Abstract
The Sertoli cell tight junction (TJ) is the key component of the blood-testis barrier, where it sequesters developing germ cells undergoing spermatogenesis within the seminiferous tubules. Hormonally regulated claudin-11 is a critical transmembrane protein involved in barrier function and its murine knockout results in infertility. We aimed to assess quantitatively the significance of the contribution of claudin-11 to TJ function, in vitro, using siRNA-mediated gene silencing. We also conducted an analysis of the contribution of occludin, another intrinsic transmembrane protein of the TJ. Silencing of claudin-11 and/or occludin was conducted using siRNA in an immature rat Sertoli cell culture model. Transepithelial electrical resistance was used to assess quantitatively TJ function throughout the culture. Two days after siRNA treatment, cells were fixed for immunocytochemical localization of junction proteins or lyzed for RT-PCR assessment of mRNA expression. Silencing of claudin-11, occludin, or both resulted in significant decreases in TJ function of 55% (P < 0.01), 51% (P < 0.01), and 62% (P < 0.01), respectively. Data were concomitant with significant decreases in mRNA expression and marked reductions in the localization of targeted proteins to the Sertoli cell TJ. We provide quantitative evidence that claudin-11 contributes significantly (P < 0.01) to Sertoli cell TJ function in vitro. Interestingly, occludin, which is hormonally regulated but not implicated in infertility until late adulthood, is also a significant (P < 0.01) contributor to barrier function. Our data are consistent with in vivo studies that clearly demonstrate a role for these proteins in maintaining normal TJ barrier structure and function.
Keywords: blood-testis barrier, claudin-11, occludin, Sertoli cell tight junction, siRNA
INTRODUCTION
The Sertoli cell tight junction (TJ) plays an essential role in spermatogenesis as the major component of the blood-testis barrier (BTB) of the seminiferous epithelium. Its transmembrane components form an impermeable “seal” between adjacent Sertoli cells, thereby separating and protecting the adluminal germ cells from the general circulation.1 TJs form in mammals at puberty, concomitant with an increase in the circulating gonadotropins follicle stimulating hormone (FSH) and luteinizing hormone (LH), with the latter stimulating testosterone (T) production. Low levels of circulating gonadotropins leading up to puberty or due to some pathological conditions result in the delay or prevention of TJ formation and a lack of spermatogenic activity, a phenotype which in several species including humans, can be reversed, at least partially, by the exogenous application of gonadotropins.2,3,4,5,6
Claudin-11 is a critical transmembrane protein component of the Sertoli cell TJ. While other claudins have been detected in the seminiferous epithelium including claudin-1, -3, -5, -8, -13, and -19,7,8,9,10,11 as well as other transmembrane proteins such as junctional adhesion molecule (JAM)-A,12 coxsackievirus and adenovirus receptor (CAR),13 and occludin,14,15 claudin-11 is the best studied and when knocked out, leads to congenital infertility in mice.16 We and others have demonstrated its importance for the initiation4 and maintenance3,5,6,17,18 of functional Sertoli cell TJs in various animal models. Claudin-11 is present at functional Sertoli cell TJs which can exclude the small molecular weight tracer biotin in vivo,3,4,5,6 but its presence at the TJ diminishes when gonadotropins are suppressed, coincident with a dysfunctional barrier as shown by biotin tracer permeability through the seminiferous tubules.3,6 In men with testicular disorders such as intraepithelial neoplasia, hypospermatogenesis, spermatogenic arrest, and Sertoli cell only testes, claudin-11 redistributes cytoplasmically away from the TJ.19,20,21 Claudin-11 and TJ function can also be down-regulated by local testicular regulators such as TGF-β322 and GDF9,23 further underscoring the importance of this protein in TJ function. Occludin also appears to be hormonally regulated and behaves similarly in animal models of hormone suppression; however, its knockout is not infertile until at least late adulthood.24
Thus, functional data for these proteins are compelling, albeit qualitative. As such, we aimed to assess quantitatively the impact of silencing claudin-11 using siRNA on TJ function in vitro and extend an analysis to occludin as well in an established culture model.
MATERIALS AND METHODS
Animals
Sprague-Dawley outbred rats aged 19–21 days were used for all cultures. Rats were obtained from Monash Animal Services, Monash University Clayton, Victoria, Australia, as approved by the Animal Ethics Committee (ethics number: MMCB 2002/04), Monash Medical Centre Clayton.
Immature Sertoli cell culture
Primary Sertoli cells were isolated from immature rat testes for cell culture as previously described.25,26 Cells were suspended in serum-free Dulbecco's Modified Eagle's Medium (DMEM)/Hams F12 medium (1:1) with supplements. Cell plating was conducted at 1.25 × 106 cells cm−2 into either 24-well culture plates (Nunc, Nalge Nunc International, Denmark) for total RNA or protein isolation, or into Millicell PCF bicameral chambers (Millipore, Bedford, MA, USA) for transepithelial electrical resistance (TER) measurements and immunocytochemistry. Culture wells and bicameral chambers were coated with Matrigel (BD Biosciences, Bedford, MA, USA; 1:8 in DMEM/F12) beforehand. Cells were incubated at 37°C in 5% CO2 for the duration of the culture (7–8 days) with the day of plating designated day 0. Media was replaced every second day and on the third day, contaminating germ cells were hypotonically shocked with 10% culture media in water for 45 s to allow for their removal.27
TER measurements
The development of Sertoli cell TJs and their response to siRNA treatment was assessed quantitatively daily by TER from the day of plating, using a Millipore Millicell-electrical resistance system (Millipore), as previously described.23,26
siRNA treatment
Two specific siRNA fragments of 21 nucleotides in length were designed for rat claudin-11 (NM_053457) and also rat occludin (NM_031329) through the Whitehead Institute's “siRNA Selection Program” (website no longer maintained)28 and obtained from Qiagen (Germany). These were designated; Clau-11-A (5’- AAACCGTTTCTATTACTCTTC-3’), Clau-11-B (5’- GACTGCGTCATGGCCACTGGT-3’), Occ-A (5’- AACGATAACCTAGAGACACCT-3’) and Occ-B (5’- AATTATCACACATCAAGAGGA-3’). According to manufacturer's instructions, a 2-day treatment of culture media containing 1 μg siRNA:3 μl of transfection reagent was applied to immature Sertoli cells at days 5 or 6 of culture. The negative control was an siRNA fragment from Qiagen (Germany) specifically targeting Lamin A/C, and the positive control was fluorescently labeled siRNA visible by fluorescent microscopy (Qiagen). Cells were treated with (i) each of the siRNAs separately, (ii) either combined claudin-11 or occludin siRNA inoculations, or (iii) a combined Clau-11-B and Occ-B regimen. The final total siRNA concentration of the mixed samples equaled that of the individual samples. Following culture, cells were harvested for RT-PCR and immunocytochemical analyses.
Total RNA extraction, reverse transcription and quantitative real-time PCR
Total RNA was extracted from the cells using the Qiagen RNeasy total RNA Isolation Kit (Qiagen) according to the manufacturer's directions. Reverse transcription was performed on 500 ng total RNA/sample using AMV-reverse transcriptase (8 U; Roche, Switzerland), random hexamer primers (200 ng; GE Healthcare, Australia), dNTPs (20 nmol each; Roche), RNasin (20 U; Promega, USA), and 5X reaction buffer (Roche).
mRNA expression was quantified using the Roche Light Cycler 380 (Roche) and the FastStart DNA Master Sybr Green 1 systems (Roche). Oligonucleotide primer pairs and PCR conditions (Table 1) were obtained from published sources and ordered from Sigma Genosys (Castle Hill, Australia).22,29,30 Standard curves for PCR analyses were generated using dilutions of an adult rat testicular cDNA preparation of arbitrary unitage. PCR of all samples was performed using triplicate reactions for 38 cycles, after which a melting curve analysis was performed to monitor product purity.
Table 1.
Primer-specific conditions used for quantitative PCR analysis
Immunocytochemistry
Cells on PCF membrane filters were fixed in 3% paraformaldehyde for 30 min at room temperature and permeabilized with 0.05% Triton-X100. Non-specific binding sites were blocked with CAS block (Zymed, San Francisco, CA, USA) containing 10% normal sheep serum before overnight incubations in primary antibodies; rabbit anti-claudin-11 (2.0 μg ml−1, catalogue #36–4500, Zymed, South San Francisco, CA, USA), rabbit anti-occludin (2.5 μg ml−1, catalogue #71–1500, Zymed), and mouse anti-β-catenin (adherens junction protein, 0.5 μg ml−1, catalogue #610154, BD Transduction Laboratories, New Jersey, USA). Negative controls substituted the primary antibodies with normal rabbit or mouse IgG at the equivalent concentration. Secondary antibody goat anti rabbit or mouse Alexa-488 (Molecular Probes, Oregon, USA) was then added for 1 h and nuclei were labeled with the nuclear stains DAPI (100 nM in PBS/BSA) and TOPRO-3 (1:100 in DAPI/PBS/BSA solution from a 1 mM stock in DMSO; Molecular Probes, Oregon, USA). After mounting, sections were visualized and photographed using a confocal microscope (FluoView, FV300, Olympus Australia, Mt Waverley, VIC, Australia).
Western blot analysis
Protein was extracted from cell lysates using boiling 2X non-reduced sample buffer and quantified using the bicinchoninic acid protein assay (Life Technologies Australia). Despite the use of multiple antibodies and extraction techniques, we were unable to detect claudin-11 in Sertoli cell extracts although positive bands were seen in whole rat testis and mouse brain protein extracts. For occludin Westerns, 15 μg of total protein was separated by SDS-PAGE on 4%–20% precast gels (Bio-Rad, Hercules, CA, USA) and transferred onto PVDF membranes by Western blot. After overnight blocking (5% skim-milk), rabbit-anti-occludin antibody (Zymed, 71–1500, 1:125) was applied to the membranes for 2 h, followed by a 2 h secondary antibody incubation (goat anti-rabbit, HRP-conjugated, Silenus, Melbourne, Australia). Protein detection was achieved using enhanced chemiluminescence (GE Healthcare) and photographic films (GE Healthcare).
Statistical analysis
Statistical analysis was performed using the SigmaStat program for Windows Version 3.5 (Jandel Corporation, CA, USA). Triplicate culture wells were used per treatment with 2–3 cultures conducted for each endpoint. Differences in TER and RT-PCR between treatments were measured by ANOVA following tests for normal distribution and equal variance. Upon the determination of differences, the post-hoc Student Newman–Keuls test was applied to determine where the differences lay. P < 0.05 was used to determine if results were statistically significant, with data expressed as mean ± s.d.
RESULTS
Effect of claudin-11 siRNA on tight junction function and mRNA expression
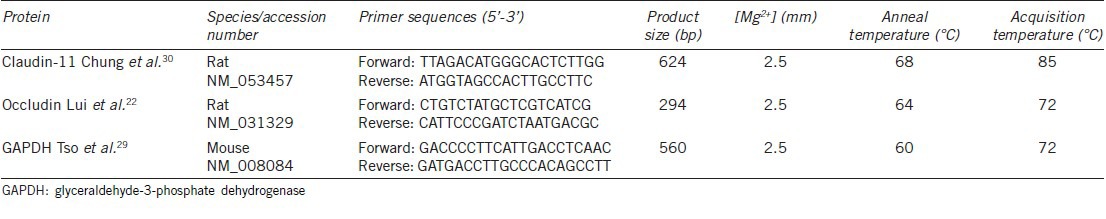
Figure 1a and 1b presents a representative result of one of our claudin-11 siRNA culture experiments. In the culture shown, TJ function increased as junctions formed from the day of cell plating to the day of claudin-11 siRNA addition (day 6) (Figure 1a). The drop in TER at day 4 is expected and is due to a hypotonic shock protocol at day 3 which removes contaminating germ cells from the culture.26 When Clau-11-A and Clau-11-B siRNA were added to Sertoli cells either separately or in combination, decreases of 47%, 76%, and 58% respectively (P < 0.01, n = triplicate wells/treatment) in TJ function over 2 days of treatment were observed (Figure 1a), with no statistical difference between the treatments. Two repeat cultures gave highly similar results (data not shown). The total decrease in TER in response to claudin-11 siRNA treatment over three cultures was 55% (P < 0.01). Medium plus transfection reagent and Lamin A/C siRNA had minor, non-significant effects on TER (Figure 1b), with a 22% decrease observed.
Figure 1.
Effect of claudin-11 siRNA on TJ function and mRNA expression. TJ function was assayed daily by transepithelial electrical resistance from the day of culturing immature rat Sertoli cells through to the cessation of culture (7–8 days later). Up until day 6 (panel a) or day 5 (panel b), all cells were left to form TJs without any treatment. At day 6 as indicated by the arrow (panel a), one group of cells was treated with medium plus transfection reagent (○), while the other three treatments received Clau-11-A siRNA (□), Clau-11-B siRNA (Δ) or both siRNA strands together (◊) for 2 days. In a separate culture at day 5 as indicated by an arrow (panel b), cells either received medium plus transfection reagent alone (○) or the Lamin A/C specific siRNA (X) for 2 days. Two days after siRNA had been transfected into cells exhibiting stable TJs as determined by TER, total RNA was extracted for RT-PCR analysis of claudin-11 (panels c and e) and occludin (panels d and f) expression. The treatment groups analyzed in panels c and d were the medium plus transfection reagent (○), Lamin A/C siRNA (X), Clau-11-A siRNA (□) and Clau-11-B siRNA (Δ). In the lower panels are medium plus transfection reagent (○) and the effects of combined claudin-11 siRNA strands (◊). Data are mean ± s.d., n = 3 wells/treatment and statistical analyses are comparisons between the day of siRNA addition and the cessation of culture. Note for RT-PCR these days are listed as days 0 and 2, respectively. ns: not significant; *P < 0.05, **P < 0.01.
Decreases in TER were coincident with decreases in claudin-11 mRNA expression (Figure 1c–1f). The top two panels as shown in Figure 1 (c and d) are for cells treated with Clau-11-A or Clau-11-B siRNA separately while the bottom two panels (e and f) are for cells treated with Clau-11-A/B siRNA combined. Over three cultures, the decreases in claudin-11 mRNA expression in response to the three siRNA treatments were not significantly different to each other, thereby giving a final decrease in claudin-11 mRNA expression of 71% (P < 0.01, n = triplicate wells/treatment, n = 3 cultures (Figure 1c and 1e)). In addition, it was observed that claudin-11 siRNA had no effect on TJ protein occludin mRNA expression (Figure 1d and 1f). A significant decrease (P < 0.01) which is observed when combining the two claudin-11 siRNA treatments (Figure 1f) was non-specific as the magnitude in decrease was comparable to that seen with the medium plus transfection reagent control. This seems to be due to some minor variability in the system (no decrease was seen in the control experiments depicted in the top panels of Figure 1). Note that the decrease in claudin-11 mRNA in cells treated with Clau-11-A/B combined was significantly (P < 0.01) greater than that seen for the medium plus transfection reagent (Figure 1e).
Effect of claudin-11 siRNA on protein localization
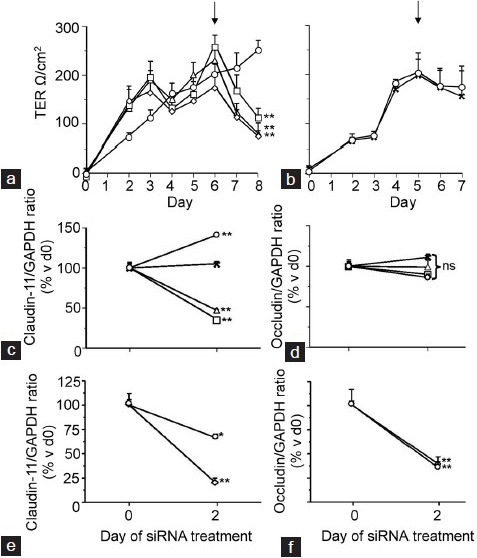
Sertoli cells were fixed 2 days after claudin-11 specific siRNA treatment for immunocytochemical analysis. Successful siRNA transfection was clearly indicated by the presence of fluorescent siRNA staining in Sertoli cell cytoplasms by microscopy (data not shown). Claudin-11 staining was extensive at inter-Sertoli cell junctions in cells treated with medium only, medium plus transfection reagent, and the Lamin A/C specific siRNA (Figure 2). Treatment with Clau-11-A or Clau-11-B siRNA either separately or in combination resulted in a marked reduction in claudin-11 localization at the TJ, with only punctate staining apparent (Figure 2).
Figure 2.
Effect of claudin-11 siRNA on claudin-11 localization to the TJ. Immature rat Sertoli cells were fixed in paraformaldehyde 2 days after claudin-11 siRNA treatment and analyzed by confocal microscopy. Claudin-11 (green) localization to the TJ around the periphery of Sertoli cells (nuclei in red) was analyzed in control cells which received medium alone (panel a), medium plus transfection reagent (panel b), Lamin A/C specific siRNA (panel c), as well as in cells which received Clau-11-A siRNA (panel d), Clau-11-B siRNA (panel e), or both siRNA strands in combination with each other (panel f). Bar = 50 μm. Inset = negative control for claudin-11.
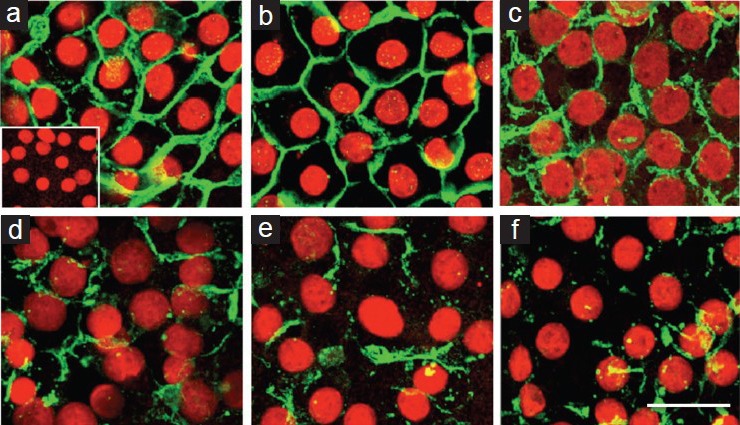
Occludin staining at the TJ and β-catenin staining at the nearby adherens junction were also extensive after treatment with medium plus transfection reagent (Figure 3). Staining was partially reduced after claudin-11 siRNA treatment for both proteins although not to the same extent as was observed for claudin-11 (Figure 3). Occludin protein (from two separate cultures) was detected at 64 kDa, and changes in expression were negligible and comparable over 2 days in both the control and Clau-11-A/B siRNA treated groups (Figure 3).
Figure 3.
Effect of claudin-11 siRNA on the localization of occludin to the TJ and β-catenin to the adherens junction, and occludin protein expression. Cultured immature rat Sertoli cells were fixed after 2 days of claudin-11 siRNA treatment. Immunocytochemical analysis was conducted on claudin-11 (green) localization around the periphery of Sertoli cells (nuclei in red) at the TJ in cells which received medium plus transfection reagent (panel a) or Clau-11-A/B siRNA treatment (panel b). Staining for occludin (green) at the Sertoli cell TJ was also conducted in medium plus transfection reagent (panel c) and Clau-11-A/B siRNA treated cells (panel d). To test for any effects of claudin-11 siRNA on other junctional types, β-catenin (green) of the adherens junction was also stained in medium plus transfection reagent (panel f) and siRNA (panel g) treated cells. Western blot analysis for occludin (panel e; arrow) was conducted on cell lysates on the day of Clau-11-A/B siRNA addition (indicated as d0) and after 2 days of treatment (indicated as d2). Note the panel on the left represents control extracts from the same time-point which did not receive siRNA. Molecular weight marker in kDa is provided to the left. For immunocytochemistry, bar = 50 μm. Inset = negative controls for claudin-11, occludin and β-catenin.
Effect of occludin and combined claudin-11/occludin siRNA on TJ function and structure
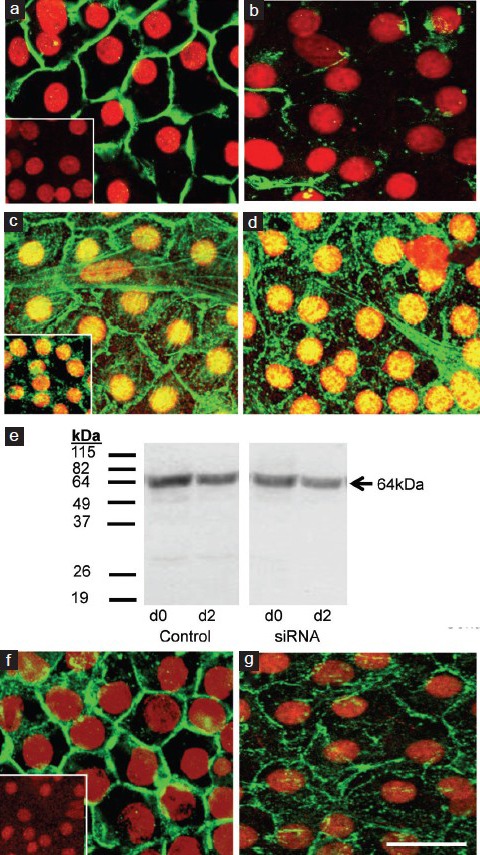
Occludin-specific Occ-A and Occ-B siRNA fragments were added separately or in combination with each other to cultured Sertoli cells with established TJs (Figure 4a). After 2 days of treatment, TJ function had decreased by 51% for all three occludin siRNA treatments (P < 0.01, n = 3 wells/treatment, n = 2 cultures). The effect of treating Sertoli cells for 2 days with combined Clau-11-B + Occ-B siRNA was a 62% (P < 0.01, n = 3 wells/treatment, n = 1 culture) decrease in TJ function (Figure 4b). This decrease was significant (P < 0.01) compared to the total 51% and 55% decrease in TJ function induced by occludin siRNA and claudin-11 siRNA (Figure 1) respectively over multiple cultures.
Figure 4.
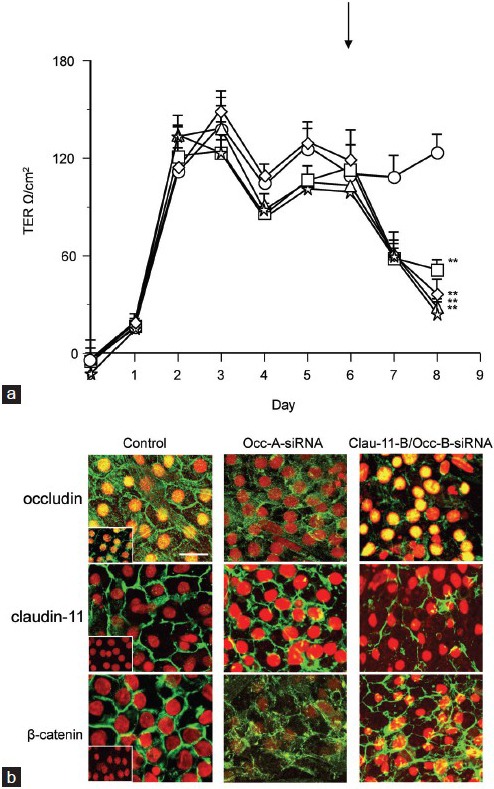
Effect of occludin ± claudin-11 siRNA on TJ function and protein localization. Immature rat Sertoli cells were cultured and allowed to develop TJs over 6 days as measured by transepithelial electrical resistance (panel a). Up until day 6, all cells within the culture were untreated. At day 6 as indicated by the arrow, one group of cells was treated with medium plus transfection reagent alone (○), while the other four treatments received Occ-A siRNA (□), Occ-B siRNA (Δ), both occludin siRNA strands together (◊) or a mixture of Clau-11-B and Occ-B siRNA (stars) for 2 days. At the end of the treatment period, cells were fixed for immunocytochemical analysis (panel b) for TJ proteins claudin-11 and occludin, as well as adherens junction protein β-catenin (all green). Sertoli cell nuclei are highlighted in red. TER data are mean ± s.d., n = triplicate wells/treatment. **P < 0.01 compared to day of treatment addition. For immunocytochemistry, bar = 50 μm. Inset = negative controls for occludin, claudin-11, and β-catenin.
Following siRNA treatment, cultured Sertoli cells were fixed for immunocytochemistry. Occludin siRNA reduced occludin localization to the TJ (Figure 4b) whereas claudin-11 staining remained extensive at the TJ and β-catenin at the adherens junction appeared slightly more punctate compared to the control, but still extensive compared to occludin (Figure 4b). The combination of claudin-11 and occludin siRNAs reduced the localization of both proteins to the TJ while β-catenin remained extensive at the adherens junction (Figure 4b).
DISCUSSION
We have quantitatively demonstrated in this study that the critical TJ transmembrane protein claudin-11 contributes significantly (P < 0.01) to Sertoli cell TJ function in vitro. This was achieved using siRNA-mediated gene silencing in an established culture model that utilizes immature (prepubertal) rat Sertoli cells, where the removal of contaminating germ cells leaves a culture of >90% Sertoli cell purity.25,26,31
Over the last decade, the number of known constituents of Sertoli cell TJs has increased, as has our understanding of the complexities behind TJ and BTB regulation (for a recent review, see Jiang et al.32). We chose to ablate claudin-11 specifically, as currently it is the only known component of the TJ barrier which when knocked out in an animal model results in infertility.16 A closer look at the molecular level in gonadotropin-suppressed rats, reveals claudin-11 redistribution away from the TJ.3 Similarly in hypogonadal mice that are congenitally infertile due to an absence of circulating gonadotropins, claudin-11 was located within the Sertoli cell cytoplasm and did not distribute to TJs until the introduction of FSH or an exogenous application of dihydrotestosterone.4 Aberrantly distributed claudin-11 in these models was associated with qualitatively dysfunctional Sertoli cell TJs as shown by permeation of the barrier by small molecular weight tracer biotin.3,4,6,33 While functional tests of the human Sertoli cell TJ in vivo with tracers is not currently feasible to our knowledge, claudin-11 staining was seen to be normal in gonadotropin suppressed men after 9 weeks despite the postmeiotic germ cell numbers reduced to 40% of control.34 The absence of anti-sperm antibodies suggested that the Sertoli cell TJ was functional. As mentioned earlier, however, men with non-gonadotropin derived testicular disorders do present with claudin-11 redistribution.19,20,21 Our culture model, while not being able to recapitulate the complex physiology and interactions seen in the in vivo situation,3,32 was sufficient to quantitatively confirm that claudin-11 is an essential contributor to Sertoli cell TJ function, and thus would be a useful model to apply to other TJ components.
Occludin is another integral transmembrane protein of the Sertoli cell TJ and like claudin-11, redistributes in the absence of circulating gonadotropins3,6 and appears to be associated with infertility, albeit late onset.24 Consistent with this is that intratesticular administration of a synthetic occludin peptide in rats results in reversible ablation of most germ cells from the seminiferous epithelium after 27 days.30 We showed that ablating occludin in vitro also led to a quantitatively significant decrease in TJ function. Decreases in TJ adhesiveness have also been observed when silencing occludin in keratinocytes.35 Interestingly, despite their apparent importance to Sertoli cell TJ competency, silencing both claudin-11 and occludin led to only a moderate further decrease in TJ function compared to silencing each gene's expression separately. This was not due to poor silencing, as shown by negligible changes in our controls compared with significantly decreased targeted mRNA expression and markedly reduced protein localization following siRNA treatment, but rather it indicates the importance that other Sertoli TJ protein constituents play in TJ function.
Other transmembrane constituents are members of the claudin and JAM families, as well as CAR. While the function of most claudins at the Sertoli cell TJ is not well understood, claudin-3 is transiently expressed at the critical moment preleptotene spermatocytes cross the TJ into the adluminal compartment in mice, before it is replaced by claudin-11.7,8,36 TJs become permeable to biotin tracer in murine knockouts of the transcription factor ets variant 5, which is critical for claudin-5 expression.37 Knockouts of either JAM-A or JAM-B are fertile, with the JAM-A knockout also being associated with histologically normal seminiferous tubules and normal sperm numbers but of reduced motility.38,39 siRNA technology may reveal quantitatively whether these genes are major contributors to Sertoli cell TJ function in vitro. Su et al.13 successfully applied siRNA-mediated silencing technology to ablate CAR from Sertoli cell TJs in vitro. The result was a significant increase in TJ permeability but knockouts are fertile.40 Tricellulin has only recently been detected at the Sertoli cell TJ11 where it may contribute to function;8 its silencing at the epididymal TJ resulted in decreased TER and expression of claudin-1, claudin-3, and occludin.41
Perhaps one of the most interesting findings to emerge from this study was the knock-on effects that ablating specific TJ components had on other TJ proteins, or other junctional types, namely β-catenin of the adherens junction. This is not a novel finding however, and simply provides further evidence of the tight integration between junctional complexes that make up the BTB. In the literature, silencing of CAR, connexin-43 of the gap junction, desmosomal components as well as cytoskeletal, actin, and microtubule regulators have all had knock-on effects on TJ proteins or components of other junctional types within the BTB.13,42,43,44,45,46,47,48,49 Thus, siRNA technology is not only useful for identifying potential protein-protein interactions but also complex-complex interactions. It also provides an alternative to previous studies which have used phthalates to assess claudin-11 and occludin in cultured Sertoli cells.50,51 Phthalates are toxicants which may impact upon other Sertoli cell or bodily functions. It is encouraging however that by using siRNA, our conclusions on the importance of these proteins are the same as the phthalate studies, thereby further validating our model.
CONCLUSION
We have quantitatively demonstrated that claudin-11 is a major contributor to Sertoli cell TJ function in vitro. We have extended this technology to the ablation of occludin and found that this protein too is a major contributor. The results are in line with published data that show that these proteins are critical for TJ function in vivo. This is a useful tool for identifying for further study (i) potentially critical components of the complex BTB and (ii) identifying complex interactions within the BTB; a barrier which is involved in the onset and maintenance of successful spermatogenesis and therefore fertility.
AUTHORS’ CONTRIBUTIONS
This work was conducted in the Male Fertility Regulation Laboratory led by P.G.S., who with P.M.S. supervised the project and provided assistance with its design and also reviewed this manuscript several times prior to submission. M.J.M. produced the study design with P.G.S. M.J.M. also produced this manuscript with guidance from M.E.D., who also provided experimental suggestions. C.F.H.F. provided expert guidance with respect to all techniques used herein.
All authors have read and approved the final version of this manuscript and agree with the order of presentation of the authors.
CONFLICT OF INTEREST
None of the authors have any conflicts to disclose.
ACKNOWLEDGEMENTS
This work was funded by National Health and Medical Research Council (Australia) Program Grants 241000 and 494802.
REFERENCES
- 1.Setchell BP. Blood-testis barrier, junctional and transport proteins and spermatogenesis. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX, USA: Landed Bioscience; 2008. p. 202. [DOI] [PubMed] [Google Scholar]
- 2.de Kretser DM, Burger H. Ultrastructural studies of the human Sertoli cell in normal men and males with hypogonadotrophic hypogonadism before and after gonadotrophic treatment. In: Saxena GG, Beling HM, editors. Gonadotrophins. New York, USA: Wiley-Interscience; 1972. p. 640. [Google Scholar]
- 3.McCabe MJ, Tarulli GA, Meachem SJ, Robertson DM, Smooker PM, et al. Gonadotrophins regulate rat testicular tight junctions in vivo. Endocrinology. 2010;151:2911–22. doi: 10.1210/en.2009-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCabe MJ, Allan CM, Foo CF, Nicholls PK, McTavish KJ, et al. Androgen initiates Sertoli cell tight junction formation in the hypogonadal (hpg) mouse. Biol Reprod. 2012;87:1–8. doi: 10.1095/biolreprod.111.094318. [DOI] [PubMed] [Google Scholar]
- 5.Tarulli GA, Stanton PG, Lerchl A, Meachem SJ. Adult Sertoli cells are not terminally differentiated in the Djungarian hamster: effect of FSH on proliferation and junction protein organization. Biol Reprod. 2006;74:798–806. doi: 10.1095/biolreprod.105.050450. [DOI] [PubMed] [Google Scholar]
- 6.Tarulli GA, Meachem SJ, Schlatt S, Stanton PG. Regulation of testicular tight junctions by gonadotrophins in the adult Djungarian hamster in vivo. Reproduction. 2008;135:867–77. doi: 10.1530/REP-07-0572. [DOI] [PubMed] [Google Scholar]
- 7.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005;102:16696–700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith BE, Braun RE. Germ cell migration across Sertoli cell tight junctions. Science. 2012;338:798–802. doi: 10.1126/science.1219969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 10.McMillan M, Andronicos N, Davey R, Stockwell S, Hinch G, et al. Claudin-8 expression in Sertoli cells and putative spermatogonial stem cells in bovine testis. Reprod Fertil Dev. 2014;26:633–44. doi: 10.1071/RD12259. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty P, William Buaas F, Sharma M, Smith BE, Greenlee AR, et al. Androgen-dependent Sertoli cell tight junction remodeling is mediated by multiple tight junction components. Mol Endocrinol. 2014;28:1055–72. doi: 10.1210/me.2013-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glick G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–4. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- 13.Su L, Mruk DD, Cheng CY. Regulation of the blood-testis barrier by coxsackievirus and adenovirus receptor. Am J Physiol Cell Physiol. 2012;303:C843–53. doi: 10.1152/ajpcell.00218.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Inazawa J, et al. Mammalian occludin in epithelial cells: its expression and subcellular distribution. Eur J Cell Biol. 1997;73:222–31. [PubMed] [Google Scholar]
- 15.Morita K, Itoh M, Saitou M, Ando-Akatsuka Y, Furuse M, et al. Subcellular distribution of tight junction-associated proteins (occludin, ZO-1, ZO-2) in rodent skin. J Invest Dermatol. 1998;110:862–6. doi: 10.1046/j.1523-1747.1998.00209.x. [DOI] [PubMed] [Google Scholar]
- 16.Mazaud-Guittot S, Meugnier E, Presenti S, Wu X, Vidal H, et al. Claudin 11 deficiency in mice results in loss of the Sertoli cell epithelial phenotype in the testis. Biol Reprod. 2010;82:2012–3. doi: 10.1095/biolreprod.109.078907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M, Hao J, Yang Q, Li G. Effects of icariin on reproductive functions in male rats. Molecules. 2014;19:9502–14. doi: 10.3390/molecules19079502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park CJ, Ha CM, Lee JE, Gye MC. Claudin-11 inter-Sertoli tight junctions in the testis of the Korean soft-shelled turtle (Pelodiscus maackii) Biol Reprod. 2015;92:96–108. doi: 10.1095/biolreprod.114.117804. [DOI] [PubMed] [Google Scholar]
- 19.Fink C, Weigel R, Fink L, Wilhelm J, Kliesch S, et al. Claudin-11 is over-expressed and dislocated from the blood-testis barrier in Sertoli cells associated with testicular intraepithelial neoplasia in men. Histochem Cell Biol. 2009;131:755–64. doi: 10.1007/s00418-009-0576-2. [DOI] [PubMed] [Google Scholar]
- 20.Nah WH, Lee JE, Park HJ, Park NC, Gye MC. Claudin-11 expression increased in spermatogenic defect in human testes. Fertil Steril. 2011;95:385–8. doi: 10.1016/j.fertnstert.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 21.Haverfield JT, Meachem SJ, O’Bryan MK, McLachlan RI, Stanton PG. Claudin-11 and connexin-43 display altered spatial patterns of organization in men with primary seminiferous tubule failure compared with controls. Fertil Steril. 2013;100:658–66. doi: 10.1016/j.fertnstert.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 22.Lui WY, Lee WM, Cheng CY. Transforming growth factor-beta3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–77. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- 23.Nicholls PK, Harrison CA, Gilchrist RB, Farnworth PG, Stanton PG. Growth differentiation factor 9 is a germ cell regulator of Sertoli cell function. Endocrinology. 2009;150:2481–90. doi: 10.1210/en.2008-1048. [DOI] [PubMed] [Google Scholar]
- 24.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–42. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perryman KJ, Stanton PG, Loveland KL, McLachlan RI, Robertson DM. Hormonal dependency of neural cadherin in the binding of round spermatids to Sertoli cells in vitro. Endocrinology. 1996;137:3877–83. doi: 10.1210/endo.137.9.8756560. [DOI] [PubMed] [Google Scholar]
- 26.Kaitu’u-Lino TJ, Sluka P, Foo CFH, Stanton PG. Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction. 2007;133:1169–79. doi: 10.1530/REP-06-0385. [DOI] [PubMed] [Google Scholar]
- 27.Galdieri M, Zani BM, Monaco L, Ziparo E, Stefanini M. Changes of Sertoli cell glycoproteins induced by removal of the associated germ cells. Exp Cell Res. 1983;145:191–8. doi: 10.1016/s0014-4827(83)80020-2. [DOI] [PubMed] [Google Scholar]
- 28.Yuan B, Latek R, Hossbach M, Tuschl T, Lewitter F. siRNA Selection Server: an automated siRNA oligonucleotide prediction server. Nucleic Acids Res. 2004;32:W130–4. doi: 10.1093/nar/gkh366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tso JY, Sun XH, Kao TH, Reece KS, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985;13:2485–502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung NP, Mruk D, Mo MY, Lee WM, Cheng CY. A 22-amino acid synthetic peptide corresponding to the second extracellular loop of rat occludin perturbs the blood-testis barrier and disrupts spermatogenesis reversibly in vivo. Biol Reprod. 2001;65:1340–51. doi: 10.1095/biolreprod65.5.1340. [DOI] [PubMed] [Google Scholar]
- 31.Chung SS, Zhu LJ, Mo MY, Silvestrini B, Lee WM, et al. Evidence for cross-talk between Sertoli and germ cells using selected cathepsins as markers. J Androl. 1998;19:686–703. [PubMed] [Google Scholar]
- 32.Jiang XH, Bukhari I, Zheng W, Yin S, Wang Z, et al. Blood-testis barrier and spermatogenesis: lessons from genetically-modified mice. Asian J Androl. 2014;16:572–80. doi: 10.4103/1008-682X.125401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haverfield JT, Meachem SJ, Nicholls PK, Rainczuk KE, Simpson ER, et al. Differential permeability of the blood-testis barrier during reinitiation of spermatogenesis in adult male rats. Endocrinology. 2014;155:1131–44. doi: 10.1210/en.2013-1878. [DOI] [PubMed] [Google Scholar]
- 34.Ilani N, Armanious N, Lue YH, Swerdloff RS, Baravarian S, et al. Integrity of the blood-testis barrier in healthy men after suppression of spermatogenesis with testosterone and levonorgestrel. Hum Reprod. 2012;27:3403–11. doi: 10.1093/humrep/des340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rachow S, Zorn-Kruppa M, Ohnemus U, Kirschner N, Vidal-y-Sy S, et al. Occludin is involved in adhesion, apoptosis, differentiation and Ca2+-homeostasis of human keratinocytes: implications for tumorigenesis. PLoS One. 2013;8:e55116–28. doi: 10.1371/journal.pone.0055116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lie PP, Cheng CY, Mruk DD. Signalling pathways regulating the blood-testis barrier. Int J Biochem Cell Biol. 2013;45:621–5. doi: 10.1016/j.biocel.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrow CM, Tyagi G, Simon L, Carnes K, Murphy KM, et al. Claudin 5 expression in mouse seminiferous epithelium is dependent upon the transcription factor ets variant 5 and contributes to blood-testis barrier function. Biol Reprod. 2009;81:871–9. doi: 10.1095/biolreprod.109.077040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakaguchi T, Nishimoto M, Miyagi S, Iwama A, Morita Y, et al. Putative “stemness” gene jam-B is not required for maintenance of stem cell state in embryonic, neural, or hematopoietic stem cells. Mol Cell Biol. 2006;26:6557–70. doi: 10.1128/MCB.00729-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao M, Ghosh A, Cooke VG, Naik UP, Martin-DeLeon PA. JAM-A is present in mammalian spermatozoa where it is essential for normal motility. Dev Biol. 2008;313:246–55. doi: 10.1016/j.ydbio.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sultana T, Hou M, Stukenborg JB, Töhönen V, Inzunza J, et al. Mice depleted of the coxsackievirus and adenovirus receptor display normal spermatogenesis and an intact blood-testis barrier. Reproduction. 2014;147:875–3. doi: 10.1530/REP-13-0653. [DOI] [PubMed] [Google Scholar]
- 41.Mandon M, Cyr DG. Tricellulin and its role in the epididymal epithelium of the rat. Biol Reprod. 2015;92:66–76. doi: 10.1095/biolreprod.114.120824. [DOI] [PubMed] [Google Scholar]
- 42.Carette D, Weider K, Gilleron J, Giese S, Dompierre J, et al. Major involvement of connexin 43 in seminiferous epithelial junction dynamics and male fertility. Dev Biol. 2010;346:54–67. doi: 10.1016/j.ydbio.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 43.Lie PP, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2010;42:975–86. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mok KW, Mruk DD, Silvestrini B, Cheng CY. RpS6 regulates blood-testis barrier dynamics by affecting F-actin organization and protein recruitment. Endocrinology. 2012;153:5036–48. doi: 10.1210/en.2012-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su W, Wong EW, Mruk DD, Cheng CY. The Scribble/Lgl/Dlg polarity protein complex is a regulator of blood-testis barrier dynamics and spermatid polarity during spermatogenesis. Endocrinology. 2012;153:6041–53. doi: 10.1210/en.2012-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerber J, Weider K, Hambruch N, Brehm R. Loss of connexin43 (Cx43) in Sertoli cells leads to spatio-temporal alterations in occludin expression. Histol Histopathol. 2014;29:935–48. doi: 10.14670/HH-29.935. [DOI] [PubMed] [Google Scholar]
- 47.Gungor-Ordueri NE, Celik-Ozenci C, Cheng CY. Fascin 1 is an actin filament-bundling protein that regulates ectoplasmic specialization dynamics in the rat testis. Am J Physiol Endocrinol Metab. 2014;307:E738–53. doi: 10.1152/ajpendo.00113.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gungor-Ordueri NE, Tang EI, Celik-Ozenci C, Cheng CY. Ezrin is an actin binding protein that regulates Sertoli cell and spermatid adhesion during spermatogenesis. Endocrinology. 2014;155:3981–95. doi: 10.1210/en.2014-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang EI, Mok KW, Lee WM, Cheng CY. EB1 regulates tubulin and actin cytoskeletal networks at the Sertoli cell blood-testis-barrier in male rats: an in vitro study. Endocrinology. 2015;156:680–93. doi: 10.1210/en.2014-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang YH, Lin L, Liu ZW, Jiang XZ, Chen BH. Disruption effects of monophthalate exposures on inter-Sertoli tight junction in a two-compartment culture model. Environ Toxicol. 2008;23:302–8. doi: 10.1002/tox.20343. [DOI] [PubMed] [Google Scholar]
- 51.Chiba K, Kondo Y, Yamaguchi K, Miyake H, Fujisawa M. Inhibition of claudin-11 and occludin expression in rat Sertoli cells by mono-(2-ethylhexyl) phthalate through p44/42 mitogen-activated protein kinase pathway. J Androl. 2012;33:368–74. doi: 10.2164/jandrol.111.013664. [DOI] [PubMed] [Google Scholar]


