Abstract
The [-2]proPSA (p2PSA) and its derivatives, the p2PSA-to-free PSA ratio (%p2PSA), and the Prostate Health Index (PHI) have greatly improved discrimination between men with and without prostate cancer (PCa) in prostate biopsies. However, little is known about their performance in cases where a digital rectal examination (DRE) and transrectal ultrasonography (TRUS) are negative. A prospective cohort of 261 consecutive patients in China with negative DRE and TRUS were recruited and underwent prostate biopsies. A serum sample had collected before the biopsy was used to measure various PSA derivatives, including total prostate-specific antigen (tPSA), free PSA, and p2PSA. For each patient, the free-to-total PSA ratio (%fPSA), PSA density (PSAD), p2PSA-to-free PSA ratio (%p2PSA), and PHI were calculated. Discriminative performance was assessed using the area under the receiver operating characteristic curve (AUC) and the biopsy rate at 91% sensitivity. The AUC scores within the entire cohort with respect to age, tPSA, %fPSA, PSAD, p2PSA, %p2PSA, and PHI were 0.598, 0.751, 0.646, 0.789, 0.814, 0.808, and 0.853, respectively. PHI was the best predictor of prostate biopsy results, especially in patients with a tPSA of 10.1–20 ng ml−1. Compared with other markers, at a sensitivity of 91%, PHI was the most useful for determining which men did not need to undergo biopsy, thereby avoiding unnecessary procedures. The use of PHI could improve the accuracy of PCa detection by predicting prostate biopsy outcomes among men with a negative DRE and TRUS in China.
Keywords: [-2]proPSA, prostate cancer, Prostate Health Index, prostate-specific antigen, receiver operating curve
INTRODUCTION
Prostate cancer (PCa) is one of the most common solid neoplasms and the second leading cause of death due to cancer among men in both the United States and Europe.1,2 In China, the incidence of PCa was low historically but has increased substantially in the last two decades. In urban Shanghai, the estimated age-standardized incidence rate (ASIR) of PCa increased from 2.3 per 100 000 during 1988–1992 to 6.9 per 100 000 during 1998–2002.3
Currently, the prostate-specific antigen (PSA) assay is widely used for the early detection of PCa and its increased use in China in recent years has indicated to an increased incidence of PCa. However, the increase in PSA screening has also led to an increase in the diagnosis of clinically insignificant tumors (over-diagnosis) and their treatment (over-treatment).4 This is primarily due to the fact that total PSA (tPSA) is not PCa-specific; noncancerous conditions such as benign prostatic hyperplasia (BPH) and prostatitis may also lead to elevated tPSA levels. Several novel serum biomarkers, including [-2]proPSA (p2PSA) and its derivatives, the p2PSA-to-free PSA ratio (%p2PSA), and Prostate Health Index (PHI), have been developed and have significantly increased the possibility of detecting PCa, especially at an initial biopsy.5 The secretion of p2PSA appears to be more specific to tumor cells, and the combination of this PSA isoform with tPSA and free PSA (fPSA) to yield PHI has greatly improved the possibility of discriminating between men with and without PCa.6,7,8 In 2012, the Food and Drug Administration approved PHI for use in men with serum PSA values of 4–10 ng ml−1. PHI has also been recommended in the National Comprehensive Cancer Network (NCCN) as a blood test to improve the specificity of PCa detection in its updated Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Prostate Cancer Early Detection.9
A prostate biopsy is routinely recommended following a suspicious digital rectal examination (DRE) or transrectal ultrasonography (TRUS) regardless of PSA levels.10,11 However, clinical guidelines are unclear regarding the next step for men with negative DRE and TRUS results but with an elevated PSA level. Biopsy may also be recommended for men with a PSA of 2.5–4.0 ng ml−1.12,13 However, this may lead to unnecessary biopsies and possibility over detection of some cancers.13,14
To investigate this issue, we tested the hypothesis that novel PSA markers (p2PSA, %p2PSA, and PHI), particularly PHI, are more accurate than age, tPSA, and free-to-total PSA ratio (%fPSA) at detecting prostate cancer. This was done in a prospective trial, using consecutively diagnosed patients with a negative DRE and TRUS who underwent prostate biopsy in China. Clear conclusions regarding this hypothesis may result in the avoidance of unnecessary biopsies.
MATERIALS AND METHODS
Patients
This study included 638 consecutive patients who underwent prostate biopsies between April 2012 and August 2013 for the detection of PCa using the current standard of care at three tertiary hospitals in Shanghai, China. Typical indications for prostate biopsy at these three hospitals were: (1) tPSA >4.0 ng ml−1; (2) %fPSA ratio <0.16; (3) PSAD >0.15; or (4) presence of prostate nodules detected by DRE or ultrasound. The TRUS-guided biopsies were performed using a 10-core scheme. All biopsy specimens were reviewed in the Pathology Department of the respective hospital. Before the biopsy, demographic and clinical variables were collected, and PSA was measured. After excluding all patients with a positive DRE or TRUS, the study included 261 patients with a negative DRE and TRUS. The primary outcome was a diagnosis of PCa based on a biopsy. The secondary outcome was a diagnosis of high-grade PCa (Gleason score 4 + 3 and ≥8).
Specimens and laboratory analysis
Blood samples collected from consenting patients were stored immediately at 4°C and then centrifuged and refrigerated within 2 h collection. The serum was frozen at −70°C for future analysis. For each patient, the serum p2PSA, tPSA, and fPSA were measured centrally using the Beckman Coulter DxI 800 Immunoassay System. PSAD was calculated by dividing the serum tPSA level by the prostate volume (PV) as determined by TRUS during the biopsy. The %fPSA and %p2PSA were calculated. The Beckman Coulter PHI was determined using the formula PHI = ([-2]proPSA/free PSA) × sqrt(PSA).
Statistical methods
Two types of biopsy outcome were tested in the study: PCa versus non-PCa and high-grade PCa versus everything else. The differences between the two types of biopsy outcome with respect to age, PV, tPSA, %fPSA, PSAD, p2PSA, %p2PSA, and PHI were assessed using the Student's t-test for normal data and the Wilcoxon rank sum test for skewed data. Due to nonnormal distributions of age, PV, tPSA, %fPSA, PSAD, p2PSA, %p2PSA, and PHI, these variables were log-transformed before any statistical analysis. The areas under the receiver operating characteristic (ROC) curves (AUC) of different variables (age, PV, tPSA, %fPSA, PSAD, p2PSA, %p2PSA, and PHI) were calculated in univariate regression analyses. AUC of PHI was compared separately with the AUC of age, tPSA, and %fPSA and multivariate analysis was used to assess the value of PHI in the diagnosis of PCa. The sensitivity and specificity of each variable were calculated to assess the diagnostic performance of the various assays in terms of PCa detection. All descriptive statistics and comparisons were performed using Stata/SE 13.0 software (StataCorp., College Station, TX, USA). A two-sided P < 0.05 was considered statistically significant in all of the analyses.
Ethics
Due to the fact that the China Food and Drug Administration has not approved p2PSA and PHI, this study was performed as a research project. Serum p2PSA was measured but not used for clinical decisions. The written informed consent was obtained from each patient. This study was approved by the Institutional Review Board at each hospital.
RESULTS
Baseline characteristics
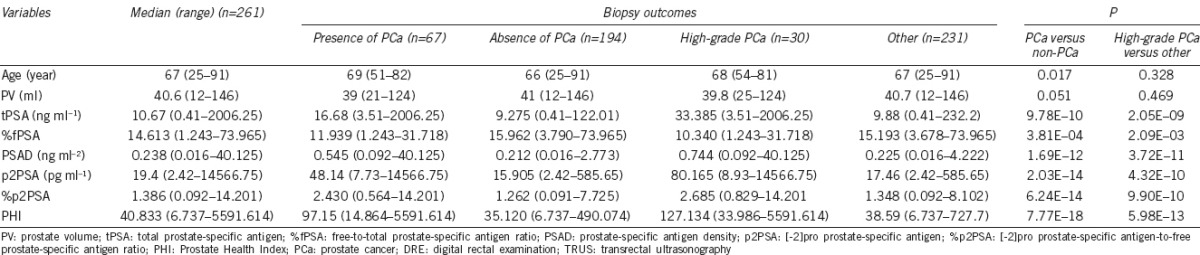
Among 261 patients with a negative DRE and TRUS who underwent prostate biopsy, 67 (26.05%) patients were diagnosed with PCa and 30 (11.49%) were diagnosed with high-grade PCa. Table 1 summarizes the clinical parameters and novel PSA markers for patients according to biopsy outcomes (PCa vs non-PCa and high-grade PCa vs everything else) for the entire cohort. Patients with PCa had a significantly higher median age (P = 0.017), tPSA (P = 9.78E-10), PSAD (P = 1.69E-12), p2PSA (P = 2.03E-14), %p2PSA (P = 6.24E-14), and PHI (P = 7.77E-18), and a lower median %fPSA (P = 3.81E-04) than non-PCa patients. Patients with high-grade PCa had a significantly higher median tPSA (P = 2.05E-09), PSAD (P = 3.72E-11), p2PSA (P = 4.32E-10), %p2PSA (P = 9.90E-10), and PHI (P = 5.98E-13), and a lower median %fPSA (P = 2.09E-03) than everyone else.
Table 1.
Characteristics of patients in the biopsy cohort in China when the results of DRE and TRUS tests are negative
ROC curves analysis
Data regarding the predictiveness of existing clinical variables and novel PSA markers for predicting PCa, measured using the AUC, are presented in Table 2 and Figure 1. Within the entire cohort, as well as within subsets of patients with tPSA at 10.1–20 ng ml−1 and tPSA >20 ng ml−1, AUC scores were consistently highest for PHI, which performed the best in terms of predicting the results of the initial prostate biopsy in our population. The difference in AUC scores between PHI and age was 0.255 (P = 5.36E-07) within the entire cohort. This difference was larger in the higher PSA level subset analysis: 0.269 (P = 0.006) in patients with tPSA at 10.1–20 ng ml−1 and 0.357 (P = 1.00E-04) in patients with tPSA at >20 ng ml−1. The difference in AUC scores between PHI and tPSA was 0.102 (P = 0.001) for the entire cohort. This difference was larger in the higher PSA level subset analysis: 0.234 (P = 0.003) in patients with tPSA at 10.1–20 ng ml−1 and 0.119 (P = 0.013) in patients with tPSA >20 ng ml−1. The difference in AUC scores between the PHI and %fPSA was 0.207 (P = 1.92E-08) for the entire cohort. This difference was similar to the one obtained in the higher PSA level subset analysis: 0.199 (P = 0.005) in patients with tPSA at 10.1–20 ng ml−1 and 0.210 (P = 0.009) in patients with tPSA >20 ng ml−1. Multivariate analysis was used to assess the value of PHI in the diagnosis of PCa. Age, tPSA, and %fPSA were entered into the multivariate analysis as the base prediction model (Table 3 and Supplementary Table 1 (605.1KB, tif) ). When PHI was added to the base model, the difference in AUC between the base model and base model + PHI was 0.069 (P = 1.25E-04) for the entire cohort. This difference was larger for the subset analysis of tPSA at 10.1–20 ng ml−1 (0.137 [P = 0.003]) and similar to the difference observed in the tPSA >20 ng ml−1 level subset analysis (0.051 [P = 0.047]).
Table 2.
Performance of PSA measurements and other clinical variables for predicting PCa when the DRE and TRUS are negative
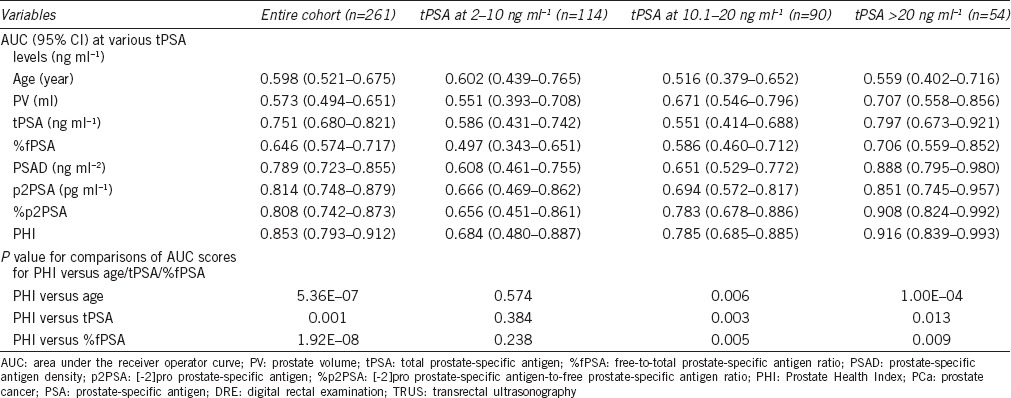
Figure 1.
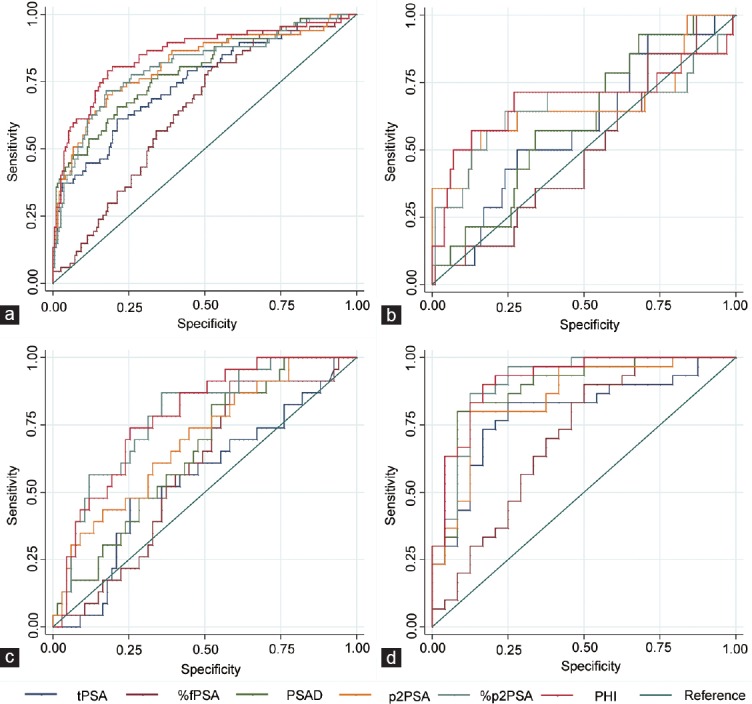
Receiver operating characteristic (ROC) curves of the various prostate-specific antigen (PSA) derivatives. (a) In the entire cohort (n = 261); (b) in patients with tPSA at 2–10 ng ml−1 (n = 114); (c) in patients with tPSA at 10.1–20 ng ml−1 (n = 90); (d) in patients with tPSA > 20 ng ml−1 (n = 54). tPSA: total PSA; %fPSA: free-to-total PSA ratio; PSAD: prostate-specific antigen density; p2PSA: [-2]proPSA; %p2PSA: p2PSA-to-free PSA ratio; PHI: Prostate Health Index.
Table 3.
The performance of two different multivariable models for predicting PCa with a negative DRE and TRUS
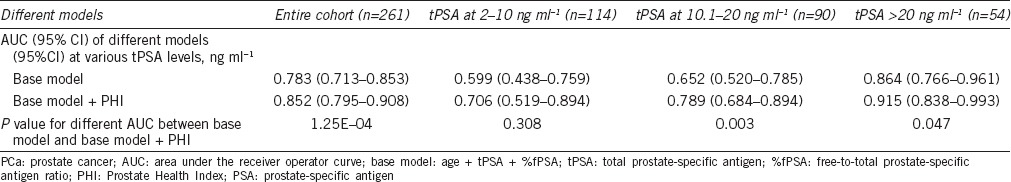
Univariate and Multivariate analyses of the performance of the PHI and other clinical variables with respect to predicting PCa with a negative DRE and TRUS
With respect to predicting high-grade PCa, the results of the AUC analyses generally supported the superior performance of the PHI at discriminating high-grade PCa from everything else in the entire cohort. The AUC score of the PHI was the highest when it came to discriminating high-grade PCa from other cases (Supplementary Table 2). In the multivariate analyses, when PHI was added to the base model (age + tPSA + %fPSA), the difference in AUC scores between the base model and base model + PHI was 0.025 (P = 0.034) for the entire cohort (Supplementary Table 2 (514.1KB, tif) ).
Performance of PSA measurements and other clinical variables with respect to predicting high-grade PCa when the DRE and TRUS are negative
Clinical significance of guiding biopsy using various tumor markers
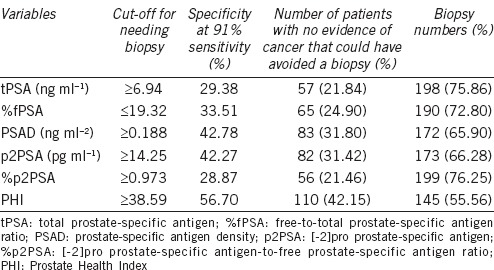
To further assess the performance of the various parameters, we set the sensitivity at 91%, which eliminated 6 of the 67 cancer cases. At this level, the cut-off values for variables associated with the need for biopsy are shown in Table 4. The cut-offs were tPSA ≥6.94 ng ml−1, %fPSA ≤19.32, PSAD ≥0.188 ng ml−2, p2PSA ≥14.25 pg ml−1, %p2PSA ≥0.973, and PHI ≥38.59. At this same sensitivity level, PHI had the highest specificity of 56.70% (Table 4), while the specificities at this level for tPSA, %fPSA, PSAD, p2PSA, and %p2PSA were 29.38%, 33.51%, 42.78%, 42.27%, and 28.87%, respectively. If we applied PHI to the cohort during the initial assessment, 110 (42.15%) patients with no evidence of PCa would avoid undergoing a biopsy. The numbers of biopsies that would have been avoided using tPSA, %fPSA, PSAD, p2PSA, and %p2PSA were 57 (21.84%), 65 (24.90%), 83 (31.80%), 82 (31.42%), and 56 (21.46%), respectively (Table 4).
Table 4.
Performance characteristics in patients with a negative digital rectal examination and transrectal ultrasonography at a preset sensitivity of 91%
DISCUSSION
The low accuracy of opportunistic PSA-based screening has led to the development of more specific plasma-based biomarkers, including p2PSA, %p2PSA, and PHI, which have been widely described as improving the detection of PCa compared to classic PSA testing.5,6,7,8,15,16,17,18,19,20 PHI is a new formula that combines tPSA, fPSA, and p2PSA into a single score that can be used to aid clinical decision-making.21 PHI is calculated as ([-2]proPSA/free PSA) × sprt(PSA). Intuitively, this formula is logical, in that men with a higher tPSA and p2PSA and with a lower fPSA are more likely to have clinically significant prostate cancer.
In addition to PHI, prostate cancer antigen 3 (PCA3) has also been assessed as a potential new screening marker,22,23 and several groups have compared the performance of PHI with PCA3 leading up to a prostate biopsy. For example, one study reported a head-to-head comparison between PHI and PCA3 in European men undergoing initial or repeat biopsies. Overall, PHI had a higher AUC (0.70) than either PCA3 (0.59) or %fPSA (0.60).24 Another recent study also compared PHI with PCA3 and found that the PHI outperformed PCA3 for predicting clinically significant prostate cancer.25
One rationale for this study design was the fact that there are considerable differences in the characteristics of PCa between patients from China and Western countries. The PSA “gray zone” is 4–10 ng ml−1 in Western countries where the detection rate of PCa is ~34% among these patients,26 while evidence from Chinese studies8,27,28 suggests that the PSA “gray zone” in China is 10–20 ng ml−1. The reported PCa detection rate among patients with a tPSA of 10.1–20 ng ml−1 in Chinese studies is 29.8%–36.5%.8,29 Hence, the performance of PHI with respect to discriminating biopsy outcomes among men with a negative DRE and TRUS at a tPSA of 10.1–20 ng ml−1 is important for the Chinese population. In this study, we found that among men undergoing their first prostate biopsy with a negative DRE and TRUS, PHI was a significant predictor of PCa for the entire cohort. PHI also performs better than tPSA and other PSA markers in discriminating which men will be diagnosed with clinically significant PCa. More specifically, the accuracy of PHI over tPSA at discriminating biopsy outcomes among men with a negative DRE and TRUS was greater in men with higher PSA levels, especially in the subset with tPSA at 10.1–20 ng ml−1. The AUC score was 0.785 for the PHI and 0.551 for tPSA in patients with tPSA of 10.1–20 ng ml−1; it was 0.916 for the PHI and 0.797 for tPSA in patients with tPSA >20 ng ml−1. The superior performance of the PHI in a sample of men with a negative DRE and TRUS with tPSA >10 ng ml−1 may have important clinical implications for Chinese men.
From the perspective of translational medicine, a PHI-based strategy may considerably reduce the number of unnecessary biopsies compared to other markers, while maintaining the same PCa detection rate among men with a negative DRE and TRUS. At a sensitivity level of 91%, which eliminated 6 of the 67 cancer cases, the cut-off of PHI with regard to the need for a biopsy was ≥38.59. Of the cases eliminated from the analysis, five patients involved T1c disease, four had Gleason scores of 3 + 3, and one had a Gleason score of 3 + 4. These are considered low-risk cases.17,30 Nevertheless, this level eliminated one higher-grade cancer patient with a Gleason score of 4 + 3 and a tPSA of 16.8 ng ml−1. At this level, the cut-off of tPSA with regard to the need for a biopsy was ≥6.94 ng ml−1, therefore a combination of the PHI with tPSA would prevent the elimination of this case. PHI had the best specificity (56.70%) compared with tPSA and other variables. To detect 91% of all PCa in the cohort, we would need to biopsy 198 (75.86%) patients with higher tPSA values (≥6.94 ng ml−1), or only 145 (55.56%) patients with higher PHI values (≥38.59), a 20.31% reduction in the number of biopsies. Similar results were found when comparing the PHI with %fPSA, PSAD, p2PSA, and %p2PSA.
Our results are slightly different from those observed in a retrospective study of 230 Asian men with tPSA levels of 4–10 ng ml−1, which used >26.54 as the PHI cut-off with regard to the need for a biopsy at 90% sensitivity but found the specificity to be 49.76.17 These discrepant results are likely to be due to differences between the study population. However, both of these Asian studies are consistent with previous international studies that specify a cut-off for the PHI with regard to the need for a biopsy ranging from 22.8 to 48.5 at 90% sensitivity.6,17,20,31,32,33
With respect to predicting the probability of higher-grade cancers, our results support the superior performance of the PHI in discriminating high-grade PCa from everything else in the entire cohort. The AUC score of PHI was higher than that of all the other variables with respect to discriminating high-grade PCa from everything else. As the overall sample size was small, there were only 30 high-grade cancers. This is one limitation in our study that might be overcome by a larger sample cohort study. Besides the relatively small cohort, the inclusion of only Chinese men may affect its external validity. Therefore, our results should be interpreted with caution, and investigations of other Chinese and non-Chinese study populations are warranted before applying our conclusions to clinical practice. Nevertheless, with well-documented cases and no missing data, the data collection proved to be reliable.
The introduction of PSA as a screening tool has contributed to the early detection of prostate cancer. However, it has also resulted in over-diagnosis and over-treatment. Promising novel biomarkers are in development. Our findings indicate that PHI may help in screening for PCa among men undergoing their first prostate biopsy with a negative DRE and TRUS, thereby avoiding over-diagnosis and over-treatment, especially in patients with a tPSA of 10.1–20 ng ml−1, which is considered to be the Chinese PSA “gray zone.” Additional multi-center studies in China with a larger sample size are needed before the PHI can be used in clinical practice.
AUTHOR CONTRIBUTIONS
GPY, RN, DWY, YHS, QD, and JFX designed the study. GPY, RN, and FL carried out the experiments. GPY, RN, DWY, JQ, FL, YSW, GMZ, YZ, and LQH participated in acquisition of data. GPY, RN, HTC, JLS, and SCR analyzed the experimental data, and then drafted the article. DKJ, SLZ, HWJ, YHS, QD, and JFX revised the paper. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declared no competing interests.
ACKNOWLEDGMENTS
We would like to thank all the study participants, urologists, and study coordinators for participating in the study. This work was partially funded by the National Key Basic Research Program Grant 973 (2012CB518301), the Key Project of the National Natural Science Foundation of China (81130047), National Natural Science Foundation of China (81202001, 81272835), China Scholarship Council (CSC), intramural grants from Fudan University and Huashan Hospital, and a research grant from Beckman Coulter, Inc.
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Cullen J, Elsamanoudi S, Brassell SA, Chen Y, Colombo M, et al. The burden of prostate cancer in Asian nations. J Carcinog. 2012;11:7. doi: 10.4103/1477-3163.94025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364:1708–17. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 5.Lazzeri M, Haese A, de la Taille A, Palou Redorta J, McNicholas T, et al. Serum isoform [-2]proPSA derivatives significantly improve prediction of prostate cancer at initial biopsy in a total PSA range of 2-10 ng/ml: a multicentric European study. Eur Urol. 2013;63:986–94. doi: 10.1016/j.eururo.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Guazzoni G, Nava L, Lazzeri M, Scattoni V, Lughezzani G, et al. Prostate-specific antigen (PSA) isoform p2PSA significantly improves the prediction of prostate cancer at initial extended prostate biopsies in patients with total PSA between 2.0 and 10 ng/ml: results of a prospective study in a clinical setting. Eur Urol. 2011;60:214–22. doi: 10.1016/j.eururo.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 7.Stephan C, Vincendeau S, Houlgatte A, Cammann H, Jung K, et al. Multicenter evaluation of [-2]proprostate-specific antigen and the prostate health index for detecting prostate cancer. Clin Chem. 2013;59:306–14. doi: 10.1373/clinchem.2012.195784. [DOI] [PubMed] [Google Scholar]
- 8.Na R, Ye D, Liu F, Chen H, Qi J, et al. Performance of serum prostate-specific antigen isoform [-2]proPSA (p2PSA) and the prostate health index (PHI) in a Chinese hospital-based biopsy population. Prostate. 2014;74:1569–75. doi: 10.1002/pros.22876. [DOI] [PubMed] [Google Scholar]
- 9.Carroll PR, Parsons JK, Andriole G, Bahnson RR, Barocas DA, et al. Prostate cancer early detection, version 1. 2014. Featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2014;12:1211–9. doi: 10.6004/jnccn.2014.0120. Quiz 9. [DOI] [PubMed] [Google Scholar]
- 10.Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283–90. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 11.Men S, Cakar B, Conkbayir I, Hekimoglu B. Detection of prostatic carcinoma: the role of TRUS, TRUS guided biopsy, digital rectal examination, PSA and PSA density. J Exp Clin Cancer Res. 2001;20:473–80. [PubMed] [Google Scholar]
- 12.Krumholtz JS, Carvalhal GF, Ramos CG, Smith DS, Thorson P, et al. Prostate-specific antigen cutoff of 2.6 ng/mL for prostate cancer screening is associated with favorable pathologic tumor features. Urology. 2002;60:469–73. doi: 10.1016/s0090-4295(02)01875-7. Discussion 73–4. [DOI] [PubMed] [Google Scholar]
- 13.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 14.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeb S, Sokoll LJ, Broyles DL, Bangma CH, van Schaik RH, et al. Prospective multicenter evaluation of the Beckman Coulter prostate health index using WHO calibration. J Urol. 2013;189:1702–6. doi: 10.1016/j.juro.2012.11.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruzhanskaya AV, Evgina SA, Skibo II. The practical application of marker -2proPSA and health index of prostate phi in diagnostics of prostate cancer. Klin Lab Diagn. 2014;1:4–8. Article in Russian. [PubMed] [Google Scholar]
- 17.Ng CF, Chiu PK, Lam NY, Lam HC, Lee KW, et al. The prostate health index in predicting initial prostate biopsy outcomes in Asian men with prostate-specific antigen levels of 4-10 ng/mL. Int Urol Nephrol. 2014;46:711–7. doi: 10.1007/s11255-013-0582-0. [DOI] [PubMed] [Google Scholar]
- 18.Loeb S, Catalona WJ. The prostate health index: a new test for the detection of prostate cancer. Ther Adv Urol. 2014;6:74–7. doi: 10.1177/1756287213513488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang YQ, Sun T, Zhong WD, Wu CL. Clinical performance of serum [-2]proPSA derivatives, %p2PSA and PHI, in the detection and management of prostate cancer. Am J Clin Exp Urol. 2014;2:343–50. [PMC free article] [PubMed] [Google Scholar]
- 20.Filella X, Foj L, Auge JM, Molina R, Alcover J. Clinical utility of %p2PSA and prostate health index in the detection of prostate cancer. Clin Chem Lab Med. 2014;52:1347–55. doi: 10.1515/cclm-2014-0027. [DOI] [PubMed] [Google Scholar]
- 21.Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, et al. A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol. 2011;185:1650–5. doi: 10.1016/j.juro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marks LS, Fradet Y, Deras IL, Blase A, Mathis J, et al. PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology. 2007;69:532–5. doi: 10.1016/j.urology.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Wei HM, Chen HT, Wang P, Wu YS, Na R, et al. Prostate cancer antigen 3 and genetic risk score as markers for the detection of prostate cancer in the Chinese population. Asian J Androl. 2015;17:168–70. doi: 10.4103/1008-682X.143245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scattoni V, Lazzeri M, Lughezzani G, De Luca S, Passera R, et al. Head-to-head comparison of prostate health index and urinary PCA3 for predicting cancer at initial or repeat biopsy. J Urol. 2013;190:496–501. doi: 10.1016/j.juro.2013.02.3184. [DOI] [PubMed] [Google Scholar]
- 25.Loeb S. Prostate cancer: predicting prostate biopsy results – PCA3 versus phi. Nat Rev Urol. 2015;12:130–1. doi: 10.1038/nrurol.2015.1. [DOI] [PubMed] [Google Scholar]
- 26.Ankerst DP, Boeck A, Freedland SJ, Thompson IM, Cronin AM, et al. Evaluating the PCPT risk calculator in ten international biopsy cohorts: results from the prostate biopsy collaborative group. World J Urol. 2012;30:181–7. doi: 10.1007/s00345-011-0818-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YR, Wei XH, Uhlman M, Lin XT, Wu SF, et al. PSA density improves the rate of prostate cancer detection in Chinese men with a PSA between 2.5-10.0 ng ml and 10.1-20.0 ng ml: a multicenter study. Asian J Androl. 2015;17:503–7. doi: 10.4103/1008-682X.142129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang P, Du W, Xie K, Deng X, Fu J, et al. Transition zone PSA density improves the prostate cancer detection rate both in PSA 4.0–10.0 and 10.1–20.0 ng/ml in Chinese men. Urol Oncol. 2013;31:744–8. doi: 10.1016/j.urolonc.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Na R, Jiang H, Kim ST, Wu Y, Tong S, et al. Outcomes and trends of prostate biopsy for prostate cancer in Chinese men from 2003 to 2011. PLoS One. 2012;7:e49914. doi: 10.1371/journal.pone.0049914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues G, Warde P, Pickles T, Crook J, Brundage M, et al. Pre-treatment risk stratification of prostate cancer patients: a critical review. Can Urol Assoc J. 2012;6:121–7. doi: 10.5489/cuaj.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perdona S, Bruzzese D, Ferro M, Autorino R, Marino A, et al. Prostate health index (phi) and prostate cancer antigen 3 (PCA3) significantly improve diagnostic accuracy in patients undergoing prostate biopsy. Prostate. 2013;73:227–35. doi: 10.1002/pros.22561. [DOI] [PubMed] [Google Scholar]
- 32.Lazzeri M, Briganti A, Scattoni V, Lughezzani G, Larcher A, et al. Serum index test %[-2]proPSA and Prostate Health Index are more accurate than prostate specific antigen and %fPSA in predicting a positive repeat prostate biopsy. J Urol. 2012;188:1137–43. doi: 10.1016/j.juro.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Ito K, Miyakubo M, Sekine Y, Koike H, Matsui H, et al. Diagnostic significance of [-2]pro-PSA and prostate dimension-adjusted PSA-related indices in men with total PSA in the 2.0-10.0 ng/mL range. World J Urol. 2013;31:305–11. doi: 10.1007/s00345-012-0927-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Univariate and Multivariate analyses of the performance of the PHI and other clinical variables with respect to predicting PCa with a negative DRE and TRUS
Performance of PSA measurements and other clinical variables with respect to predicting high-grade PCa when the DRE and TRUS are negative


