Abstract
Objective:
This study sought to confirm the relationship between the degree of blood–brain barrier (BBB) damage and the severity of intracranial hemorrhage (ICH) in a population of patients who received endovascular therapy.
Methods:
The degree of BBB disruption on pretreatment MRI scans was analyzed, blinded to follow-up data, in the DEFUSE 2 cohort in which patients had endovascular therapy within 12 hours of stroke onset. BBB disruption was compared with ICH grade previously established by the DEFUSE 2 core lab. A prespecified threshold for predicting parenchymal hematoma (PH) was tested.
Results:
Of the 108 patients in the DEFUSE 2 trial, 100 had adequate imaging and outcome data and were included in this study; 24 developed PH. Increasing amounts of BBB disruption on pretreatment MRIs was associated with increasing severity of ICH grade (p = 0.004). BBB disruption on the pretreatment scan was associated with PH (p = 0.020) with an odds ratio for developing PH of 1.69 for each 10% increase in BBB disruption (95% confidence interval 1.09–2.64), although a reliably predictive threshold was not identified.
Conclusions:
The amount of BBB disruption on pretreatment MRI is associated with the severity of ICH after acute intervention. This relationship has now been identified in patients receiving IV, endovascular, and combined therapies. Further study is needed to determine its role in guiding treatment.
Recent studies have validated the use of endovascular therapy in the treatment of acute stroke.1–5 Initial studies that failed to show benefit of endovascular therapy omitted imaging-based selection criteria.6,7 The first trial to demonstrate that imaging could be used to select a population of patients who would benefit from endovascular therapy was the DEFUSE 2 trial.8 Although the MR RESCUE study subsequently challenged the utility of imaging selection,9 post hoc comparisons of these 2 trials have identified several limitations to the MR RESCUE trial that likely accounted for its negative results.10,11
The first randomized trial to show benefit simply used large vessel occlusion for imaging selection.1 The absolute benefit of endovascular therapy was greater in subsequent trials that used imaging to exclude patients with large ischemic core or poor collateral blood flow.2,3,5 The DEFUSE 2 trial identified an MRI profile most likely to benefit from endovascular therapy simply using lesion volumes from diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI). However, multimodal MRI contains additional information that may be harnessed to improve patient selection. One such measure is the integrity of the blood–brain barrier (BBB).
BBB disruption of the ischemic core has been associated with hemorrhagic transformation and poor outcome.12–14 In patients receiving IV tissue plasminogen activator (tPA), an association between the amount of pretreatment BBB disruption and the severity of intracranial hemorrhage (ICH) after treatment has been identified.15 The purpose of this study was to determine whether such a relationship exists in patients undergoing endovascular therapy.
METHODS
This collaborative study was initiated in an attempt to validate a prior finding. To do so, one investigator, R.L., who was not involved in the DEFUSE 2 trial and who did not have access to outcome data from the trial, performed the BBB calculations using the previously described methodology.15 This investigator remained blinded to outcome data until quantitative BBB results were submitted to the DEFUSE 2 investigators. This report describes the relationship between that blinded analysis and the imaging outcome measures of the DEFUSE 2 core lab, which had been previously determined.
Standard protocol approvals, registrations, and patient consents.
Approval for the study was obtained from local institutional review boards. Written informed consent was provided by all patients or a legally authorized representative. The DEFUES 2 clinical trial identifier number is NCT01327989. Deidentified analysis of the DEFUSE 2 dataset was excluded from institutional review board review by the Office of Human Subjects Research Protections of the NIH.
Population.
The DEFUSE 2 study population has been previously described in detail.8 Briefly, the DEFUSE 2 trial was a multicenter prospective cohort study in which MRI was collected before endovascular therapy but not used to make treatment decisions. Patients could receive IV tPA before enrollment, and the time window for enrollment was up to 12 hours from symptom onset. MRI scanning included DWI and PWI. Patients who received IV tPA before their MRI were eligible for the study, and the type of endovascular care delivered was at the discretion of the clinical team treating the patient.
Blood–brain permeability analysis.
PWI is performed using dynamic susceptibility contrast (DSC) imaging. This method collects T2*-weighted images of the brain at a frequency of 1 to 2 seconds during the injection of a gadolinium contrast agent. Intravascular contrast causes a drop in the recorded signal because of susceptibility effects of the contrast agent. The recorded signal can be processed to yield a gadolinium concentration curve from which blood flow measures can be extracted. This curve typically shows an initial peak with the first pass of the bolus, sometimes a second smaller peak with recirculation, and typically thereafter a constant progression to the baseline for the remainder of the sequence acquisition.
When contrast crosses the BBB, the recorded signal is altered in a manner that is proportional to the concentration of gadolinium accumulation in the tissue.16 This signal is caused by the mild T1-weighting of a predominantly T2*-weighted sequence. When present, this signal is opposite to the T2* effect. Thus, when compared to normal tissue, the concentration curve in a voxel with BBB damage will approach the baseline faster and may fall below it. Using normal tissue as a comparison, the effect of BBB disruption can be modeled and a measure of permeability, often referred to as K2, can be derived.17
In patients with a perfusion deficit, using DSC permeability methods is more challenging. Comparing the recorded signal of normal tissue with tissue that has both diminished blood flow and BBB disruption requires additional processing to separate the effects. To address this issue, an arrival time correction method was developed to remove the effects of blood flow before calculating the permeability signal.18 The resulting blood–brain permeability image (BBPI) is a modified version of K2 in which percent leakage is generated on a voxel-by-voxel basis. Although these values are unitless, they reflect what fraction of the measured cerebral blood volume was lost because of the T1 effect.
For this study, the DSC source data from the pre-endovascular MRI PWI scans were provided to the blinded investigator. Using the previously described method, BBPIs were generated for each patient.15 Patients with inadequate or unsuccessful PWI were excluded. Regions of interest (ROIs) generated by the DEFUSE 2 core lab based on coregistered DWIs were provided to the blinded investigator. A DWI-based ROI was used in this study instead of a PWI-based ROI that was used in the prior study.15 The rationale for this was that BBB disruption related to ischemia should occur in DWI-positive tissue. Mean permeability derangement was calculated within the ROI for each patient. Mean permeability derangement was the mean value of voxels within the ROI whose permeability was greater than 2 SDs above that of normal tissue. This method was used, as in the prior study, to capture areas of focal BBB disruption rather than a diffuse mean of the entire ROI. Mean permeability derangement values for each patient were submitted to the DEFUSE 2 investigators before unblinding.
Outcome analysis.
The primary outcome for this study was severity of ICH. ICH was graded by the DEFUSE 2 core lab based on ECASS (European Cooperative Acute Stroke Study) criteria.19 Patients were separated into 4 groups based on this outcome: no ICH (no-ICH), hemorrhagic infarct type 1 (HI1), hemorrhagic infarct type 2 (HI2), parenchymal hematoma type 1 (PH1), and parenchymal hematoma type 2 (PH2). These gradings were based on hemosiderin-weighted MRI scans that are more sensitive than head CT in detecting hemorrhagic transformation. Thus, this classification is not directly comparable to that of the original ECASS trial that used head CT.
Statistical analysis.
The mean permeability derangement (independent variable) of the outcome groups (dependent variable) was compared to detect statistically significant differences. As in the prior study, the relationship between these 2 variables was assessed using linear regression. However, since the relationship between these variables may not be linear because the dependent variable is ordinal but not necessarily interval, the relationship was also tested using a multinomial logistic regression in which each hemorrhage grade was independently compared with the no-hemorrhage group.
Since the development of a PH is more likely to be clinically significant than other patterns of hemorrhagic transformation,20 the data were also dichotomized into 2 groups, PH1 + PH2 and no-ICH + HI1 + HI2. Using these 2 groups, a logistic regression was performed to assess the risk of PH with increasing permeability measures.
A prespecified permeability threshold of 21%, identified in the prior study as being potentially predicative of developing PH,15 was tested for sensitivity, specificity, and positive predictive value. In addition, a receiver operator characteristic (ROC) curve was calculated to test the performance of all possible thresholds in predicting PH.
RESULTS
Imaging data were provided on 108 patients from the DEFUSE 2 trial. Of these MRI scans, 102 had adequate PWI source images to perform the BBPI analysis. Of the 102 patients processed, 100 patients had outcome data available and were included in the final analysis. Table 1 shows the distribution of the population broken down into ICH classifications. HI occurred in 33 patients and PH in 24. Fifty-three percent of the patients received IV tPA before their MRI scan and before endovascular therapy.
Table 1.
Distribution of the population across hemorrhage grades and the median permeability and IQR for each group
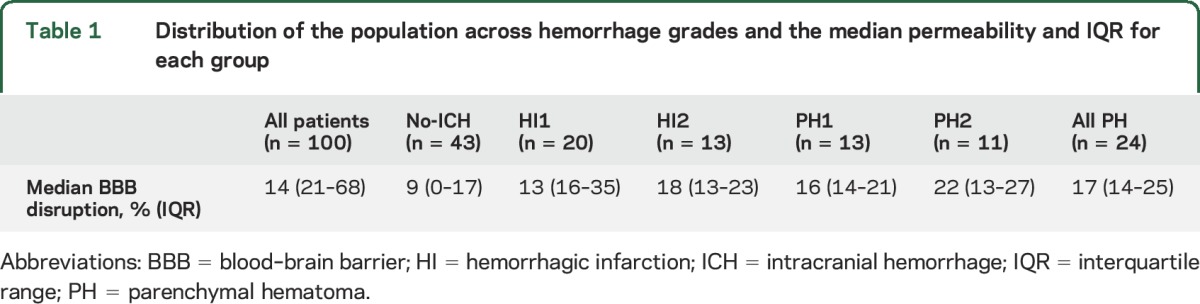
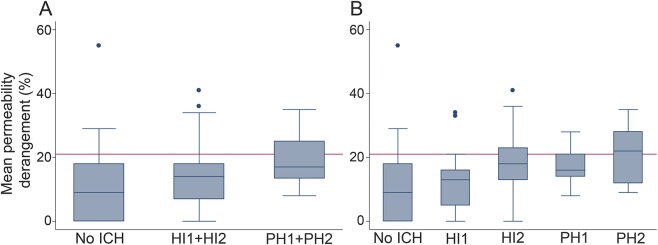
Figure 1A shows a boxplot for the mean permeability grouped by no-ICH, HI1 + HI2, and PH1 + PH2 as was performed in the prior IV tPA study.15 The relationship between increasing permeability and severity of ICH was again significant when evaluated with linear regression (p = 0.004). However, in the current analysis, the previously identified threshold of 21% (shown as a red line in figure 1) for separating PH from HI is above the median value for PH, suggesting that lower levels of BBB disruption are associated with PH formation after endovascular therapy in this later time window.
Figure 1. A comparison of the pretreatment blood–brain barrier disruption with the posttreatment hemorrhage grade.
(A) Boxplots for the mean permeability grouped by no-ICH, HI1 + HI2, and PH1 + PH2. (B) Boxplots for each group individually. The red line on both graphs is the 21% threshold identified in the prior study as potentially being predictive of PH. HI = hemorrhagic Infarction; ICH = intracranial hemorrhage; PH = parenchymal hematoma.
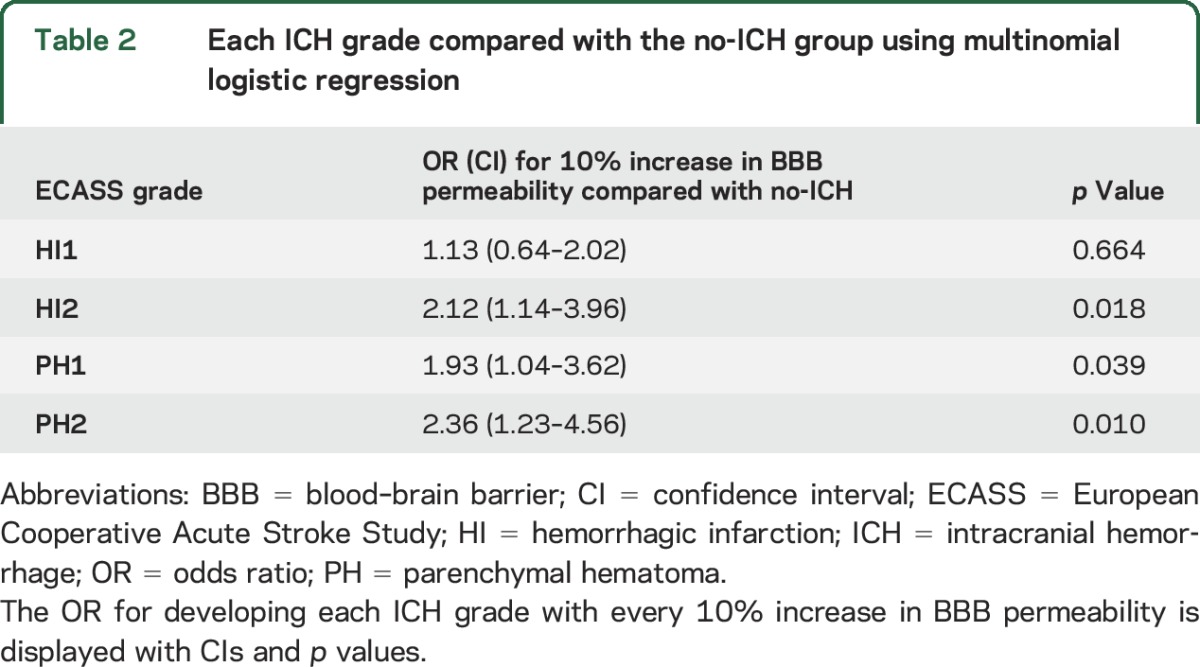
Figure 1B shows a further separation of the outcomes by individual ICH gradings. In this boxplot, it is evident that mean permeability does not differentiate well between HI2 and PH1, as the median for PH1 falls below that of HI2. However, comparing each ICH grade with the no-ICH group using multinomial logistic regression found all groups to be at significantly increased risk of hemorrhage with the exception of HI1, the mildest form of ICH. Table 2 shows the odd ratios of each ICH grade compared with no-ICH.
Table 2.
Each ICH grade compared with the no-ICH group using multinomial logistic regression
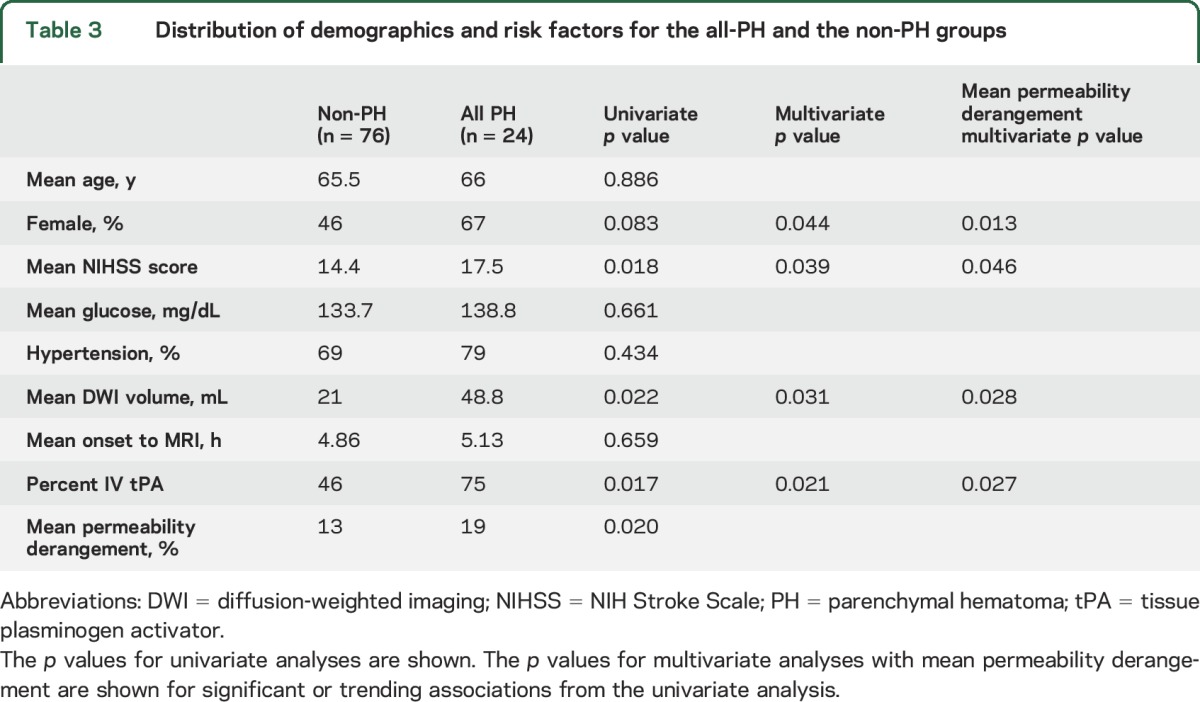
To compare the group of ICHs that are more likely to be clinically significant with all others, PH (PH1 + PH2) was compared with no PH (no-ICH + HI1 + HI2) using logistic regression. The odds ratio for each 10% increase in BBB disruption was 1.69 (95% confidence interval 1.09–2.64, p = 0.020). BBB disruption was also compared with several other potential predictors of PH detailed in table 3. Of these, NIH Stroke Scale, DWI stroke volume, pretreatment IV tPA, and female sex showed a possible association (p < 0.10) with PH. Multivariate analysis of each of these with BBB permeability found all to be independent predictors of PH as was BBB permeability in each case. When all of these were combined into one multivariate model, only BBB permeability remained significant (p = 0.048).
Table 3.
Distribution of demographics and risk factors for the all-PH and the non-PH groups
Using the prespecified permeability threshold of 21% to predict PH resulted in a sensitivity of 0.375, specificity of 0.803, and positive predictive value of 0.375. However, these results must be taken in the context of the incidence of PH, which was 24/100 = 0.24. ROC analysis found an area under the curve of 0.68.
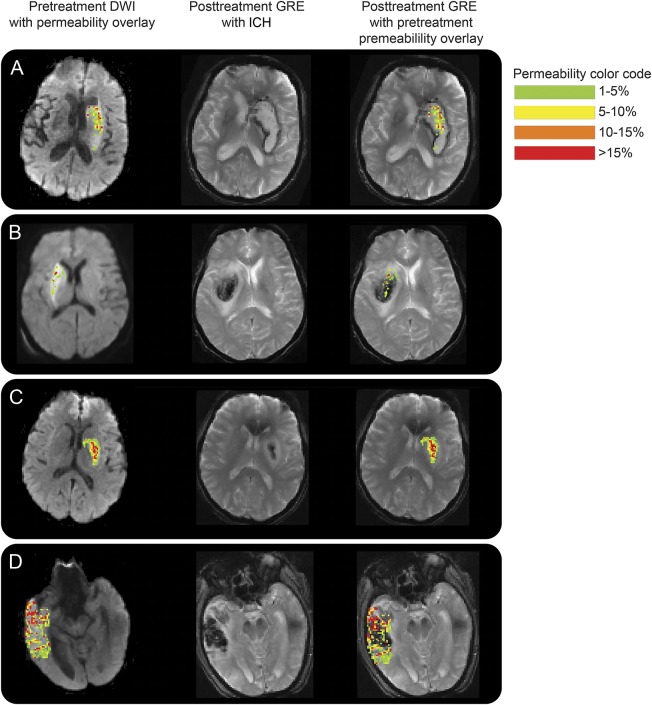
Figure 2 shows examples of the BBPI of 4 patients who went on to develop ICH after endovascular therapy. The pretreatment DWI, pretreatment BBPI, and follow-up gradient echo (GRE) images are all coregistered. The first column shows the acute DWI scans with the color permeability map overlaid on top. The second column shows the follow-up GRE images demonstrating ICH. The third column has the pretreatment color permeability maps overlaid on the follow-up GRE. Patient A had a mean permeability derangement of 28% and experienced a PH as predicted by the 21% threshold from the prior study. Patient B had a lower mean permeability derangement of 9% but still experienced a PH. Patient C had a mean permeability of 18%, thus was below the PH threshold, and only had HI. Patient D, however, had a very high mean permeability of 41% but yet did not have PH, only HI. These cases demonstrate that while BBPI may not be predictive of PH when interpreted using a threshold, the locations of the BBB disruption align well with the locations of subsequent ICH.
Figure 2. Examples of pretreatment blood–brain barrier disruption and posttreatment ICH.
This figure shows examples of the blood–brain permeability imaging of 4 patients who went on to develop ICH after endovascular therapy. The first column shows the acute DWI scans with the color permeability map overlaid on top. The second column shows the follow-up GRE images with ICH. The third column has the pretreatment color permeability maps overlaid on the follow-up GRE. (A) This patient had a mean permeability derangement of 28% and experienced a PH. (B) This patient had a lower mean permeability derangement of 9% but still experienced a PH. (C) This patient had a mean permeability of 18%, thus was below the PH threshold, and only had HI. (D) This patient had a very high mean permeability of 41% but did not have PH, only HI. DWI = diffusion-weighted imaging, GRE = gradient recalled echo; HI = hemorrhagic infarction; ICH = intracranial hemorrhage; PH = parenchymal hematoma.
DISCUSSION
This study supports the previously identified dose-dependent relationship between the amount of pretreatment BBB disruption and the severity of posttreatment ICH by analyzing a larger number of patients entered into a carefully controlled trial. Furthermore, by analyzing a new population and treatment type, we have shown that this association appears to be independent of the type of acute treatment delivered or the time window of treatment since IV, IA, and IV + IA therapies have now been examined. Whether or not this relationship exists in the absence of any acute intervention has not been studied.
Unlike the previous study,15 in which there were very few PH1s, this cohort had a fairly even distribution of HI1s, HI2s, PH1s, and PH2s, which allowed for a comparison of these individual groups. This revealed that permeability measures did not discriminate well between HI2s and PH1s. This may reflect the difficulty in visually discriminating between these gradings when assessing outcome on follow-up imaging. Alternately, this finding may suggest that the underlying tissue injury for these 2 types of ICH is similar.
The exact mechanism of BBB disruption in acute stroke remains unclear and may involve multiple pathways. Animal models of ischemia have identified a biphasic pattern to BBB disruption.21 Within hours, there is an early, potentially reversible, BBB disruption that is distinct from the delayed, inflammatory BBB disruption that occurs on the order of days.22 The clinical implications of these 2 types of BBB disruption may be quite different. The existing literature on BBB disruption in human acute ischemic stroke often does not give attention to this distinction, thereby making interpretation of the results difficult.
The present study measured BBB disruption within 12 hours of onset, thus it is most likely representative of the early phase. As also shown in the prior IV tPA study,15 the detection of BBB disruption was not uncommon, even in patients who did not go on to develop any hemorrhage at all. However, the key finding in both of these studies is that severe BBB disruption is associated with PH formation, an imaging outcome that carries the greatest risk of clinical deterioration. One interpretation is that mild BBB disruption reflects a reversible BBB dysfunction while severe BBB disruption is indicative of BBB rupture. While the former may be related to failure of energy-dependent BBB machinery, the latter may reflect destruction of the endothelium.
Ideally, BBB disruption detected on MRI before treatment would be used to guide treatment decisions. Specifically, patients at high risk of PH formation would be excluded from therapy. Conversely, patients who currently may go untreated because of other factors may be identified as safe for treatment based on BBB analysis. In the current study, however, a BBB “rupture threshold” suggested by the earlier study15 was not found and an ROC analysis of all thresholds did not perform in a manner that would be likely to guide therapy because of a low positive predictive value. This may be attributable in part to factors unlikely to be captured in a pretreatment MRI such as procedural complications or degree of recanalization. We also identified several other predictors of PH formation that were independent of the BBB effect.
It also may be that the exact method of BBB quantification used in this study is not the best measure of ICH risk. The approach used focused on focal areas of severe disruption rather than the total volume of tissue with increased BBB permeability. This was based on observations from the prior study in which even small areas of focal, severe BBB disruption carried a high risk of PH formation with IV tPA. The current study was not designed to identify the best method for BBB detection but rather to test a prespecified hypothesis. Future analyses will focus on understanding the relationship between BBB and ICH in an attempt to improve the predictive value of this measure. Combining BBB permeability with other measures of tissue injury may yield the best model for predicting ICH grade after treatment.
The strength of this study was in the use of a blinded analysis to evaluate a prior finding in a unique and well-documented dataset. However, there are some limitations to this study. BBPI is a relatively new method for quantifying BBB disruption. Although several studies have used PWI source images to measure BBB leakage of gadolinium,12,14 the method used in this and the prior study has not yet been replicated by other investigators. Studies are under way to validate this method against dynamic contrast enhance imaging and to determine the role that scan parameters, such as echo time, have in the measured signal.
This study expands our appreciation of the relationship between BBB disruption and posttreatment hemorrhagic transformation. More research is needed to understand this new information such that it may be clinically applicable.
Supplementary Material
GLOSSARY
- BBB
blood–brain barrier
- BBPI
blood–brain permeability image
- DSC
dynamic susceptibility contrast
- DWI
diffusion-weighted imaging
- ECASS
European Cooperative Acute Stroke Study
- GRE
gradient echo
- HI
hemorrhagic infarct
- ICH
intracranial hemorrhage
- PH
parenchymal hematoma
- PWI
perfusion-weighted imaging
- ROC
receiver operator characteristic
- ROI
region of interest
- tPA
tissue plasminogen activator
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: DEFUSE 2 Investigators, Jeffery Saver, Pierre Fayad, George Howard, Thomas Tomsick, Tudor Jovin, Lawrence Wechsler, Sharon DeCesare, Gregory Albers, Maarten Lansberg, Stephanie Kemp, D Thai, Michael Wilder, A Tricot, A Sherr, Helmi Lutsep, Logan McDaneld, Darren Larsen, Todd Czartoski, Bart Keogh, Amer Malik, A Brown, Richard Bernstein, K Muskovich, Cherylee Chang, T Stern, Steven Warach, Lisa Davis, Franz Fazekas, Thomas Seifert-Held, Gregory Albers, Greg Zaharchuk, Michael Marks, Aaryani Tipirneni, and Michael Mlynash
AUTHOR CONTRIBUTIONS
Dr. Leigh: primary author, analysis and interpretation. Dr. Christensen: analysis and interpretation. Dr. Campbell: analysis and interpretation. Dr. Marks: study design and supervision. Dr. Albers: study design and supervision. Dr. Lansberg: study concept, design and supervision, analysis and interpretation.
STUDY FUNDING
Dr. Leigh is supported by the Intramural Program of NIH, NINDS. Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2 (DEFUSE 2) was funded by grants from the National Institute for Neurological Disorders and Stroke (R01 NS03932505 to Dr. Albers and K23 NS051372 to Dr. Lansberg). Dr. Campbell reports funding of the National Health and Medical Research Council of Australia.
DISCLOSURE
R. Leigh reports no disclosures relevant to the manuscript. S. Christensen is a consultant for IschemaView Inc. B. Campbell reports no disclosures relevant to the manuscript. M. Marks reports no disclosures relevant to the manuscript. G. Albers is a consultant for iSchemaView Inc. and a shareholder of iSchemaView Inc.; advisory board: Covidien and Lundbeck. M. Lansberg reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. New Engl J Med 2015;372:1009–1030. [DOI] [PubMed] [Google Scholar]
- 4.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-pa vs. t-pa alone in stroke. N Engl J Med 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 6.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-pa versus t-pa alone for stroke. N Engl J Med 2013;368:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med 2013;368:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 2012;11:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013;368:914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsons MW, Albers GW. MR RESCUE: is the glass half-full or half-empty? Stroke 2013;44:2055–2057. [DOI] [PubMed] [Google Scholar]
- 11.Leigh R, Urrutia VC, Llinas RH, Gottesman RF, Krakauer JW, Hillis AE. A comparison of two methods for MRI classification of at-risk tissue and core infarction. Front Neurol 2014;5:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bang OY, Buck BH, Saver JL, et al. Prediction of hemorrhagic transformation after recanalization therapy using T2*-permeability magnetic resonance imaging. Ann Neurol 2007;62:170–176. [DOI] [PubMed] [Google Scholar]
- 13.Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke 2004;35:2659–2661. [DOI] [PubMed] [Google Scholar]
- 14.Scalzo F, Alger JR, Hu X, et al. Multi-center prediction of hemorrhagic transformation in acute ischemic stroke using permeability imaging features. Magn Reson Imaging 2013;31:961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leigh R, Jen SS, Hillis AE, et al. Pretreatment blood-brain barrier damage and post-treatment intracranial hemorrhage in patients receiving intravenous tissue-type plasminogen activator. Stroke 2014;45:2030–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaharchuk G. Theoretical basis of hemodynamic MR imaging techniques to measure cerebral blood volume, cerebral blood flow, and permeability. AJNR Am J Neuroradiol 2007;28:1850–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 2006;27:859–867. [PMC free article] [PubMed] [Google Scholar]
- 18.Leigh R, Jen SS, Varma DD, Hillis AE, Barker PB. Arrival time correction for dynamic susceptibility contrast MR permeability imaging in stroke patients. PLoS One 2012;7:e52656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–1025. [PubMed] [Google Scholar]
- 20.Thomalla G, Sobesky J, Kohrmann M, et al. Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke 2007;38:313–318. [DOI] [PubMed] [Google Scholar]
- 21.Kuroiwa T, Ting P, Martinez H, Klatzo I. The biphasic opening of the blood-brain barrier to proteins following temporary middle cerebral artery occlusion. Acta Neuropathol 1985;68:122–129. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 2011;42:3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.