Abstract
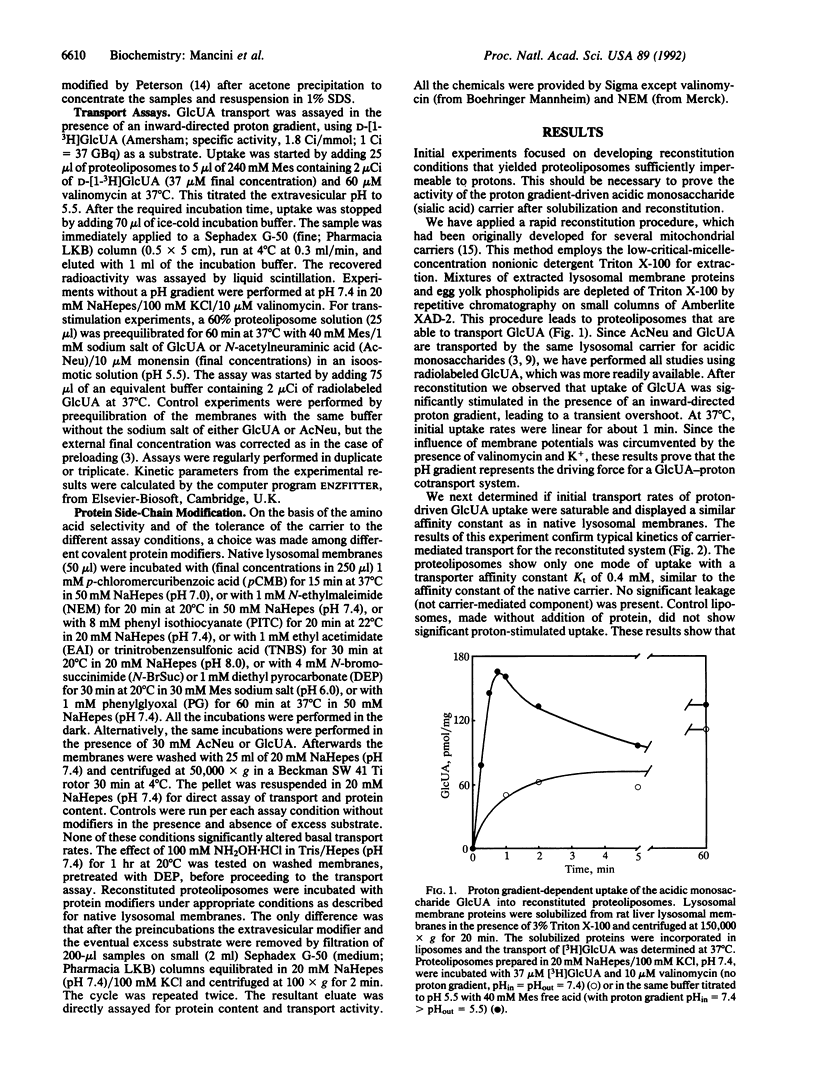
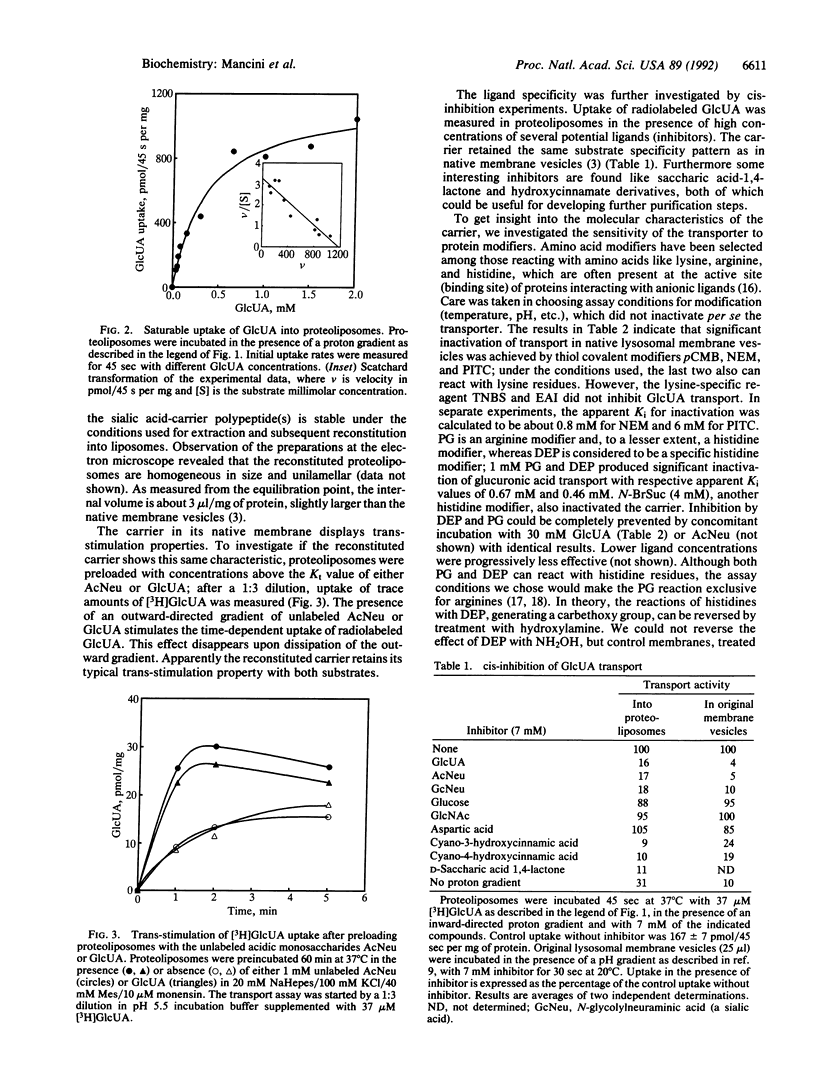
The lysosomal carrier for the acidic monosaccharides sialic acid and glucuronic acid was solubilized from rat liver lysosomal membranes and reconstituted into phospholipid vesicles. Membrane proteins were extracted from lysosomal membranes with Triton X-100. Upon removal of the detergent by absorption on Amberlite XAD-2 beads, the solubilized proteins were incorporated in egg yolk phospholipids. The reconstituted proteoliposomes show proton-driven carrier-mediated uptake of acidic monosaccharides. The reconstituted carrier was compared in several characteristics with the transporter as present in the native lysosomal membrane. Transporter substrate affinity (Kt for glucuronic acid = 0.4 mM) and specificity for acidic monosaccharides are completely retained. The proteoliposomes also demonstrate trans-stimulation properties with both substrates sialic acid and glucuronic acid. The transporter is inhibited, both in its native and in the reconstituted state, by the sulfhydryl-modifying agents p-chloromercuribenzoic acid, N-ethylmaleimide, and phenyl isothiocyanate. In native membrane vesicles, arginine and histidine modifiers phenylglyoxal and diethyl pyrocarbonate inactivated transport in a substrate-protectable manner. In reconstituted proteoliposomes, similar inhibition was observed. However, protection by substrates was achieved only after treatment with phenylglyoxal. These data suggest that arginine or histidine residues or both are present at or near the substrate binding site of the carrier. Possibly, other essential histidines become exposed in the reconstituted state. The successful functional reconstitution of the lysosomal sialic acid carrier represents an important step towards its purification and its detailed molecular characterization.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bame K. J., Rome L. H. Acetyl coenzyme A: alpha-glucosaminide N-acetyltransferase. Evidence for a transmembrane acetylation mechanism. J Biol Chem. 1985 Sep 15;260(20):11293–11299. [PubMed] [Google Scholar]
- Bateman R. C., Jr, Jackson D., Slaughter C. A., Unnithan S., Chai Y. G., Moomaw C., Hersh L. B. Identification of the active-site arginine in rat neutral endopeptidase 24.11 (enkephalinase) as arginine 102 and analysis of a glutamine 102 mutant. J Biol Chem. 1989 Apr 15;264(11):6151–6157. [PubMed] [Google Scholar]
- Bolli R., Nałecz K. A., Azzi A. Monocarboxylate and alpha-ketoglutarate carriers from bovine heart mitochondria. Purification by affinity chromatography on immobilized 2-cyano-4-hydroxycinnamate. J Biol Chem. 1989 Oct 25;264(30):18024–18030. [PubMed] [Google Scholar]
- D'Souza M. P., Ambudkar S. V., August J. T., Maloney P. C. Reconstitution of the lysosomal proton pump. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6980–6984. doi: 10.1073/pnas.84.20.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahl W. A. Lysosomal membrane transport in cellular nutrition. Annu Rev Nutr. 1989;9:39–61. doi: 10.1146/annurev.nu.09.070189.000351. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. Biochem J. 1975 Apr;148(1):85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idriss J. M., Jonas A. J. Vitamin B12 transport by rat liver lysosomal membrane vesicles. J Biol Chem. 1991 May 25;266(15):9438–9441. [PubMed] [Google Scholar]
- Krämer R., Palmieri F. Molecular aspects of isolated and reconstituted carrier proteins from animal mitochondria. Biochim Biophys Acta. 1989 Apr 17;974(1):1–23. doi: 10.1016/s0005-2728(89)80160-4. [DOI] [PubMed] [Google Scholar]
- LEVVY G. A. The preparation and properties of beta-glucuronidase. IV. Inhibition by sugar acids and their lactones. Biochem J. 1952 Nov;52(3):464–472. doi: 10.1042/bj0520464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch A. M., McGivan J. D. A rapid method for the reconstitution of Na+-dependent neutral amino acid transport from bovine renal brush-border membranes. Biochem J. 1987 Jun 15;244(3):503–508. doi: 10.1042/bj2440503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G. M., Beerens C. E., Aula P. P., Verheijen F. W. Sialic acid storage diseases. A multiple lysosomal transport defect for acidic monosaccharides. J Clin Invest. 1991 Apr;87(4):1329–1335. doi: 10.1172/JCI115136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G. M., Beerens C. E., Verheijen F. W. Glucose transport in lysosomal membrane vesicles. Kinetic demonstration of a carrier for neutral hexoses. J Biol Chem. 1990 Jul 25;265(21):12380–12387. [PubMed] [Google Scholar]
- Mancini G. M., Verheijen F. W., Galjaard H. Free N-acetylneuraminic acid (NANA) storage disorders: evidence for defective NANA transport across the lysosomal membrane. Hum Genet. 1986 Jul;73(3):214–217. doi: 10.1007/BF00401229. [DOI] [PubMed] [Google Scholar]
- Mancini G. M., de Jonge H. R., Galjaard H., Verheijen F. W. Characterization of a proton-driven carrier for sialic acid in the lysosomal membrane. Evidence for a group-specific transport system for acidic monosaccharides. J Biol Chem. 1989 Sep 15;264(26):15247–15254. [PubMed] [Google Scholar]
- Peterson G. L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem. 1979 Dec;100(2):201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- Pisoni R. L., Thoene J. G. Detection and characterization of a nucleoside transport system in human fibroblast lysosomes. J Biol Chem. 1989 Mar 25;264(9):4850–4856. [PubMed] [Google Scholar]
- Renlund M., Tietze F., Gahl W. A. Defective sialic acid egress from isolated fibroblast lysosomes of patients with Salla disease. Science. 1986 May 9;232(4751):759–762. doi: 10.1126/science.3961501. [DOI] [PubMed] [Google Scholar]
- Riordan J. F. Arginyl residues and anion binding sites in proteins. Mol Cell Biochem. 1979 Jul 31;26(2):71–92. doi: 10.1007/BF00232886. [DOI] [PubMed] [Google Scholar]
- Riordan J. F., McElvany K. D., Borders C. L., Jr Arginyl residues: anion recognition sites in enzymes. Science. 1977 Mar 4;195(4281):884–886. doi: 10.1126/science.190679. [DOI] [PubMed] [Google Scholar]
- Takahashi K. The reactions of phenylglyoxal and related reagents with amino acids. J Biochem. 1977 Feb;81(2):395–402. doi: 10.1093/oxfordjournals.jbchem.a131471. [DOI] [PubMed] [Google Scholar]
- Wendland M., Waheed A., von Figura K., Pohlmann R. Mr 46,000 mannose 6-phosphate receptor. The role of histidine and arginine residues for binding of ligand. J Biol Chem. 1991 Feb 15;266(5):2917–2923. [PubMed] [Google Scholar]



