Abdin M.Z. Plant signaling: Response to reactive oxygen species. In Plant Signaling: Understanding the Molecular Crosstalk. http://link.springer.com/10.1007/978-81-322-1542- 4_1. 2014. pp. 1–38.
- 40.Davies W.J., Zhang J. Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Biol. 1991;42(1):55–76. [Google Scholar]
- 41.Mohamed M.F., Keutgen N., Tawfika A.A., Noga G. Dehydration-avoidance responses of tepary bean lines differing in drought resistance. J. Plant Physiol. 2002;159(1):31–38. [Google Scholar]
- 42.Hammer G.L., Dong Z., McLean G., Doherty A., Messina C., Schussler J., Zinselmeier C., Paszkiewicz S., Mark Cooper M. Can changes in canopy and/or root system architecture explain historical maize yield trends in the US corn belt? Crop Sci. 2009;49(1):299–312. [Google Scholar]
- 43.Forster B.P., Thomas W.T., Chloupek O. Genetic controls of barley root systems and their associations with plant performance. Asp. Appl. Biol. 2005;73:199–204. [Google Scholar]
- 44.Hu H., Xiong L. Genetic engineering and breeding of drought-resistant crops. Annu. Rev. Plant Biol. 2014;65:715–741. doi: 10.1146/annurev-arplant-050213-040000. [DOI] [PubMed] [Google Scholar]
- 45.Osakabe Y., Osakabe K., Shinozaki K., Tran L-S. Response of plants to water stress. Front. Plant Sci. 2014;5 doi: 10.3389/fpls.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou J-J., Wei F-J., Wang C., Wu J-J., Ratnasekera D., Liu W-X., Wu W-H. Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 2010;154(3):1232–1243. doi: 10.1104/pp.110.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iuchi S., Kobayashi M., Taji T., Naramoto M., Seki M., Kato T., Tabata S., Kakubari Y., Yamaguchi-Shinozaki K., Shinozaki K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001;27(4):325–333. doi: 10.1046/j.1365-313x.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- 48.Endo A., Sawada Y., Takahashi H., Okamoto M., Ikegami K., Koiwai H., Seo M., Toyomasu T., Mitsuhashi W., Shinozaki K., Nakazono M., Kamiya Y., Koshiba T., Nambara E. Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol. 2008;147(4):1984–1993. doi: 10.1104/pp.108.116632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang J., Hwang J-U., Lee M., Kim Y-Y., Assmann S.M., Martinoia E., Lee Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA. 2010;107(5):2355–2360. doi: 10.1073/pnas.0909222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanno Y., Hanada A., Chiba Y., Ichikawa T., Nakazawa M., Matsui M., Koshiba T., Kamiya Y., Seo M. Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc. Natl. Acad. Sci. USA. 2012;109(24):9653–9658. doi: 10.1073/pnas.1203567109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu J-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002;53:247. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamaguchi-Shinozaki K., Shinozaki K. Organization of cis-acting regulatory elements in osmotic and cold-stress-responsive promoters. Trends Plant Sci. 2005;10(2):88–94. doi: 10.1016/j.tplants.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi-Shinozaki K., Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- 54.Li W-X., Oono Y., Zhu J., He X-J., Wu J-M., Iida K., Lu X-Y., Cui X., Jin H., Zhu J-K. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and post-transcriptionally to promote drought resistance. Plant Cell. 2008;20(8):2238–2251. doi: 10.1105/tpc.108.059444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu H., Dai M., Yao J., Xiao B., Li X., Zhang Q., Xiong L. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl. Acad. Sci. USA. 2006;103(35):12987–12992. doi: 10.1073/pnas.0604882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karpinski S., Escobar C., Karpinska B., Creissen G., Mullineaux P.M. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell. 1997;9(4):627–640. doi: 10.1105/tpc.9.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davletova S., Schlauch K., Coutu J., Mittler R. The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 2005;139(2):847–856. doi: 10.1104/pp.105.068254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang F-Z., Wang Q-B., Kwon S-Y., Kwak S-S., Su W-A. Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J. Plant Physiol. 2005;162(4):465–472. doi: 10.1016/j.jplph.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Miao Y., Lv D., Wang P., Wang X-C., Chen J., Miao C., Song C-P. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell. 2006;18(10):2749–2766. [Google Scholar]
- 60.You J., Zong W., Li X., Ning J., Hu H., Li X., Xiao J., Xiong L. The SNAC1-targeted gene OsSRO1c modulates stomatal closure and oxidative stress tolerance by regulating hydrogen peroxide in rice. J. Exp. Bot. 2013;64(2):569–583. doi: 10.1093/jxb/ers349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verbruggen N., Villarroel R., Van Montagu M. Osmoregulation of a pyrroline-5-carboxylate reductase gene in Arabidopsis thaliana. Plant Physiol. 1993;103(3):771–781. doi: 10.1104/pp.103.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delauney A.J., Hu C.A., Kishor P.B., Verma D.P. Cloning of ornithine delta-aminotransferase cDNA from Vigna aconitifolia by trans-complementation in Escherichia Coli and regulation of proline biosynthesis. J. Biol. Chem. 1993;268(25):18673–18678. [PubMed] [Google Scholar]
- 63.Roosens N.H., Thu T.T., Iskandar H.M., Jacobs M. Isolation of the ornithine-δ-aminotransferase cDNA and effect of salt stress on its expression in Arabidopsis thaliana. Plant Physiol. 1998;117(1):263–271. doi: 10.1104/pp.117.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeo E-T., Kwon H-B., Han S-E., Lee J-T., Ryu J-C., Byu M.O. M.O. Genetic engineering of drought resistant potato plants by introduction of the trehalose-6-phosphate synthase (TPS1) gene from Saccharomyces cerevisiae. Mol. Cells. 2000;10(3):263–268. [PubMed] [Google Scholar]
- 65.Zhang N., Si H-J., Wen G., Du H-H., Liu B-L., Wang D. Enhanced drought and salinity tolerance in transgenic potato plants with a BADH gene from spinach. Plant Biotechnol. Rep. 2011;5(1):71–77. [Google Scholar]
- 66.Wilson P.B., Estavillo G.M., Field K.J., Pornsiriwong W., Carroll A.J., Howell K.A., Woo N.S., Lake J.A., Smith S.M., Harvey Miller A., von Caemmerer S., Poqson B.J. The nucleotidase/phosphatase SAL1 is a negative regulator of drought tolerance in Arabidopsis. Plant J. 2009;58(2):299–317. doi: 10.1111/j.1365-313X.2008.03780.x. [DOI] [PubMed] [Google Scholar]
- 67.Estavillo G.M., Crisp P.A., Pornsiriwong W., Wirtz M., Collinge D., Carrie C., Giraud E., Whelan J., David P., Javot H., Brearly C., Hell R., Marin E., Poqson B.J. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell. 2011;23(11):3992–4012. doi: 10.1105/tpc.111.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao Y., Savchenko T., Baidoo E.E., Chehab W.E., Hayden D.M., Tolstikov V., Corwin J.A., Kliebenstein D.J., Keasling J.D., Dehesh K. Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell. 2012;149(7):1525–1535. doi: 10.1016/j.cell.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 69.Tuberosa R. Phenotyping for drought tolerance of crops in the genomics era. Front. Physiol.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3446691/. 2012;3 doi: 10.3389/fphys.2012.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thudi M., Gaur P.M., Krishnamurthy L., Mir R.R., Kudapa H., Fikre A., Kimurto P., Tripathi S., Solen K.R., Mulwa R., Bharadwaj C., Datta S., Chaturvedi S.K., Varshney R.K. Genomics-assisted breeding for drought tolerance in chickpea. Funct. Plant Biol. 2014;41(11):1178–1190. doi: 10.1071/FP13318. [DOI] [PubMed] [Google Scholar]
- 71.White J.W., Andrade-Sanchez P., Gore M.A., Bronson K.F., Coffelt T.A., Conley M.M., Feldmann K.A., French A.N., Heun J.T., Hunsaker D.J., Jenks M.A., Kimball B.A., Roth R.L., Strand R.J., Thorp K.R., Wall G.W., Wang G. Field-based phenomics for plant genetics research. Field Crops Res. 2012;133:101–112. [Google Scholar]
- 72.Mittler R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006;11(1):15–19. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 73.Salekdeh G.H., Reynolds M., Bennett J., Boyer J. Conceptual framework for drought phenotyping during molecular breeding. Trends Plant Sci. 2009;14(9):488–496. doi: 10.1016/j.tplants.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 74.Shinozaki K., Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007;58(2):221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- 75.Xu Y., Crouch J.H. Marker-assisted selection in plant breeding: From publications to practice. Crop Sci. 2008;48(2):391–407. [Google Scholar]
- 76.Passioura J.B. Scaling up: The essence of effective agricultural research. Funct. Plant Biol. 2010;37(7):585–591. [Google Scholar]
- 77.Cairns J.E., Impa S.M., O’Toole J.C., Jagadish S.V., Price A.H. Influence of the soil physical environment on rice (Oryza Sativa L.) response to drought stress and its implications for drought research. Field Crops Res. 2011;121(3):303–310. [Google Scholar]
- 78.Kahinda J.M., Lillie E.S., Taigbenu A.E., Taute M., Boroto R.J. Developing suitability maps for rainwater harvesting in South Africa. Phys. Chem. Earth. 2008;33(8):788–799. [Google Scholar]
- 79.Reshmidevi T.V., Jana R., Eldho T.I. Geospatial estimation of soil moisture in rain-fed paddy fields using SCS-CN-based model. Agric. Water Manage. 2008;95(4):447–457. [Google Scholar]
- 80.Edmeades G.O. Progress in Achieving and Delivering Drought Tolerance in Maize—an Update. Ithaca, NY: ISAAA; 2013. [Google Scholar]
- 81.Barker T., Campos H., Cooper M., Dolan D., Edmeades G., Habben J., Schussler J., Wright D., Zinselmeier C. Improving drought tolerance in maize. Plant Breed. Rev. 2005;25:173–253. [Google Scholar]
- 82.Reynolds M., Tuberosa R. Translational research impacting on crop productivity in drought-prone environments. Curr. Opin. Plant Biol. 2008;11(2):171–179. doi: 10.1016/j.pbi.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 83.Bengough A.G., Gordon D.C., Al-Menaie H., Ellis R.P., Allan D., Keith R., Thomas W.T., Forster B.P. Gel observation chamber for rapid screening of root traits in cereal seedlings. Plant Soil. 2004;262(1-2):63–70. [Google Scholar]
- 84.Hargreaves C.E., Gregory P.J., Bengough A.G. Measuring root traits in barley (Hordeum Vulgare Ssp. Vulgare and Ssp. Spontaneum) seedlings using gel chambers, soil sacs and X-Ray microtomography. Plant Soil. 2009;316(1-2):285–297. [Google Scholar]
- 85.Araus J.L., Cairns J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014;19(1):52–61. doi: 10.1016/j.tplants.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 86.Passioura J.B. Phenotyping for drought tolerance in grain crops: When is it useful to breeders? Funct. Plant Biol. 2012;39(11):851–859. doi: 10.1071/FP12079. [DOI] [PubMed] [Google Scholar]
- 87.Rebetzke G.J., Chenu K., Biddulph B., Moeller C., Deery D.M., Rattey A.R., Bennett D., Barrett-Lennard G., Mayer J.E. A multisite managed environment facility for targeted trait and germplasm phenotyping. Funct. Plant Biol. 2013;40(1):1–13. doi: 10.1071/FP12180. [DOI] [PubMed] [Google Scholar]
- 88.Blum A. Drought adaptation in cereal crops: A prologue. Drought Adaptation in Cereals; 2006. pp. 3–15. [Google Scholar]
- 89.Blum A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009;112(2):119–123. [Google Scholar]
- 90.Monneveux P., Ribaut J-M. Secondary Traits for Drought Tolerance Improvement in Cereals. Drought Adaptation in Cereals; 2006. pp. 97–143. [Google Scholar]
- 91.Himmelbauer M.L., Loiskandl W., Kastanek F. Estimating length, average diameter and surface area of roots using two different image analyses systems. Plant Soil. 2004;260(1-2):111–120. [Google Scholar]
- 92.Lynch J.P., Nielsen K.L., Davis R.D., Jablokow A.G. SimRoot: Modelling and visualization of root systems. Plant Soil. 1997;188(1):139–151. [Google Scholar]
- 93.Villordon A.Q., Ginzberg I., Firon N. Root architecture and root and tuber crop productivity. Trends Plant Sci. 2014;19(7):419–425. doi: 10.1016/j.tplants.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 94.Hirano Y., Yamamoto R., Dannoura M., Aono K., Igarashi T., Ishii M., Yamase K., Makita N., Kanazawa Y. Detection frequency of Pinus thunbergii roots by ground-penetrating radar is related to root biomass. Plant Soil. 2012;360(1-2):363–373. [Google Scholar]
- 95.Rascher U., Blossfeld S., Fiorani F., Jahnke S., Jansen M., Kuhn A.J., Matsubara S., Martin L.L.A., Merchant A., Metzner R., Muller-Linow M., Nagel K.A., Pierushka R., Pinto F., Schreiber C.M., Temperton V.M., Thorpe M.R., van Dusschoten D., van Volkenburgh E., Windt C.W., Schurr U. Non-invasive approaches for phenotyping of enhanced performance traits in bean. Funct.Plant Biol. 2011;38(12):968–983. doi: 10.1071/FP11164. [DOI] [PubMed] [Google Scholar]
- 96.Carlson T.N., Ripley D.A. On the relation between NDVI, fractional vegetation cover, and leaf area index. Remote Sens. Environ. 1997;62(3):241–252. [Google Scholar]
- 97.Gray S.B., Dermody O., DeLucia E.H. Spectral reflectance from a soybean canopy exposed to elevated CO2 and O3. J. Exp. Bot. 2010:erq244. doi: 10.1093/jxb/erq244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arunyanark A., Jogloy S., Akkasaeng C., Vorasoot N., Kesmala T., Rao R.C., Wright G.C., Patanothai A. Chlorophyll stability is an indicator of drought tolerance in peanut. J. Agron. Crop Sci. 2008;194(2):113–125. [Google Scholar]
- 99.Guo P., Baum M., Varshney R.K., Graner A., Grando S., Ceccarelli S. QTLs for chlorophyll and chlorophyll fluorescence parameters in barley under post-flowering drought. Euphytica. 2008;163(2):203–214. [Google Scholar]
- 100.Passioura J.B. Grain yield, harvest index, and water use of wheat. J. Austr. Inst. Agric. Sci.http://agris.fao.org/agrissearch/ search.do?recordID=AU19780321350. 1977.
- 101.Fahlgren N., Gehan A.M., Baxter I. Lights, camera, action: High-throughput plant phenotyping is ready for a close-up. Curr. Opin. Plant Biol. 2015;24:93–99. doi: 10.1016/j.pbi.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 102.Hartmann A., Czauderna T., Hoffmann R., Stein N., Schreiber F. HTPheno: an image analysis pipeline for high-throughput plant phenotyping. BMC Bioinformatics. 2011;12:148. doi: 10.1186/1471-2105-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lobet G., Draye X., Perilleux C. An online database for plant image analysis software tools. Plant Methods. 2013;9:38. doi: 10.1186/1746-4811-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klukas C., Chen D., Pape J-M. Integrated analysis platform: an open-source information system for high-throughput plant phenotyping. Plant Physiol. 2014;165:506–518. doi: 10.1104/pp.113.233932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varshney R.K., Terauchi R., McCouch S.R. Harvesting the promising fruits of genomics: Applying genome sequencing technologies to crop breeding. PLoS Biology. 2014 doi: 10.1371/journal.pbio.1001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown P.J., Upadyayula N., Mahone G.S., Tian F., Bradbury P.J., Myles S., Holland J.B., Flint-Garcia S., McMullen M.D., Buckler E.S., Rocheford T.R. Distinct genetic architectures for male and female inflorescence traits of maize. PLoS Genetics. 2011. [DOI] [PMC free article] [PubMed]
- 107.Huang X., Wei X., Sang T., Zhao Q., Feng Q., Zhao Y., Li C., Zhu C., Lu T., Zhang Z., Li M., Fan D., Guo Y., Wang A., Wang L., Deng L., Lu Y., Weng Q., Liu K., Huang T., Zhou T., Jing Y., Li W., Lin Z., Buckler E.S., Qian Q., zhang Q., Li J., Han B. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 2010;42(11):961–967. doi: 10.1038/ng.695. [DOI] [PubMed] [Google Scholar]
- 108.Kollers S., Rodemann B., Ling J., Korzun V., Ebmeyer E., Argillier O., Hinze M., Plieske J., Kulosa D., Ganal M.W., Roder M.S. Whole genome association mapping of Fusarium head blight resistance in European winter wheat (Triticum aestivum L.). PLoS One. 2013;8(2):e57500. doi: 10.1371/journal.pone.0057500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hwang E-Y., Song Q., Jia G., Specht J.E., Hyten D.L., Costa J., Cregan P.B. A Genome-wide association study of seed protein and oil content in soybean. BMC Genomics. 2014;15(1):1. doi: 10.1186/1471-2164-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morris G.P., Ramu P., Deshpande S.P., Hash C.T., Shah T., Upadhyaya H.D., Riera-Lizarazu O., Brown P.J., Acharya C.B., Mitchell S.E., Harriman J., Glaubitz J.C., Buckler E.S., Kresovich S. Population genomic and genome-wide association studies of agroclimatic traits in sorghum. Proc. Natl. Acad. Sci. USA. 2013;110(2):453–458. doi: 10.1073/pnas.1215985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jia G., Huang X., Zhi H., Zhao Y., Zhao Q., Li W., Chai Y., Yang L., Liu K., Lu H., Zhu C., Lu Y., Zhou C., Fan D., Weng Q., Guo Y., Huang T., Zhang L., Lu T., Feng Q., Hao H., Liu H., Lu P., Zhang N., Li Y. A haplotype map of genomic variations and genome-wide association studies of agronomic traits in foxtail millet (Setaria italica). Nat. Genet. 2013;45(8):957–961. doi: 10.1038/ng.2673. [DOI] [PubMed] [Google Scholar]
- 112.Bidinger F.R., Nepolean T., Hash C.T., Yadav R.S., Howarth C.J. Quantitative trait loci for grain yield in pearl millet under variable post-flowering moisture conditions. Crop Sci. 2007;47(3):969–980. [Google Scholar]
- 113.Collins N.C., Tardieu F., Tuberosa R. Quantitative trait loci and crop performance under abiotic stress: Where do we stand? Plant Physiol. 2008;147(2):469–486. doi: 10.1104/pp.108.118117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Varshney R.K., Thudi M., Nayak S.N., Gaur P.M., Kashiwagi J., Krishnamurthy L., Jaganathan D., Koppolu J., Bohra A., Tripathi S., Rathore A., Jukanti A.K., Jayalakshmi V., Vemula A., Singh S.J., Yasin M., Sheshshayee M.S., Viswanatha K.P. Genetic dissection of drought tolerance in chickpea (Cicer Arietinum L.). Theor. Appl. Genet. 2014;127(2):445–462. doi: 10.1007/s00122-013-2230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marino R., Ponnaiah M., Krajewski P., Frova C., Gianfranceschi L., Pè M.E., Sari-Gorla M. Addressing drought tolerance in maize by transcriptional profiling and mapping. Mol. Genet. Genomics. 2009;281(2):163–179. doi: 10.1007/s00438-008-0401-y. [DOI] [PubMed] [Google Scholar]
- 116.Kumar A., Dixit S., Ram T., Yadaw R.B., Mishra K.K., Mandal N.P. Breeding high-yielding drought-tolerant rice: genetic variations and conventional and molecular approaches. J. Exp. Bot. 2014:eru363. doi: 10.1093/jxb/eru363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Henry A., Gowda V.R., Torres R.O., McNally K.L., Serraj R. Variation in root system architecture and drought response in rice (Oryza sativa): Phenotyping of the OryzaSNP panel in rainfed lowland fields. Field Crops Res. 2011;120(2):205–214. [Google Scholar]
- 118.Ahmed H.U., Henry A., Mauleon R., Dixit S., Vikram P., Tilatto R., Verulkar S.B., Perraju P., Mandal N.P., Variar M., Robin S., Chandrababu R., Singh O.N., Dwivedi J.L., Das S.P., Mishra K.K., Yadaw R.B., Aditya T.L., Karmakar B., Satoh K., Moumeni A., Kikuch S., Leung H., Arvind K. physiological, and gene expression analyses reveal that multiple QTL enhance yield of rice mega-variety IR64 under drought. PLoS. 2013 doi: 10.1371/journal.pone.0062795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Henry A., Swamy B.P., Dixit S., Torres R.D., Batoto T.C., Manalili M., Anantha M.S., Mandal N.P., Kumar A. Physiological mechanisms contributing to the QTL-combination effects on improved performance of IR64 rice NILs under drought. J. Exp. Bot. 2015:eru506. doi: 10.1093/jxb/eru506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eathington S.R., Crosbie T.M., Edwards M.D., Reiter R.S., Bull J.K. Molecular markers in a commercial breeding program. Crop Sci. 2007;47(3):154. [Google Scholar]
- 121.Reddy N., Rama R., Ragimasalawada M., Sabbavarapu M.M., Nadoor S., Patil J.V. Detection and validation of stay-green QTL in post-rainy sorghum involving widely adapted cultivar, M35-1 and a popular stay-green genotype B35. BMC Genomics. 2015;15(1):909. doi: 10.1186/1471-2164-15-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Borrell A.K., Mullet J.E., George-Jaeggli B., van Oosterom E.J., Hammer G.L., Klein P.E., Jordan D.R. Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake. J. Exp. Bot. 2014:eru232. doi: 10.1093/jxb/eru232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schafleitner R., Tincopa L.R., Palomino O., Rossel G., Robles R.F., Alagon R., Rivera C., Quispe C., Rojas L., Pacheco J.A., Solis J., Cerna D., Kim J.W., Hou J., Rheinhardt S. A sweetpotato gene index established by de novo assembly of pyrosequencing and sanger sequences and mining for gene-based microsatellite markers. BMC Genomics. 2010;11(1):604. doi: 10.1186/1471-2164-11-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Parchman T.L., Geist K.S., Grahnen J.A., Benkman C.W., Buerkle C.A. Transcriptome sequencing in an ecologically important tree species: Assembly, annotation, and marker discovery. BMC Genomics. 2010;11(1):180. doi: 10.1186/1471-2164-11-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barakat A., DiLoreto D.S., Zhang Y., Smith C., Baier K., Powell W.A., Wheeler N., Sederoff R., Carlson J.E. Comparison of the transcriptomes of American chestnut (Castanea dentata) and Chinese chestnut (Castanea mollissima) in response to the chestnut blight infection. BMC Plant Biol. 2009;9(1):51. doi: 10.1186/1471-2229-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Z., Fang B., Chen J., Zhang X., Luo Z., Huang L., Chen X., Li Y. De novo assembly and characterization of root transcriptome using illumina paired-end sequencing and development of cSSR markers in sweetpotato (Ipomoea batatas). BMC Genomics. 2010;11(1):726. doi: 10.1186/1471-2164-11-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Molina C., Rotter B., Horres R., Udupa S.M., Besser B., Bellarmino L., Baum M., Matsumura H., Terauchi R., Kahl G., Winter P. SuperSAGE: The drought stress-responsive transcriptome of chickpea roots. BMC Genomics. 2008;9(1):553. doi: 10.1186/1471-2164-9-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Utsumi Y., Tanaka M.A., Morosawa T., Kurotani A., Yoshida T., Mochida K., Matsui A., Umemura Y., Ishitani M., Shinozaki K., Sakurai T., Seki M. Transcriptome analysis using a high-density oligomicroarray under drought stress in various genotypes of cassava: An important tropical crop. DNA Res. 2012;19(4):335–345. doi: 10.1093/dnares/dss016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chapman S.C. Use of crop models to understand gGenotype by environment interactions for drought in real-world and simulated plant breeding trials. Euphytica. 2008;161(1-2):195–208. [Google Scholar]
- 130.Reymond M., Muller B., Leonardi A., Charcosset A., Tardieu F. Combining quantitative trait loci analysis and an ecophysiological model to analyze the genetic variability of the responses of maize leaf growth to temperature and water deficit. Plant Physiol. 2003;131(2):664–675. doi: 10.1104/pp.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Varshney R.K., Nayak S.N., May G.D., Jackson S.A. Next-generation sequencing technologies and their implications for crop genetics and breeding. Trends Biotechnol. 2009;27(9):522–530. doi: 10.1016/j.tibtech.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 132.Elshire R.J., Glaubitz J.C., Sun Q., Poland J.A., Kawamoto K., Buckler E.S., Mitchell S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One. 2011;6(5):e19379. doi: 10.1371/journal.pone.0019379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Von Mark V.C., Kilian A., Dierig D.A. Development of DArT marker platforms and genetic diversity assessment of the US collection of the new oilseed crop Lesquerella and related species. PLoS. 2013 doi: 10.1371/journal.pone.0064062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nakaya A., Isobe S.N. Will genomic selection be a practical method for plant breeding? Ann. Bot. (Lond.) 2012:mcs109. doi: 10.1093/aob/mcs109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meuwissen T.H., Hayes B.J., Goddard M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics. 2001;157(4):1819–1829. doi: 10.1093/genetics/157.4.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Crossa J., Pérez P., Hickey J., Burgueño J., Ornella L., Cerón-Rojas J., Zhang X., Dreisigacker S., Babu R., Li Y., Bonett T., Matthews K. Genomic prediction in CIMMYT maize and wheat breeding programs. Heredity. 2014;112(1):48–60. doi: 10.1038/hdy.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gouy M., Rousselle Y., Bastianelli D., Lecomte P., Bonnal L., Roques D., Efile J-C., Rocher S., Daugrois J., Toubi L., Nabeneza S., Hervouet C., Telismart H., Denis M., Thong-Chane A., Glaszmann J.C., Hoarau J-Y., Nibouche S., Costet L. Experimental assessment of the accuracy of genomic selection in sugarcane. Theor. Appl. Genet. 2013;126(10):2575–2586. doi: 10.1007/s00122-013-2156-z. [DOI] [PubMed] [Google Scholar]
- 138.Varshney R.K., Mohan S.M., Gaur P.M., Gangarao N.V., Pandey M.K., Bohra A., Sawargaonkar S.L., Chitikineni A., Kimurto P.K., Janila P., Saxena K.B., Fikre A., Sharma M., Rathore A., Pratap A., Tripathi S., Datta S., Chaturvedi S.K., Mallikarjuna N., Anuradha G., Babbar A., Choudhary A.K., Mhase M.B., Bharadwaj Ch., Mannur D.M., Harer P.N., Guo B., Liang X., Nadarajan N., Gowda C.L. Achievements and prospects of genomics-assisted breeding in three legume crops of the semi-arid tropics. Biotechnol. Adv. 2013;31(8):1120–1134. doi: 10.1016/j.biotechadv.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 139.Beaulieu J., Doerksen T., Clément S., MacKay J., Bousquet J. Accuracy of genomic selection models in a large population of open-pollinated families in white spruce. Heredity. 2014;113(4):343–352. doi: 10.1038/hdy.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.de Oliveira E.J., de Resende M.D., Santos V.D., Ferreira C.F., Oliveira G.A., da Silva M.S., de Oliveira L.A., Aguilar-Vildoso C.I. Genome-wide selection in cassava. Euphytica. 2012;187(2):263–276. [Google Scholar]
- 141.Ly D., Hamblin M., Rabbi I., Melaku G., Bakare M., Gauch H.G., Okechukwu R., Dixon A.G., Kulakow P., Jannink J-L. Relatedness and genotype-by- environment interaction affect prediction accuracies in genomic selection: A study in cassava. Crop Sci. 2013;53(4):1312–1325. [Google Scholar]
- 142.Kumar S., Bink M.C., Volz R.K., Bus V.G., Chagné D. Towards genomic selection in apple (Malus Domestica Borkh.) breeding programmes: Prospects, challenges and strategies. Tree Genet. Genomes. 2012;8(1):1–14. [Google Scholar]
- 143.Rutkoski J.E., Heffner E.L., Sorrells M.E. Genomic selection for durable stem rust resistance in wheat. Euphytica. 2011;179(1):161–173. [Google Scholar]
- 144.Torres R.O., McNally K.L., Cruz C.V., Serraj R., Henry A. Screening of rice genebank germplasm for yield and selection of new drought tolerance donors. Field Crops Res. 2013;147(June):12–22. doi: 10.1016/j.fcr.2013.03.016. [DOI] [Google Scholar]
- 145.Ortiz R., Taba S., Tovar V.H., Mezzalama M., Xu Y., Yan J., Jonathan H., Crouch J.H. Conserving and enhancing maize genetic resources as global public goods-a perspective from CIMMYT. Crop Sci. 2010;50(1):13–28. [Google Scholar]
- 146.Gregorio G.B., Senadhira D., Mendoza R.D., Manigbas N.L., Roxas J.P., Guerta C.Q. Progress in breeding for salinity tolerance and associated abiotic stresses in rice. Field Crops Res. 2002;76(2):91–101. [Google Scholar]
- 147.Trethowan R.M., Mujeeb-Kazi A. Novel germplasm resources for improving environmental stress tolerance of hexaploid wheat. Crop Sci. 2008;48(4):1255–1265. [Google Scholar]
- 148.Cabello R., Monneveux P., De Mendiburu F., Bonierbale M. Comparison of yield based drought tolerance indices in improved varieties, genetic stocks and landraces of potato (Solanum tuberosum L.). Euphytica. 2013;193(2):147–156. [Google Scholar]
- 149.Lu Y., Hao Z., Xie C., Crossa J., Araus J-L., Gao S., Vivek B.S., Magorokosho C., Mugo S., Makumbi D., Taba S., Pan G., Li X., Rong T., Zhang S., Xu Y. Large-scale screening for maize drought resistance using multiple selection criteria evaluated under water-stressed and well-watered environments. Field Crops Res. 2011;124(1):37–45. [Google Scholar]
- 150.Beardmore J.A. Transgenics: Autotransgenics and allotransgenics. Transgenic Res. 1997;6(1):107–108. [Google Scholar]
- 151.Valliyodan B., Nguyen H.T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Biol. 2006;9(2):189–195. doi: 10.1016/j.pbi.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 152.Saijo Y., Hata S., Kyozuka J., Shimamoto K., Izui K. Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 2000;23(3):319–327. doi: 10.1046/j.1365-313x.2000.00787.x. [DOI] [PubMed] [Google Scholar]
- 153.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Makarova K.S., Haft D.H., Barrangou R., Brouns S.J., Charpentier E., Horvath P., Moineau S., Mojica F.J., Wolf Y.I., Yakunin A.F., Van der Oost J., Koonin E.V. Evolution and classification of the CRISPR-Cas systems. Nat. Rev. Microbiol. 2011;9(6):467–477. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]