Abstract
A recently published article described how a fertility center in the United States implemented air quality control to newly designed in vitro fertilization (IVF) laboratory.1 A highly-efficient air filtration was achieved by installing a centered system supplying filtered air to the IVF laboratory and related critical areas, combining air particulate and volatile organic compound (VOC) filtration. As a consequence, live birth rates were increased by improvements in air quality. This article highlights the key aspects of air contamination in the IVF context. The topic is important not only to IVF specialists but also to Andrologists due to the great number of male infertility patients referred to assisted reproductive technology (ART) treatments. The evidence is growing that laboratory air quality is paramount importance for improved IVF outcome.
IMPORTANCE OF LABORATORY AIR QUALITY TO EMBRYO DEVELOPMENT IN VITRO
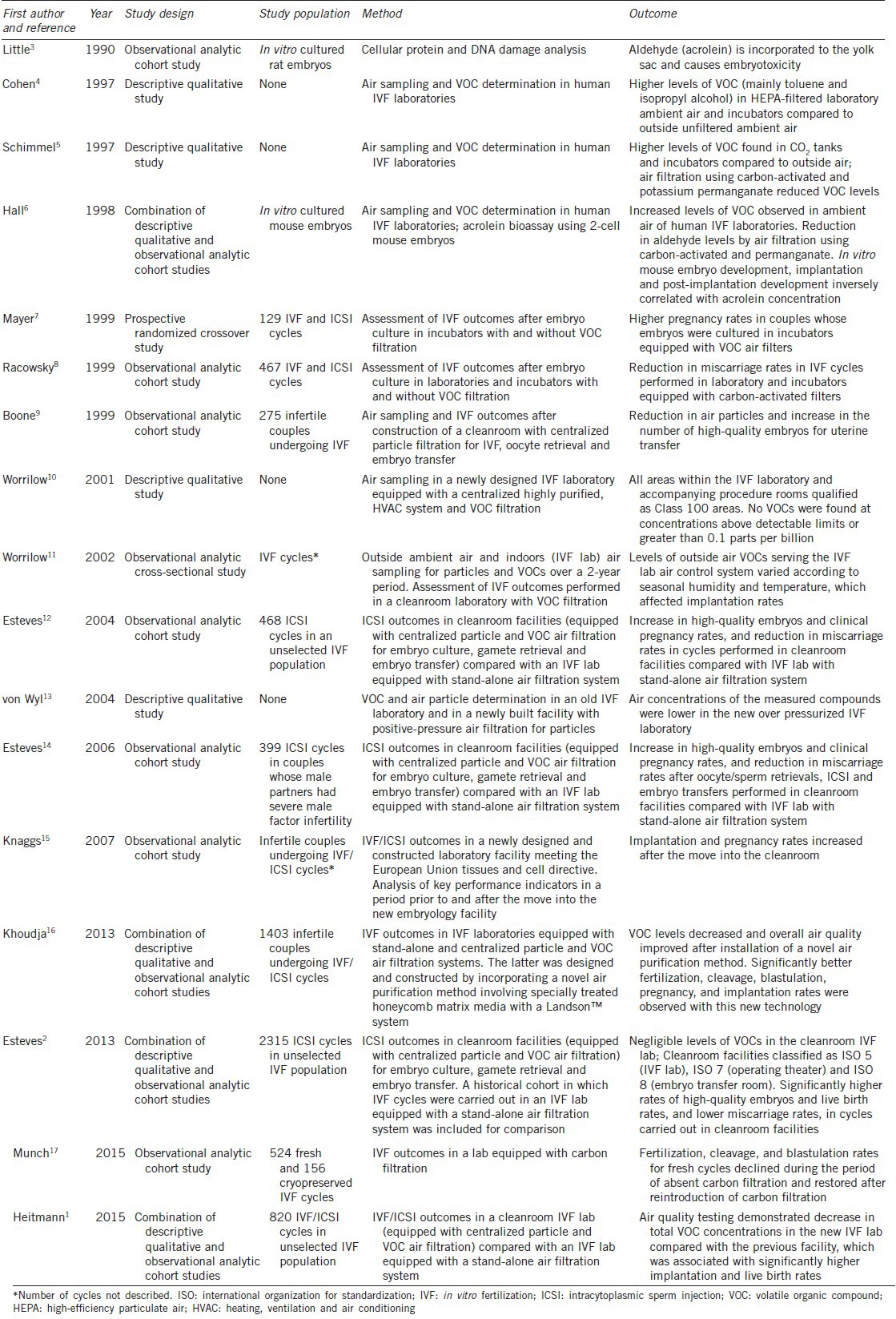
Both animal and human studies have suggested an association between poor laboratory air quality conditions and impaired embryo development, resulting in decreased implantation and pregnancy rates. The deleterious effects of poor air quality to embryo development and implantation and how controlling laboratory air quality can minimize such effects have been investigated over the last 15 years (Table 1).1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17 Recognizing the importance of laboratory air quality to the safety of IVF treatments, regulatory directives in the European Union and Brazil dictate specific requirements for air quality control within reproductive laboratories.18,19 Such regulatory directives aim to safeguard public health in line with the precautionary principle, but they require different strategies to mitigate the air-related risks (revised in Esteves and Bento, 2013).2 Little attention has been given, however, to how IVF laboratories should implement air quality control.2,20
Table 1.
Summary of evidence assessing the impact of laboratory air quality in IVF outcomes
A RISK ASSESSMENT IS CRITICAL BEFORE INSTALLING AIR FILTRATION SYSTEMS
Is air particle filtration enough or do we need to combine it with volatile organic compound (VOC) filtration? Are commercially available stand-alone filters sufficient or is it necessary to implement centralized built-in air filtration systems? How often do we have to replace the filters? What periodic testing is needed to ensure conformity? Will the implementation of air filtration change IVF outcomes? With so many uncertainties but recognizing the importance of laboratory air quality, many of us working in this field have chosen to install commercially available filtration systems without proper risk assessment and validation procedures. Little attention is given, for instance, to other critical issues that affect indoor air quality, such as laboratory premises (e.g., age and size of laboratory, equipment/furniture and construction materials atmospheric air pollution, and proximity to anesthetic gases), room humidity and temperature, disposable materials and cleaning agents used inside the laboratory, and personnel (number per workspace and use of protective clothing and cosmetics).
IMPORTANCE OF AIR QUALITY CONTROL IN ASSISTED REPRODUCTIVE TECHNOLOGY (ART) LABORATORY
One of the goals of air filtration in the IVF environment is to decrease the number of air particles through the use of high-efficiency filtration systems. This is important because microorganisms can attach themselves to these particles. Removal of airborne particulates is achieved by forced movement of air using positive air pressurization through a series of filters of increasing efficiency.21 On the other hand, VOCs are much smaller than the effective pore size of high-efficiency particulate air (HEPA) filters and cannot be trapped by HEPA filters.22 Volatile organic compounds, which are constantly generated by materials and cleaning agents used in the laboratory, react with the indoor ozone. These chemical reactions produce submicron-sized particles and harmful by-products that have been associated with poorer IVF outcomes.3,4,5,6,8,17 In the IVF setting, VOCs can be found in CO2 gas cylinders, insulation used in air handling systems, refrigerant gases, cleaning agents, plastic ware, constructing materials, and furniture.
HOW TO IMPLEMENT AN EFFICIENT AIR FILTRATION SYSTEM – LESSONS LEARNED FROM NOVEL RESEARCH
VOC removal should be an integral element of air cleanness in IVF. Removal of VOCs is achieved by potassium permanganate-impregnated, pelletized coconut shell-based activated carbon filters. The spaces between the carbon particles contain a cloud of delocalized electrons that acts as electronic glue, thus forcing the chemical contaminants to bind to the carbon.23 Alcohols and ketones that are not normally removed by the pore structure of coconut shell-based carbons can be oxidized, and thereby detoxified by potassium permanganate.6
In vitro fertilization laboratories aiming to control air pollution should integrate both air particle and VOC filtration. An example of a laboratory with the aforesaid combination is depicted in Figure 1. Evaluating results over 9-year period, we demonstrated the benefit of operating under these optimum environmental conditions, which resulted not only in an increase in live birth but also reduction in miscarriage rates.2 Along these lines, a better definition for IVF cleanrooms would be “a room, in which the concentration of airborne particles and VOC is controlled and which is constructed and used in a manner to minimize the introduction, generation, and retention of particles and VOCs, and in which, temperature, humidity, and pressure are controlled.” Equally important are the methods set up for training laboratory personnel and validating/monitoring the installations while in operation, that is, during normal routine workload. In general, expensive filters, such as HEPA, are not replaced unless they show nonconformance during periodic inspections. VOC filter efficiency is monitored periodically by sending chemical module samples to the manufacturer to determine remaining chemical bed activity, thus guiding how often filters should be replaced. Filter saturation levels depend on outside air quality and levels of indoor VOC generation, and replacement of filters by analyzing objective data helps minimize operational costs.
Figure 1.
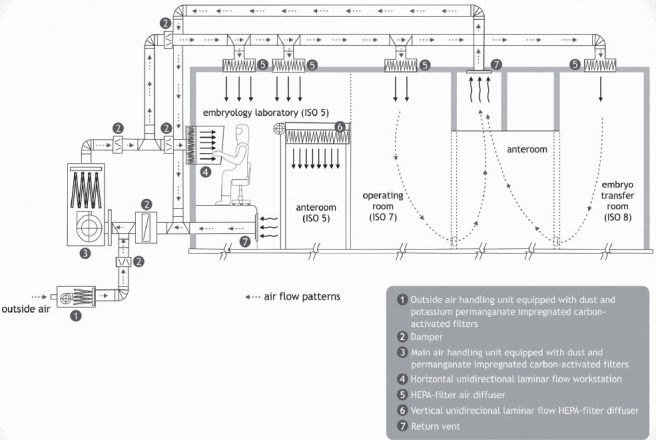
Schematic representation of cleanroom IVF facilities, including airflow patterns and filtration units. The air handling ventilation unit room has a roof-top air-handling unit that draws outside air through coarse and charcoal prefilters before it enters into the main ventilation unit. A free-standing main ventilation unit pulls prefiltered outside air and cleanrooms’ return air through coarse filters, past a 16-unit potassium permanganate impregnated pelletized coal-based activated carbon filters, and then through fine dust filters. Lastly, filtered air enters the cleanrooms through high-efficiency particulate air (HEPA) filter diffusers. Floor and ceiling-level vents in the cleanrooms’ return air to the main ventilation unit, to be remixed with the existing air. Differential positive pressure is maintained between rooms. The embryology laboratory/anteroom is positive to the operating room, which is positive to both the embryo transfer room and the dressing room/hallways. Reprinted from Esteves and Bento, Reprod Biomed Online 2013; 26: 9–21, with permission from Elsevier.
Heitmann and colleagues also contributed a detailed description of their filtration system and construction methods, which included removal of both particulate matter and VOC.1 Better air quality conditions were associated with significantly higher embryo development, implantation, and live birth rates in couples undertaking treatment in their new facility. In both aforementioned studies, an air filtration system controlling indoor particulate and VOC was implemented using a centralized system supplying filtered air to the IVF laboratory and adjacent critical areas.1,2
Installation of centralized air filtration such as the highlighted ones is costly. A less expensive but yet to be proven effective alternative, particularly for existing IVF laboratories, would be to incorporate portable freestanding commercial units. However, it is unlikely that portable units would provide the same air quality than a robust, centralized air filtration system. Notwithstanding, risk minimization and quality management should be considered equally powerful tools to improve IVF laboratory air quality.22
In conclusion, accumulating evidence indicates that laboratory air quality plays a significant role in IVF outcome, which is of broad interest for practitioners dealing with male infertility and patients alike. Implementation of air quality control by the combination of particulate matter and chemical filtration seems sound, but guidelines on the target limits and best practice statements on how to implement air quality control to IVF are still lacking. At present, built-in systems supplying filtered air to the IVF lab and adjacent areas seems to be the best alternative to mitigate the risks of poor IVF outcomes related to laboratory air quality. Good laboratory practices are also critical for improved IVF outcomes.
COMPETING INTERESTS
The authors declared that they had no conflict of interest.
REFERENCES
- 1.Heitmann RJ, Hill MJ, James AN, Schimmel T, Segars JH, et al. Live births achieved via IVF are increased by improvements in air quality and laboratory environment. Reprod Biomed Online. 2015;31:364–71. doi: 10.1016/j.rbmo.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esteves SC, Bento FC. Implementation of air quality control in reproductive laboratories in full compliance with the Brazilian Cells and Germinative Tissue Directive. Reprod Biomed Online. 2013;26:9–21. doi: 10.1016/j.rbmo.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Little SA, Mirkes PE. Relationship of DNA damage and embryotoxicity induced by 4-hydroperoxydechosphamine in postimplantation rat embryos. Teratology. 1990;41:223–31. doi: 10.1002/tera.1420410214. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J, Gilligan A, Esposito W, Schimmel T, Dale B. Ambient air and its potential effects on conception in vitro. Hum Reprod. 1997;12:1742–9. doi: 10.1093/humrep/12.8.1742. [DOI] [PubMed] [Google Scholar]
- 5.Schimmel T, Gilligan A, Garrisi GJ, Esposito B, Jr, Cecchi M, et al. Removal of volatile organic compounds from incubators used for gamete and embryo culture. Fertil Steril. 1997;67:S165. [Google Scholar]
- 6.Hall J, Gilligan A, Schimmel T, Cecchi M, Cohen J. The origin, effects and control of air pollution in laboratories used for human embryo culture. Hum Reprod. 1998;13:146–55. doi: 10.1093/humrep/13.suppl_4.146. [DOI] [PubMed] [Google Scholar]
- 7.Mayer JF, Nehchiri F, Weedon VM, Jones EL, Kalin HL, et al. Prospective randomized crossover analysis of the impact of an incubator air filtration on IVF outcomes. Fertil Steril. 1999;72:S42. [Google Scholar]
- 8.Racowsky C, Jackson KV, Nureddin A, de los Santos MJ, Kelley JR, et al. Sydney, Australia: 1999. Carbon-Activated Air Filtration Results in Reduced Spontaneous Abortion Rates Following IVF. Proceedings of the 11th World Congress on In Vitro Fertilization and Human Reproductive Genetics. [Google Scholar]
- 9.Boone WR, Johnson JE, Locke AJ, Crane MM, Price TM. Control of air quality in an assisted reproductive technology laboratory. Fertil Steril. 1999;71:150–4. doi: 10.1016/s0015-0282(98)00395-1. [DOI] [PubMed] [Google Scholar]
- 10.Worrilow KC, Huynh HT, Gwozdziewicz JB, Schillings W, Peters AJ. A retrospective analysis: the examination of a potential relationship between particulate (P) and volatile organic compound (VOC) levels in a class 100 IVF laboratory cleanroom (CR) and specific parameters of embryogenesis and rates of implantation (IR) Fertil Steril. 2001;76:S15–6. [Google Scholar]
- 11.Worrilow KC, Huynh HT, Bower JB, Schillings W, Peters AJ. A retrospective analysis: seasonal decline in implantation rates (IR) and its correlation with increased levels of volatile organic compounds (VOC) Fertil Steril. 2002;78:S39. [Google Scholar]
- 12.Esteves SC, Gomes AP, Verza S., Jr Control of air pollution in assisted reproductive technology laboratory and adjacent areas improves embryo formation, cleavage and pregnancy rates and decreases abortion rate: comparison between a class 100 (ISO 5) and a class 1.000 (ISO 6) cleanroom for micromanipulation and embryo culture. Fertil Steril. 2004;82:S259–60. [Google Scholar]
- 13.von Wyl S, Bersinger NA. Air quality in the IVF laboratory: results and survey. J Assist Reprod Genet. 2004;21:283–4. doi: 10.1023/b:jarg.0000043700.70912.0b. [DOI] [PubMed] [Google Scholar]
- 14.Esteves S, Verza S, Jr, Gomes AP. Comparison between International Standard Organization (ISO) type 5 and type 6 cleanrooms combined with volatile organic compounds filtration system for micromanipulation and embryo culture in severe male factor infertility. Fertil Steril. 2006;86:S353–4. [Google Scholar]
- 15.Knaggs P, Birch D, Drury S, Morgan M, Kumari S, et al. Full compliance with the EU directive air quality standards does not compromise IVF outcome. Hum Reprod. 2007;22:i164–5. [Google Scholar]
- 16.Khoudja RY, Xu Y, Li T, Zhou C. Better IVF outcomes following improvements in laboratory air quality. J Assist Reprod Genet. 2013;30:69–76. doi: 10.1007/s10815-012-9900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munch EM, Sparks AE, Duran HE, Van Voorhis BJ. Lack of carbon air filtration impacts early embryo development. J Assist Reprod Genet. 2015;32:1009–17. doi: 10.1007/s10815-015-0495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Commission of the European Parliament. Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on Setting Standards of Quality and Safety for the Donation, Procurement, Testing, Processing, Preservation, Storage and Distribution of Human Tissues and Cells; 2004. [Last accessed on 2015 Oct 12]. Available from: http://eur-lex.europa.eu/Lex-UriServ/LexUriServ.do?uri=OJ:L:2004:102:0048:0058:en:PDF .
- 19.ANVISA. Brazilian National Agency for Sanitary Surveillance Resolução no. 33 da Diretoria colegiada da Agência Nacional de Vigilância Sanitária (amended by RDC23 of 27 May 2011 on Setting Standards of Quality and Safety for the Donation, Procurement, Testing, Processing, Preservation, Storage and Distribution of Human Tissues and Cells); 2006. [Last accessed on 2015 Sep 12]. Available from: http://www.bvsms.saude.gov.br/bvs/saudelegis/anvisa/2011/res0023_27_05_2011.html .
- 20.Commission of the European Union Communities. Communication from the Commission on the Precautionary Principle; 2000. [Last accessed on 2015 May 14]. Available from: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=URISERV:l32042 .
- 21.National Environmental Balancing Bureau. Procedural Standards for Certified Testing of Cleanrooms, Vienna, Virginia, USA; 1998. [Last accessed on 2014 Feb 14]. Available from: http://www.nebb.org .
- 22.Esteves SC, Agarwal A. Explaining how reproductive laboratories work. In: Bento F, Esteves SC, Agarwal A, editors. Quality Management in ART Clinics: A Practical Guide. 1st ed. New York: Springer Science+Business Media; 2013. pp. 79–127. [Google Scholar]
- 23.Chiang YC, Chiang PC, Huang CP. Effects of pore structure and temperature on VOC adsorption on activated carbon. Carbon. 2001;39:523–34. [Google Scholar]


