Abstract
Background
Hemichorea/hemiballism associated with nonketotic hyperglycemia is a well-recognized syndrome, but few case series have been reported in the literature.
Case Report
We describe 20 patients with hemichorea/hemiballism associated with hyperglycemia (9 males and 11 females) with mean age of 67.8 years. Ten patients had a previous diagnosis of type 2 diabetes mellitus, and one had type 1 diabetes mellitus. Six of them had documentation of poor diabetic control over at least the last 3 months. Nine patients had new-onset hyperglycemia with a diagnosis of diabetes mellitus made after discharge. Seventeen patients had unilateral chorea/ballism, while three had bilateral chorea/ballism. Eighteen cases had striatal hyperdensities on computed tomography (CT) and/or hyperintense signals on magnetic resonance imaging (MRI). The putamen was affected in all cases, and the caudate nucleus was involved in nine.
Discussion
Hemichorea/hemiballism associated with nonketotic hyperglycemia can be the presenting sign of diabetes mellitus in almost half of cases or can occur after a few months of poor glycemic control in patients with diagnosed diabetes. This case series is one of the largest to date and adds valuable information about clinical and neuroimaging features that are comparable with published data but also emphasize the role of adequate diabetes mellitus control.
Keywords: Chorea, ballism, hyperglycemia
Introduction
Hemichorea/hemiballism associated with nonketotic hyperglycemia is a well-recognized syndrome characterized by the sudden occurrence of hemichorea or its more severe expression, hemiballism. It typically affects older adults, especially females, with poorly controlled type 2 diabetes mellitus in a nonketotic hyperglycemic state. Insidious onset and more generalized movements have occasionally been described. These patients often exhibit a contralateral striatal hyperdensity on computed tomography (CT) and/or a striatal hyperintense signal on magnetic resonance imaging (MRI).1
Although the posthyperglycemic complication of chorea or ballism is considered to be self-limited, the extent to which patients improve may range from none to complete over days to months. Besides blood glucose correction, symptomatic antichorea treatments may be used in those with severe deficits.2
We describe the clinical, biochemical, and neuroimaging features of a series of 20 consecutive Peruvian patients who met the criteria of hemichorea/hemiballism associated with hyperglycemia.
Patients and methods
We performed a retrospective study of patients with hemichorea/hemiballism associated with hyperglycemia admitted to our institution between January 2011 and December 2014. All patients were admitted to a neurology ward and were clinically assessed by neurologists. Demographic data and clinical, biochemical, and neuroimaging findings were obtained from the medical charts. Details of the involuntary movements such as type and side(s) involved were noted. Mild to moderate cases with low-amplitude, random, flowing movements were considered as chorea, and severe cases with proximal and large-amplitude movements were qualified as ballism. Neuroimaging was performed in all patients except one because of economic constraints. Unenhanced CT studies were performed with a helical scanner. MRI comprised at least T1-, T2-, and diffusion-weighted images, and was performed when CT was considered normal or doubtful.
Results
Twenty patients with chorea/ballism associated with hyperglycemia were identified, including 9 (45%) males and 11 (55%) females with a mean age of 67.8 years (range 29–87 years).
Ten patients had a previous diagnosis of type 2 diabetes mellitus, and one had a previous diagnosis of type 1 diabetes mellitus. Six (54.5%) had documented poor diabetic control over the last several months. Nine patients had new-onset hyperglycemia with a diagnosis of diabetes mellitus made after discharge. Eleven patients also had arterial hypertension. None had a family history of movement disorders, history of exposure to potentially offending drugs, or polycythemia.
The estimated mean interval between chorea/ballism onset and admission was 33 days (range, 4–180 days), but this was difficult to determine in some cases.
All chorea/ballism patients had hyperglycemia on admission with a mean blood glucose of 306.7 mg/mL (range: 125–600 mg/mL). Patient 6 had a blood glucose value of 125 mg/mL on admission but was seen 4 weeks after involuntary movement onset. Urinalysis was negative for ketones in all patients.
Seventeen patients had unilateral (upper and/or lower limb involvement) chorea/ballism, while three had bilateral involvement. The right and left sides were affected in nine (45%) and eight (40%) patients, respectively (Table 1). In 10 patients, the involuntary movement was classified as pure ballism, in four as pure chorea, and in six patients the movement disorder was considered a combination of chorea and ballism. None had tremor, dystonia, or other involuntary movements.
Table 1. Demographic Data.
| No. | Age of Onset | Sex | DM | Arterial Hypertension | Movement Disorder | Side Affected | Glucose on Admission (mg/mL) | Neuroimaging | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chorea | Ballism | R | L | CT | MRI | ||||||
| 1 | 80 | M | + | + | + | + | − | + | 191 | Abnormal | ND |
| 2 | 71 | F | − | + | − | + | + | + | 250 | Abnormal | ND |
| 3 | 76 | M | + | − | + | − | + | − | 234 | Abnormal | ND |
| 4 | 29 | F | + | − | + | + | + | − | 348 | ND | ND |
| 5 | 55 | F | + | − | + | + | + | − | 125 | Normal | ND |
| 6 | 71 | F | − | + | − | + | − | + | 407 | Abnormal | ND |
| 7 | 77 | F | − | + | + | − | + | + | 310 | Abnormal | Abnormal |
| 8 | 65 | M | − | + | − | + | + | + | 600 | Abnormal | ND |
| 9 | 83 | M | − | + | − | + | − | + | 300 | ND | Abnormal |
| 10 | 65 | F | + | + | − | + | − | + | 416 | Abnormal | ND |
| 11 | 56 | F | − | − | + | + | − | + | 320 | Normal | Abnormal |
| 12 | 72 | M | + | + | + | + | + | − | 131 | Normal | Abnormal |
| 13 | 76 | F | + | − | − | + | + | − | 251 | ND | Abnormal |
| 14 | 57 | F | + | − | + | − | − | + | 280 | Abnormal | Abnormal |
| 15 | 75 | M | − | − | + | + | + | − | 259 | Abnormal | ND |
| 16 | 87 | F | − | + | − | + | + | − | 470 | ND | Abnormal |
| 17 | 64 | F | − | − | − | + | + | − | 379 | Abnormal | Abnormal |
| 18 | 74 | M | + | + | − | + | + | − | 189 | Abnormal | ND |
| 19 | 56 | M | + | − | + | − | − | + | 450 | Normal | Abnormal |
| 20 | 67 | M | + | + | − | + | − | + | 224 | Normal | Abnormal |
Abbreviations: CT, computed tomography; DM, Diabetes Mellitus; F, Female; L, Left; M, Male; MRI, Magnetic Resonance Imaging; ND, Not Done; R, Right.
As blood glucose normalization did not lead to immediate resolution of the involuntary movements, low-dose haloperidol (2–4 mg/day) was administered to all patients and then tapered off after several weeks after complete resolution of the involuntary movement. The mean latency of time to resolution was approximately 2 weeks, although the exact number of days was difficult to establish from the charts of some cases.
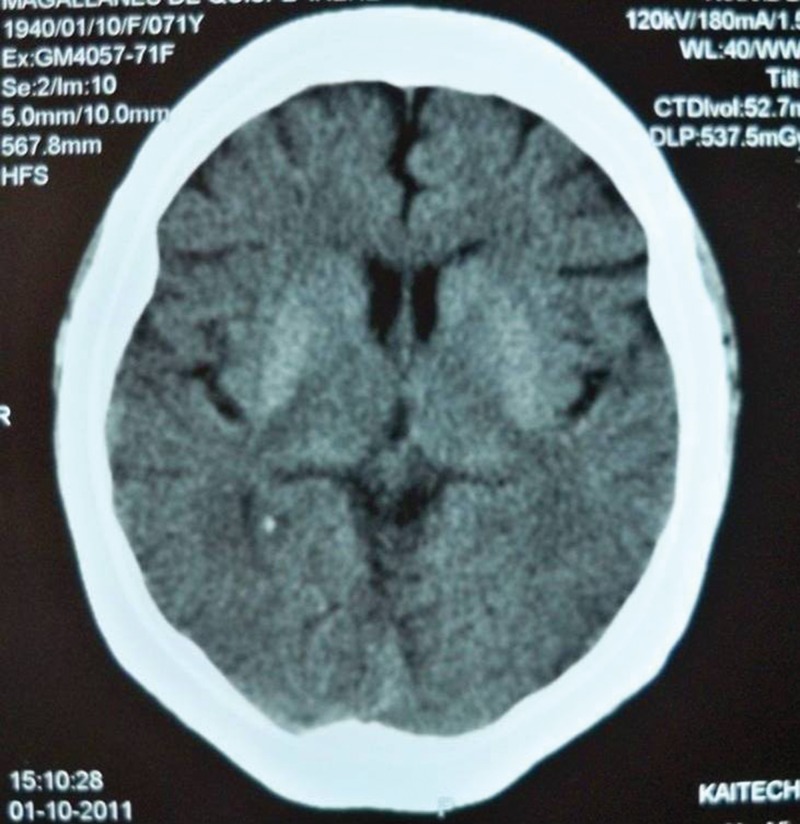
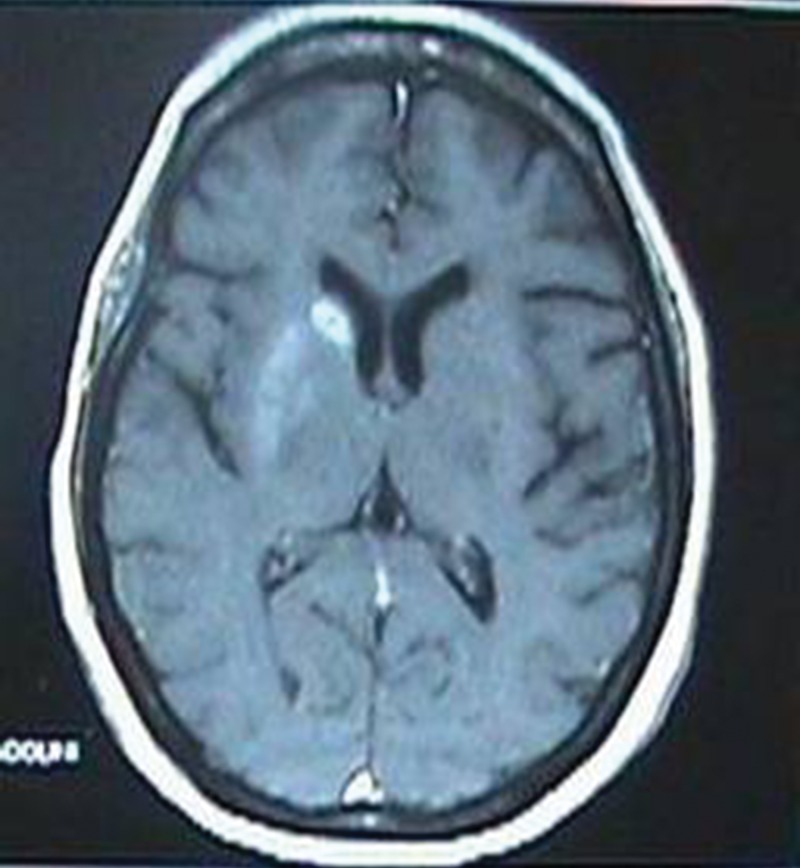
Nineteen patients had neuroimaging (CT and/or MRI) performed within the following days after admission. CT was performed in thirteen patients and MRI in nine patients. From the nineteen patients only one had normal cerebral images (only CT scan as MRI was not performed because of economic constraints). Eighteen cases had striatal hyperdensity on CT and/or hyperintensity signal on MRI. The putamen was involved in all cases (seven right, six left and five bilateral) and the caudate nucleus in nine cases (four right, two left and three bilateral). No patient had abnormalities in the globus pallidus, thalamus or subthalamus (Figures 1 and 2).
Figure 1. Computed tomography scan showing bilateral putaminal hyperdensities.
Figure 2. Magnetic resonance imaging showing unilateral hyperintensity in the putamen and caudate nucleus.
Discussion
Chorea/ballism associated with hyperglycemia was first described by Bedwell in 1960,3 but fewer than 200 cases and very few case series have been published since.4,5 The typical triad includes unilateral (or bilateral) involuntary movements, contralateral (or bilateral) striatal abnormalities on neuroimaging, and hyperglycemia in patients with known or previously unrecognized diabetes mellitus.
There have been no epidemiological studies, but the estimated prevalence of this disorder is less than 1 in 100,000.1 The female:male ratio is 1.8:1, and older age appears to be the greatest risk factor.2 The mean age at onset is about 70 years (range 22–90). We found a 1.3:1 female:male ratio with a mean age of 67.8 years. One of our patients was just 29 years old, one of the youngest patients reported.6,7 Most of the literature originates from Asian countries, but cases have been reported in all ethnic backgrounds. There are a few case reports from South America, but the current report is the first case series from Peru and one of the largest to date. Fifty-five percent of our cohort had arterial hypertension. We were not able to determine if arterial hypertension is a comorbidity or a predisposing factor.
The involuntary movements begin acutely to subacutely, often worsening over several days. The abnormal involuntary movements can be classified from mild chorea to severe ballism based on their type and severity. Most published reports use the term hemichorea or hemiballism because bilateral involvement occurs in less than 10% of cases.2 Nevertheless, we had three bilateral cases, representing 15% of the cohort. As a result, we suggest describing this syndrome as chorea/ballism associated with hyperglycemia.
All but one patient had striatal abnormalities on neuroimaging. Striatal hyperdensities and/or hyperintensities were contralateral to the hemichorea/hemiballism in patients with unilateral syndrome but bilateral in the three patients with bilateral chorea/ballism. It must be mentioned that bilateral and nearly symmetric striatal abnormalities were associated with unilateral chorea/ballism in two other cases. Also, there is one case in the literature without chorea/ballism but with typical neuroimaging abnormalities.8 As previously reported, the putamen was involved in all 20 patients, while the head of the caudate nucleus was affected in less than 50% of cases. There was no correlation between involvement of the putamen and/or caudate nucleus head and clinical chorea and/or ballism. Lesions are always well delineated and do not follow a vascular distribution. The lesions typically resolve over time with eventual normalization of CT or MRI, but in some cases lesions persist despite clinical resolution of hemichorea/hemiballism. Further studies are needed to clarify this discrepancy. The exact pathophysiology of these lesions remains enigmatic. Petechial hemorrhage, ischemia, and hyperviscosity are among the possible suggested mechanisms.9,10
By definition, all subjects have hyperglycemia prior to chorea/ballism onset. Type 2 and less frequently type 1 diabetes are associated with this disorder. Notably, diabetes was newly diagnosed in 45% of our patients. Hemichorea/hemiballism slowly improves in the days after serum glucose correction, but neuroleptics like haloperidol are often used to expedite symptomatic resolution. We did not find any relationship between the presence of chorea and/or ballism and poorly controlled or newly diagnosed diabetes mellitus.
In conclusion, although uncommon, chorea/ballism associated with nonketotic hyperglycemia can be the first presenting sign of unknown diabetes mellitus or can occur after weeks or even months of poor glycemic control in diabetic patients. This case series is one of the largest published so far and adds valuable information about the clinical and neuroimaging features of this condition. While our results are comparable with published data, they further emphasize the role of adequate control of diabetes mellitus.
Footnotes
Funding: None.
Financial Disclosures: None.
Conflict of Interests: The authors report no conflict of interest.
Ethics Statement: This study was reviewed by the authors' institutional ethics committee and was considered exempted from further review.
References
- 1.Ondo WG. Hyperglycemic nonketotic states and other metabolic imbalances. Handb Clin Neurol. 2011;100:287–291. doi: 10.1016/B978-0-444-52014-2.00021-5. doi: 10.1016/B978-0-444-52014-2.00021-5. [DOI] [PubMed] [Google Scholar]
- 2.Oh SH, Lee KY, Im JH, Lee MS. Chorea associated with nonketonic hyperglycemia and hyperintensity basal ganglia lesion on T1 weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neurol Sci. 2002;200:57–62. doi: 10.1016/s0022-510x(02)00133-8. doi: 10.1016/S0022-510X(02)00133-8. [DOI] [PubMed] [Google Scholar]
- 3.Bedwell SF. Some observations on hemiballismus. Neurology. 1960;10:619–622. doi: 10.1212/wnl.10.6.619. doi: 10.1212/WNL.10.6.619. [DOI] [PubMed] [Google Scholar]
- 4.Guo Y, Miao YW, Ji XF, Liu X, Sun XP. Hemichorea associated with nonketonic hyperglycemia: clinical and neuroimaging features in 12 patients. Eur Neurol. 2014;71:299–304. doi: 10.1159/000357210. doi: 10.1159/000357210. [DOI] [PubMed] [Google Scholar]
- 5.Lee BC, Hwang SH, Chang GY. Hemiballismus-hemichorea in older diabetic women: a clinical syndrome with MRI correlation. Neurology. 1999;52:646–648. doi: 10.1212/wnl.52.3.646. doi: 10.1212/WNL.52.3.646. [DOI] [PubMed] [Google Scholar]
- 6.Oerlemans WG, Moll LC. Non-ketotic hyperglycemia in a young woman, presenting as hemiballism – hemichorea. Acta Neurol Scand. 1999;100:411–414. doi: 10.1111/j.1600-0404.1999.tb01062.x. doi: 10.1111/j.1600-0404.1999.tb01062.x. [DOI] [PubMed] [Google Scholar]
- 7.Aquino JH, Spitz M, Pereira JS. Hemichorea-hemiballismus as the first sign of type 1b diabetes during adolescence and its recurrence in the setting of infection. J Child Neurol. 2015;10:1362–1365. doi: 10.1177/0883073814553972. doi: 10.1177/0883073814553972. [DOI] [PubMed] [Google Scholar]
- 8.Hansford B, Albert D, Yang E. Classic neuroimaging findings of nonketotic hyperglycemia on computed tomography and magnetic resonance imaging with absence of typical movement disorder symptoms (hemichorea-hemiballism) Radiology Case. 2013;8:1–9. doi: 10.3941/jrcr.v7i8.1470. doi: 10.3941/jrcr.v7i8.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mestre TA, Ferreira JJ, Pimentel J. Putaminal petechial haemorrhage as the cause of non-ketotic hyperglycaemic chorea: a neuropathological case correlated with MRI findings. J Neurol Neurosurg Psychiatry. 2007;78:549–550. doi: 10.1136/jnnp.2006.105387. doi: 10.1136/jnnp.2006.105387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu K, Kang DW, Kim DE, Park SH, Roh JK. Diffusion-weighted and gradient echo magnetic resonance findings of hemichorea-hemiballismus associated with diabetic hyperglycemia: a hyperviscosity syndrome? Arch Neurol. 2002;59:448–452. doi: 10.1001/archneur.59.3.448. doi: 10.1001/archneur.59.3.448. [DOI] [PubMed] [Google Scholar]