Abstract
Mechanical sensitivity is commonly affected in chronic pain and other neurological disorders. To discover mechanisms of individual differences in punctate mechanosensation, we performed quantitative trait locus (QTL) mapping of the response to von Frey monofilament stimulation in BXD recombinant inbred (BXD) mice. Significant loci were detected on mouse chromosome (Chr) 5 and 15, indicating the location of underlying polymorphisms that cause heritable variation in von Frey response. Convergent evidence from public gene expression data implicates candidate genes within the loci: von Frey thresholds were strongly correlated with baseline expression of Cacna2d1, Ift27 and Csnk1e in multiple brain regions of BXD strains. Systemic gabapentin and PF-670462, which target the protein products of Cacna2d1 and Csnk1e, respectively, significantly increased von Frey thresholds in a genotype-dependent manner in progenitors and BXD strains. Real-time polymerase chain reaction confirmed differential expression of Cacna2d1 and Csnk1e in multiple brain regions in progenitors and showed differential expression of Cacna2d1 and Csnk1e in the dorsal root ganglia of the progenitors and BXD strains grouped by QTL genotype. Thus, linkage mapping, transcript covariance and pharmacological testing suggest that genetic variation affecting Cacna2d1 and Csnk1e may contribute to individual differences in von Frey filament response. This study implicates Cacna2d1 and Ift27 in basal mechanosensation in line with their previously suspected role in mechanical hypersensitivity. Csnk1e is implicated for von Frey response for the first time. Further investigation is warranted to identify the specific polymorphisms involved and assess the relevance of these findings to clinical conditions of disturbed mechanosensation.
Keywords: Casein kinase 1, linkage mapping, microarray, quantitative trait locus, transcript abundance, voltage-gated calcium channels, von Frey
Mechanosensation is a fundamental sensory trait that is often affected in patients with neurological disorders including chronic pain of inflammatory or neuropathic origin (Baron 2000). As such, understanding the molecular mechanisms underlying mechanosensation may have clinical importance and utility. Punctate mechanical sensitivity, hypersensitivity and painful hypersensitivity (allodynia) are commonly assessed in the clinic and in animal models of chronic pain with application of nylon von Frey monofilaments or other similar instruments to the skin within affected regions (Baron 2000). Mechanosensitivity in the von Frey test is highly variable and heritable, with 69% of the variability in sensitivity of standard inbred mouse strains explained by genetic factors (Lariviere et al. 2001, 2002; Mogil 1999; Mogil et al. 1999a). Similarly, nerve injury-induced mechanical hypersensitivity is also highly variable and heritable (Mogil et al. 1999a) and moderately genetically correlated with baseline mechanosensation in standard inbred mouse strains (Mogil et al. 1999b). Mechanosensitive receptors, ion channels and afferent fibers have been identified (Shin et al. 2003), but the genetic and molecular mechanisms underlying individual differences in mechanosensation have yet to be identified. Thus, the identification of polymorphic genes with functional effects on mechanosensation could provide new opportunities for the treatment of pain that involves altered mechanosensation.
Using mouse genetic reference populations (GRPs), genetic susceptibility loci and candidate genes underlying individual differences in pain traits have been identified and the responsible genes subsequently determined (Lacroix-Fralish & Mogil 2009). The GRPs such as recombinant inbred (RI) mice enable the simultaneous detection of genetic susceptibility loci, through quantitative trait locus (QTL) mapping of behavior, and correlated gene expression variation in tissues that may play a role in individual differences in complex traits (Chesler et al. 2003b). This study employs BXD RI mice to perform genome-wide QTL mapping, genetic correlation analysis with other behavioral and neurobiological traits, expression QTL and trait-transcript correlation analysis to identify genomic loci and candidate genes underlying individual differences in von Frey mechanosensitivity. Genomic regions containing candidate genes were significantly linked to mechanical sensitivity in the von Frey test, and convergent transcript covariance evidence was obtained for a subset of the candidate genes. Expression assays and pharmacological testing was performed to assess the role of compelling candidate genes.
Materials and methods
Experimental subjects
For QTL mapping and genetic correlation analysis, adult male mice of 26 BXD RI strains were tested for mechanical thresholds in the von Frey test (N = 204; n = 6–9/strain). The BXD RI strains were all ‘/TyJ’ substrains obtained from The Jackson Laboratory (Bar Harbor, ME, USA). The BXD RI strains have been created by crossing the standard inbred strains C57BL/6J and DBA/2J and re-inbreeding the F2 hybrid offspring (Taylor 1978) and have been maintained inbred since their creation.
Mice acclimated to the animal facilities for a minimum of 5 days before testing. Each procedure was approved by the Institutional Animal Care and Use Committee (IACUC) where it was performed. The testing of the BXD RI strains in the initial mapping study was performed at University of Texas Medical Branch-Galveston, pharmacological testing and tissue dissection from BXD strains was performed at The Jackson Laboratory, gene expression quantitative PCR was performed at University of Pittsburgh and pharmacological testing of congenic strains was conducted at University of Chicago. All experiments adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (Zimmermann 1983) and of the National Institutes of Health of the USA.
Assessment of mechanical sensitivity using von Frey withdrawal threshold testing
Mice were placed in transparent acrylic compartments (4 × 4 × 7 cm3) on a metal mesh floor for 20–30 min prior to testing. Mechanical sensitivity was measured on the plantar surface of the hind paws of habituated mice by application of von Frey-type monofilaments with bending forces of 0.03, 0.07, 0.16, 0.41, 1.00, 2.24, 5.50 g (Stoelting, Wood Dale, IL, USA) for 1–2 seconds with an inter-trial interval of 10 seconds. The up-down method (Chaplan et al. 1994) was used to determine the bending force to evoke paw withdrawal 50% of the time. One paw was completely tested prior to testing the other paw. The baseline thresholds for each hind paw were determined on 3 separate days. Because no effect of paw side or repeated testing was observed (see Results), the average of values for both paws and 3 days of testing were calculated and used for QTL mapping and genetic correlation analyses.
Heritability and QTL mapping
The effect of BXD RI mouse strain on mean von Frey withdrawal threshold was determined by repeated-measures analysis of variance (anova) with test day as the repeated measure, and by one-way anova of the 3-day averaged thresholds. Heritability was estimated by h2 = VA/(VA + VE), where VA is the additive genetic variation estimated by the between-strain variance and VE is the environmental variance estimated by the within-strain variance from the anova results (Falconer & Mackay 1996). Note that the additive genetic variance, when estimated using isogenic lines, also contains gene × gene and gene × environment interactions, and that environmental variance is confounded with other technical variation. While this estimate does not directly estimate the transmission of phenotypic values from parent to offspring, it provides an estimate of the resemblance among isogenic relatives.
To determine the location of genomic loci linked to variability in mechanosensation, whole-genome single locus QTL mapping using the BXD RI strain phenotypic means was performed in GeneNetwork.org, where the data have been deposited (GeneNetwork Record ID 11296). Regions of the genome containing polymorphisms associated with mechanical sensitivity were determined by Haley–Knott marker-trait regression analysis using the QTL detection software integrated with the GeneNetwork/WebQTL website (www.genenetwork.org; www.webqtl.org) (Wang et al. 2003) to construct an interval map. A total of 3795 non-redundant DNA markers polymorphic between the progenitor strains and among BXD RI strains and at loci throughout the genome were considered (June 2005 version; http://www.genenetwork.org/dbdoc/BXDGeno.html). The threshold logarithm of the odds (LOD) score for a significant QTL was determined using an empirical P-value from 1000 permutations of the strain means with a genome-wide error rate α = 0.05 (Churchill & Doerge 1994).
Genetic correlation analyses with traits and transcript levels
Because of the inbred status of the RI strains, their unchanging genotypes are allowing von Frey threshold means to be directly compared, using genetic correlation analysis, with all other traits obtained since the inception of the BXD RI lines, including expression of genes and many behaviors. Strong significant correlations among phenotypic values from large inbred strain panels indicate shared genetic regulatory mechanisms between the traits and correlations close to zero indicate genetic distinctiveness of the traits (Crabbe et al. 1990). To assess the genetic relatedness of mechanosensation to other traits, Pearson correlation coefficients (rp) were calculated between BXD RI von Frey threshold strain means and strain means of >4600 other complex traits available on the GeneNetwork website (http://www.genenetwork.org/dbdoc/BXDPublish.html) (Chesler et al. 2004; Wang et al. 2003). All phenotypic correlations were exported from GeneNetwork, and P-values were imported into the R/qvalue package (R Core Team 2013) for pointwise estimation of the false discovery rate for each result (Storey & Tibshirani 2003).
The QTL positional candidates were evaluated to identify polymorphic genes within the QTL region, and co-expressed genes within the region. Tissue-specific mRNA transcript levels provide the transcriptional context in which sensitivity to a stimulus is determined (McClearn 2006). Transcript covariance analysis was used to empirically reduce the list of positional candidate genes from QTL mapping by comparing von Frey mechanical threshold strain means of the BXD RI mice with mRNA expression in nine brain areas and the adrenal glands for which transcript expression profiles are available for numerous BXD RI strains (Chesler & Williams 2004; Chesler et al. 2003b, 2005; Peirce et al. 2003) (see Table S1, Supporting Information, for description of microarray datasets). Pearson product–moment correlations were calculated between von Frey thresholds and transcript expression in publicly available Affymetrix (Santa Clara, CA, USA), Illumina (San Diego, CA, USA) or Agilent (Santa Clara, CA, USA) whole mouse genome mRNA microarray data for the adrenal gland, cerebellum, hippocampus, hypothalamus, pituitary, whole neocortex, whole midbrain, prefrontal cortex, nucleus accumbens and the striatum, from up to 24 of the BXD RI strains. The data sets and complete methodological details for each study are available on the GeneNetwork website. Several of these publicly available data sets contain unbalanced combined samples from both genders, although more recent samples contain gender-balanced or gender-specific data. These data are part of a greater repository of BXD phenotypic data and cannot readily be broken out by gender. Where possible we selected male-specific data to complement our male-specific von Frey data set – in the midbrain, pituitary, amygdala, hypothalamus and adrenals. Gender differences and gender-specific genetic mechanisms will be missed in this analysis. Correlations were retrieved from GeneNetwork using the most recent version of the gene expression data for each tissue. The calculations were performed using each of the normalization methods reported (Microarray Suite 5 [MAS5], Position-Dependent Nearest Neighbor [PDNN] or Robust Multi-array Average [RMA]) to protect against potentially spurious findings related to specific normalizations. The maximum number of transcript probes assessed for a single brain region is over 45 000, which renders the critical P = 1.1 × 10−6 with Bonferroni correction. This comparison-wise type I error rate comes with a substantial rate of false negative results. Thus, a moderately conservative critical P = 0.001 was chosen based on previous whole-genome transcript expression studies of sensory traits (Costigan et al. 2002; Griffin et al. 2007). Each thresholded set of correlates was exported from GeneNetwork directly to GeneWeaver (Baker et al. 2012), a tool that facilitates comparison of gene set data across heterogeneous species and experimental platforms. Using the Boolean tool in GeneWeaver, the union of gene expression correlates with P < 0.001 observed for each normalization was obtained within tissue to construct a single gene list per tissue. The GeneSet Graph tool was then applied to the resulting gene lists to visualize the frequently correlated genes among all tissues. The resulting transcripts are those under multi-tissue regulation by allelic variation within the QTL region. The strategy may omit transcripts that vary specifically in functionally relevant tissue, and will also exclude those genes for which polymorphisms will alter function but not abundance. For those genes and mechanisms identified, functional validation is required.
Candidate gene testing
The roles of the candidate genes were tested in the two progenitor strains of the BXD RI mice as well as selected BXD strains to determine if targeting the protein product of the gene affects von Frey withdrawal thresholds, a critical step in establishing that the genes are possible candidates for mechanosensation. Candidate gene pharmacological testing was performed in male progenitor strains and a subset of the BXD strains (The Jackson Laboratory). The BXD strains were chosen based on single nucleotide polymorphism genotypes at the peak of each of the detected QTL such that strains with the high and low predicted von Frey sensitivity were used for validation testing, though they were not necessarily the strains with the most extreme observed phenotypes in the mapping population (Fig. 1). The allelic effects at the two loci were in opposite directions, so half of the strains had C57BL/6J genotype and the other half the DBA/2J genotype at the QTL peak on Chr 5 (rs29681564) with the opposite on Chr 15 (rs3665030). The low sensitivity strains were BXD1, BXD6, BXD12, BXD19 (Chr 5 rs29681564 = D2/Chr 15 rs3665030 = B6) and the high sensitivity strains were BXD15, BXD18, BXD21 (Chr 5 rs29681564 = B6/Chr 15 rs3665030 = D2).
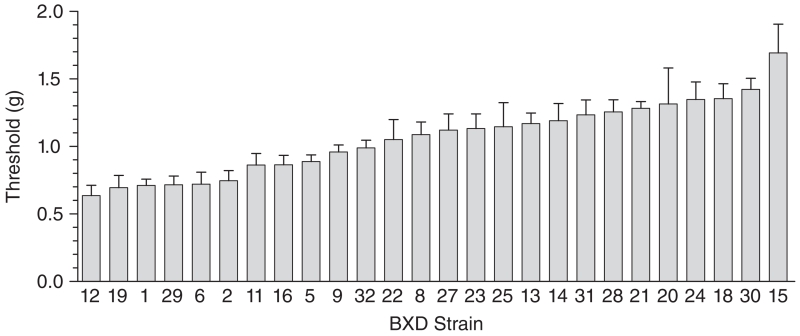
Figure 1. Overall von Frey threshold means for the 26 BXD RI strains surveyed show a significant effect of strain (P < 0.001) and a wide range of sensitivity among the strains (N = 204; n = 6–9/strain).
The calculated heritability estimate (h2 = 0.44) indicates that 44% of the overall trait variability is because of additive allelic variance (polymorphisms) among the inbred strains. Error bars represent standard error of the mean (SEM).
These strains were evaluated to assess whether allele status determines the relative effectiveness of targeted pharmacological manipulations to alter von Frey withdrawal thresholds. The method of von Frey mechanical sensitivity testing was as described above; however, an intraperitoneal (IP) saline injection was given 30 min prior to baseline testing to minimize the effect of the injection procedure on the test day (day 4). Gabapentin targets the protein product of Cacna2d1, the α2/δ1 subunit of L-type Voltage-gated Calcium Channel (VGCCs) (Marais et al. 2001). On the test day, gabapentin (25–50 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) or saline vehicle was injected (IP, 20 μl/g). von Frey testing was performed 45 and 150 min after injection, with time of testing based on our previous study (Chesler et al. 2003a). The investigator administering injections and performing von Frey testing was blind to the treatment. To evaluate the effects of gabapentin in BXD strains, the most effective dose from the study in the progenitor strains (50 mg/kg) was used and all mice were evaluated for mechanical sensitivity at both 45 and 150 min.
To test the role of Csnk1e in mechanosensation, the effect of PF-670462 (Pfizer Global R&D, Groton, CT, USA; and subsequently Tocris Bioscience, Ellisville, MO, USA), an inhibitor of casein kinase (CK)-1δ/ε, was tested in progenitor and selected BXD RI strains similar to the method described above for Cacna2d1 testing. The method of von Frey testing was as described above but with an IP saline injection given 5 min prior to baseline testing. On the test day, PF-670462 (20–40 mg/kg) or saline vehicle was injected IP (10 μl/g) and von Frey testing was performed 5 min after injection. When evaluating the effects of PF-670462 in BXD strains, the effective dose from the progenitor strain study (20 mg/kg) was administered and mechanical sensitivity evaluated at 5, 20 and 30 min post-injection. The investigator administering injections and performing von Frey testing was blind to the treatment.
As a confirmatory test of transcript covariance and to evaluate whether the general pattern of expression covariance is also found in a pain-relevant tissue, basal Cacna2d1 and Csnk1e transcript expression in pain-related brain regions and L3–L4 dorsal root ganglia (DRGs) in the two progenitor strains using quantitative, real-time polymerase chain reaction (RT-PCR). Naïve, male C57BL/6J and DBA/2J mice (5–6/strain, The Jackson Laboratory) were briefly anesthetized with isoflurane, immediately decapitated and their brains removed and dissected to remove brain regions of interest and DRGs were removed after isoflurane anesthesia and intra-cardiac perfusion with saline. The DRGs from two male mice of the same strain were pooled per sample to provide sufficient RNA yield.
The RNA was extracted from the tissues using 1.0 ml trizol (Invitrogen, Carlsbad, CA, USA). The cDNA was created using SuperScript II reverse transcriptase according to manufacturer’s instructions. The thermal cycling conditions for quantitative reverse transcription-PCR were 10 min at 95°C followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 min on an ABI PRISM 7000 Sequence Detection System or an ABI StepOne Plus System. Forward and reverse primers were as follows: 5 μm of ATGTGTCCGTCGTGGATCTGA and ATGCCTGCTTCACCACCTTCT T for Glyceraldehyde-3-Phosphate Dehydrogenase (Gapdh); 5 μm of ACAGACGGTGGGATTACGAG and GCACCAGGTCCACTTTTGTT for Cacna2d1; and 10 μm GACTGGAACATGCTCAAATTCG and CCGTCTTTCCCGGTCTACATC for Csnk1e (Palmer et al. 2005) (Integrated DNA Technologies, Inc., Coralville, IA, USA). Statistical significance of strain differences in target gene expression relative to Gapdh was determined with Student’s t-test. Fold difference in expression is reported relative to the C57BL/6 strain. In a data set collected with the same strains, but not presented in this manuscript, we were able to complete a comparison of GAPDH expression levels across BXD strains. We found no significant difference in expression of GAPDH for the strains used herein for any of the tissues used.
Mixed model anova was used to test for group differences because of genotype at the location of interest and drug treatment. The effect of pharmacological agent administration was calculated by adjusting for saline treatment effects at each time point relative to the start of injection. The peak effect of drug over time was fit in a mixed model anova using jmp 10 (SAS institute, Cary, NC, USA). The random effect of strain was fit within allele group. The fixed effect of allele group was evaluated to determine the impact of allele (Cacna2d1 or Csnk1e, as appropriate) on sensitivity to pharmacological targeting of the appropriate gene product in representative strains.
Results
Repeated-measures anova showed no significant interaction of BXD RI strain with day of testing (F50,356 = 0.99; P = 0.51), no main effect of day of testing (F2,356 = 0.17; P = 0.85) (data not shown), and a significant effect of BXD RI mouse strain on mechanical threshold (F25,178 = 5.5; P < 0.001). The BXD RI strain means of von Frey withdrawal thresholds averaged over 3 days for left and right hind paws separately were highly correlated with each other (rp = 0.96) and with the average threshold for both paws (rp = 0.99, 0.99). Because no effect of paw side or repeated testing was observed, the average of values for both hind paws and 3 days of testing was calculated, are presented in Fig. 1, and were used for QTL mapping and genetic correlation analyses. Analyses using left or right hind paw means produced similar results. The calculated heritability estimate (h2 = 0.44) indicates that 44% of the total trait variability is because of the genotype of the strains.
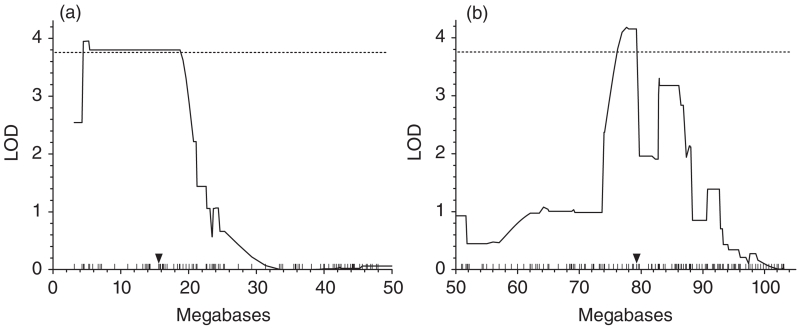
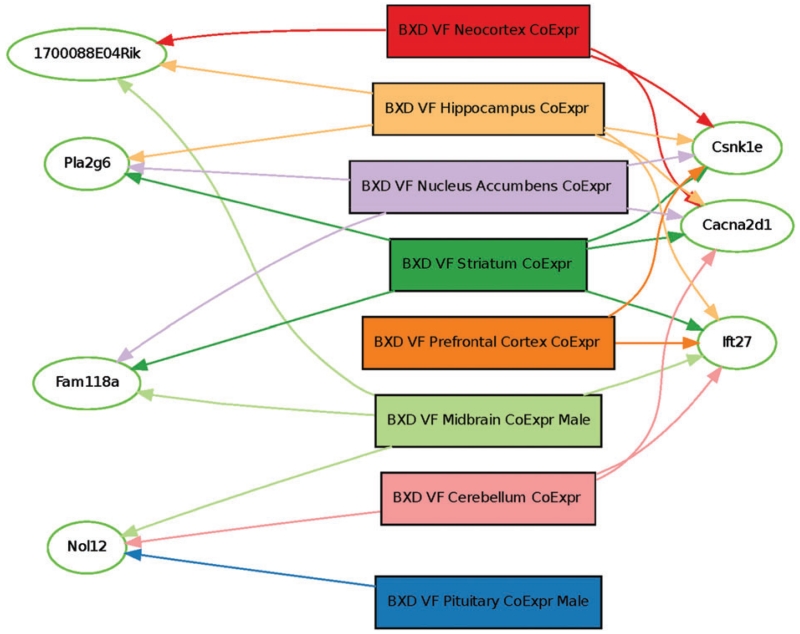
The QTL mapping of the BXD strain means showed two statistically significant QTL peaks on proximal Chr 5 (LOD = 4.0) and distal Chr 15 (LOD = 4.2) (significant LOD by permutation = 3.76; Fig. 2; see Fig. S1). Based on a 1.0 LOD drop-off from the peak LOD score (Lander & Botstein 1989), there are 64 genes in the region of the QTL on Chr 5 [4.468–19.63 Mb, 95% confidence interval (CI); Fig. 2] and 153 genes in the QTL region on Chr 15 (74.791–79.503 Mb; Fig. 2) according to a search of the Mouse Genome Database (1 May 2015; GRCm38/mm10 assembly). Transcript covariance analysis was used to prioritize subsequent testing of the positional candidate genes. Expression of three genes, Cacna2d1, Csnk1e and Ift27 (Rabl4) was highly correlated with von Frey withdrawal thresholds in five of eight brain areas for which data are available in the BXD RI mouse strains. Furthermore, Cacna2d1, Csnk1e and Ift27 are located in the detected QTLs on Chr 5: 15.4–15.9 Mb, Chr15: 79.2–79.3 Mb and Chr 15: 78.15–78.17, respectively. Expression was significantly correlated (P < 0.001) in one or more brain areas for 1470 genes or microarray probe sets, two or more brain regions for 44 transcripts, three or more brain regions for 7 transcripts (see Fig. 3). These results can be replicated on the GeneNetwork and GeneWeaver systems, where the analyses can be reproduced and results examined in detail (see Appendix S3, Supporting Information). Each of the brain areas examined has a known role in this sensory trait and evidence from each brain area was equally considered in this analysis. While using the number of brain regions in which gene expression is altered is not a perfect standard for determination of a gene’s influence on a phenotype of interest, it seemed reasonable to prioritize genes exhibiting differential expression across multiple brain regions with a known role in nociceptive sensory processing and/or modulation for this study. The co-expression across multiple brain regions implies a general mechanism of regulation that may imply similar correlation in other more relevant tissues (e.g. DRG, dorsal spinal cord, etc.) whereas a correlation with expression in an irrelevant tissue is less likely to generalize and would be a less compelling criterion for prioritization.
Figure 2. Significant QTLs associated with mechanical sensitivity were identified on Chr 5 (panel a) and Chr 15 (panel b).
Interval mapping of von Frey mechanical threshold means of BXD RI mice indicate in (a) a significant QTL on proximal Chr 5 (peak LOD score = 4.0; only 0–50 Mb from centromere shown) and in (b) a significant QTL on distal Chr 15 (peak LOD score = 4.2; only 50–103 Mb from centromere shown). The 95% CIs of the QTLs (based on a 1.0 LOD drop-off from the peak) indicate that polymorphic genes responsible for variation in mechanical sensitivity are located in the genomic regions from 4.468 to 19.63 Mb on Chr 5 and from 74.791 to 79.503 Mb on Chr 15. The dotted lines indicate the significant LOD score threshold of 3.76 determined by 1000 permutations of the strain means and a genome-wide error rate of 0.05. Vertical hatch marks on the x-axis indicate DNA marker positions. Arrowheads indicate the position of the candidate genes Cacna2d1 and Csnk1e on Chr 5 and Chr 15, respectively.
Figure 3. Transcript-trait covariance across brain regions.
Genes with transcription covariance to von Frey sensitivity are depicted by oval shaped nodes. Brain regions are represented by rectangular nodes. The most highly connected genes based on correlation P < 0.001 are represented on the right side of the graph. One-thousand four-hundred and seventy genes are significantly correlated to von Frey responding in any tissue, with only 44 genes correlated in two or more tissues. Only those genes represented here are correlated in three or more tissues.
A second criterion that may be applied to candidate genes exhibiting high trait covariance is whether there is sufficient evidence of a genetic mechanism with expression that is regulated by the same QTL as the behavioral trait. It is possible, though not likely given the pattern of results seen herein, that differential gene expression is not the underlying functional mechanism of action of the regulatory variants. The QTL mapping of transcript abundance enables the detection of polymorphisms that act through regulation of expression or stability of RNA. Such loci should exist for transcripts that have high genetic correlation to behavioral phenotypes. Expression QTL was retrieved for each of the three transcripts in the M430 INIA brain data set in GeneNetwork.org. The peak cis QTL for each transcript is Chr 5: 14.32 Mb at rs3687916 (P < 0.000055) for Cacna2d1; Chr 15: 78.74 Mb at rs3667755 (P < 0.000001) for Csnk1e and Chr 15: 77.99 Mb at rs6342608 (P < 0.000004) for Ift27.
To verify that the strain differences in the abundance of Cacna2d1, Csnk1e and Ift27 were attributable to genetic effects and not to polymorphisms within the probe target sequence (Walter et al. 2007, 2009), we utilized the brain M430 INIA data set in GeneNetwork.org and identified the probe sets for each of the candidate genes. After omitting the probe(s) in which a B6 vs. D2 Single-nucleotide polymorphism (SNP) was identified, QTL mapping was performed for each transcript. A significant cis expression QTL remained detectable for all genes, indicating that the abundance of each transcript is regulated by a genetic polymorphism at or near its coding gene (see Appendix S1 for probe level analysis).
Each of the three genes makes compelling candidates, with potential regulatory variants, and it is entirely possible that multiple-linked genes influence mechanical sensitivity. A role for Ift27 in mechanosensation is already known because of its association with the congenital ciliopathy Bardet–Biedl syndrome (Tan et al. 2007). Deletion of other BBS-related genes in mice and worms has been shown to affect von Frey sensitivity (Tan et al. 2007). Thus, Ift27 remains a plausible candidate gene. Unfortunately, no selective pharmacological agents target the Ift27 gene product. However, Cacna2d1 and Csnk1e are amenable to specific pharmacological targeting and to date have no known role in punctate mechanosensation. Thus, they were considered the highest priority for candidate genes testing. Additional candidates from these intervals may also exist within the QTL intervals, so these tests are not exclusionary.
To further interpret the nature of the observed genetic effects on the von Frey assay of mechanosenation, we examined the correlation of von Frey scores to over 4600 traits in GeneNetwork.org. Correlation P-values were subject to q-value estimation to control for false discovery rate. It is robust to the dependencies among multiple tests and therefore suitable to application in GeneNetwork, for which many repeated observations are included. von Frey withdrawal threshold means of the BXD RI strains were significantly correlated with dopamine (DA) transporter expression in the caudate putamen (striatum) of (Jones et al. 1999) (rp = 0.79, P = 3.5 × 10−5, q < 0.05, 18 strains in common; RecordID 10276 in www.genenetwork.org). Cocaine induced stereotypy from this study was also correlated with von Frey threshold (rp = 0.69, P = 1.6 × 10−4, q < 0.13, 23 strains in common; RecordID 10295). The stereotypy trait is regulated by a QTL on Chromosome 15 at 82.95 Mb. This is upstream of the Ift27/Csnk1e region, but likely to be in linkage disequilibrium with it, introducing the potential for spurious genetic association of DA-related phenotypes with von Frey response.
The von Frey mechanosensitivity was correlated with measures of anxiety (rp = 0.75, P = 6.0 × 10−5, q < 0.09, 20 strains in common; RecordID 12395) and habituation (rp = 0.67, P = 8.2 × 10−4, q < 0.29, 20 strains in common; RecordID 10038) both regulated by a genetic locus on Chr 15 near Csnk1e and consistent with the previously established role of Csnk1e in anxiety and habituation. No trends of correlation were observed to traits regulated by the Chr 5 locus, indicating that variation in Cacna2d1 modulates punctate mechanosensation and not a known confounding trait. Furthermore, von Frey withdrawal thresholds were not significantly correlated with previously examined thermal, inflammatory and electrical footshock-induced nociception (rp =−0.02 to 0.32, 12–24 strains in common; data on www.genenetwork.org), further indicating the genetic distinctiveness of mechanical sensitivity among sensory traits examined in BXD RI mice.
To determine whether the candidate genes are involved in modulating baseline mechanical sensitivity, pharmacological ligands acting at the protein products of Cacna2d1 and Csnk1e were tested in the progenitor strains of the BXD RI mice, C57BL/6J and DBA/2J. The IP injection of low doses of the α2/δ1 subunit-targeting drug gabapentin (Field et al. 2006; Marais et al. 2001) significantly and dose-dependently increased von Frey mechanical thresholds compared with baseline (Fig. 4, P < 0.05). Gabapentin was less effective in DBA/2J mice as reflected by a significant effect of strain on change in mechanical threshold after treatment (P < 0.05). DBA/2J mice also exhibit greater Cacna2d1 expression in the cerebellum, prefrontal cortex and striatum and less expression in the lumbar DRGs (Table 1), which suggests that the effects may be because of the effects of gabapentin acting at the peripheral afferent level rather than in the brain. However, when tested in selected BXD strains, presence of the C57BL/6J and/or DBA/2J allele for Cacna2d1 did not significantly affect the response to gabapentin (F1,13 = 0.023; P = 0.882; see Fig. 5) though comparison of mRNA expression means across strains with the same allele status shows significant DRG expression differences of both candidate genes (C57BL/6J > DBA/2J, P < 0.01) (see Table 2). This may be because of confounding sedating effects with independent genetic control (though the dose in this study is subthreshold for inducing sedation) and/or strain differences in gabapentin metabolism separate from the effects of Cacna2d1 genotype.
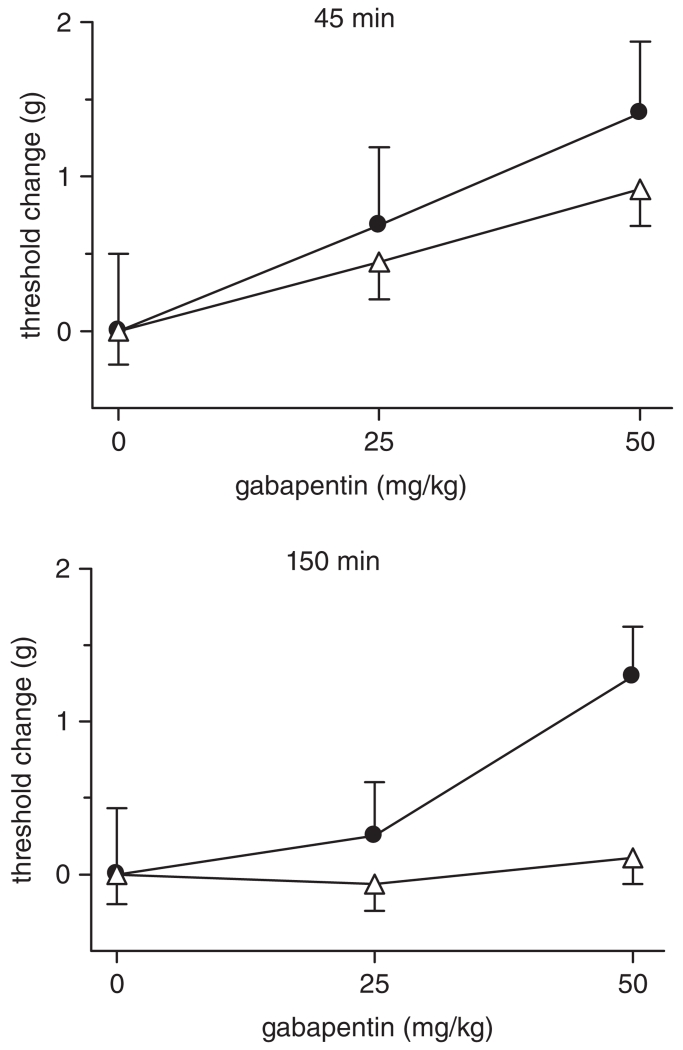
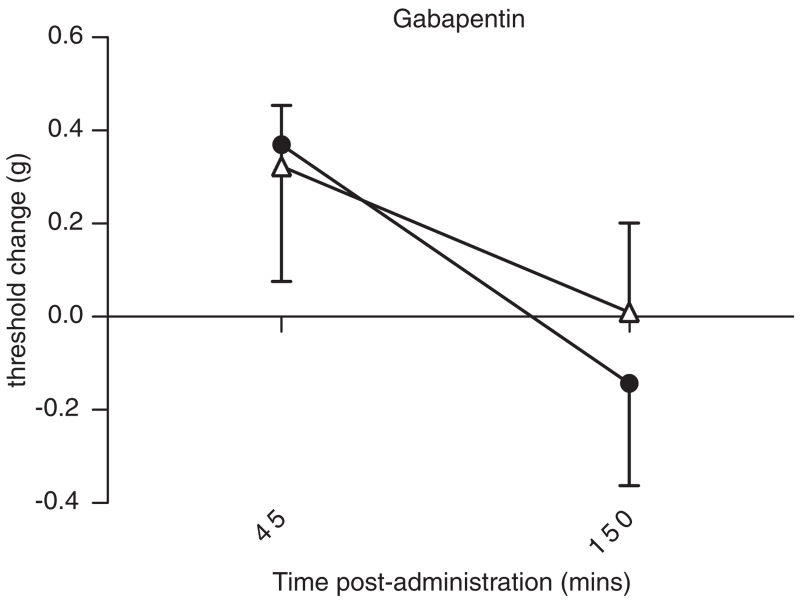
Figure 4. Gabapentin dose-dependently decreases baseline mechanical sensitivity (increases threshold) with differential effectiveness observed between the progenitor strains of BXD RI mice, C57BL/6J (filled circles) and DBA/2J (open triangles) (n = 6).
These strains show significant differences in expression of Cacna2d1 as determined by the transcript-trait covariance (Fig. 3) and confirmed using RT-PCR (see Table 1). At 45 min post-injection, gabapentin dose-dependently increases von Frey thresholds (P < 0.05) and does not show significant strain differences (P > 0.05). However, at 150 min post-injection, only C57BL/6J mice display dose-dependence of the effect of gabapentin on von Frey thresholds compared with vehicle-treated mice (P < 0.05). Threshold change is plotted for clarity as vehicle-treated C57BL/6J mice exhibited greater mechanical thresholds than vehicle-treated DBA/2J mice (greatest difference at 150 min, 1.1 ± 0.3 vs. 0.9 ± 0.1 g; P > 0.05). Error bars represent SEM.
Table 1.
Transcript expression measured by RT-PCR of candidate genes in selected brain areas and DRG of BXD parental strains, C57BL/6J and DBA/2J
| Cerebellum |
Hippocampus |
Prefrontal cortex |
Striatum |
DRG |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Transcript | C57BL/6J | DBA/2J | C57BL/6J | DBA/2J | C57BL/6J | DBA/2J | C57BL/6J | DBA/2J | C57BL/6J | DBA/2J |
| Cacna2d1 | 1.00 ± 0.07 | 1.42 ± 0.08* | 1.00 ± 0.14 | 1.16 ± 0.15 | 1.00 ± 0.10 | 1.37 ± 0.11* | 1.00 ± 0.10 | 1.60 ± 0.22* | 1.00 ± 0.11 | 0.38 ± 0.03** |
| Csnk1e | 1.00 ± 0.07 | 1.03 ± 0.04 | 1.00 ± 0.10 | 1.93 ± 0.23** | 1.00 ± 0.18 | 1.96 ± 0.32* | 1.00 ± 0.18 | 1.95 ± 0.28* | 1.00 ± 0.09 | 0.66 ± 0.07* |
Values are relative to the normalized C57BL/6J strain mean ± SEM.
DBA/2J significantly different from C57BL/6J, P < 0.05.
P< 0.01, n = 5–6 for brain areas, n = 3 for DRG (seven to eight DRGs pooled from two mice per sample).
Figure 5. Gabapentin (50 mg/kg) decreases baseline mechanical sensitivity regardless of Cacna2d1 allele status, C57BL/6J (filled circles) and DBA/2J (open triangles), in BXD RI mouse strains (n = 6–9 per genotype) (P < 0.05).
Threshold change was analyzed and presented because of differences in baseline thresholds by strain and/or genotype. Error bars represent SEM.
Table 2.
Transcript expression measured by RT-PCR of candidate genes in DRG of BXD recombinant inbred strains based on presence of C57BL/6J (four strains) and DBA/2J (four strains) alleles for the region of interest
| DRG |
||
|---|---|---|
| Transcript | C57BL/6J allele | DBA/2J allele |
| Cacna2d1 | 2.87 ± 0.46 | 1.11 ± 0.14** |
| Csnk1e | 0.613 ± 0.12 | 1.04 ± 0.07** |
Values are relative to the BXD6 strain (C57BL/6J allele) mean ± SEM.
BXD strains with DBA/2J allele significantly different from strains with C57BL/6J allele.
P<0.05, n = 4–5 (four DRG pooled from a single mouse per sample, four to five mice per strain).
The IP injection of PF-670462, the selective inhibitor of CK-1δ/ε, produced a greater dose-dependent (P < 0.0001) increase in von Frey withdrawal thresholds in DBA/2J mice than in C57BL/6J mice (P < 0.05 for effect of strain; Fig. 6) that is particularly apparent at the lowest dose of PF-670462. The DBA/2J mice, which are more sensitive to the CK-1δ/ε inhibitor, have greater expression of Csnk1e in the hippocampus, prefrontal cortex and striatum and less expression in the DRGs compared with C57BL/6J mice (Table 1). The IP injection of PF-670462 (20 mg/kg) produced a similar genotype-dependent increase in von Frey withdrawal thresholds in BXD strains such that strains with the DBA/2J allele showed a significant increase in mechanical threshold compared with those strains with the C57BL/6J allele (F1,24 = 7.456; P = 0.012) for effect of strain; Fig. 7). Because this dose of PF-670462 does not have motor or sedative effects (Bryant et al. 2009), the results are more likely a primary effect of Csnk1e on mechanosensitivity. Gabapentin and PF-670462 did not have the same pattern of effects against thermal nociception in Hargreaves’ thermal plantar test, in which the same behavioral response of paw withdrawal is elicited [shown for gabapentin in (Chesler et al. 2003a) see also Supporting Information for Hargreaves’ test methods and data; Figs. S2,S3]. Together, these findings implicate both genes in modulating von Frey response.
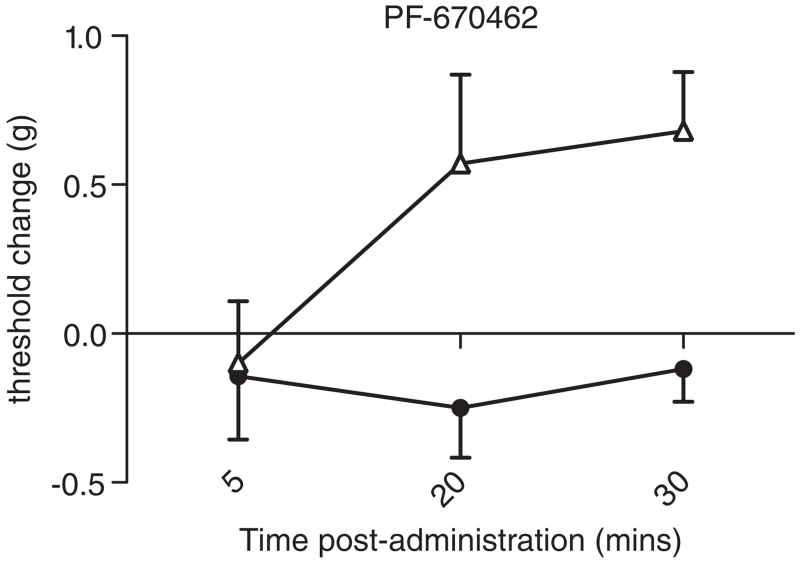
Figure 6. The CK-1δ/ε inhibitor dose-dependently decreases mechanical sensitivity with differential effectiveness observed between C57BL/6J (n = 6–9; filled circles) and DBA/2J (n = 7–9; open triangles) strains.
These strains show significant differences in expression of Csnk1e (see Tables 1 and 2). PF-670462 produced a dose-dependent increase in von Frey thresholds in both parental strains (P < 0.0001). The effect was significantly greater in DBA/2J mice (P < 0.05). The lowest dose administered (20 mg/kg) was effective only in DBA/2J mice (P < 0.05). Threshold change is plotted for clarity as vehicle-treated C57BL/6J mice exhibited greater mechanical thresholds than vehicle-treated DBA/2J mice (1.3 ± 0.3 vs. 1.2 ± 0.2 g; P < 0.05). Error bars represent SEM.
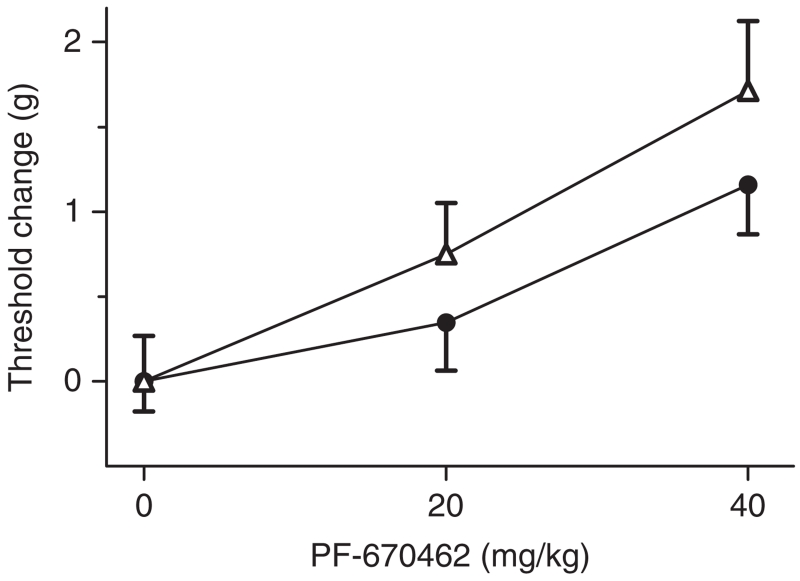
Figure 7. PF-670462 (20 mg/kg) decreases mechanical sensitivity with differential effectiveness observed across BXD strains with C57BL/6J (filled circles) and DBA/2J (open triangles) Csnk1e alleles (n = 12–14 per genotype), in line with findings presented from progenitor stains (see Fig. 4).
Threshold change was analyzed and presented due differences in baseline thresholds by strain and/or genotype. Error bars represent SEM.
Discussion
This study identified regions on Chr 5 and 15 containing genes underlying heritable differences in mechanosensation. Three types of evidence were evaluated in addressing whether these genes were involved in heritable trait variation: (1) is the gene polymorphic? (2) is the gene regulated by variation within the region? (3) is there functional evidence implicating the gene product in trait variation? The first two are addressed through analyses in GeneNetwork, followed by more focused expression studies. The third was addressed through pharmacological experimentation.
Consistent and robust positive genetic correlations of von Frey thresholds with expression of Cacna2d1, Ift27 and Csnk1e were detected in five of eight brain areas with gene expression data available in BXD RI mice. Although these tissues are not all widely accepted as substrates of classical pain pathways, these areas have been previously implicated in sensory processes, emotionality and/or responses to, or the treatment of, neuropathic pain including mechanical hypersensitivity in neuropathic pain models and from imaging studies of human patients (Ansah et al. 2007; Bian et al. 2006; Duquette et al. 2007; Hagelberg et al. 2004; Saab & Willis 2003). The convergence of expression correlations across multiple gene expression data sets suggests that the genetic effects on gene expression and correlation to von Frey thresholds may be manifested in other tissues. Further investigation of the anatomical location of action of the candidate genes and their precise role in von Frey mechanosensation is warranted, particularly because these results do not preclude other anatomical sites of action. Furthermore, although we evaluated multiple microarray data sets and multiple tissues, the possibility of false negative results remains, and therefore, other polymorphic loci within the QTL may influence von Frey tests of mechanosensation. However, experimental validation of the candidate genes provide strengthened support for their role in von Frey response. The expression covariance results together with pharmacological targeting of the genes’ protein products suggest that expression levels of Cacna2d1, Ift27 and Csnk1e across BXD RI strains and their progenitor strains may contribute to heritable differences in mechanical sensitivity.
The neuropathic pain medications gabapentin and pregabalin reduce neuronal excitability and neurotransmission of affected neurons by acting at the VGCC α2/δ1 subunit encoded by Cacna2d1, and thereby, counter mechanical allodynia in neuropathic pain patients and animal models of neuropathic pain (Bian et al. 2006; Field et al. 2000, 2006; Joshi & Taylor 2006). This study shows the ability of gabapentin to increase baseline von Frey thresholds in naïve mice from the two progenitor strains, indicating that Cacna2d1 is a reasonable candidate gene for basal mechanosensation, and that the effect is genotype-dependent as expected from previous studies (Chesler et al. 2003a; Rode et al. 2007; Zhou et al. 2014). Prior studies have shown that Cacna2d1 expression is increased in the DRG and dorsal horn of the spinal cord after nerve injury (Costigan et al. 2002; Field et al. 2000, 2006; Luo et al. 2002; Matsumoto et al. 2006) and this increase has been shown to be essential to the development of mechanical, but not thermal, hypersensitivity after nerve injury (Patel et al. 2013). Thus, in addition, this study suggests that drugs that act at the α2/δ1 subunit may act directly on the fundamental and heritable mechanisms of mechanosensation, and hence, Cacna2d1 may represent a molecular mechanism common to baseline mechanosensation and mechanical hypersensitivity after nerve injury.
Cacna2d1 expression in the investigated central nervous system (CNS) areas of BXD RI mouse strains is positively correlated with von Frey thresholds (or negatively correlated with mechanosensitivity). This appears in contrast with the increase in Cacna2d1 expression in DRGs and dorsal horn that is correlated with mechanical allodynia after nerve injury and with the current results of gabapentin being less effective to increase von Frey thresholds in DBA/2J mice that display more Cacna2d1 expression in the CNS areas than C57BL/6J mice. Potentially explaining the latter, it is possible that the genotype-dependent effects of systemic gabapentin on von Frey thresholds reported here are mediated by cells in the DRG including peripheral afferent neurons because, paradoxically, we show that DBA/2J mice exhibit less Cacna2d1 expression in lumbar DRGs than C57BL/6J mice. Comparisons across multiple measures with only two inbred strains have inherent limitations that caution against concluding the presence or absence of a relationship between the measures (of DRG expression and von Frey thresholds) especially when one is a behavioral measure (Crabbe et al. 1999). To further investigate this issue, we evaluated the effect of gabapentin on mechanical sensitivity in a subset of BXD strains and found that Cacna2d1 genotype did not predict the magnitude of change in mechanical sensitivity following gabapentin; the difference may be because of a number of factors related to strain differences in other genes influencing drug metabolism and/or issues related to competing behaviors also induced by gabapentin, namely sedation which could mask subtle effects on mechanical threshold. Prior studies have identified sedation as a primary side effect of gabapentin administration at analgesic doses (O’Connor & Dworkin 2009) and this may alter the response to the test stimulus apart from any effects at our site of interest. At present, the opposite directions of C57BL/6J and DBA/2J strain differences in expression of Cacna2d1 between the lumbar DRGs and CNS areas cannot be resolved, although one could speculate that Cacna2d1 might be differentially regulated in the peripheral nervous system as observed with other calcium channels (Shin et al. 2003). Regarding the primary goal of this study, which was to determine candidate mechanisms of heritable variation, data are not yet available from a sufficient number of inbred strains to determine whether Cacna2d1 expression differences in the DRG contribute specifically to heritable differences in mechanosensation but the findings herein support this hypothesis.
Although the QTL for mechanosensation on Chr 15 overlaps with the previously identified pain1 QTL for the neuropathic pain model of autotomy behavior following sciatic and saphenous nerve transection (Devor et al. 2005; Lariviere 2009; Seltzer et al. 2001), it is likely that different genes are involved in the effects observed here. A recent report by Nissenbaum and colleagues localized pain1 to an interval on Chr 15 (75.27–79.5 Mb) that almost precisely matches the CI of the significant QTL for von Frey thresholds on Chr 15 (75.3–79.4) despite the use of RI segregation test and recombinant progeny test experiments with different progenitor strains than those of the BXD RI strains (Nissenbaum et al. 2010). Cacng2 was identified as the single highest priority candidate from pain1 based on significant correlation between DRG mRNA expression and autotomy behavior in five inbred mouse strains. We found no convergent evidence of CNS Cacng2 expression covariance with von Frey thresholds across BXD RI strains (P > 0.02; data not shown). Though this study does not rule it out, our findings are in agreement with prior research showing variation in sensitivity to neuropathic pain, thermal nociception and mechanosensation depend on genetically distinct mechanisms of heritability (Lariviere 2002; Young 2014).
Pharmacological testing of the CK-1 inhibitor PF-670462 indicates for the first time that Csnk1e and its protein product, CK-1ε, have a role in mechanosensation, and that the drug effect is genotype-dependent and greater in DBA/2J mice that exhibit greater Csnk1e expression in several CNS areas. Csnk1e is involved in a diverse array of signaling pathways, including dopaminergic and glutamatergic neurotransmission in the striatum and other brain areas (Chergui et al. 2005; Greengard 2001). In this study, von Frey thresholds were highly positively correlated with Csnk1e expression in five brain areas including the striatum across BXD strains. Utz et al. (2010) report strong staining within nerve cells of the trigeminal and DRG, in addition to widespread neuronal expression of CK-1ε in the brain. Similar to the pattern seen with Cacna2d1, Csnk1e expression has been shown to increase in DRG and the spinal cord after nerve injury (Sakurai et al. 2009) and could, therefore, represent a mechanism underlying mechanical hypersensitivity at this location. More recently, Kurihara et al. (2014) reported that CK-1 inhibition attenuated mechanical allodynia and decreased excitatory post-synaptic potentials in the dorsal spinal cord following inflammation. In the CNS, CK-1 is also known for regulating circadian rhythms via the phosphorylation of Period proteins (Gallego & Virshup 2007) and influencing Wnt signaling and GSK-3 formation (Knippschild et al. 2005; Price 2006). In QTL mapping studies, the genomic location of Csnk1e has been implicated in sensitivity to circadian function (Meng et al. 2008) and the stimulant effects of methamphetamine (Bryant et al. 2009, 2012; Palmer et al. 2005). Subsequently, polymorphisms in the human CSNK1E gene have been implicated in differential sensitivity to the euphoric effects of amphetamine (Veenstra-Vanderweele et al. 2006), though a subsequent replication of this finding was not successful (Hart et al. 2013), risk for heroin addiction (Levran et al. 2008) as well as with risk for schizophrenia (Huang et al. 2012). These studies attest to the pleiotropic properties of CK-1ε and highlight both the presence of functional polymorphisms of Csnk1e that may influence mechanosensation in humans and the need for further study of the potential pharmacological inhibition of CK-1ε to treat patients suffering from mechanoallodynia and/or hypersensitivity.
Genetic variation and co-expression of mechanosensation with Ift27 implicates a cilia protein in pain sensitivity. Previous findings indicate that murine DRG neurons are ciliated and that disruption of the normal structure and function of the cilia results in alterations in thermal and mechanical sensitivity (Tan et al. 2007). Moreover, ciliary function in other cell types, namely keratinocytes (Elofsson et al. 1984) and Schwann cells (Grillo & Palay 1963), could also play a role in mechanosensation. Recent genetic analysis of thermal nociception in the Diversity Outbred mapping population showed hydin, which encodes a ciliary protein as a likely candidate gene (Recla et al. 2014), further highlighting the potential role of variation within ciliary structure and function in peripheral sensory processing. Given the lack of pharmacological agents capable of selectively targeting the protein product of Ift27, we could not further evaluate its role at this time.
This study presents not only evidence for candidate genes underlying heritable differences in mechanosensation but also serves as a model for the combination of tools and techniques that can be used to elucidate new biological mechanisms and identify novel targets for drug development. Using RI strains to map QTL, eQTL to identify putative candidates within the identified genomic region(s), and pharmacological manipulation of the protein products of these genes, we identified genetic mechanisms with a previously unknown role in mechanical sensitivity. While it is possible that polymorphisms in other candidate genes, both from within and outside of this region of interest, may contribute to variation in mechanosensation, the current transcript covariance results, their convergence with the QTL mapping results and pharmacological testing suggest that Cacna2d1 and Csnk1e play a significant role in heritable variation in baseline mechanosensitivity and response to pharmacotherapeutics. Identification and manipulations of the precise causal genetic mechanisms may lead to development of better preclinical models of mechanical sensitivity and identification of novel therapeutic agents for the treatment of neurological disorders that feature mechanical sensitivity.
Supplementary Material
Acknowledgments
Funding for this study was obtained from: the Department of Anesthesiology of the University of Pittsburgh School of Medicine, the University of Pittsburgh School of Health Sciences Bridge Funding (W.R.L.); NIH grants 1R01DA021198 (W.R.L); T32DA007255 and 1F32DA026697 (C.D.B); R01DA021336 (A.A.P.); 5R01NS031680 and 2P01NS011255 (J.M.C.); R01DA15191 (J.S.M.); DA020677 (E.J.C.), R01AA18776 (E.J.C.); and the Office of Biological and Environmental Research, Office of Science, U.S. Department of Energy, under Contract DE-AC05-00OR22725 with UT-Battelle, LLC (E.J.C.). We thank Pfizer Global R&D (Groton, CT, USA) for the generous gift of a portion of the PF-670462 used.
Footnotes
Additional supporting information may be found in the online version of this article at the publisher’s web-site:
Figure S1: Genome-wide interval mapping results for mechanical sensitivity in the von Frey test of BXD RI mice indicate statistically significant QTLs on proximal Chr 5 (LOD = 4.0) and distal Chr 15 (LOD = 4.2) within which polymorphic genes responsible for variation in mechanical sensitivity are located. Statistical significance was determined empirically from 1000 permutations of the strain means and is indicated by the pink horizontal line (significant LOD = 3.76) and pale blue horizontal line (suggestive LOD = 2.34). Numbers and the letter X along the top of the figure refer to chromosomes. A total of 3795 non-redundant DNA markers polymorphic between the progenitor strains and at loci throughout the genome were considered. Table 2 lists the significantly associated markers. Figures 3,4 show the significant QTLs in greater detail.
Figure S2: Gabapentin dose-dependently decreases baseline thermal nociceptive sensitivity with differential effectiveness observed between the progenitor strains of BXD RI mice, C57BL/6J (filled circles) and DBA/2J (open triangles) (n = 5–6). These strains show significant differences in expression of Cacna2d1 (see Tables 1 and 2). At 45 min post-injection, gabapentin dose-dependently increases von Frey thresholds only in DBA/2J mice (P < 0.05). At 150 min post-injection, only DBA/2J mice display dose-dependence of the effect of gabapentin on thermal latencies compared to vehicle-treated mice (P < 0.05). Error bars represent SEM.
Figure S3: PF-670462, a selective inhibitor of the protein product of Csnk1e CK-1ε did not significantly affect thermal nociceptive sensitivity (P > 0.05) in C57BL/6J (n = 5–6; filled circles) and DBA/2J (n = 5–6; open triangles) strains. Error bars represent SEM.
Table S1: Details of mRNA microarray data sets to which von Frey threshold means were compared.
Appendix S1: Methods and results of effects of gabapentin and PF-670462 on Hargreaves’ thermal plantar nociception test.
Appendix S2: Probe level mapping of von Frey QTL candidate genes.
Appendix S3: Summary of transcript covariance across pain-relevant tissues.
References
- Ansah OB, Leite-Almeida H, Wei H, Pertovaara A. Striatal dopamine D2 receptors attenuate neuropathic hypersensitivity in the rat. Exp Neurol. 2007;205:536–546. doi: 10.1016/j.expneurol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Baker EJ, Jay JJ, Bubier JA, Langston MA, Chesler EJ. GeneWeaver: a web-based system for integrative functional genomics. Nucleic Acids Res. 2012;40:D1067–D1076. doi: 10.1093/nar/gkr968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R. Peripheral neuropathic pain: from mechanisms to symptoms. Clin J Pain. 2000;16:S12–S20. doi: 10.1097/00002508-200006001-00004. [DOI] [PubMed] [Google Scholar]
- Bian F, Li Z, Offord J, Davis MD, McCormick J, Taylor CP, Walker LC. Calcium channel alpha2-delta type 1 subunit is the major binding protein for pregabalin in neocortex, hippocampus, amygdala, and spinal cord: an ex vivo autoradiographic study in alpha2-delta type 1 genetically modified mice. Brain Res. 2006;1075:68–80. doi: 10.1016/j.brainres.2005.12.084. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Graham ME, Distler MG, Munoz MB, Li D, Vezina P, Sokoloff G, Palmer AA. A role for casein kinase 1 epsilon in the locomotor stimulant response to methamphetamine. Psychopharmacology. 2009;203:703–711. doi: 10.1007/s00213-008-1417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Kole LA, Guido MA, Sokoloff G, Palmer AA. Congenic dissection of a major QTL for methamphetamine sensitivity implicates epistasis. Genes Brain Behav. 2012;11:623–632. doi: 10.1111/j.1601-183X.2012.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chergui K, Svenningsson P, Greengard P. Physiological role for casein kinase 1 in glutamatergic synaptic transmission. J Neurosci. 2005;25:6601–6609. doi: 10.1523/JNEUROSCI.1082-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Williams RW. Brain gene expression: genomics and genetics. Int Rev Neurobiol. 2004;60:59–95. doi: 10.1016/S0074-7742(04)60003-1. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Ritchie J, Kokayeff A, Lariviere WR, Wilson SG, Mogil JS. Genotype-dependence of gabapentin and pregabalin sensitivity: the pharmacogenetic mediation of analgesia is specific to the type of pain being inhibited. Pain. 2003a;106:325–335. doi: 10.1016/S0304-3959(03)00330-0. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Wang J, Lu L, Qu Y, Manly KF, Williams RW. Genetic correlates of gene expression in recombinant inbred strains: a relational model system to explore neurobehavioral phenotypes. Neuroinformatics. 2003b;1:343–357. doi: 10.1385/NI:1:4:343. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Lu L, Wang J, Williams RW, Manly KF. WebQTL: rapid exploratory analysis of gene expression and genetic networks for brain and behavior. Nat Neurosci. 2004;7:485–486. doi: 10.1038/nn0504-485. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Lu L, Shou S, Qu Y, Gu J, Wang J, Hsu HC, Mountz JD, Baldwin NE, Langston MA, Threadgill DW, Manly KF, Williams RW. Complex trait analysis of gene expression uncovers polygenic and pleiotropic networks that modulate nervous system function. Nat Genet. 2005;37:233–242. doi: 10.1038/ng1518. [DOI] [PubMed] [Google Scholar]
- Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol Clin Exp Res. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Devor M, Gilad A, Arbilly M, Yakir B, Raber P, Pisante A, Darvasi A. pain1: a neuropathic pain QTL on mouse chromosome 15 in a C3HxC58 backcross. Pain. 2005;116:289–293. doi: 10.1016/j.pain.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Duquette M, Roy M, Lepore F, Peretz I, Rainville P. Cerebral mechanisms involved in the interaction between pain and emotion. Rev Neurol. 2007;163:169–179. doi: 10.1016/s0035-3787(07)90388-4. [DOI] [PubMed] [Google Scholar]
- Elofsson R, Andersson A, Falck B, Sjoborg S. The ciliated human keratinocyte. J Ultrastruct Res. 1984;87:212–220. doi: 10.1016/s0022-5320(84)80061-1. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. Longman; Essex: 1996. [Google Scholar]
- Field MJ, Hughes J, Singh L. Further evidence for the role of the alpha(2)delta subunit of voltage dependent calcium channels in models of neuropathic pain. Br J Pharmacol. 2000;131:282–286. doi: 10.1038/sj.bjp.0703604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell S, Corradini L, England S, Winks J, Kinloch RA, Hendrich J, Dolphin AC, Webb T, Williams D. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A. 2006;103:17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139–148. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Griffin RS, Costigan M, Brenner GJ, Ma CH, Scholz J, Moss A, Allchorne AJ, Stahl GL, Woolf CJ. Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J Neurosci. 2007;27:8699–8708. doi: 10.1523/JNEUROSCI.2018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo MA, Palay SL. Ciliated Schwann cells in the autonomic nervous system of the adult rat. J Cell Biol. 1963;16:430–436. doi: 10.1083/jcb.16.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagelberg N, Jaaskelainen SK, Martikainen IK, Mansikka H, Forssell H, Scheinin H, Hietala J, Pertovaara A. Striatal dopamine D2 receptors in modulation of pain in humans: a review. Eur J Pharmacol. 2004;500:187–192. doi: 10.1016/j.ejphar.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Hart AB, De Wit H, Palmer AA. Candidate gene studies of a promising intermediate phenotype: failure to replicate. Neuropsychopharmacology. 2013;38:802–816. doi: 10.1038/npp.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BC, Tarantino LM, Rodriguez LA, Reed CL, McClearn GE, Plomin R, Erwin VG. Quantitative-trait loci analysis of cocaine-related behaviours and neurochemistry. Pharmacogenetics. 1999;9:607–617. [PubMed] [Google Scholar]
- Joshi I, Taylor CP. Pregabalin action at a model synapse: binding to presynaptic calcium channel alpha2-delta subunit reduces neurotransmission in mice. Eur J Pharmacol. 2006;553:82–88. doi: 10.1016/j.ejphar.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Knippschild U, Gocht A, Wolff S, Huber N, Lohler J, Stoter M. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Sakurai E, Toyomoto M, Kii I, Kawamoto D, Asada T, Tanabe T, Yoshimura M, Hagiwara M, Miyata A. Alleviation of behavioral hypersensitivity in mouse models of inflammatory pain with two structurally different casein kinase 1 (CK1) inhibitors. Mol. 2014;10:17. doi: 10.1186/1744-8069-10-17. doi: 10.1186/1744-8069-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix-Fralish ML, Mogil JS. Progress in genetic studies of pain and analgesia. Annu Rev Pharmacol Toxicol. 2009;49:97–121. doi: 10.1146/annurev-pharmtox-061008-103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Botstein D. Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989;121:185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariviere WR, Wilson SG, Laughlin TM, Kokayeff A, West EE, Adhikari SM, Wan Y, Mogil JS. Heritability of nociception. III. Genetic relationship among commonly used assays of nociception and hypersensitivity. Pain. 2002;97:75–86. doi: 10.1016/s0304-3959(01)00492-4. [DOI] [PubMed] [Google Scholar]
- Lariviere WR. Genetics of neuropathic pain. In: Dobretsov M, Zhang JM, editors. Mechanisms of Pain in Peripheral Neuropathy. Research Signpost; Trivandrum: 2009. [Google Scholar]
- Lariviere WR, Chesler EJ, Mogil JS. Transgenic studies of pain and analgesia: mutation or background genotype? J Pharmacol Exp Ther. 2001;297:467–473. [PubMed] [Google Scholar]
- Lariviere WR, Wilson SG, Laughlin TM, Kokayeff A, West EE, Adhikari SM, Wan Y, Mogil JS. Heritability of nociception. III. Genetic relationships among commonly used assays of nociception and hypersensitivity. Pain. 2002;97:75–86. doi: 10.1016/s0304-3959(01)00492-4. [DOI] [PubMed] [Google Scholar]
- Levran O, Londono D, O’Hara K, Nielsen DA, Peles E, Rotrosen J, Casadonte P, Linzy S, Randesi M, Ott J, Adelson M, Kreek MJ. Genetic susceptibility to heroin addiction: a candidate gene association study. Genes Brain Behav. 2008;7:720–729. doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, Myers RR. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002;303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- Marais E, Klugbauer N, Hofmann F. Calcium channel alpha(2)delta subunits-structure and gabapentin binding. Mol Pharmacol. 2001;59:1243–1248. doi: 10.1124/mol.59.5.1243. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Inoue M, Hald A, Xie W, Ueda H. Inhibition of paclitaxel-induced A-fiber hypersensitization by gabapentin. J Pharmacol Exp Ther. 2006;318:735–740. doi: 10.1124/jpet.106.103614. [DOI] [PubMed] [Google Scholar]
- McClearn GE. Contextual genetics. Trends Genet. 2006;22:314–319. doi: 10.1016/j.tig.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Meng QJ, Logunova L, Maywood ES, Gallego M, Lebiecki J, Brown TM, Sladek M, Semikhodskii AS, Glossop NR, Piggins HD, Chesham JE, Bechtold DA, Yoo SH, Takahashi JS, Virshup DM, Boot-Handford RP, Hastings MH, Loudon AS. Setting clock speed in mammals: the CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron. 2008;58:78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proc Natl Acad Sci U S A. 1999;96:7744–7751. doi: 10.1073/pnas.96.14.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999a;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception II. ‘Types’ of nociception revealed by genetic correlation analysis. Pain. 1999b;80:83–93. doi: 10.1016/s0304-3959(98)00196-1. [DOI] [PubMed] [Google Scholar]
- Nissenbaum J, Devor M, Seltzer Z, Gebauer M, Michaelis M, Tal M, Dorfman R, Abitbul-Yarkoni M, Lu Y, Elahipanah T, Delcanho S, Minert A, Fried K, Persson AK, Shpigler H, Shabo E, Yakir B, Pisante A, Darvasi A. Susceptibility to chronic pain following nerve injury is genetically affected by CACNG2. Genome Res. 2010;20:1180–1190. doi: 10.1101/gr.104976.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(10 Suppl):S22–S32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Verbitsky M, Suresh R, Kamens HM, Reed CL, Li N, Burkhart-Kasch S, McKinnon CS, Belknap JK, Gilliam TC, Phillips TJ. Gene expression differences in mice divergently selected for methamphetamine sensitivity. Mamm Genome. 2005;16:291–305. doi: 10.1007/s00335-004-2451-8. [DOI] [PubMed] [Google Scholar]
- Patel R, Bauer CS, Nieto-Rostro M, Margas W, Ferron L, Chaggar K, Crews K, Ramirez JD, Bennett DL, Schwartz A, Dickenson AH, Dolphin AC. alpha 2delta-1 gene deletion affects somatosensory neuron function and delays mechanical hypersensitivity in response to peripheral nerve damage. J Neurosci. 2013;33:16412–16426. doi: 10.1523/JNEUROSCI.1026-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JL, Chesler EJ, Williams RW, Lu L. Genetic architecture of the mouse hippocampus: identification of gene loci with selective regional effects. Genes Brain Behav. 2003;2:238–252. doi: 10.1034/j.1601-183x.2003.00030.x. [DOI] [PubMed] [Google Scholar]
- Price MA. CKI, there’s more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev. 2006;20:399–410. doi: 10.1101/gad.1394306. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2013. URL http://www.R-project.org/ [Google Scholar]
- Recla JM, Robledo RF, Gatti DM, Bult CJ, Churchill GA, Chesler EJ. Precise genetic mapping and integrative bioinformatics in Diversity Outbred mice reveals Hydin as a novel pain gene. Mamm Genome. 2014;25:211–222. doi: 10.1007/s00335-014-9508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode F, Thomsen M, Brolos T, Jensen DG, Blackburn-Munro G, Bjerrum OJ. The importance of genetic background on pain behaviours and pharmacological sensitivity in the rat spared serve injury model of peripheral neuropathic pain. Eur J Pharmacol. 2007;564:103–111. doi: 10.1016/j.ejphar.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Saab CY, Willis WD. The cerebellum: organization, functions and its role in nociception. Brain Res Rev. 2003;42:85–95. doi: 10.1016/s0165-0173(03)00151-6. [DOI] [PubMed] [Google Scholar]
- Sakurai E, Kurihara T, Kouchi K, Saegusa H, Zong S, Tanabe T. Upregulation of casein kinase 1epsilon in dorsal root ganglia and spinal cord after mouse spinal nerve injury contributes to neuropathic pain. Mol Pain. 2009;5:74. doi: 10.1186/1744-8069-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer Z, Wu T, Max MB, Diehl SR. Mapping a gene for neuropathic pain-related behavior following peripheral neurectomy in the mouse. Pain. 2001;93:101–106. doi: 10.1016/S0304-3959(01)00295-0. [DOI] [PubMed] [Google Scholar]
- Shin JB, Martinez-Salgado C, Heppenstall PA, Lewin GR. A T-type calcium channel required for normal function of a mammalian mechanoreceptor. Nat Neurosci. 2003;6:724–730. doi: 10.1038/nn1076. [DOI] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PL, Barr T, Inglis PN. Loss of Bardet-Biedl syndrome proteins causes defects in peripheral sensory innervation and function. Proc Natl Acad Sci U S A. 2007;104:17524–17529. doi: 10.1073/pnas.0706618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BA. Recombinant inbred strains: use in gene mapping. In: Morse HCI, editor. Origins of Inbred Mice. Academic Press; New York, NY: 1978. [Google Scholar]
- Utz AC, Hirner H, Blatz A, Hillenbrand A, Schmidt B, Deppert W, Henne-Bruns D, Fischer D, Thal DR, Leithäuser F, Knippschild U. Analysis of cell type-specific expression of CK1 epsilon in various tissues of young adult BALB/c mice and in mammary tumors of SV40 T-Ag-transgenic mice. J Histochem Cytochem. 2010;58:1–15. doi: 10.1369/jhc.2009.954628. DOI:10.1369/jhc.2009.954628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-Vanderweele J, Qaadir A, Palmer AA, Cook EH, Jr., De Wit H. Association between the casein kinase 1 epsilon gene region and subjective response to D-amphetamine. Neuropsychopharmacology. 2006;31:1056–1063. doi: 10.1038/sj.npp.1300936. [DOI] [PubMed] [Google Scholar]
- Walter NA, McWeeney SK, Peters ST, Belknap JK, Hitzemann R, Buck KJ. SNPs matter: impact on detection of differential expression. Nat Methods. 2007;4:679–680. doi: 10.1038/nmeth0907-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter NA, Bottomly D, Ladera T, Mooney MA, Darakjian P, Searles RP, Harrington CA, McWeeney SK, Hitzemann R, Buck KJ. High throughput sequencing in mice: a platform comparison identifies a preponderance of cryptic SNPs. BMC Genomics. 2009;10:379. doi: 10.1186/1471-2164-10-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Williams RW, Manly KF. WebQTL: web-based complex trait analysis. Neuroinformatics. 2003;1:299–308. doi: 10.1385/NI:1:4:299. [DOI] [PubMed] [Google Scholar]
- Young EE, Costigan M, Herbert TA, Lariviere WR. Heritability of nociception IV: neuropathic pain assays are genetically distinct across methods of peripheral nerve injury. Pain. 2014;155:868–880. doi: 10.1016/j.pain.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Bryant CD, Loudon A, Palmer AA, Vitaterna MH, Turek FW. The circadian clock gene Csnk1e regulates rapid eye movement sleep amount, and nonrapid eye movement sleep architecture in mice. Sleep. 2014;37:785–793. doi: 10.5665/sleep.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.