Abstract
Basigin is a member of the immunoglobulin superfamily and plays various important roles in biological events including spermatogenesis. To examine the basigin molecular variants during spermatogenesis and sperm maturation in the mouse, immunoprecipitated basigin samples from testis and epididymal spermatozoa were analyzed by liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS). The results demonstrated that basigin molecules from the testis and spermatozoa were separable into two major bands and that the differences in the molecular sizes were possibly because of an endoproteolytic cleavage. Since basigin is known to be a chaperone for the monocarboxylate transporter 1 (MCT1), the localization of basigin, MCT1 and MCT2 was examined during postnatal testicular development. Immunohistochemical studies showed different expression patterns of MCT1 and MCT2. MCT1 was localized on the surface of spermatogonia, spermatocytes, and spermatids. In contrast, MCT2 appeared on the principal piece of spermatozoa in the testis, where basigin was also observed. In mature epididymal spermatozoa, MCT2 was located on the midpiece, where basigin co-localized with MCT2 but not with MCT1. Furthermore, MCT2 was immunoprecipitated with basigin in mouse testes and sperm. These results suggest that basigin has a functional role as a binding partner with MCT2 in testicular and epididymal spermatozoa.
Keywords: basigin, monocarboxylate transporter, sperm maturation, spermatogenesis, spermatozoa, testis
INTRODUCTION
Basigin (also called CD147 and EMMPRIN) is a transmembrane protein belonging to the immunoglobulin superfamily, which is involved in many biological events including reproduction.1,2 In tumor cells, basigin induces an elevated expression of matrix metalloproteinases (MMPs), resulting in tumor invasion and metastasis.3 Basigin is also involved in inflammatory processes owing to its association with cyclophilin A.4
Mice deficient in the Basigin gene (Bsg) show various phenotypes.5,6 The mice exhibit abnormalities in sensory and memory functions,7 and defects in vision because of deletion of basigin in the retina.8 Moreover, Bsg-deficient mice are sterile in both sexes.5 In Bsg-knockout male mice, spermatocytes are arrested at the metaphase stage of the first meiotic division.9 Basigin in the mouse testes is localized on the surface of spermatocytes, spermatids and the principal pieces of spermatozoa.10 During sperm maturation in the epididymis, the localization of basigin in sperm cells shifts from the principal piece in the caput epididymis to the middle piece in the corpus and cauda epididymis.11 Furthermore, the molecular weight of basigin also changes during sperm maturation.11 Basigin is also present in the head of sperm cells, where it may be involved in the sperm-egg interaction.12
Several proteins are known to be associated with basigin, including monocarboxylate transporters (MCTs). MCTs catalyze the proton-linked transport of monocarboxylates, such as lactate, pyruvate and ketone bodies, across the plasma membrane. MCTs require an ancillary protein for their translocation to the plasma membrane and transporter activity.13 Basigin is the preferred binding partner for MCT1, as confirmed by expression studies in Xenopus oocytes.14 In contrast, expression of MCT2 in the plasma membrane of mammalian cells requires co-expression with embigin rather than basigin.15 These data indicate that embigin, which belongs to the same family as basigin, is the preferred binding partner of MCT2. MCT2 has a higher affinity than MCT1 for substrates such as pyruvate and L-lactate.16
MCTs are expressed in the testes and spermatozoa;16,17,18,19,20 however, their localization is rather controversial, especially in the case of MCT1. In this report, we examined the expression of MCT1, MCT2 and basigin by immunohistochemical and Western blot analyses to elucidate their function in mouse testes and sperm cells. We report that basigin behaves together with MCT2 but not with MCT1 in the maturing process of spermatozoa.
MATERIALS AND METHODS
Animals
Male ICR mice were purchased from Clea Japan Inc., (Tokyo, Japan) and kept in an air-conditioned room with free access to food and water. The present study was conducted according to the guidelines for the care and use of laboratory animals of the Chiba University Graduate School of Medicine (Approved No. A26-16).
Western blot analysis
Testes from 1-, 2-, 3-, 4-, and 5-week-old mice were removed and sonicated in sodium dodecylsulfate (SDS)-sample buffer (2% (w/v) SDS, 6% (v/v) b-mercaptoethanol, 10% (v/v) glycerol, 0.005% (w/v) Bromophenol Blue). Testes and cauda epididymidal spermatozoa from adult mice were also removed and extracted with the SDS-sample buffer. After centrifugation at 20 000 g for 15 min at 4°C, the lysates were electrophoresed on a 12.5% (w/v) SDS-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane (PVDF; Millipore, Bedford, MA, USA).
Western blot analysis was performed according to a standard protocol using TBS-T (20 mmol l−1 Tris-HCl, pH 7.6, 137 mmol l−1 NaCl, and 0.1% (v/v) Tween 20) containing 5% (w/v) skimmed milk as a blocking solution and for antibody dilution. Primary antibodies were diluted at 1:2000 for anti-basigin antibody (sc-9757; Santa Cruz Biotechnology, Dallas, TX, USA) or at 1:1000 for anti-MCT1 (AB1286; Millipore) and anti-MCT2 (sc-50323; Santa Cruz) antibodies. Horseradish peroxidase (HRP)-conjugated secondary antibodies were diluted at a ratio of 1:10 000. The blots were developed with enhanced chemiluminescence (ECL Plus, GE Healthcare, Buckinghamshire, UK) and exposed to an X-ray film.
Immunoprecipitation and LC-MS/MS analysis
Testes from adult mice and spermatozoa from the cauda epididymis were extracted with NP-40 buffer (1% (v/v) Nonidet P-40, 150 mmol l−1 NaCl, 50 mmol l−1 Tris-HCl, pH 8.0) containing protease inhibitors (Complete Mini, Roche Diagnostics, Mannheim, Germany) and centrifuged at 20 000 g for 10 min to remove insoluble debris. The lysates were incubated with anti-basigin antibody for 1 h with slow tilt rotation (15 rpm), and Dynabeads-Protein G (Invitrogen, Carlsbad, CA, USA) was then added and incubated for 1 h at 4°C. The immunoprecipitate was eluted with 1% (w/v) SDS in 50 mmol l−1 Tris-HCl at pH 7.4 and concentrated by centrifugation in a Vivaspin 500 (GE Healthcare). After separation by SDS-PAGE, the protein samples were detected using Oriole fluorescent stain (BioRad, Hercules, CA, USA). The bands of interest were excised from the Oriole-stained gel and digested with trypsin. The digested peptides were analyzed by nano liquid chromatography (LC)-MS/MS system composed of an LTQ Orvitrap Velos (Thermo Fisher Scientific, Waltham, MA, USA) coupled with Advanced UHPLC (Michrom Bioresources, Auburn, CA, USA) and HTC-PAL-xt autosampler (CTC Analytics, Zwingen, Switzerland). All MS/MS spectra were analyzed in a Mascot Server (Matrix Science, Boston, MA, USA).
Immunohistochemistry
Testes from 1-, 2-, and 3-week-old mice were immersed in Bouin solution for 2 h after being made a cut in the capsule. Four-week-old and older mice were fixed with Bouin solution by perfusion through the left ventricle for 20 min. Testes and epididymides were removed and immersed in the same fixative for 2 h. After being dehydrated in a graded ethanol series and xylene, the samples were processed for paraffin embedding and sectioned at 4 μm thickness. The sections were incubated in blocking buffer (PBS containing 10% (v/v) fetal bovine serum) for 30 min at room temperature. The sections were then incubated with anti-basigin antibody at a 1:400 dilution, anti-MCT1 antibody at a 1:200 dilution, or anti-MCT2 antibody at a 1:200 dilution for 1 h at room temperature. After a wash in PBS, the samples were incubated with the following HRP-conjugated secondary antibodies: donkey anti-goat IgG antibody (Jackson Immunoresearch Laboratories, West Grove, PA, USA) for basigin, bovine anti-chicken IgY antibody (Santa Cruz Biotechnology) for MCT1, and anti-rabbit IgG antibody (GE Healthcare) for MCT2 detection. Immunohistochemical reactions were visualized using 3,3’-diaminobenzidine (DAB) and H2 O2. Negative controls were prepared by the omission of the primary antibodies. Some sections were counterstained with Hematoxylin, and adjacent sections were stained with periodic-acid-Schiff and Hematoxylin (PAS-H) to evaluate stage of the seminiferous epithelium.
Indirect immunofluorescence (IIF)
Sperm from the caput and cauda epididymis were collected, fixed with 2% (w/v) paraformaldehyde for 15 min, and dried on glass slides. The samples were double-stained with anti-basigin and anti-MCT2 antibodies, or anti-MCT1 and anti-MCT2 antibodies. After incubation with primary antibodies, the sperm were treated for 1 h at room temperature with secondary antibodies; Alexa Fluor 488-conjugated donkey anti-goat IgG (0.5 μg ml−1) for basigin, Alexa Fluor 488-conjugated donkey anti-chicken IgY (0.5 μg ml−1) for MCT1, and Alexa Fluor 546-conjugated goat anti-rabbit IgG (0. 5 μg ml−1) antibodies and Hoechst 33 258 (5 μg ml−1) were employed. Observations were made using a confocal laser microscope (FV10i; Olympus, Tokyo, Japan).
Silver-intensified immunogold method for electron microscopy
The formaldehyde-fixed tissues were dipped in 30% (w/v) sucrose solution overnight at 4°C, embedded in OCT compound (Tissue-Tek, Sakura, Tokyo, Japan), and quickly frozen in liquid nitrogen. Frozen sections of 15 μm in thickness were mounted on poly-L-lysine-coated glass slides, incubated with the rabbit anti-MCT2 antibody (1 μg ml−1) overnight, and subsequently reacted with goat anti-rabbit IgG covalently linked with 1-nm gold particles (1:200 in dilution; Nanoprobes, Yaphank, NY, USA). Following silver enhancement with a kit (HQ silver; Nanoprobes), the sections were osmicated, dehydrated and directly embedded in Epon (Nisshin EM, Tokyo, Japan). Ultrathin sections were prepared and stained with both uranyl acetate and lead citrate for observation in an electron microscope (H-7100; Hitachi, Tokyo, Japan). The specificity of the immunoreactions was confirmed by the disappearance of immunolabeling when the antibody was preincubated with the antigen.
RESULTS
Basigin molecules in the testis and spermatozoa
We immunoprecipitated testis and sperm extracts with anti-basigin antibody to examine the difference between the basigin molecules in the testes and spermatozoa of mice. As shown in Figure 1a, there were two major basigin bands at around 40 kDa (T-1) and 38 kDa (T-2) in the testis and at 29 kDa (S-1) and 26 kDa (S-2) in spermatozoa. LS-MS/MS analyses of these bands (Figure 1b) revealed possible proteolytic molecular changes. Testicular peptides migrating with basigin from the T-1 band were matched up to the 265th amino acid; however, those from the T-2 band were matched only up to the 254th amino acid. These data demonstrate that T-2 had lost several amino acids from the C terminus compared with T-1. Similarly, when the matched sperm peptides were examined, those in S-2 appeared to have lost more amino acids from the N terminus than those in S-1. Further, sperm-type basigin proteins S-1 and S-2 had lost amino acids from the N terminus compared with testis-type basigin proteins T-1 and T-2. These results suggest that endoproteolytic cleavage had occurred during sperm maturation.
Figure 1.

(a) Proteins from adult testes (T) or cauda epididymidal spermatozoa (S) immunoprecipitated by an anti-basigin antibody. T-1, T-2, S-1 and S-2 bands were cut from the gel and analyzed by LC-MS/MS. (b) Amino acid sequence of basigin. The peptides corresponding to basigin protein detection by LC-MS/MS spectra were analyzed using Mascot Server, and matched peptides are shown in red. 1–21: signal peptide, 24–103: Ig-like C2-type domain, 105–203: Ig-like V-type domain, 210–233: transmembrane, 234–273: cytoplasmic.
Western blot analysis during postnatal testicular development
Proteins from the testes of 1-, 2-, 3-, 4- and 5-week-old mice, as well as adult mice were separated by SDS-PAGE, and Western blotting was carried out with anti-basigin, anti-MCT1 and anti-MCT2 antibodies Figure 2. Basigin was not detected in the testes of 1-week-old mice and testis type T-2 band (38 kDa) appeared faintly in those of the 2-week-old mice (Figure 2a). The reactivity became strong in 3-week-old mice, and thereafter gradually increased in intensity. On the other hand, testis type T-1 basigin (40 kDa) first appeared at 5 weeks of age. Although an additional band was detectable at around 43 kDa (a small arrow in Figure 2a) in the testes of 1- and 2-week-old mice, it may have come from the blood, since the blood sample showed the same band (data not shown). Two bands were recognized by anti-MCT1 antibody (Figure 2b), and the intensity of the bands was stronger at younger ages of the mice. Two bands also existed at around 40 kDa and 65 kDa in the case of MCT2 (Figure 2c). The lower band (Figure 2c, arrowhead) appeared in an age-specific pattern; the band first appeared faintly in testes of 4-week-old mice, and the reactivity strengthened with age. In contrast, the higher band was seen in samples from mice of all ages.
Figure 2.
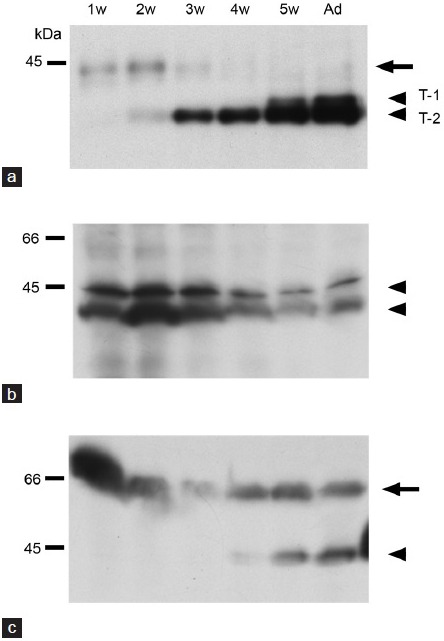
Western blot analyses of basigin (a), MCT1 (b) and MCT2 (c) during testicular development. The proteins were detected in the testes from 1- (1w), 2- (2w), 3- (3w), 4- (4w), 5-week (5w)-old and adult (Ad) mice. Arrowheads show the detected proteins. Small arrows show a possible contaminant of blood (a) or a band from interstitial cells of the testis (c). w: week.
Immunohistochemical localization of basigin, MCT1 and MCT2 in adult mouse testes
Testes from the adult mice were immunostained with anti-basigin (Figure 3a and 3b), anti-MCT1 (Figure 3d and 3e), or anti-MCT2 antibody (Figure 3g and 3h). Basigin immunoreactivity was observed in the spermatocytes, spermatids and principal piece of spermatozoa. Immunostaining indicated that MCT1 was localized in the spermatogenic cells and its expression was especially strong on the surface of spermatogonia and early spermatocytes. In contrast, MCT2 immunoreactivity was found in the cytoplasm of elongated spermatids and on the principal piece of spermatozoa, as well as in the interstitial tissues between seminiferous tubules. These results indicated that both basigin and MCT2 immunoreactivities were found on the principal piece of testicular spermatozoa. Negative controls lacking primary antibodies showed no immunoreactivity (Figure 3c, 3f, 3i).
Figure 3.
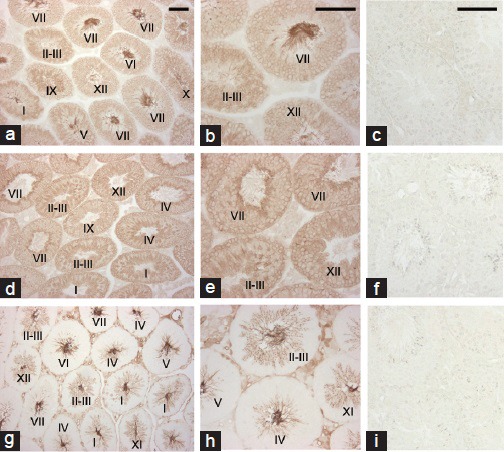
Immunohistochemical localization of basigin, MCT1 and MCT2 in mouse testes. Testes from adult mice were immunostained with anti-basigin (a and b), anti-MCT1 (d and e) or anti-MCT2 (g and h) antibody. Roman numerals indicate the stage of the seminiferous epithelium. (c, f, i) negative controls lacking primary antibodies, anti-basigin (c), anti-MCT1 (f) and anti-MCT2 (i). Scale bars: 100 μm.
Immunohistochemical localization of basigin, MCT1 and MCT2 in the testis during postnatal development
At 1 week of age, when spermatogonia and Sertoli cells were present in the seminiferous epithelium, basigin immunoreactivity was not found (Figure 4a). Basigin immunoreaction product was seen in the spermatocytes that appeared at 2 weeks of age of the mice (Figure 4b), and spermatocytes and spermatids at 4 weeks of age (Figure 4c). Strong reactivity in the sperm tail was also seen in the testes of 4-week-old (Figure 4c) and adult mice (Figure 3a and 3b). MCT1 showed weak but clear immunoreactivity in testes of 1-week-old mice (Figure 4d), and all of the spermatogenic cells seemed to be immunoreactive during postnatal development (Figure 4d–4f). In addition, Sertoli cells in the seminiferous tubules appeared to be occasionally positive for MCT1 expression. Immunoreactivity for MCT2 was not seen in the seminiferous tubules of 1- and 2-week-old mice (Figure 4g and 4h) and was restricted to the cytoplasm and tails of the elongated spermatids in the seminiferous tubules at 4 weeks (Figure 4i) as well as in interstitial cells. Negative controls lacking primary antibodies showed no positive reaction (data not shown).
Figure 4.
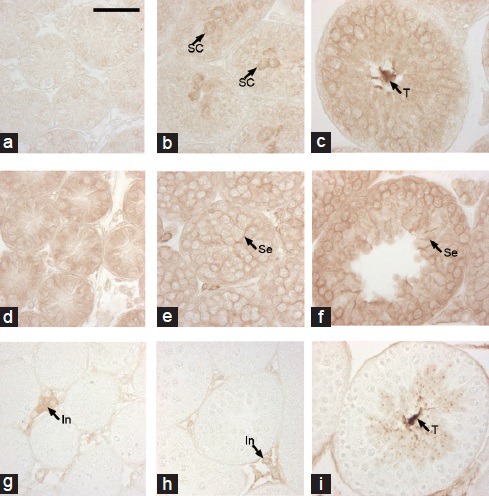
Immunolocalization of basigin, MCT1 and MCT2 during postnatal testicular development. Testes obtained from 1- (a, d, g), 2- (b, e, h) and 4-week (c, f, i)-old mice were immunostained with anti-basigin (a–c), anti-MCT1 (d–f) or anti-MCT2 (g–i) antibody. SC: spermatocyte, T: tails of testicular spermatozoa, Se: Sertoli cell, In: interstitial cells. Scale bar: 50 μm.
Immunolocalization of basigin, MCT1 and MCT2 in the epididymal spermatozoa
Indirect immunofluorescence study revealed protein localization in spermatozoa from the caput and cauda epididymis. Immunoreactivities for basigin and MCT2 were co-localized; in sperm cells from the caput epididymis, the reactions occurred on the principal piece (Figure 5a–5d). In contrast, the midpiece was positive for both basigin and MCT2 in spermatozoa from the cauda epididymis (Figure 5i–5l). Immunoelectron microscopy studies with the anti-MCT2 primary antibody confirmed these results (Figure 6). MCT2 was localized on the plasma membranes of the principal piece of caput epididymidal spermatozoa and on those of the midpiece of spermatozoa from the ductus deferens, which showed the same immunofluorescent reactivity for basigin and MCT2 as cauda epididymidal spermatozoa (data not shown). However, the localization of MCT1 was not that of basigin and MCT2; the anterior part of the principal piece was immunoreactive for MCT1 in spermatozoa from both the caput and cauda epididymis (Figure 5e–5h, 5m–5p). In addition, some reaction was found in the sperm heads. No positive reactions were observed in the negative controls lacking primary antibodies (data not shown).
Figure 5.
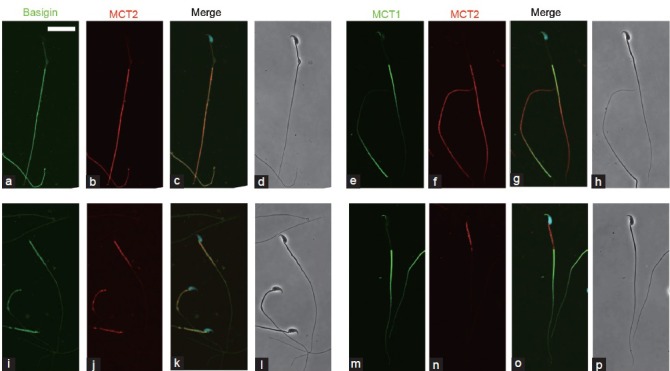
Indirect immunofluorescence localization of basigin, MCT1, and MCT2 in spermatozoa. Spermatozoa from the caput (a–h) and cauda (i–p) epididymis were immunostained with anti-basigin and anti-MCT2 antibodies (a–d, i–l) as well as with anti-MCT1 and anti-MCT2 antibodies (e–h, m–p). Immunoreaction for basigin (a and i) and MCT1 (e and m) is represented in green, and that for MCT2 (b, f, j, n) in red. (c, g, k, o) merged images with green, red and Hoechst staining (blue). More than 90% of spermatozoa showed similar immunoreactions. (d, h, l, p) phase contrast images. Scale bar: 20 μm.
Figure 6.
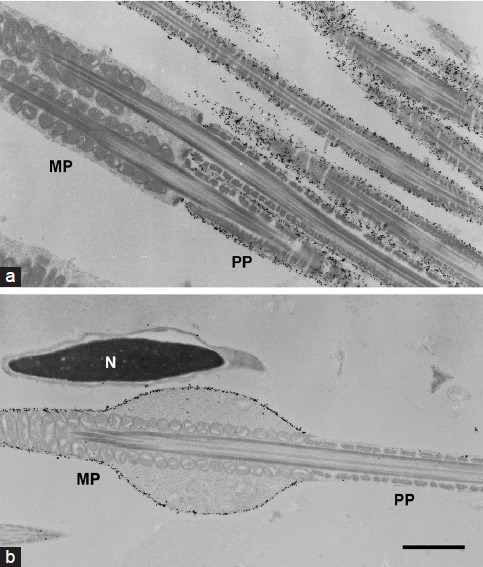
Immunoelectron micrographs of MCT2 in spermatozoa. Spermatozoa from the caput epididymis (a) and ductus deferens (b) were immunolabeled with anti-MCT2 antibody. The majority of gold particles were seen on the surface of the principal piece (PP), not on the midpiece (MP) of the sperm flagella from the caput epididymis (a). In the sperm from ductus deferens, the immunoparticles were detected on the MP, not on the PP (b). N: nucleus of the sperm head. Scale bar: 1 μm.
Co-immunoprecipitation of MCT2 with basigin
Proteins from adult testes and cauda epididymal sperm were immunoprecipitated with anti-basigin antibody, and Western blot analysis of the resulting precipitates indicated that MCT2 was co-immunoprecipitated (Figure 7).
Figure 7.
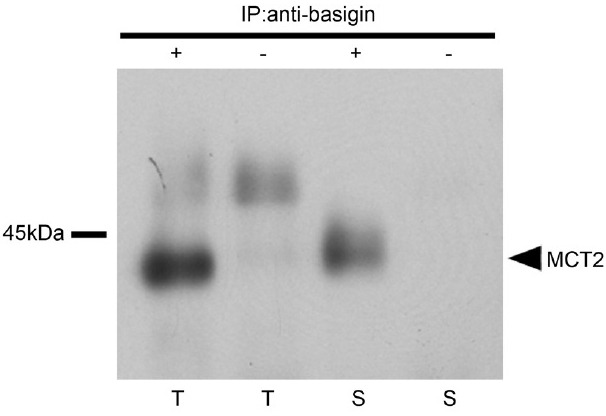
Basigin co-immunoprecipitates MCT2. Proteins from testes (T) and spermatozoa from the cauda epididymis (S) were immunoprecipitated with (+) or without (-) anti-basigin antibody. Western blot analysis involved the anti-MCT2 antibody.
DISCUSSION
In this report, we demonstrated the localization of basigin, MCT1 and MCT2 in mouse testes and epididymal spermatozoa. In the testes, basigin was localized in the spermatocytes, spermatids and sperm tails, as previously described by us.10 The localization of MCT1 and MCT2 immunoreactivities were quite different. MCT1 was detected on the cell surface of the spermatogonia and spermatocytes, and Sertoli cells occasionally appeared to show a positive reaction for MCT1. The staining results for spermatogenic cells were similar to those of Goddard et al.19 Nakai et al.18 also reported the occasional staining of Sertoli cells with MCT1 antibody in mouse testes. It is interesting to speculate on the function of MCT1 in Sertoli cells in terms of Sertoli cell-germ cell interaction, since Sertoli cells produce the lactate that postmeiotic germ cells utilize.21,22
Information on the localization of nutrient transporters is important for identifying the predominant energy source of each cell type. In the mouse seminiferous epithelium, spermatogonia expressed MCT1 most intensely. Only spermatocytes and spermatids express GLUT3 abundantly, but spermatogonia lack GLUT3 and other GLUT subtypes (Iwanaga, unpublished data). These findings suggest that lactate and other mono-carboxylates are the main fuel for spermatogonia.
An intense immunoreactivity for MCT2 was detected on the principal pieces of the testicular spermatozoa, as reported by others.16,17,19,20 Furthermore, we demonstrated that the cytoplasm of elongated spermatids was also immunoreactive for MCT2. The specific expression was confirmed during postnatal testicular development. Western blot analyses of MCT2 revealed two bands at around 40 kDa and 65 kDa. Since a 40-kDa band first appeared in testes of 4-week-old mice when MCT2 was immunohistochemically detected in the tails of spermatids, the band is thought to be derived from the MCT2 of the spermatogenic cells in the seminiferous epithelium. The other 65-kDa band is probably from interstitial cells, because the expression was observed at all ages.
There were also different patterns of MCT1 and MCT2 immunoreactivities in epididymal spermatozoa. MCT2 was immunolocalized on the principal piece of caput epididymidal spermatozoa and the middle piece of cauda epididymidal spermatozoa. These results indicate the co-localization of MCT2 and basigin. In the case of MCT1, immunolocalization on spermatozoa from both the caput and cauda epididymis was found on the anterior half of the principal piece. Some immunoreaction of MCT1 was also seen in the sperm heads. Garcia et al.16 reported that MCT1 was present on the heads but not the tails of hamster spermatozoa. On the other hand, Mannowetz et al.20 stated that MCT1 was restricted to the murine sperm midpiece. These discrepancies might result from differences in the species and antibodies used and immunostaining conditions employed.
We demonstrated that MCT2 co-immunoprecipitated with basigin in cauda epididymidal spermatozoa. Because both MCT2 and basigin were co-localized in these cells, it is likely that these proteins are associated at the molecular level. It has been reported that basigin specifically interacts with MCT1 and MCT4 but not with MCT2.23 However, our results indicate that basigin is a preferred binding partner for MCT2 rather than for MCT1 in spermatozoa. Mannowetz et al.20 demonstrated that basigin interacts with both MCT1 and MCT2 in mature mouse spermatozoa, although our immunofluorescence analyses showed that basigin was not co-localized with MCT1 in spermatozoa. MCT2 was also co-immunoprecipitated with basigin in the testes, and we suggest that the interaction of MCT2 and basigin occurs in the principal piece of testicular spermatozoa. However, MCT2 was not localized in the spermatocytes and round spermatids in which basigin was expressed. It is interesting that MCT1 was localized in those cells; thus, it is possible that basigin will interact with MCT1 in spermatocytes and round spermatids, or that MCT1 interacts with another basigin family member, embigin. Embigin is reported to be a binding partner of MCT2,13 although Mannowetz et al.20 reported that embigin was localized only in the sperm tail in mouse testes. The mechanisms underlying the functions of MCTs and their ancillary proteins may be different from those in the testes. It is reported that the genetic variation in MCT2 has functional and clinical relevance for male infertility.24 MCTs are involved in sperm energy metabolism, and consequently may affect sperm survival and mortality.
Basigin is a highly glycosylated protein, and its molecular mass differs among tissues and cells. In the present report, we examined two major bands of basigin from the testis (T-1, T-2) and spermatozoa (S-1, S-2). From the LC-MS/MS analyses, it is possible that processing of basigin molecule yields these different bands. Since basigin is highly glycosylated, the differences in its molecular mass might partially result from modification of glycosylation. Our data demonstrate the possibility of an endoproteolytic cleavage occurring at the extracellular N-terminus of the basigin molecule in testicular and cauda epididymidal spermatozoa. This is in agreement with the results in the rat.25 During postnatal testicular development, the appearance of band T-1 was delayed compared with T-2, suggesting a functional difference between them. Band T-1 was first detected in the testes of 5-week-old mice by Western blot analysis, and a clear expression of MCT2 appeared in those of 5-week-old mice. These results could indicate that basigin T-1 plays a role as a binding partner for MCT2. Basigin has diverse activities and multiple binding partners, such as integrin,26 gamma-secretase complex,27 caveolin-128 and cyclophilin A.4 It is possible that modifications of basigin molecules in specific tissues and cells are important factors in determining the partners to which they bind.
CONCLUSION
Basigin molecules changed during spermatogenesis and sperm maturation. Basigin was co-localized with MCT2 in mouse testicular and epididymal spermatozoa, and co-immunoprecipited with MCT2. The cellular localizations of MCT1 and MCT2 in the mouse testis and sperm were different. These results indicate basigin is the binding partner protein for MCT2, not MCT1 in mouse spermatozoa. Further study is necessary to clarify the role of the MCTs in spermatozoa.
AUTHOR CONTRIBUTIONS
CC carried out Western blot analyses and wrote the manuscript. MM carried out immunohistochemistry and designed the study. KY carried out co-immunoprecipitation study. MN carried out LS-MS/MS analyses. CI, TI and KT carried out immunofluorescence and immunoelectron studies and helped to draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTEREST
All authors declare no competing interest.
ACKNOWLEDGMENTS
The authors would like to thank Dr. K Saito of DNA-chip Development Center for Infectious Diseases (Research Institute for Microbial Diseases, Osaka University, Japan) for the LC-MS/MS analysis. This work was supported in part by grant from the Japan Society for the Promotion of Science to Dr. Mamiko Maekawa (22590168).
REFERENCES
- 1.Muramatsu T, Miyauchi T. Basigin (CD147): a multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion. Histol Histopathol. 2003;18:981–7. doi: 10.14670/HH-18.981. [DOI] [PubMed] [Google Scholar]
- 2.Toshimori K, Ito C, Maekawa M, Toyama Y, Suzuki-Toyota F, et al. Impairment of spermatogenesis leading to infertility. Anat Sci Int. 2004;79:101–11. doi: 10.1111/j.1447-073x.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 3.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, et al. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–9. [PubMed] [Google Scholar]
- 4.Yurchenko V, Constant S, Eisenmesser E, Bukrinsky M. Cyclophilin-CD147 interactions: a new target for anti-inflammatory therapeutics. Clin Exp Immunol. 2010;160:305–17. doi: 10.1111/j.1365-2249.2010.04115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Igakura T, Kadomatsu K, Kaname T, Muramatsu H, Fan QW, et al. A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol. 1998;194:152–65. doi: 10.1006/dbio.1997.8819. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Kadomatsu K, Kondo M, Toyama Y, Toshimori K, et al. Effects of flanking genes on the phenotypes of mice deficient in basigin/CD147. Biochem Biophys Res Commun. 2004;324:147–53. doi: 10.1016/j.bbrc.2004.08.232. [DOI] [PubMed] [Google Scholar]
- 7.Naruhashi K, Kadomatsu K, Igakura T, Fan QW, Kuno N, et al. Abnormalities of sensory and memory functions in mice lacking Bsg gene. Biochem Biophys Res Commun. 1997;236:733–7. doi: 10.1006/bbrc.1997.6993. [DOI] [PubMed] [Google Scholar]
- 8.Hori K, Katayama N, Kachi S, Kondo M, Kadomatsu K, et al. Retinal dysfunction in basigin deficiency. Invest Ophthalmol Vis Sci. 2000;41:3128–33. [PubMed] [Google Scholar]
- 9.Toyama Y, Maekawa M, Kadomatsu K, Miyauchi T, Muramatsu T, et al. Histological characterization of defective spermatogenesis in mice lacking the basigin gene. Anat Histol Embryol. 1999;28:205–13. doi: 10.1046/j.1439-0264.1999.00194.x. [DOI] [PubMed] [Google Scholar]
- 10.Maekawa M, Suzuki-Toyota F, Toyama Y, Kadomatsu K, Hagihara M, et al. Stage-specific localization of basigin, a member of the immunoglobulin superfamily, during mouse spermatogenesis. Arch Histol Cytol. 1998;61:405–15. doi: 10.1679/aohc.61.405. [DOI] [PubMed] [Google Scholar]
- 11.Saxena DK, Oh-Oka T, Kadomatsu K, Muramatsu T, Toshimori K. Behaviour of a sperm surface transmembrane glycoprotein basigin during epididymal maturation and its role in fertilization in mice. Reproduction (Camb, Engl) 2002;123:435–44. doi: 10.1530/rep.0.1230435. [DOI] [PubMed] [Google Scholar]
- 12.Saxena DK, Toshimori K. Molecular modifications of MC31/CE9, a sperm surface molecule, during sperm capacitation and the acrosome reaction in the rat: is MC31/CE9 required for fertilization? Biol Reprod. 2004;70:993–1000. doi: 10.1095/biolreprod.103.021667. [DOI] [PubMed] [Google Scholar]
- 13.Halestrap AP. The monocarboxylate transporter family – Structure and functional characterization. IUBMB Life. 2012;64:1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 14.Poole RC, Halestrap AP. Interaction of the erythrocyte lactate transporter (monocarboxylate transporter 1) with an integral 70-kDa membrane glycoprotein of the immunoglobulin superfamily. J Biol Chem. 1997;272:14624–8. doi: 10.1074/jbc.272.23.14624. [DOI] [PubMed] [Google Scholar]
- 15.Wilson MC, Meredith D, Fox JE, Manoharan C, Davies AJ, et al. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70) J Biol Chem. 2005;280:27213–21. doi: 10.1074/jbc.M411950200. [DOI] [PubMed] [Google Scholar]
- 16.Garcia CK, Brown MS, Pathak RK, Goldstein JL. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem. 1995;270:1843–9. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- 17.Boussouar F, Mauduit C, Tabone E, Pellerin L, Magistretti PJ, et al. Developmental and hormonal regulation of the monocarboxylate transporter 2 (MCT2) expression in the mouse germ cells. Biol Reprod. 2003;69:1069–78. doi: 10.1095/biolreprod.102.010074. [DOI] [PubMed] [Google Scholar]
- 18.Nakai M, Chen L, Nowak RA. Tissue distribution of basigin and monocarboxylate transporter 1 in the adult male mouse: a study using the wild-type and basigin gene knockout mice. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:527–35. doi: 10.1002/ar.a.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goddard I, Florin A, Mauduit C, Tabone E, Contard P, et al. Alteration of lactate production and transport in the adult rat testis exposed in utero to flutamide. Mol Cell Endocrinol. 2003;206:137–46. doi: 10.1016/s0303-7207(02)00433-1. [DOI] [PubMed] [Google Scholar]
- 20.Mannowetz N, Wandernoth P, Wennemuth G. Basigin interacts with both MCT1 and MCT2 in murine spermatozoa. J Cell Physiol. 2012;227:2154–62. doi: 10.1002/jcp.22949. [DOI] [PubMed] [Google Scholar]
- 21.Mita M, Hall PF. Metabolism of round spermatids from rats: lactate as the preferred substrate. Biol Reprod. 1982;26:445–55. doi: 10.1095/biolreprod26.3.445. [DOI] [PubMed] [Google Scholar]
- 22.Brauchi S, Rauch MC, Alfaro IE, Cea C, Concha II, et al. Kinetics, molecular basis, and differentiation of L-lactate transport in spermatogenic cells. Am J Physiol Cell Physiol. 2005;288:C523–34. doi: 10.1152/ajpcell.00448.2003. [DOI] [PubMed] [Google Scholar]
- 23.Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, et al. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19:3896–904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Lee DR, Lee S. The genetic variation in monocarboxylic acid transporter 2 (MCT2) has functional and clinical relevance with male infertility. Asian J Androl. 2014;16:694–7. doi: 10.4103/1008-682X.124561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petruszak JA, Nehme CL, Bartles JR. Endoproteolytic cleavage in the extracellular domain of the integral plasma membrane protein CE9 precedes its redistribution from the posterior to the anterior tail of the rat spermatozoon during epididymal maturation. J Cell Biol. 1991;114:917–27. doi: 10.1083/jcb.114.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curtin KD, Meinertzhagen IA, Wyman RJ. Basigin (EMMPRIN/CD147) interacts with integrin to affect cellular architecture. J Cell Sci. 2005;118:2649–60. doi: 10.1242/jcs.02408. [DOI] [PubMed] [Google Scholar]
- 27.Zhou S, Zhou H, Walian PJ, Jap BK. CD147 is a regulatory subunit of the gamma-secretase complex in Alzheimer's disease amyloid beta-peptide production. Proc Natl Acad Sci U S A. 2005;102:7499–504. doi: 10.1073/pnas.0502768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang W, Hemler ME. Caveolin-1 regulates matrix metalloproteinases-1 induction and CD147/EMMPRIN cell surface clustering. J Biol Chem. 2004;279:11112–8. doi: 10.1074/jbc.M312947200. [DOI] [PubMed] [Google Scholar]


