Abstract
Objective:
To estimate the risks for cancer (overall and site-specific) in an amyotrophic lateral sclerosis (ALS) cohort.
Methods:
In this observational longitudinal study, ALS and cancer cases were identified in a computerized Utah genealogy database (Utah Population Database) linked to a statewide cancer registry and death certificates. Hazard ratios (HRs) were estimated as the ratio of observed to expected number of cancers. Site-specific rates for cancer were estimated within the Utah Population Database; sex, birth year (5-year range), and birth state (Utah or not) cohorts were used to estimate the expected number of cancers among ALS cases. To account for an overall shortened lifespan, Cox regression was used to include years at risk in estimation of cancer risks for ALS cases.
Results:
An overall decreased hazard (hazard ratio [HR] 0.80, p = 0.014, 95% confidence interval [CI] 0.66–0.96) was found for cancer of any site in 1,081 deceased patients with ALS. A decreased hazard was found for lung cancer (HR 0.23, p = 0.002, CI 0.05–0.63). An increased hazard was found for salivary (HR 5.27, p = 0.041, 95% CI 1.09–15.40) and testicular (HR 3.82, p = 0.042, 95% CI 1.06–9.62) cancers. A nonsignificant hazard was observed for cutaneous malignant melanoma (HR 1.62, p = 0.12, 95% CI 0.88–2.71) for which increased risk has previously been reported.
Conclusions:
Using a unique population database, the overall risk of cancer of any site was found to be significantly reduced in cases with ALS, as was the risk of lung cancer. Significantly increased risk was observed for salivary and testicular cancers.
The pathogenesis of amyotrophic lateral sclerosis (ALS) is unknown, except in those cases with an identifiable genetic cause. A number of oncogenes have been implicated, including FUS1 and GRN,2 and potentially UBQLN1, UBQLN2,3 and SQSTM1.4 To further explore these genetic implications and investigate the findings of a few case series,5–7 several epidemiologic studies have been performed.8–14 No overall difference in the risk of developing ALS in cancer survivors has been identified,9,10,12 and no overall difference has been found for a subsequent risk of cancer following the diagnosis of ALS.8,13 However, there was an increased risk of ALS diagnosis the year following a cancer diagnosis, and a decreased risk of cancer diagnosis 2 or more years after the diagnosis of ALS.8
Several studies have examined the risk of cancer in other neurodegenerative diseases, including Parkinson disease (PD) and Alzheimer disease, and report a protective effect of PD and Alzheimer disease against overall development of cancer.13,15–24 However, this protective effect may be isolated to lung or smoking-related cancers.20–23
Given the varied biology of cancers, the risk of site-specific cancers in ALS has been investigated. Although results have been mixed, melanoma has been reported to occur at an increased risk.8–11 Tongue cancer has been associated with increased risk of ALS.9,10 An elevated rate of brain and prostate cancers has also been reported in patients with ALS.8 However, another study found an overall decreased risk of ALS death among prostate cancer survivors.9 Given conflicting findings in the literature and the low frequency of co-occurrence of ALS and cancer, and specifically site-specific cancers, further investigation using a large database with a low cohort selection bias is warranted.
METHODS
Utah Population Database Genealogy data.
The Utah Population Database (UPDB) is composed of a computerized genealogy linked with multiple medical resources. It includes genealogic data dating back to the original Utah pioneers and their descendants as well as individual data for millions of Utah residents over time.25 The UPDB currently contains data on 7.3 million individuals. We analyzed the more than 4 million individuals who are a part of at least 3 generations of genealogy, ensuring that all individuals analyzed had some successful record linkage in the resource. The Utah pioneers founded Utah in the mid-1800s and were composed of a generally unrelated mixture of Europeans.26 The continued high rates of immigration to Utah resulted in consistently low levels of inbreeding.27 Individuals in the UPDB have been record-linked to all computerized Utah death certificates dating back to 1904 and to the Utah Cancer Registry (UCR) dating back to 1966.
ALS diagnosis data.
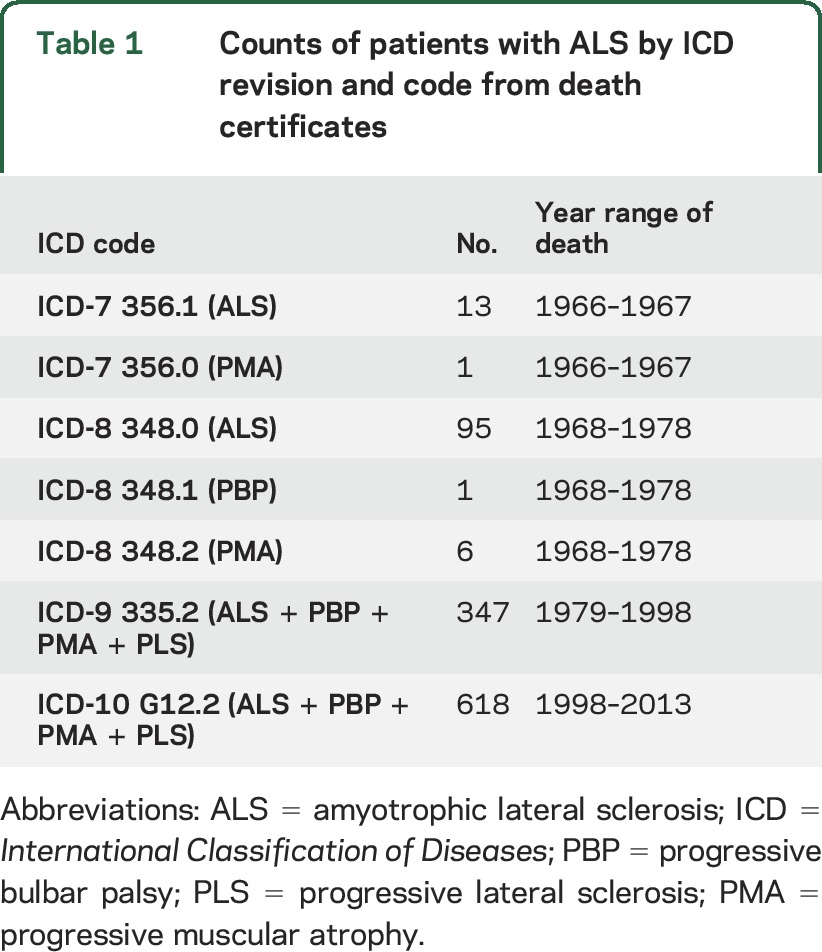
Patients with ALS were identified from among the 588,239 Utah death certificates linked to individuals in the UPDB as those which listed ALS as a primary or contributing cause of death between 1966 (after cancer data were available in Utah) and 2013 (latest data available in the UPDB). ALS-coded death records have a robust estimated accuracy of 70% to 90%,28 as ALS is clinically well recognized, rapidly progressive, and invariably fatal. The causes of death on Utah death certificates have been encoded using ICD codes, which include causes of death for ALS and other motor neuron diseases (progressive muscular atrophy, progressive bulbar palsy, and progressive lateral sclerosis, but excluding spinal muscular atrophy). This method is considered to be the most accurate method to capture patients with ALS using death certificates.29 We use “ALS” to describe the studied disease, with the understanding that we may also be including the above-mentioned less common forms of motor neuron disease. The frequency of ALS cases by ICD code is shown in table 1. We excluded deaths coded as above that occurred before the age of 14 years, as these may represent cases of miscoded spinal muscular atrophy.
Table 1.
Counts of patients with ALS by ICD revision and code from death certificates
Cancer diagnosis data.
All cancer diagnosis data originated from the UCR. The UCR was established in 1966, and in 1973 became part of the US National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program.30 The UCR follows rigorous SEER guidelines to ensure data accuracy, and includes pathology reports and follow-up, with an established case ascertainment rate of 98%.31 The 37 different cancer sites examined were defined by primary site and histology.32 All independent primary cancers except for basal and squamous carcinomas of the skin are required to be reported to the UCR. More than 190,000 Utah cancer records are linked to the 4.2 million individuals analyzed.
Statistical analysis.
Individuals with ALS have an overall decreased life expectancy, thus their time at risk for developing cancer differs from the general population. To account for this difference, we have modified the general procedure used to estimate cancer risk by site32,33 as described below. UPDB cohort-specific rates for each cancer site were estimated. The 4.2 million individuals analyzed were assigned to 1 of 136 cohorts based on sex, birth state (Utah or not), and year of birth (5-year cohorts).
To estimate cancer risks for ALS cases, each ALS case was cohort-matched to 100 controls (randomly selected without replacement). A Cox proportional hazard model was then fit in R using the coxphf package. For each cancer site analyzed, a different survival function was fit for ALS cases and controls. The time to cancer diagnosis was defined as an individual's age at cancer diagnosis (in 1-year increments). Appropriate censoring occurred for individuals who died without having the cancer of interest. Under the Cox proportional hazard model, time to diagnosis with the cancer of interest was determined by an individual's ALS case status and the covariate profile of that individual's cohort. After fitting the model, an overall cancer hazard rate was determined for ALS cases. The coefficients of the fit model were then used to estimate the hazard ratio (HR), which indicates a relative likelihood for developing a specific cancer per 1-year time period in patients with ALS compared to controls. These methods account for the risk for varying site-specific cancer risk over time.
Standard protocol approvals, registrations, and patient consents.
This research was limited to the analysis of unidentifiable data only. There was no contact with human subjects, and waiver of informed consent was approved. The University of Utah Institutional Review Board and the Resource for Genetic Epidemiological Research approved the research.
RESULTS
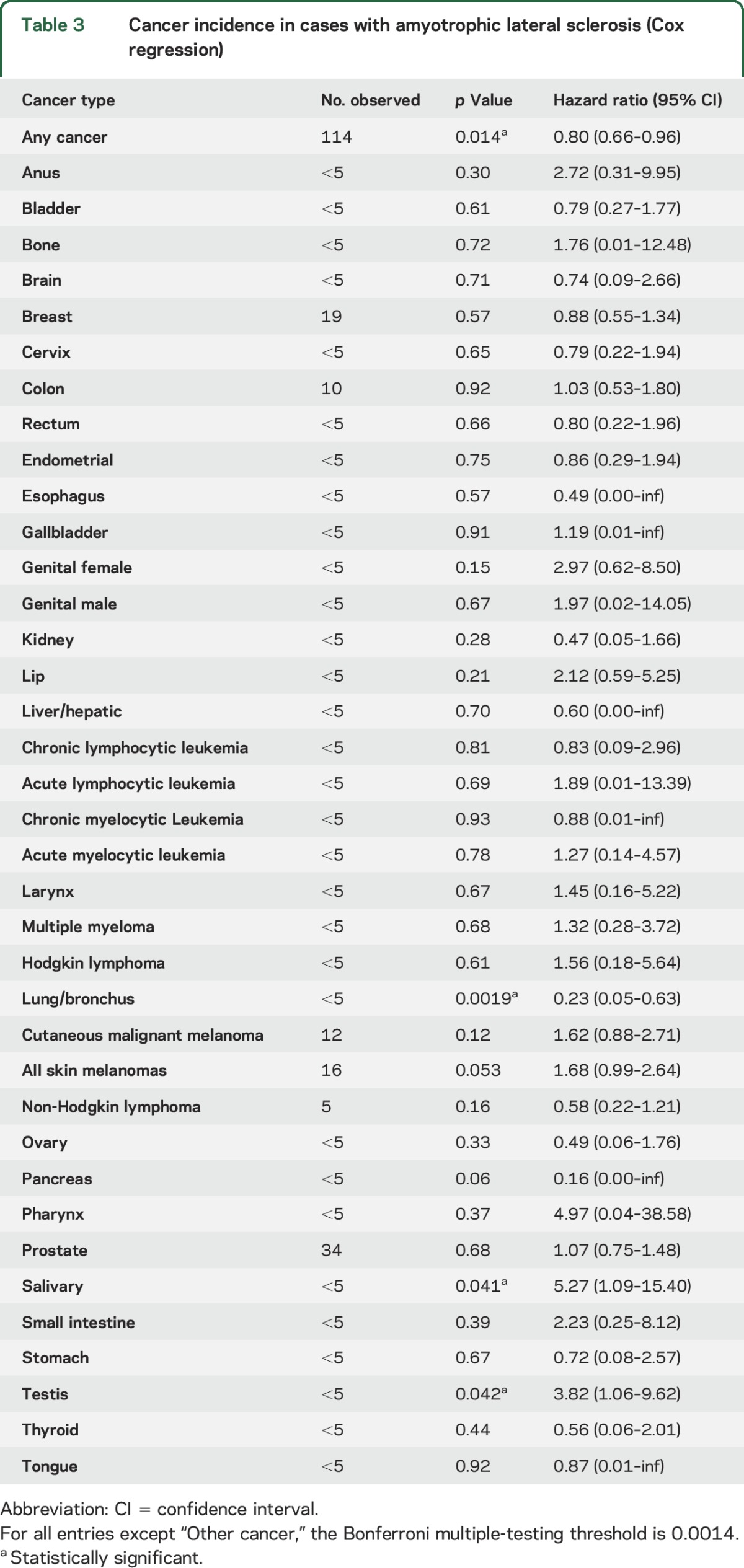
The characteristics of the 1,081 ALS cases are shown in table 2. Among the total 1,242 ALS death certificates in the UPDB individuals analyzed, 1,081 ALS cases occurred after 1966 and were older than 14 years. In the 1,081 ALS cases, there were 114 diagnoses (10.5%) of independent primary cancers observed (table 3). This is significantly lower than expected for the UPDB population (HR 0.80, p = 0.014, 95% confidence interval [CI] 0.66–0.96). The only site-specific cancer observed to have a significantly decreased risk was lung cancer (HR 0.23, p = 0.002, 95% CI 0.05–0.63). Significantly elevated HRs were observed for salivary gland cancer (HR 5.27, p = 0.041, 95% CI 1.09–15.40) and testicular cancer (HR 3.82, p = 0.042, 95% CI 1.06–9.62). The estimated HR for cutaneous malignant melanomas was elevated, but not significantly (HR 1.62, p = 0.12, 95% CI 0.88–2.71).
Table 2.
Characteristics of patients with ALS in the Utah Population Database
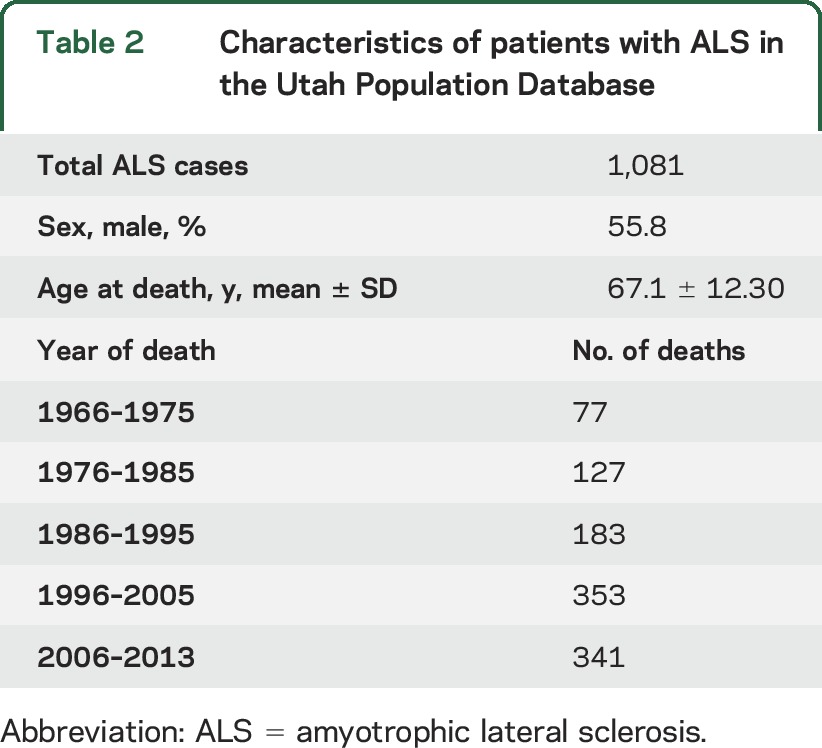
Table 3.
Cancer incidence in cases with amyotrophic lateral sclerosis (Cox regression)
The overall converse risk of death from ALS among all individuals in UPDB with a cancer diagnosis and a death certificate (91,790 deaths) showed a significant deficit of ALS deaths (HR 0.44, p < 0.0001, 95% CI 0.30–0.46).
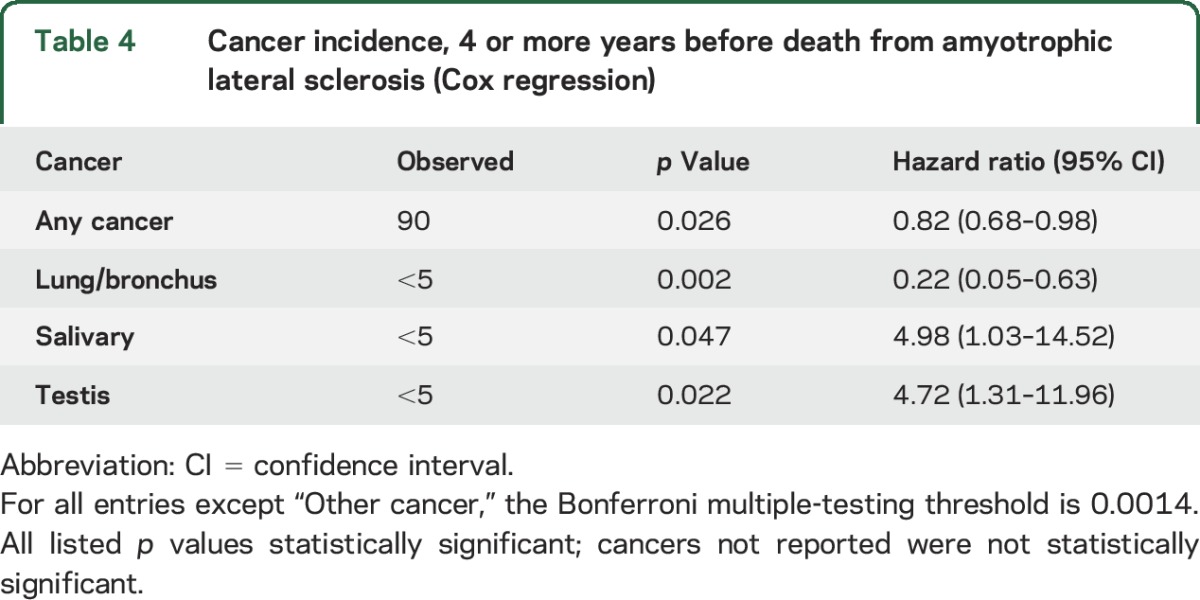
To avoid the possibility of bias from decreased cancer surveillance preceding death from ALS, we limited analysis to cancer diagnosis made 4 years or more before death from ALS (table 4). Conclusions did not change; a significantly decreased HR was observed for any cancer (HR 0.82, p = 0.026, 95% CI 0.68–0.98) and for lung cancer (HR 0.22, p = 0.002, 95% CI 0.05–0.63). Significantly increased HRs were observed for salivary cancer (HR 4.98, p = 0.047, 95% CI 1.03–14.52) and testicular cancer (HR 4.72, p = 0.022, 95% CI 1.31–11.96).
Table 4.
Cancer incidence, 4 or more years before death from amyotrophic lateral sclerosis (Cox regression)
DISCUSSION
This population-based analysis of a unique database (UPDB) controlling for sex, birth year, birth state, and age at death estimated the risk of cancer at any site among 1,081 individuals who died of ALS. We observed a decreased lifetime risk of cancer in patients with ALS (HR 0.80, p = 0.014, 95% CI 0.66–0.96), as well as decreased risk of ALS among all deceased cancer cases.
The protective effect of other neurodegenerative diseases (PD and Alzheimer disease) for cancer, particularly lung cancer, is well documented.13,15–24 However, this benefit has not been clearly identified in previous ALS-specific studies.8–14 One study found a decreased risk of cancer, but only 2 or more years after the diagnosis of ALS, and this was attributed to decreased cancer surveillance bias.8 It is unlikely that our results are attributable to a surveillance bias, since exclusion of the 4 years before death from ALS (the average time from symptom onset to death from ALS) did not affect the results. Because previous studies used the diagnosis of cancer as the pivotal event, rather than death of the patient, overestimation of the risk of cancer may have occurred.9–12
The mechanism for a protective cancer benefit in other neurodegenerative diseases (PD and Alzheimer disease) is not well understood, and a number of mechanisms have been proposed. It has been suggested that increased apoptosis may be a critical factor, as this mechanism is associated both with an increased risk of neurodegeneration but also may protect against cancer. Furthermore, mutations in tumor suppressor genes such as PTEN have been observed in patients with PD.17,18,23 An intracellular signaling molecule, Pin1, has also been implicated, as its dysregulation has critical but opposing roles in the pathogenesis of Alzheimer disease and human cancers.34 Others have pointed to the potentially neuroprotective benefits of chemotherapies as a mechanism for this effect.18
Cancer site–specific subanalysis revealed mixed results. Significantly increased hazards for salivary gland cancer (HR 5.27, p = 0.041) and testicular cancer (HR 3.82, p = 0.042) were observed. This has not been previously reported and should be interpreted with caution until further validation, particularly given the rarity of these cancers. It must be noted that if a multiple testing correction (Bonferroni method) were applied for the analyses of cancer by site, neither of these results would reach significance (p < 0.05/37 = 0.0014). The previously reported increased HR for cutaneous malignant melanoma8–11 was not observed in this study. It is noteworthy that there is strong evidence that skin melanoma is also a risk factor for PD.23 Only lung cancer showed a significantly decreased risk (HR 0.23, p = 0.002), which is not significant after correction for multiple testing.
It is unlikely that the decreased risk of lung cancer observed is directly tobacco-related. We are unable to determine tobacco use among the ALS cases analyzed, but we note that, because of the religious identity and associated cultural practices of the majority of the population of the state of Utah, this state is continuously ranked to have the lowest percentage of smokers (about 10%) and lung cancers in the United States.35 In addition, no studies have reported a reduced use of tobacco among patients with ALS, and some have reported a moderately increased use, particularly in postmenopausal women.36 In other cancers strongly related to tobacco,37–39 we observed no significantly decreased risk and some risks were elevated (cancer of the esophagus [HR 0.49, p = 0.57], cancer of the tongue [HR 0.87, p = 0.92], lip cancer [HR 2.12, p = 0.21], colon cancer [HR 1.03, p = 0.92], bladder cancer [HR 0.79, p = 0.61], pancreas cancer [HR 0.16, p = 0.06], cancer of the larynx [HR = 1.45, p = 0.67], kidney cancer [HR 0.47, p = 0.28], and stomach cancer [HR 0.72, p = 0.67], including a significantly increased risk of salivary cancer [HR 5.27, p = 0.041]). In other cancers weakly associated with tobacco use (liver cancer, ovarian cancer, cervical cancer, and leukemia), there was also no significant association observed.39 Given the historical normal to elevated rates of use of tobacco in patients with ALS, persistently low rates of use of tobacco in Utah, and our mixed tobacco related cancer results, our findings are unlikely to be explained by tobacco use alone. This protective effect, isolated to lung cancer, has also been reported in other neurodegenerative diseases,20–22 especially PD patients who use tobacco.24
The background characteristics of these Utah ALS cases are consistent with many other ALS population-based studies regarding mean age at death and the slight male predominance, which supports the internal validly of the data. As reported both in our previous study using the UPDB40 and in previous publications that used death records, the incidence of ALS is likely stable. The apparent increase over time (table 2) is likely related to other factors, including increasing awareness of ALS, increasing life-expectancy of the general population, changes to the death certificate that now allow for additional causes of death to be listed, and specific centers of care for ALS (established in Utah in 1990).
A limitation of this study, which is inherent to any study investigating the co-occurrence of 2 rare events, is that evidence for significant relationships might have been missed because of small sample size. Another limitation is the use of death certificates and ICD coding. While death certificates are generally considered accurate in ALS,28 they remain a potential source of error. Some individuals diagnosed with ALS were likely missed and other individuals were likely miscoded as ALS on a death certificate. Given the rigorous guidelines and regulation of the SEER program, the cancer diagnosis data are highly accurate.31 Strengths of the study include the use of an unparalleled regional database, allowing for a large sample size over nearly 50 years, with patient characteristics consistent with general ALS populations.
This population-based analysis of a unique database (UPDB) provides evidence that ALS, like other neurodegenerative diseases, may be protective against cancer, especially lung cancer. The pathogenic factors are not clear but are worthy of study. This study also provides suggestive evidence for increased risk of testicular cancer and salivary cancer among ALS cases that must be validated in other populations, but could be considered when deciding appropriate screening for ALS cases.
GLOSSARY
- ALS
amyotrophic lateral sclerosis
- CI
confidence interval
- HR
hazard ratio
- ICD
International Classification of Diseases
- PD
Parkinson disease
- SEER
Surveillance, Epidemiology, and End Results
- UCR
Utah Cancer Registry
- UPDB
Utah Population Database
AUTHOR CONTRIBUTIONS
Dr. Gibson has made a substantive contribution to the design and conceptualization of the study, interpretation of the data, and drafting and revising of the manuscript. Dr. Abbott has made a substantive contribution to the analysis and interpretation of the data and drafting and revising of the manuscript. Mr. Farnham has made a substantive contribution to the analysis and interpretation of the data. Ms. Thai has made a substantive contribution to the analysis and interpretation of the data and drafting of the manuscript. Ms. McLean has made a substantive contribution to the design of the study and drafting of the manuscript. Mrs. Figueroa has made a substantive contribution to the design of the study and revising of the manuscript. Dr. Bromberg has made a substantive contribution to the revising of the manuscript. Dr. Pulst has made a substantive contribution to the design and conceptualization of the study and revising of the manuscript. Dr. Cannon-Albright has made a substantive contribution to the design and conceptualization of the study, statistical analysis and interpretation of the data, and drafting and revising the manuscript.
STUDY FUNDING
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the NIH under award number TL1TR001066. Dr. Cannon-Albright acknowledges support from the Huntsman Cancer Foundation. The Utah Cancer Registry is funded by contract HHSN261201000026C from the National Cancer Institute's SEER Program with additional support from the Utah State Department of Health and the University of Utah. Partial support for all datasets within the UPDB was provided by the Huntsman Cancer Institute, University of Utah, and the Huntsman Cancer Institute's Cancer Center Support grant, P30 CA42014 from the National Cancer Institute. Partial funding support for Dr. Pulst was provided by Target ALS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURE
S. Gibson: Recursion Pharmaceuticals, share holder. D. Abbott, J. Farnham, K. Thai, H. McLean, K. Figueroa, and M. Bromberg report no disclosures relevant to the manuscript. S. Pulst: Progenitor Life Sciences, share holder; Cedars-Sinai, royalties; University of Utah, royalties; and Ataxion Therapeutics, consultant. L. Cannon-Albright reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Dormann D, Haass C. Fused in sarcoma (FUS): an oncogene goes awry in neurodegeneration. Mol Cell Neurosci 2013;56:475–486. [DOI] [PubMed] [Google Scholar]
- 2.He Z, Ismail A, Kriazhev L, Sadvakassova G, Bateman A. Progranulin (PC-cell-derived growth factor/acrogranin) regulates invasion and cell survival. Cancer Res 2002;62:5590–5596. [PubMed] [Google Scholar]
- 3.Shah PP, Lockwood WW, Saurabh K, et al. Ubiquilin1 represses migration and epithelial-to-mesenchymal transition of human non-small cell lung cancer cells. Oncogene 2015;34:1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson HG, Harris JW, Wold BJ, Lin F, Brody JP. p62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Oncogene 2003;22:2322–2333. [DOI] [PubMed] [Google Scholar]
- 5.Brain L, Croft PB, Wilkinson M. Motor neurone disease as a manifestation of neoplasm (with a note on the course of classical motor neurone disease). Brain 1965;88:479–500. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell DM, Olczak SA. Remission of a syndrome indistinguishable from motor neurone disease after resection of bronchial carcinoma. Br Med J 1979;2:176–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadot E, Carluer L, Corcia P, Delozier Y, Levy C, Viader F. Breast cancer and motor neuron disease: clinical study of seven cases. Amyotroph Lateral Scler 2007;8:288–291. [DOI] [PubMed] [Google Scholar]
- 8.Fang F, Al-Chalabi A, Ronnevi LO, et al. Amyotrophic lateral sclerosis and cancer: a register-based study in Sweden. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman DM, Curtis RE, Daugherty SE, Goedert JJ, Kuncl RW, Tucker MA. The association between cancer and amyotrophic lateral sclerosis. Cancer Causes Control 2013;24:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman DM, Travis LB, Gridley G, Kuncl RW. Amyotrophic lateral sclerosis mortality in 1.9 million US cancer survivors. Neuroepidemiology 2005;25:176–180. [DOI] [PubMed] [Google Scholar]
- 11.Baade PD, Fritschi L, Freedman DM. Mortality due to amyotrophic lateral sclerosis and Parkinson's disease among melanoma patients. Neuroepidemiology 2007;28:16–20. [DOI] [PubMed] [Google Scholar]
- 12.Freedman DM, Wu J, Daugherty SE, Kuncl RW, Enewold LR, Pfeiffer RM. The risk of amyotrophic lateral sclerosis after cancer in U.S. elderly adults: a population-based prospective study. Int J Cancer 2014;135:1745–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fois AF, Wotton CJ, Yeates D, Turner MR, Goldacre MJ. Cancer in patients with motor neuron disease, multiple sclerosis and Parkinson's disease: record linkage studies. J Neurol Neurosurg Psychiatry 2010;81:215–221. [DOI] [PubMed] [Google Scholar]
- 14.Chio A, Brignolio F, Meineri P, Rosso MG, Tribolo A, Schiffer D. Motor neuron disease and malignancies: results of a population-based study. J Neurol 1988;235:374–375. [DOI] [PubMed] [Google Scholar]
- 15.Roe CM, Fitzpatrick AL, Xiong C, et al. Cancer linked to Alzheimer disease but not vascular dementia. Neurology 2010;74:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inzelberg R, Jankovic J. Are Parkinson disease patients protected from some but not all cancers? Neurology 2007;69:1542–1550. [DOI] [PubMed] [Google Scholar]
- 17.Roe CM, Behrens MI, Xiong C, Miller JP, Morris JC. Alzheimer disease and cancer. Neurology 2005;64:895–898. [DOI] [PubMed] [Google Scholar]
- 18.Driver JA, Beiser A, Au R, et al. Inverse association between cancer and Alzheimer's disease: results from the Framingham Heart Study. BMJ 2012;344:e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moller H, Mellemkjaer L, McLaughlin JK, Olsen JH. Occurrence of different cancers in patients with Parkinson's disease. BMJ 1995;310:1500–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi HB, Tang B, Liu YW, Wang XF, Chen GJ. Alzheimer disease and cancer risk: a meta-analysis. J Cancer Res Clin Oncol 2015;141:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kareus SA, Figueroa KP, Cannon-Albright LA, Pulst SM. Shared predispositions of parkinsonism and cancer: a population-based pedigree-linked study. Arch Neurol 2012;69:1572–1577. [DOI] [PubMed] [Google Scholar]
- 22.Lo RY, Tanner CM, Van Den Eeden SK, Albers KB, Leimpeter AD, Nelson LM. Comorbid cancer in Parkinson's disease. Mov Disord 2010;25:1809–1817. [DOI] [PubMed] [Google Scholar]
- 23.Driver JA, Logroscino G, Buring JE, Gaziano JM, Kurth T. A prospective cohort study of cancer incidence following the diagnosis of Parkinson's disease. Cancer Epidemiol Biomarkers Prev 2007;16:1260–1265. [DOI] [PubMed] [Google Scholar]
- 24.Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G. Prospective case-control study of nonfatal cancer preceding the diagnosis of Parkinson's disease. Cancer Causes Control 2007;18:705–711. [DOI] [PubMed] [Google Scholar]
- 25.Skolnick M. The Utah genealogical database: a resource for genetic epidemiology. In: Cairns J, Lyon JL, Skolnick M, editors. Banbury Report No. 4: Cancer Incidence in Defined Populations. New York: Cold Spring Harbor Laboratory; 1980:285–297. [Google Scholar]
- 26.McLellan T, Jorde LB, Skolnick MH. Genetic distances between the Utah Mormons and related populations. Am J Hum Genet 1984;36:836–857. [PMC free article] [PubMed] [Google Scholar]
- 27.Jorde LB. Inbreeding in the Utah Mormons: an evaluation of estimates based on pedigrees, isonymy, and migration matrices. Ann Hum Genet 1989;53:339–355. [DOI] [PubMed] [Google Scholar]
- 28.Chio A, Magnani C, Oddenino E, Tolardo G, Schiffer D. Accuracy of death certificate diagnosis of amyotrophic lateral sclerosis. J Epidemiol Community Health 1992;46:517–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marin B, Couratier P, Preux PM, Logroscino G. Can mortality data be used to estimate amyotrophic lateral sclerosis incidence? Neuroepidemiology 2011;36:29–38. [DOI] [PubMed] [Google Scholar]
- 30.NCI SEER Registries: Utah. Available at: http://seer.cancer.gov/registries/utah.html2015. Accessed February 11, 2016.
- 31.Swan J, Wingo P, Clive R, et al. Cancer surveillance in the U.S.: can we have a national system? Cancer 1998;83:1282–1291. [DOI] [PubMed] [Google Scholar]
- 32.Teerlink CC, Albright FS, Lins L, Cannon-Albright LA. A comprehensive survey of cancer risks in extended families. Genet Med 2012;14:107–114. [DOI] [PubMed] [Google Scholar]
- 33.Cannon Albright LA. Utah family-based analysis: past, present and future. Hum Hered 2008;65:209–220. [DOI] [PubMed] [Google Scholar]
- 34.Driver JA, Zhou XZ, Lu KP. Pin1 dysregulation helps to explain the inverse association between cancer and Alzheimer's disease. Biochim Biophys Acta 2015;1850:2069–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy J. In U.S., smoking rate lowest in Utah, highest in Kentucky [online]. Available at: http://www.gallup.com/poll/167771/smoking-rate-lowest-utah-highest-kentucky.aspx. Accessed July 30, 2015.
- 36.Ingre C, Roos PM, Piehl F, Kamel F, Fang F. Risk factors for amyotrophic lateral sclerosis. Clin Epidemiol 2015;7:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doll R. Cancers weakly related to smoking. Br Med Bull 1996;52:35–49. [DOI] [PubMed] [Google Scholar]
- 38.Levitz JS, Bradley TP, Golden AL. Overview of smoking and all cancers. Med Clin North Am 2004;88:1655–1675, xiii. [DOI] [PubMed] [Google Scholar]
- 39.Surgeon General's Report. The Health Consequences of Smoking—50 Years of Progress. Available at: http://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/#booklet: CDC, 2014. Accessed November 20, 2015. [Google Scholar]
- 40.Gibson SB, Figueroa KP, Bromberg MB, Pulst SM, Cannon-Albright L. Familial clustering of ALS in a population-based resource. Neurology 2014;82:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


