Abstract
Objective:
Little information is available about sex-related differences in intracerebral hemorrhage (ICH). This is a prospective observational study to describe the sex differences in demographics, vascular risk factors, stroke care, and outcomes in primary ICH.
Methods:
BasicMar is a hospital-based registry of all stroke patients admitted to a single public hospital that covers a population of 330,000. From 2005 to 2015, there were 515 consecutive acute primary ICH patients. Outcome data were obtained at 3 months.
Results:
More men than women had ICH (52.4% vs 47.6%); the women were older and had worse previous functional status than men, who were more likely to drink alcohol and smoke and to have ischemic heart disease and peripheral arterial disease. There were no sex differences in etiology, severity, or hemorrhage volume. ICH score was greater in women than in men (p = 0.018). Women had more lobar ICH than men (odds ratio adjusted by age was 1.75 [95% confidence interval 1.18–2.58], p = 0.005). The quality of stroke care was similar in both sexes. Mortality at 3 months was 44.1% in women and 41.1% in men (p = 0.656), and 3-month poor outcome among survivors (modified Rankin Scale [mRS] score 3–5) 58.4% in women and 45.3% in men (p = 0.027). After adjustment for previous mRS and ICH score, there were no differences in 3-month mortality or poor outcome at 3 months between sexes.
Conclusions:
Patients with ICH showed sex-related differences in demographic characteristics, ICH location, and vascular risk factors, but not in stroke care, 3-month mortality, or adjusted poor outcome.
Intracerebral hemorrhage (ICH) is the most severe stroke subtype, with about 50% mortality within the first month after the event and only about 20%–25% of survivors able to live independently at 6 months.1–4 Sex differences in ICH characteristics and outcome have not been fully studied and the reported results have been inconclusive. A meta-analysis in 20104 and a review published in 20155 both emphasize the conflicting results reported about sex differences in ICH outcomes. Both studies concluded that more data on functional outcome after ICH are needed. Some methodologic problems that would account for the inconsistencies and heterogeneity of the available data have been noted, such as differences in metrics used, length of study period, race/ethnicity of participants, etiology, or the adjustments made in the statistical analysis.4–6 Moreover, most of the available data are from retrospective stroke registries6–12 or from studies published more than a decade ago.13,14 The most recent published series comes from Asian countries,15,16 and probably have some bias and racial/ethnic specificity. Finally, it is not clear whether these series are exhaustive, taking into account, for instance, early deaths (i.e., during the first hours at the emergency room). According to a recent review,5 the reported ICH case fatalities range from 16.2% to 51.8% in women and 18.8% to 51.8% in men, with outcome data showing even less consistency.
Our aim was to assess whether sex differences could be observed in demographics, etiology, location, stroke care, and 3-month mortality and disability after primary ICH in a comprehensive and prospective 11-year study, taking into account the covariables that could influence outcome data.
METHODS
From May 2005 to April 2015, 660 patients with acute ICH were admitted to our hospital. Patients with no outcome data (n = 23), with inaccurate clinical information or who denied to participate in the BasicMar database (n = 13), and with secondary ICH (n = 109) were excluded. The BasicMar database17 is an ongoing prospective register of patients with acute stroke at University Hospital del Mar, a tertiary public hospital serving a population of 330,000 in 3 districts of Barcelona. The final cohort was 515 patients with acute primary ICH. All patients received a CT scan in the emergency room.
All patients were evaluated at hospital admission by a vascular-trained neurologist who established initial severity using the NIH Stroke Scale (NIHSS) and the Glasgow Coma Scale (GCS). Assessment by a neurosurgeon or intensive medicine specialist was considered, according to the clinical situation, age, previous functional status, and comorbidities. Additional CT, MRI evaluations, or angiographic studies were done, if needed, during hospitalization.
Vascular risk factors, as defined by international guidelines, were obtained from the patient, relatives, caregivers, or previous medical records. A structured questionnaire was used to record the following variables: arterial hypertension (evidence of at least 2 raised blood pressure measurements, systolic >140 mm Hg or diastolic >90 mm Hg, recorded on different days before stroke onset; a physician's diagnosis; or use of medication); diabetes (previous physician diagnosis or use of medication); hyperlipidemia (physician diagnosis, use of medication, serum cholesterol concentration >220 mg/dL, low-density lipoprotein cholesterol >130 mg/dL, or serum triglyceride concentration >150 mg/dL); ischemic heart disease (documented history of angina pectoris or myocardial infarction); peripheral arterial disease (documented current intermittent claudication with an ankle brachial index of less than 0.9 or a history of intermittent claudication, together with a previous related intervention, such as amputation); atrial fibrillation (physician diagnosis, use of medication, or conclusive ECG data); current smoking habit; alcohol overuse (>60 g/d); and illicit drug use.
ICH was classified as primary if arterial hypertension or cerebral amyloid angiopathy (CAA) was the suspected cause. Patients without a demonstrable cause of ICH, including those on anticoagulant or antiplatelet treatments, also were considered primary ICH. Secondary ICH hemorrhage was considered when the ICH was due to cerebral vascular malformations, brain tumors, brain diseases caused by infection, systemic diseases, coagulopathies, or ICH related to alcohol or illicit drug use. Patients with secondary ICH were excluded from the study.
To analyze ICH location, the first available CT was used and was evaluated by vascular neurologists blinded to clinical data. Involvement of the thalamus, basal ganglia, or internal capsule was defined as deep ICH; of the cortex and cortical-subcortical junction as lobar ICH; and of the cerebellum as cerebellar ICH. If more than one territory, pure intraventricular ICH, or brainstem hemorrhages were involved, it was classified as “other locations.” ICH volume was measured on CT using the ABC/2 formula, where A is the greatest hemorrhage diameter, B is the diameter 90° to A, and C is the approximate number of CT slices with hemorrhage multiplied by the slice thickness.18
Time interval from ICH symptom onset to hospital admission, time interval from hospital admission to death, stroke care characteristics (admission to monitored acute stroke unit or neurointensive acute unit vs general ward, neurosurgical treatment, and rehabilitation therapy), and discharge destination were also analyzed according to sex.
The main outcome endpoints were mortality and poor outcome (modified Rankin Scale [mRS] score from 3 to 5) at 3 months. Mortality data were obtained from electronic medical records, hospital admissions records, or telephone contact with primary care physicians or family members. Disability data were obtained from a clinic visit at 3-month follow-up or by telephone contact from a trained staff member to assess functional status using the mRS.
Statistical analysis.
Age, NIHSS score, GCS, hematoma volume, and ICH score presented a non-normal distribution and were expressed as medians and interquartile ranges (IQR 25–75). Categorical data were expressed as counts and percentages. Differences in parametric and nonparametric continuous variables were evaluated using the t test and Mann-Whitney U test, respectively, and the χ2 test was used for proportional analysis. We compared the demographic, vascular risk factor, and stroke care differences between the sexes by bivariate analysis.
Finally, we compared 3-month mortality and 3-month poor outcome between sexes. The analysis was bivariate and multivariate, adjusted by previous mRS and ICH score. We chose to adjust by ICH score rather than adjusting separately by age, clinical severity, or ICH volume because ICH score is a validated method, is widely used, and includes in a single score the most relevant mortality predictors.
All analyses were 2-tailed. The significance level was set at 0.05.
Standard protocol approvals, registrations, and patient consents.
The information used in this study was collected from the prospective BasicMar register, with the approval of our local ethics committee. All patients gave informed consent prior to their inclusion in the study.
RESULTS
From May 2005 to April 2015, 515 patients (245 women, 270 men) with a spontaneous primary ICH were studied. Median age was 78 years. Demographics of the study population are summarized in table 1. Missing data included previous history of arterial hypertension (n = 3), diabetes (n = 6), hyperlipidemia (n = 8), stroke (n = 7), coronary artery disease (n = 4), peripheral arterial disease (n = 12), smoking (n = 19), alcohol overuse (n = 32), NIHSS score (n = 11), hematoma volume (n = 31), ICH score (n = 36), and GCS (n = 7).
Table 1.
Demographics and differences according to sex (n = 515)
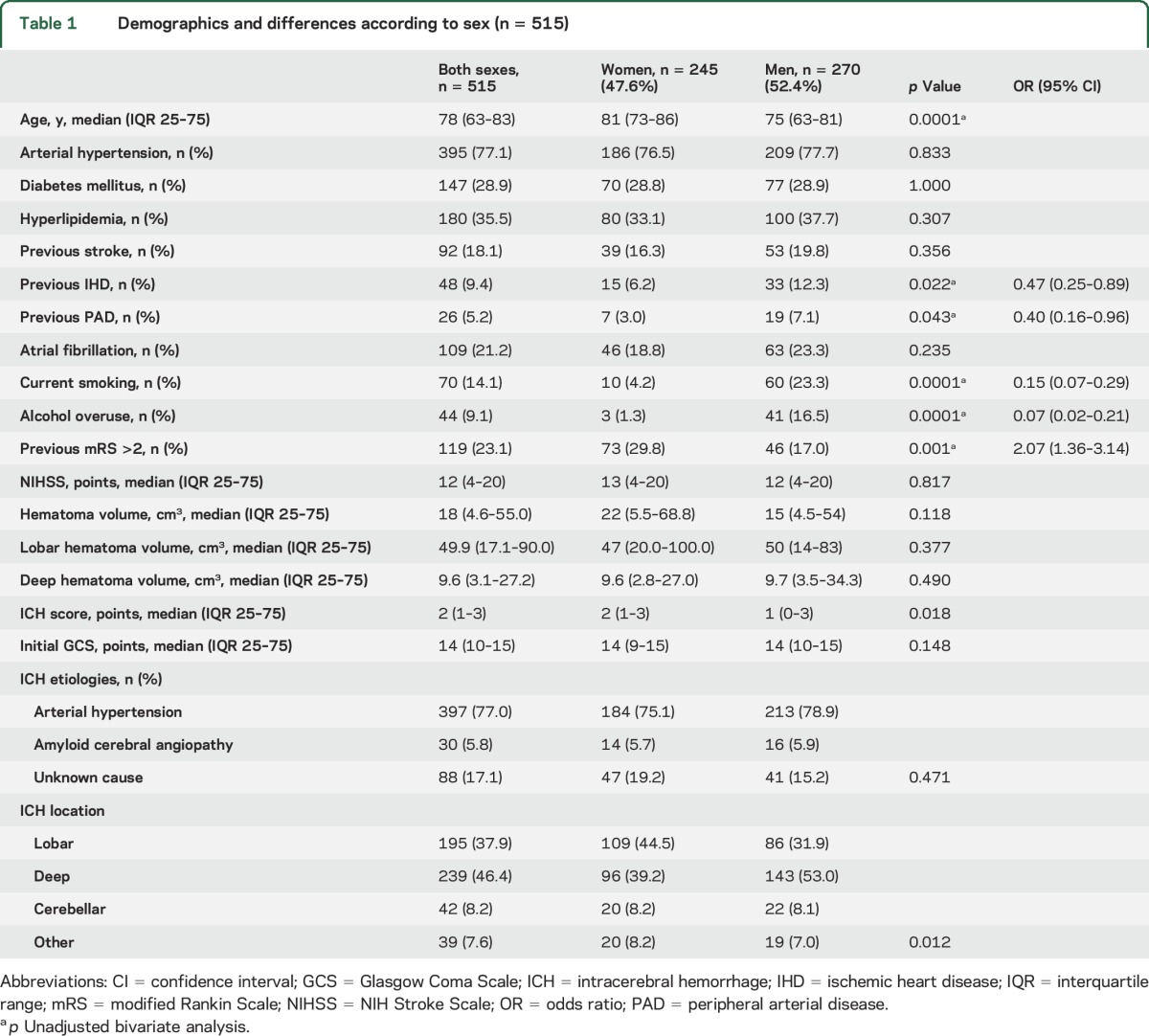
Differences between sexes (table 1) were the following: the women were older than the men (81 vs 75 years, p < 0.0001) and more likely to have previous poor functional status (29.8% vs 17%, p < 0.001). Adjusted by age, the functional status difference disappeared (p = 0.324). More men than women were current smokers (23.3% vs 4.2%, p < 0.0001), were alcohol drinkers (16.5% vs 1.3%, p < 0.0001), and had peripheral arterial disease (7.1% vs 3.0%, p = 0.043) and ischemic heart disease (12.3% vs 6.2%, p = 0.022). ICH severity (assessed by NIHSS and GCS) was similar in the sexes, as well as hematoma volume. Lobar ICH volumes were larger than deep volumes: median (IQR 25–75) = 49.9 cm3 (17.1–90.0) vs 9.6 cm3 (3.1–27.2), p < 0.0001. There were no differences between women and men in hematoma volume in lobar hematomas (median [IQR 25–75] = 47 cm3 [20–100] vs 50 cm3 [14–83], p = 0.377) or deep hematomas (median [IQR 25–75] = 9.6 cm3 [2.8–27] vs 9.7 cm3 [3.5–34.3], p = 0.490), respectively.
ICH score was higher in women than in men (p = 0.0018) but, adjusted by age, the difference disappeared (p = 0.456). There was a clear sex difference in lobar or deep ICH location (p = 0.012); after adjustment by age, the difference remained for lobar ICH (odds ratio [OR] 1.75 [95% confidence interval (CI) 1.18–2.58]) in women compared to men (p = 0.005).
Arterial hypertension was the most common etiology (77.0%), without sex differences (p = 0.471). Although women were older and had more lobar ICH than men, we found no differences in CAA etiology. However, age was similar in women, median (IQR 25–75) = 77.0 (72–83) years, and men 79.5 (75–82) with CAA (p = 0.525).
There were no significant sex differences between time interval from ICH symptom onset to hospital admission: in 125 cases (24.3%) the time to presentation was unknown (25.6% of women, 23.0% of men, p = 0.537); in the remaining cases, the interval was less than 6 hours in 256 cases (65.5%), 116 women (63.4%) and 140 men (67.6%); from 7 to 12 hours, 66 cases (16.9%), 35 women (19.1%) and 31 men (15.0%); from 13 to 24 hours, 37 cases (9.5%), 17 women (9.3%) and 20 men (9.7%); and >24 hours, 31 cases (7.9%), 15 women (8.2%) and 16 men (7.67%). These differences were not significant (p = 0.730).
Regarding stroke care analysis, 467 patients were admitted to hospital (45 died in the emergency room, 2 were discharged home, and one was discharged to another hospital). Among these 467 patients, 376 (80.5%) were admitted to the monitored acute stroke unit or neurointensive acute unit, 162 women (74.7%) and 214 men (85.6%); 91 (19.5%) were admitted to the neurologic ward or other hospital departments, 55 women (25.3%) and 36 men (14.4%). Comparing women to men, the OR for admission to a monitored acute stroke unit or neurointensive acute unit was 0.50 (95% CI 0.31–0.79), p = 0.003. After age and previous mRS adjustment, the difference disappeared (female sex, OR 0.62 [95% CI 0.38–1.01], p = 0.056; age, OR: 0.98 [95% CI 0.96–1.00], p = 0.052; and previous mRS, OR 0.73 [95% CI 0.62–0.086], p < 0.0001). There were no sex differences in mortality among patients admitted to the monitored acute stroke unit or neurointensive acute unit (36.9% of men and 32.7% of women died; p = 0.445) or to other medical wards (41.7% of men and 49.1% of women died), p = 0.525. In total, 44 patients (8.5%) were neurosurgically treated, with no significant sex differences: 19 (7.7%) women and 25 (9.3%) men, p = 0.533. There were no sex differences in referral for in-hospital rehabilitation therapy (65.2% vs 65.8%, respectively; p = 0.910).
Among survivors, 47.8% of women and 42.6% of men returned home, 21.7% and 34.6% went to rehabilitation departments, and 30.4% and 22.8% were transferred to nursing homes, respectively. The differences were statistically significant (p = 0.041), but disappeared after adjusting for age (p = 0.201) or ICH score (p = 0.122).
Outcome data are summarized in table 2. ICH 3-month mortality was 42.5%, with no sex differences. Adjusted by previous mRS and ICH score, 3-month mortality rates were lower in women than in men, but this observation was not significant (OR 0.71 [95% CI 0.42–1.24], p = 0.211). There were no sex differences regarding the time interval from hospital admission to death, divided into 4 groups (within 24 hours of admission [41.1% in women vs 34.2% in men], 25 hours to 7 days [32.7% vs 37.8%], 7–30 days [24.3% vs 23.4%], and more that 30 days [1.9% vs 4.5%]; p = 0.515). The differences remained nonsignificant after adjustment by ICH score (p = 0.456) or by NIHSS (p = 0.506).
Table 2.
Outcome according to sex in patients with primary intracerebral hemorrhage
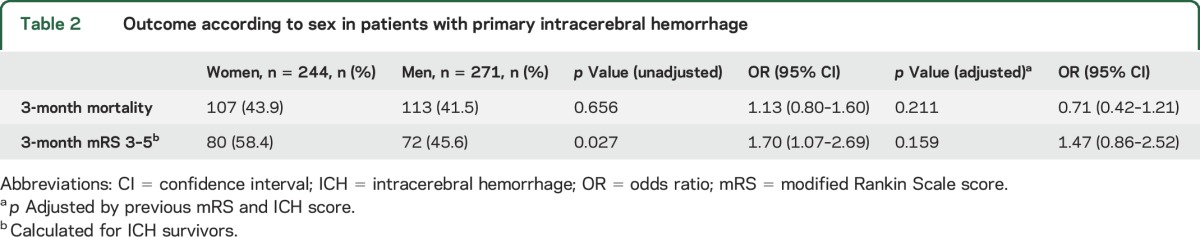
Regarding disability, 152 patients (28%) were functionally dependent at 3 months, with worse outcomes in women than in men (OR 1.70 [95% CI 1.07–2.69], p = 0.027). Adjusted by previous mRS and ICH score, the statistical differences disappeared (OR 1.47 [95% CI 0.86–2.52], p = 0.159).
DISCUSSION
Our study demonstrated some sex-related clinical differences in a large database of ICH patients, and adds accurate data to a topic with scant information available in the current literature.
Women constituted 47.6% of all primary ICH patients admitted to our center, were an average 6 years older than men, and were more likely to have previous poor functional status than men. Men reported more alcohol and tobacco use and had more atherosclerotic disease. Most of these data have been reported previously, including the higher ICH prevalence in men,19 although this finding is not uniform in all studies.4 In most of the previous reports, women with ICH were older than their male counterparts,12,18,20 similar to the findings for ischemic stroke.21 The higher tobacco and alcohol use we observed in men also has been described previously22 and probably reflects behavioral and cultural sex differences rather than a sex-specific risk factor. We found no previous reports of worse previous functional status in women than men, but this finding was probably due to the age difference because the difference disappeared after age adjustment. Similarly, the higher prevalence of peripheral arterial disease in men has not been described, but it seems logical because of the higher atherosclerotic prevalence in smokers.23 Despite sex-related differences in age and previous functional status, ICH severity (NIHSS score) was equal in both sexes. However, the ICH score was higher in women than in men (p = 0.018); again, this was probably due to the age difference because the difference disappears when adjusted by age (p = 0.456). Lobar ICH volumes were larger than deep volumes (median [IQR 25–75] = 49.9 cm3 [17.1–90.0] vs 9.6 cm3 [3.1–27.2]; p < 0.0001). Although ICH volume was slightly greater in women than in men, when we analyzed lobar or deep hematoma volumes separately, we found no sex differences, either in lobar or in deep hematomas. That means that the slight sex difference merely reflects the higher number of lobar hematomas in women compared with men.
We also found sex differences in hematoma location, with a predilection for deep (basal ganglia, thalamus, or putamen) hematomas (53.0% vs 39.2%) in men and lobar hematomas (44.5% vs 31.9%) in women (p = 0.012). We found no sex differences in cerebellar or other ICH locations. Similar results have been reported,24 although sex differences in hematoma location seem to vary by population.5 In our population, lobar ICH in women had an OR of 1.75 (95% CI 1.18–2.58) compared to men (p = 0.005) after age adjustment, suggesting a sex-related difference that merits further exploration. Concerning ICH etiology, there were no sex differences, and the frequency of hypertensive hemorrhage and CAA was similar in the sexes. Although women were older and had more lobar ICH than men, we found no differences in CAA etiology. We cannot explain this finding, but CAA may be underdiagnosed in women, because the percentage of lobar ICH of unknown etiology was greater in women (23.6%) than in men (16.3%). On the other hand, there was no age difference between sexes in patients with CAA diagnoses.
Studies analyzing sex differences in stroke care among patients with ICH are scarce and mainly centered on the use of do not resuscitate orders, with conflicting results.20,25 This information was not available in our study. We found that women were admitted to the monitored acute stroke unit or neurointensive acute unit less often than men (74.7% vs 85.6%, OR 0.50 [95% CI 0.31–0.79], p = 0.003); however, this difference disappeared after adjusting for age and previous mRS (p = 0.056). No sex differences were seen in other stroke care parameters, such as neurosurgical treatment (p = 0.533) or in-hospital rehabilitation therapy (p = 0.910). Regarding discharge destination, we found no sex differences after adjusting by age (p = 0.201) or ICH score (p = 0.122).
Published outcomes data show a wide range of mortality rates, from 16.2% to 51.8%,5 with conflicting results regarding sex differences. While a recent meta-analysis found no sex differences in mortality rates,4 other studies have shown higher mortality in women than in men,14,26 or in men than in women.11 In our study, mortality at 3 months was 42.5%, within the range (median 10-month case fatality of 40.4%) described in the literature for occidental patients.4 We found no absolute (44.1% vs 41.1%, p = 0.515) or adjusted differences in 3-month mortality between sexes, also agreeing with the results described in the literature.4 After adjustment for previous mRS and ICH score, however, there was a nonsignificant lower mortality in women. Concerning outcome, women remained more dependent than men at 3 months in the bivariate analysis. However, this difference was not significant after adjusting for previous mRS and ICH score (OR 1.47 [95% CI 0.86–2.52], p = 0.152). When we analyzed the percentage of patients who were functionally independent at 3 months, our results were similar to those described in the literature4: 27.9% in the whole series, with fewer women (23.3%) than men (32.2%). This information is important because of the paucity of studies on this question.4,5
The limitations of this study are its single-center design and the lack of long-term outcome data. The strengths are its prospective design, the comprehensive data, and the inclusion of all patients admitted to our hospital in the analysis, even those who died during the first hours in the emergency room and those admitted to departments other than neurology.
Sex-related differences in demographic and vascular risk factors and location exist. Although ICH score is higher in women, 3-month mortality and adjusted 3-month disability did not differ between sexes.
Supplementary Material
ACKNOWLEDGMENT
Elaine M. Lilly, PhD, Writers First Aid, provided assistance in the English translation and copyediting of the manuscript.
GLOSSARY
- CAA
cerebral amyloid angiopathy
- CI
confidence interval
- GCS
Glasgow Coma Scale
- ICH
intracerebral hemorrhage
- IQR
interquartile range
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- OR
odds ratio
Footnotes
Editorial, page 244
AUTHOR CONTRIBUTIONS
Jaume Roquer: design, data interpretation, statistical analysis, writing, and revising the manuscript. Ana Rodríguez-Campello, Jordi Jiménez-Conde, Elisa Cuadrado-Godia, Eva Giralt-Steinhauer, Rosa M. Vivanco Hidalgo, Carol Soriano, and Angel Ois contributed to data interpretation and revising the manuscript.
STUDY FUNDING
Supported in part by Spain's Ministry of Health (Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III FEDER, RD12/0042/0020).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med 2001;344:1450–1460. [DOI] [PubMed] [Google Scholar]
- 2.Flaherty ML, Haverbusch M, Sekar P, et al. Long-term mortality after intracerebral hemorrhage. Neurology 2006;66:1182–1186. [DOI] [PubMed] [Google Scholar]
- 3.Sansing LH, Messe SR, Cucchiara BL, Cohen SN, Lyden PD, Kasner SE; for the CHANT Investigators. Prior antiplatelet use does not affect hemorrhage growth or outcome after ICH. Neurology 2009;72:1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–176. [DOI] [PubMed] [Google Scholar]
- 5.Gokhale S, Caplan LR, James ML. Sex differences in incidence, pathophysiology, and outcome of primary intracerebral hemorrhage. Stroke 2015;46:886–892. [DOI] [PubMed] [Google Scholar]
- 6.Umeano O, Phillips-Bute B, Hailey CE, et al. Gender and age interact to affect early outcome after intracerebral hemorrhage. Plos One 2013;8:e81664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franke CL, van Swieten JC, Algra A, van Gijn J. Prognostic factors in patients with intracerebral haematoma. J Neurol Neurosurg Psychiatry 1992;55:653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang LF, Yang J, Hong Z, et al. Collaborative Group of China Multicenter Study of Cardiovascular Epidemiology. Proportion of different subtypes of stroke in China. Stroke 2003;34:2091–2096. [DOI] [PubMed] [Google Scholar]
- 9.Sheikh K, Bullock CM. Effect of measurement on sex difference in stroke mortality. Stroke 2007;38:1085–1087. [DOI] [PubMed] [Google Scholar]
- 10.Vaartjes I, Reitsma JB, Berger-van Sijl M, Bots ML. Gender differences in mortality after hospital admission for stroke. Cerebrovasc Dis 2009;28:564–571. [DOI] [PubMed] [Google Scholar]
- 11.Zia E, Engström G, Svensson PJ, Norrving B, Pessah-Rasmussen H. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke 2009;40:3567–3573. [DOI] [PubMed] [Google Scholar]
- 12.Ganti L, Jain A, Yerragondu N, et al. Female gender remains an independent risk factor for poor outcome after acute nontraumatic intracerebral hemorrhage. Neurol Res Int 2013;2013:219097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura Y, Takishita S, Muratani H, et al. Demographic study of first-ever stroke and acute myocardial infarction in Okinawa, Japan. Intern Med 1998;37:736–745. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson OG, Lindgren A, Brandt L, Säveland H. Prediction of death in patients with primary intracerebral hemorrhage: a prospective study of a defined population. J Neurosurg 2002;97:531–536. [DOI] [PubMed] [Google Scholar]
- 15.Yesilot NF, Koyuncu BA, Coban O, Tuncay R, Bahar SZ. Gender differences in acute stroke: Istanbul medical school stroke registry. Neurol India 2011;59:174–179. [DOI] [PubMed] [Google Scholar]
- 16.Wang WJ, Lu JJ, Wang YJ, et al. China National Stroke Registry (CNSR). Clinical characteristics, management, and functional outcomes in Chinese patients within the first year after intracerebral hemorrhage: analysis from China National Stroke Registry. CNS Neurosci Ther 2012;18:773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roquer J, Rodríguez-Campello A, Gomis M, et al. Acute stroke unit care and early neurological deterioration in ischemic stroke. J Neurol 2008;255:1012–1017. [DOI] [PubMed] [Google Scholar]
- 18.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996:27:1304–1305. [DOI] [PubMed] [Google Scholar]
- 19.Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke 2009;40:1082–1090. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa K, Vento MA, Seto TB, et al. Sex differences in the use of early do-not-resuscitate orders after intracerebral hemorrhage. Stroke 2013;44:3229–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roquer J, Rodríguez Campello A, Gomis M. Sex differences in first-ever acute stroke. Stroke 2003;34:1581–1585. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, Zhang Y, Arima H, et al. Sex differences in clinical characteristics and outcomes after intracerebral haemorrhage: results from a 12-month prospective stroke registry in Nanjing, China. BMC Neurol 2014;14:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014;34:509–515. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Sandoval JL, Chiquete E, Gárate-Carrillo A, et al. RENAMEVASC investigators. Spontaneous intracerebral hemorrhage in Mexico: results from a multicenter nationwide hospital-based registry on cerebrovascular disease (RENAMEVASC). Rev Neurol 2011;53:705–712. [PubMed] [Google Scholar]
- 25.Zahuranec DB, Brown DL, Lisabeth LD, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology 2007;68:1651–1657. [DOI] [PubMed] [Google Scholar]
- 26.Thrift AG, Dewey HM, Sturm JW, et al. Incidence of stroke subtypes in the North East Melbourne Stroke Incidence Study (NEMESIS): differences between men and women. Neuroepidemiology 2009;32:11–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


