Summary
Temperature is a potent inducer of fungal dimorphism. Multiple signalling pathways control the response to growth at high temperature, but the sensors that regulate these pathways are poorly defined. We show here that the signalling mucin Msb2 is a global regulator of temperature stress in the fungal pathogen Candida albicans. Msb2 was required for survival and hyphae formation at 42°C. The cytoplasmic signalling domain of Msb2 regulated temperature-dependent activation of the CEK mitogen activated proteins kinase (MAPK) pathway. The extracellular glycosylated domain of Msb2 (100–900 amino acid residues) had a new and unexpected role in regulating the protein kinase C (PKC) pathway. Msb2 also regulated temperature-dependent induction of genes encoding regulators and targets of the unfolded protein response (UPR), which is a protein quality control (QC) pathway in the endoplasmic reticulum that controls protein folding/degradation in response to high temperature and other stresses. The heat shock protein and cell wall component Ssa1 was also required for hyphae formation and survival at 42° C and regulated the CEK and PKC pathways.
Introduction
An important environmental stimulus encountered by most organisms is temperature. The adaptation to thermal stress is a common feature of animals, plants and microorganisms. The response to temperature stress involves the regulation of protein folding by molecular chaperones, and the turnover of mis-folded proteins in the cytosol and other compartments. Cells respond to temperature stress in different ways, including the reorganization of the cytoskeleton, alteration of membrane fluidity, and the regulated arrest of cell-cycle progression (Zeuthen, 1971; Lindquist, 1980; Yost and Lindquist, 1986; Richter et al., 2010). Temperature sensing and thermal adaptation mechanisms have been well-established in bacteria (Klinkert and Narberhaus, 2009) and fungal microorganisms (Nemecek et al., 2006). Temperature sensing has been intensively studied in pathogens, which are thought to detect high temperature as an indicator of entry into a warm-blooded host. Accordingly, much effort has been made to identify the signalling pathways and proteins that regulate the adaptation to high temperature in microbial pathogens.
Fungal species undergo distinctive morphogenetic and developmental behaviours in response to environmental challenges (Madhani and Fink, 1998; Lengeler et al., 2000; Heitman, 2005; d’Enfert, 2009; Leach and Brown, 2012). Candida albicans is an opportunistic fungal species present as a normal flora in the majority of the human population. Under pre-disposing conditions in the host, this species can be pathogenic and cause mucosal and systemic candidiasis (Scully et al., 1994; Moran et al., 2011; Leach and Brown, 2012; Polvi et al., 2015). One of the major virulence factors involved in C. albicans pathogenesis is its conversion from the yeast to the hyphal form (Sudbery et al., 2004). Other virulence traits include genome plasticity (Selmecki et al., 2010; Goodenough and Heitman, 2014), cell-surface variegation (Nather and Munro, 2008; Latge, 2010), and biofilm formation (Kumamoto and Vinces, 2005; Nobile and Mitchell, 2006). Multiple environmental signals induce morphogenetic responses in C. albicans, such as altered nutrient levels, temperature, pH, CO2, pheromones and the release of quorum-sensing compounds (Biswas et al., 2007). These diverse stimuli are sensed by a variety of different sensors, which regulate signal transduction pathways that control morphogenetic and biochemical changes to promote host invasion and colonization (Rispail et al., 2009). Among the major signalling pathways that regulate dimorphism in C. albicans are Mitogen Activated Protein Kinase (MAPK) pathways, the RAS pathway, the calcium-calcineurin pathway and amino acid sensing and signalling pathways (Biswas et al., 2007; Zhao et al., 2007; Rispail et al., 2009).
The response to temperature stress and filamentation in C. albicans has been well characterized (Shapiro et al., 2009; Brown et al., 2010; Shapiro and Cowen, 2010; Shapiro and Cowen, 2012a,b; Shapiro et al., 2012a; Leach and Cowen, 2013; Leach and Cowen, 2014a,b; O’Meara and Cowen, 2014). One aspect of thermoregulation is mediated by an evolutionarily conserved heat shock response (HSR). The major role of the response is to promote protein folding by the action of heat shock proteins (HSPs) such as the major molecular chaperone Hsp90 (Morimoto, 1998). Other pathways sense and respond to changes in protein folding, such as the unfolded protein response (UPR) that operates in the endoplasmic reticulum (Patil and Walter, 2001; Wimalasena et al., 2008; Gardner et al., 2013). Short-term responses include stabilization of the essential transcription factor Cta8/Hsf1, which is a ‘client protein’ for Hsp90 (Leach et al., 2012a). Prolonged thermal adaptation requires coordination between Hsp90 and MAPK pathways (Liu and Chang, 2008; Leach et al., 2012a,). In this case, Hsp90 stabilizes other client proteins that include Cek1 (Leach et al., 2012b), the MAP kinase that regulates the CEK pathway (Leberer et al., 1996; Csank et al., 1998). Hsp90 also stabilizes the MAP kinase Hog1 (Diezmann et al., 2012), which regulates the high osmolarity glycerol response (HOG) pathway (Alonso-Monge et al., 1999; Saito, 2010). In addition, Hsp90 stabilizes the MAP kinase Mkc1 (LaFayette et al., 2010; Leach et al., 2012a), which regulates the protein kinase C (PKC) pathway (Navarro-Garcia et al., 1998). The RAS pathway is also a global nutrient-sensing pathway that regulates the response to high temperature (Shapiro et al., 2009; Huang et al., 2010; Langford et al., 2013; Rao et al., 2013).
Although much work has been done to characterize the signalling pathways that regulate thermoregulation in C. albicans and other species, the ‘sensors’ that detect changes in temperature have not been well defined. One candidate protein is the signalling mucin Msb2. Signalling mucins are transmembrane (TM) glycoproteins that regulate signalling pathways in eukaryotes (Kufe, 2009; Tian and Ten Hagen, 2009; Bafna et al., 2010; Cullen, 2011; Birchenough et al., 2015). In fungal species, Msb2 is a regulator of environmental stress, cell wall biogenesis and the CEK pathway (Cullen et al., 2004; Roman et al., 2009; Puri et al., 2012). Because the CEK pathway is activated by growth in high temperatures, Msb2 may be a candidate regulator of thermal stress. We show here that Msb2 plays an important role in thermo-adaptation in C. albicans. Msb2 was required for hyphae formation and growth at 42°C Msb2 regulated the response to high temperatures by regulating the CEK pathway. In addition, Msb2 regulated the activity of the PKC pathway and the expression of genes that encode proteins that regulate the UPR. Ssa1, a Hsp70 chaperone and abundant cell wall protein (Sun et al., 2010), was also required for thermal adaptation and MAP kinase (CEK and PKC) pathway regulation. These results extend the understanding of the signalling pathways that regulate thermo-tolerance in C. albicans to include signalling mucins and cell-wall-associated HSP-type sensors. The identification of such regulatory proteins is critical to understand thermo-tolerance regulation in fungi and possibly other systems.
Results
The signalling mucin Msb2 is required for hyphae formation and survival at 42°C
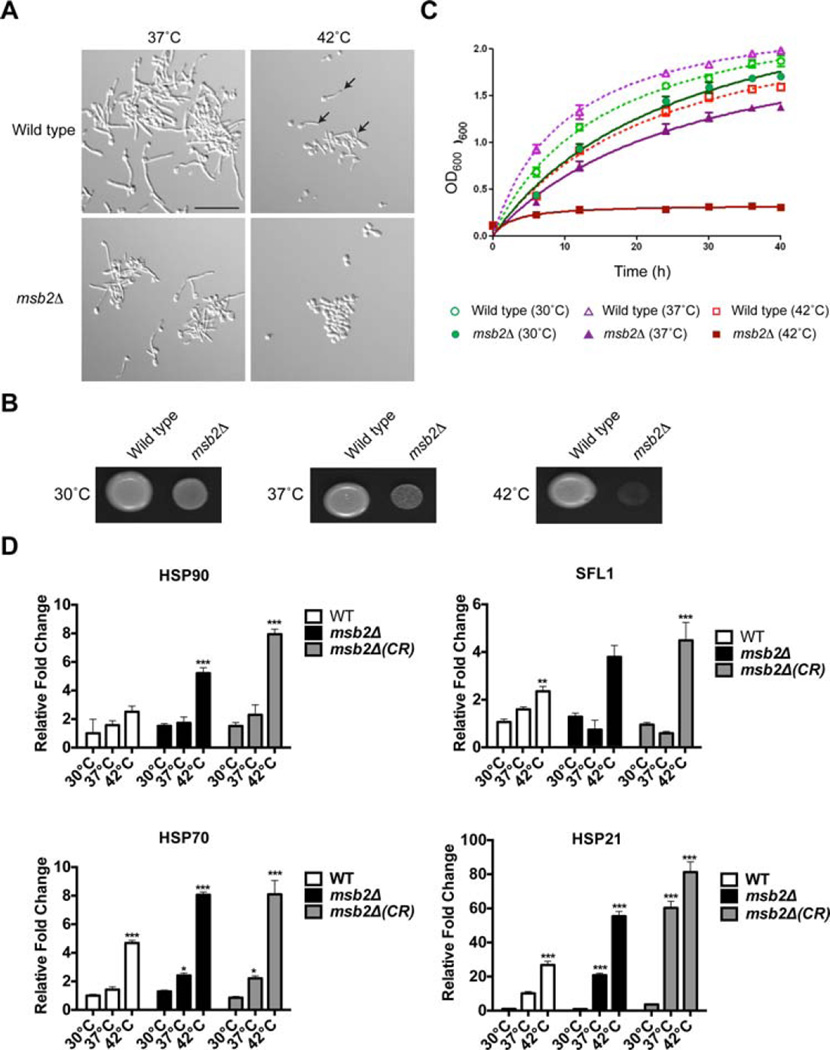
Msb2 is a signalling glycoprotein that regulates the CEK MAP kinase pathway (Cullen et al., 2004; Roman et al., 2009; Puri et al., 2012). To better understand the role of Msb2 in regulating signalling and behavioural responses in C. albicans, wild-type cells and the msb2Δ mutant were examined by microscopy under different growth conditions. Nutrient limitation and growth at elevated temperatures are potent inducers of fungal dimorphism in C. albicans (Puri et al., 2012). The formation of hyphae was therefore examined in response to growth in different carbon sources and different temperatures. Growth of C. albicans is optimal at 37°C, but the organism can tolerate temperature stresses exceeding 55°C. We found that the msb2Δ mutant was defective for hyphae formation at 42°C Wild-type cells showed a reduction in the formation of hyphae at 42°C, but the msb2Δ mutant was completely defective (Fig. 1A, arrows point to examples of hyphae at 42°C; see Supporting Information Fig. S1 for quantitation). These results show that Msb2 plays a role in hyphae formation at 42°C.
Fig. 1. Role of Msb2 in hyphae formation and survival at 42°C.
A. Wild type and the msb2Δ mutant were grown in YNB + 2% Glu for 3 h at 37°C and 42°C and observed by microscopy at 20X magnification. Arrows, examples of hyphae at 42°C. Bar, 10 microns.
B. Growth of wild-type cells and the msb2Δ mutant in YPD media at 30°C, 37°C, 42°C. Equal concentrations of cells were spotted onto plates for 48 h.
C. Growth curves of wild-type cells and the msb2Δ mutant in YPD media at 30°C, 37°C, 42°C. Results are the average of two independent experiments. Error bars represent the standard deviation between experiments.
D. Quantification of HSP gene expression was measured by quantitative RT-PCR of RNA extracted from cells grown in 30°C, 37°C, 42°C for 3 h. Transcript levels were normalized to GAPDH2 gene expression. Levels are expressed relative to wild type, which was set to 1. Data are the mean and SD of three independent biological replicates. Error bars refer to the standard deviation between trials. The *** indicates P value < 0.001, ** indicates P < 0.01 and * indicates P < 0.5. CR, congo red.
To better define the basis for the defect in hyphae production at 42°C, the msb2Δ mutant was examined in detail. The msb2Δ mutant showed a growth defect at 30°C and 37°C and had a striking growth defect at 42°C (Fig. 1B). The growth (OD600) of wild-type cells and the msb2Δ mutant was assessed by growth curves in liquid media. Wild-type cells grew optimally at 37°C and showed a reduced growth rate at 30°C and 42°C (Fig. 1C). By comparison, the msb2Δ was severely defective for growth at 42°C (Fig. 1C). It may be that cells compromised for viability at 42°C are unable to produce hyphae. This is probably in part true. However, measuring colony forming units (CFU) at the time point tested showed that the msb2Δ mutant was viable at 42°C under conditions when it was defective for hyphae formation (Supporting Information Fig. S2A). Furthermore, at an intermediate temperature (39°C), the msb2Δ mutant was defective for hyphae formation but viable (Supporting Information Fig. S2B). Therefore, in addition to its growth defect at 42°C, the msb2Δ mutant is defective for hyphae formation at 42°C.
In response to growth at high temperatures, cells induce a Heat Shock Response (HSR). One feature of this response is the transcriptional induction of genes encoding heat shock proteins (HSPs) that include Hsp90 (Leach et al., 2012a), Hsp70 (Leach et al., 2012a), Hsp21 (Mayer et al., 2012) and the transcription factor Sfl1 (Znaidi et al., 2013). Quantitative PCR (qPCR) analysis was used to measure the expression of HSP90, HSP21, HSP70 and SFL1 in wild-type cells and the msb2Δ mutant at 30°C, 37°C and 42°C. The expression of heat shock genes was elevated by more than twofold in wild-type cells grown at 42°C in YPD medium for 3 h (Fig. 1D, white bars). Thus, growth of C. albicans at 42°C induces thermal stress. The expression of heat shock genes was also elevated in the msb2Δ mutant, by more than twofold above that seen in wild-type cells (Fig. 1D, black bars). These results indicate that the msb2Δ mutant experiences an elevated HSR compared to wild-type cells. We also examined HSR targets in response to Congo Red (CR) stress at three temperatures (Fig. 1D, CR). For at least some targets, an additive effect of temperature and cell wall stress was observed. Elevated HSR in the msb2Δ mutant did not rescue the growth defect, which suggests that Msb2 regulates a specific pathway. Below we show that Msb2 regulates the CEK, PKC, and UPR. Altogether, the findings identify a new role for Msb2 in regulating hyphae formation and survival at 42°C.
The extracellular domain of Msb2 is required for survival and hyphae formation at 42°C
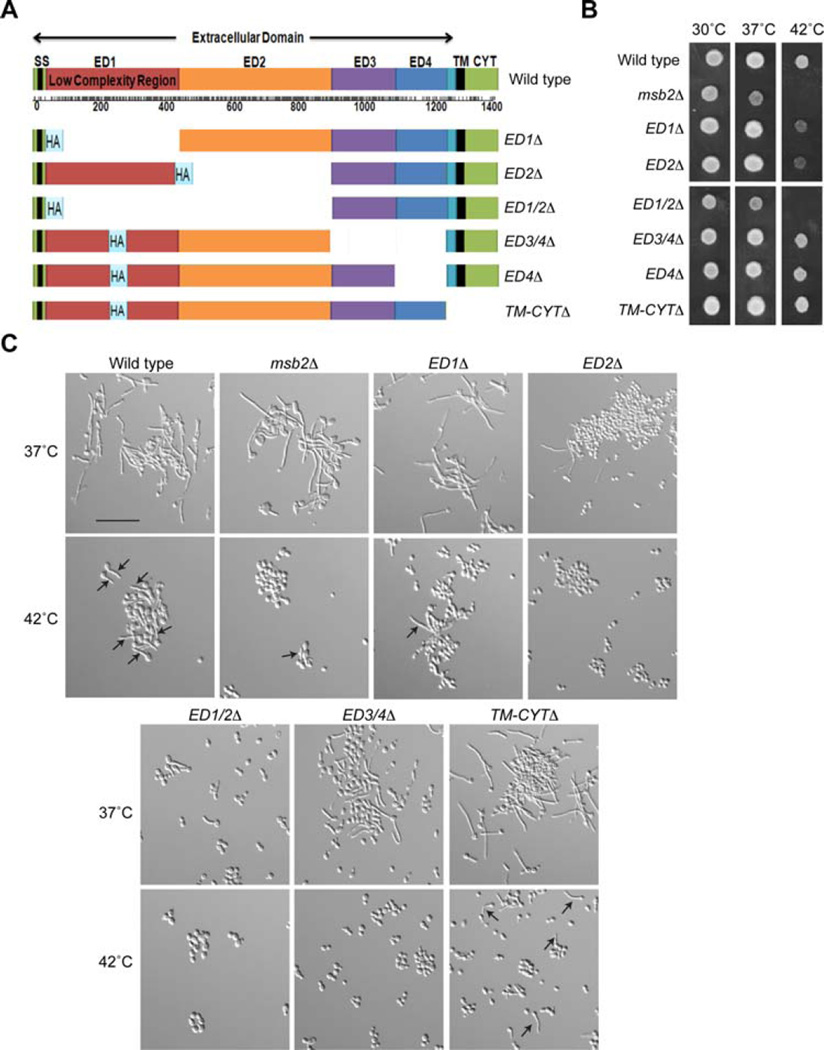
Like other signalling mucins (Singh and Hollingsworth, 2006; Tian and Ten Hagen, 2009; Kufe, 2013), Msb2 is a type I single-pass transmembrane (TM) protein that contains a highly glycosylated extracellular domain, a cleavage domain (CD) and a cytosolic signalling domain (CYT; Fig. 2A). The domain structure of Msb2 has been well characterized (Szafranski-Schneider et al., 2012; Swidergall et al., 2015). The extracellular domain of Msb2 is highly modified by O-linked glycans in a repeat region that is rich in proline, threonine and serine residues (PTS region, 100–900 aa residues). A highly glycosylated PTS region is shared with all mucin members (Desseyn et al., 2008; Tarp and Clausen, 2008). The PTS region of Msb2 also contains a Low Complexity Region [LCR (Coletta et al., 2010)] in its N-terminus (aa 100–450). Versions of Msb2 were constructed that lacked each of the major functional domains, including the PTS region (ED1Δ, ED2Δ and ED1/2Δ), the CD (ED3/4Δ and ED4Δ) and the TM and CYT domains (TM-CYTΔ Fig. 2A). The deletion derivatives were tested for growth at 30°C, 37°C and 42°C Versions of Msb2 lacking the PTS region (ED1Δ, ED2Δ and particularly ED1/2Δ) were required for growth at high temperatures (Fig. 2B). The CD (ED3/4Δ and ED4Δ) and TM-CYT domains (TM-CYTΔ) were not required (Fig. 2B). A similar phenotype was observed by examining the formation of hyphae at 42°C The ED1Δ, ED2Δ, ED1/2Δ and ED3/4Δ mutants were defective for hyphae production at 42°C, but the TM-CYTΔ mutant was not (Fig. 2C, Supporting Information Fig. S1). The ED2 domain also contributed to hyphae formation at 37°C (Fig. 2C, Supporting Information Fig. S1). CFU analysis showed that the cells were viable (Supporting Information Fig. S2C). These results identify the extracellular domain of Msb2 (primarily the PTS region) as being required for survival and hyphae formation at 42°C.
Fig. 2. Role of different domains of Msb2 in regulating survival and hyphae production at high temperatures.
A. Full length Msb2 and deletion derivatives used in the study. The signal sequence (ss), low-complexity region, extracellular domain, transmembrane domain (TM) and cytosolic (CYT) domains are indicated. The deletions are shown as indicated in the figure, with corresponding replacement of the domains of the Msb2 protein by the hemagglutinin (HA) epitope.
B. Strains containing Msb2 deletions were spotted on YPD media at 30°C, 37°C and 42°C for 48 h.
C. Strains containing Msb2 deletions were grown in YNB + 2% Glu for 3 h at 37°C and 42°C and observed by microscopy at 20X magnification. Arrows, examples of hyphae at 42°C. Bar, 10 microns.
Msb2 regulates temperature-dependent activation of the CEK pathway
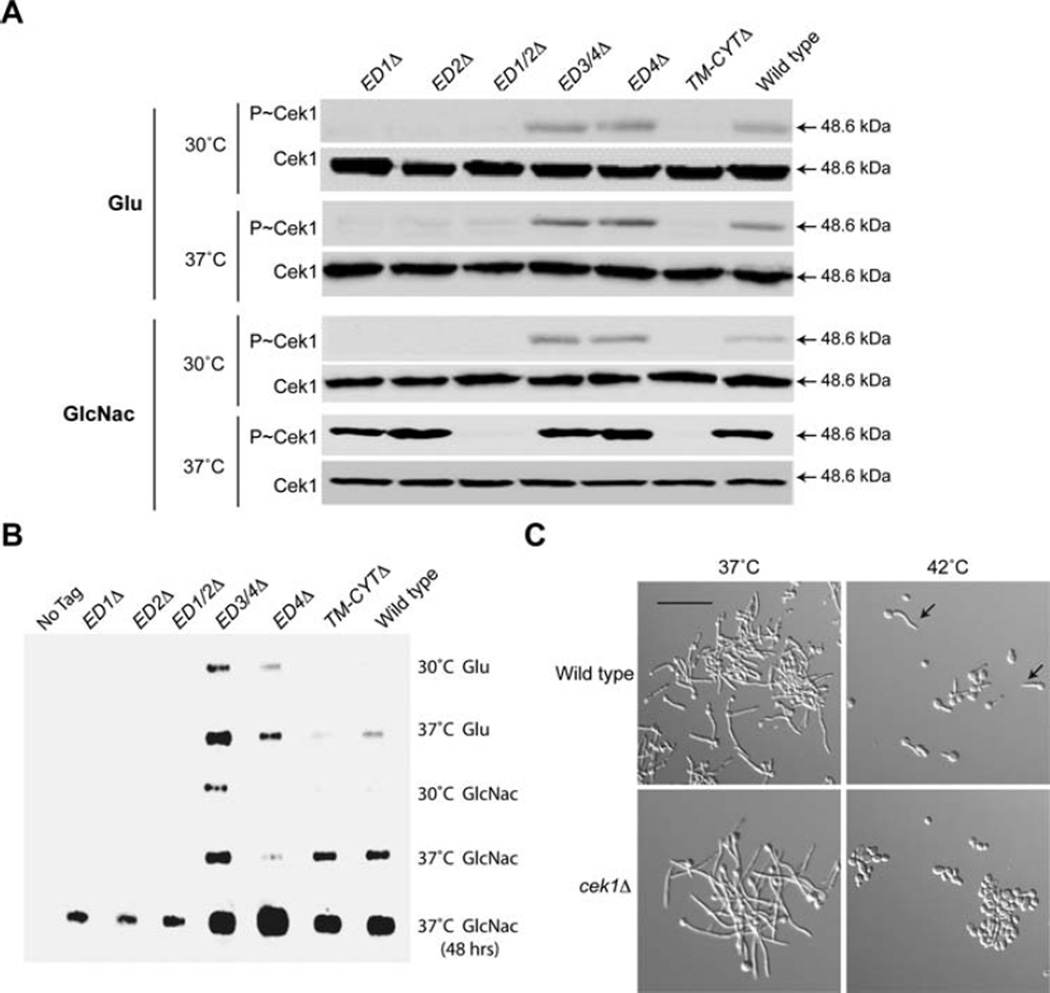
Msb2 is an established regulator of the CEK MAP kinase pathway (Roman et al., 2009; Puri et al., 2012). The CEK pathway requires both limiting nutrients [e.g. growth in the poor carbon source N-Acetyl-Glucosamine (GlcNAc)] and high temperature (37°C) for activation (Puri et al., 2012). Deletion derivatives of Msb2 were tested for the regulation of the CEK pathway by measuring phosphorylation of the MAP kinase Cek1 (P~Cek1). Consistent with a previous report (Puri et al., 2012), growth of wild-type cells in glucose (Glu) did not induce P~Cek1 at 30°C or 37°C (Fig. 3A, wild type). However, growth of cells in GlcNac induced P~Cek1 at 37°C (Fig. 3A, wild type). Growth of cells at 42°C also induced the CEK pathway but to a lesser extent than growth at 37°C (see below). Two mutants were defective for this induction, the ED1/2Δ and the TM-CYTΔ domain (Fig. 3A). Thus, Msb2 regulates temperature-dependent CEK pathway activation through the ED1/2 and TM-CYT domains. Moreover, the TM-CYT domain is dispensable for survival (Fig. 2B) and hyphae formation at 42°C (Fig. 2C, Supporting Information Fig. S1) but is required for CEK pathway activation at 37°C (Fig. 3A) and at 42°C (see below).
Fig. 3. Msb2 regulates the response to high temperature through the CEK pathway.
A. Immunoblot of P~Cek1 levels in Msb2 deletions. Strains were incubated in pre-warmed YNB media with 1.25% GlcNac at 30°C or 37°C for 3 h. Total cellular protein (20 µg) from cell lysates was examined by immunoblot analysis with α-phospho p42/44 MAPK antibodies or α-Cek1 antibodies as a control.
B. To determine Msb2 shedding, control (CAI4) or cells expressing HA-Msb2 and derivatives were grown in YNB + Glu or YNB + GlcNac media (pre-warmed to 30°C or 37°C) for 3 h. Cells were removed by centrifugation, and proteins in the supernatant were precipitated by TCA. Normalized protein (20 µg) was examined by slot blot using anti-HA antibodies.
C. Cells were grown in YNB + 2% Glu for 3 h at 37°C and 42°C and observed by microscopy at 20X magnification. Arrows, examples of hyphae at 42°C Bar, 10 microns.
The activation of the CEK pathway involves Sap-dependent Msb2 processing and shedding of the extracellular domain (Puri et al., 2012; Vadaie et al., 2008). Growth at 37°C resulted in elevated shedding of HA-Msb2 in wild-type cells (Fig. 3B, wild type), which correlates with the activation of the CEK pathway. The deletion derivatives were all detected by slot blot, indicating that they are all expressed (Fig. 3B). ED1Δ, ED2Δ and ED1/2Δ were present at lower levels, which may indicate that these domains contribute to the stability of the protein. The reduced levels of these versions of Msb2 may account for the phenotypes described above. Slot blot analysis showed that ED3/4Δ and ED4Δ were shed at higher levels than wild-type Msb2 in glucose, which may indicate this region is inhibitory to shedding in some contexts. It is possible that different Saps differentially impact Msb2 shedding in a condition-dependent manner. In related studies (Szafranski-Schneider et al., 2012; Swidergall et al., 2015), deletion derivatives of Msb2 showed functional differences to those reported here. This may result, in part, from differences in MSB2 expression, because MSB2 was expressed from an exogenous (strong) promoter in (Szafranski-Schneider et al., 2012; Swidergall et al., 2015) or from functional differences arising from the precise deletions constructed.
The MAP kinase Cek1 was itself required for hyphae formation at 42°C (Fig. 3C, Supporting Information Figs. S1 and S2A). The cek1Δ mutant [and other CEK pathway mutants, like sho1Δ (Roman et al., 2009)] also showed a growth defect at 42°C, but not to the same extent as the msb2Δ mutant, because the growth defect was detected at early (24 h) but not late (48 h) time points (Supporting Information Fig. S3). These results agree with other studies where Msb2 deletions were constructed and analyzed (Szafranski-Schneider et al., 2012; Swidergall et al., 2015). Altogether, the results show that one aspect of Msb2’s temperature response is mediated through its canonical role in regulating the CEK pathway.
Msb2 also regulates the PKC pathway
Several observations indicate that Msb2 regulates survival at 42°C beyond its role in regulating the CEK pathway. First, the extracellular domain (but not the TM-CYT domain) is required for survival at 42°C (Fig. 2B). Second, the msb2Δ mutant has a more severe growth defect than other CEK pathway mutants (Supporting Information Fig. S3). To identify how Msb2 might regulate thermo-tolerance outside of its role in regulating the CEK pathway, a panel of mutants was examined that were defective for pathways that impact stress signalling and dimorphism including the HOG (sln1Δ, sho1Δ, ssk1Δ, ssk2Δ and hog1Δ), RAS (ras1Δ, cyr1Δ, tpk1Δ, tpk2Δ and efg1Δ), PKC (wsc1Δ, wsc2Δ, wsc4Δ, bck1Δ, mkk2Δ and mkc1Δ) and UPR (ire1Δ and hac1Δ) pathways, as well as several proteins that function in the cell wall (mp65Δ, mu1cΔ, hyp1Δ, hwp2Δ, ssa2Δ, ssa1Δ and sgt1Δ). Several mutants showed growth defects after 24 h incubation at 42°C, including mkc1Δ, ire1Δ, and ssa1Δ (Fig. S3), which were examined in detail.
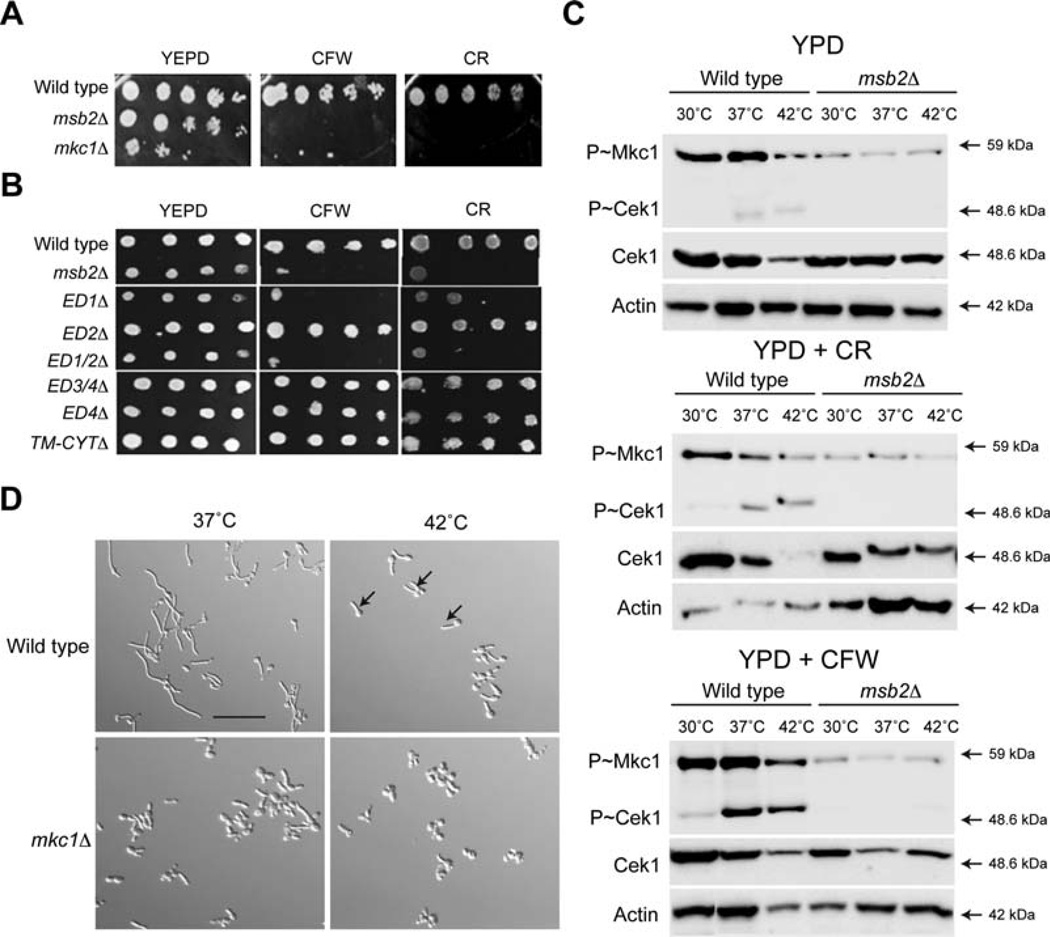
The first mutant that showed a growth defect at 42°C was the mkc1Δ mutant. The growth defect of the rnkc1Δ mutant resembled that of the cek1Δ mutant, which had slow growth at 24 h but grew normally at 48 h (Supporting Information Fig. S3). Mkd is the MAP kinase that regulates the PKC or cell wall integrity pathway and is an established regulator of the response to high-temperature stress (Levin, 2005; LaFayette et al., 2010; Diezmann et al., 2012). Thus, we tested whether Msb2 regulates the PKC pathway. The PKC pathway protects cells against a variety of stresses to the cell wall (Diezmann et al., 2012). The msb2Δ mutant was sensitive to cell wall perturbing agents such as Congo Red (CR) and Calcofluor White (CFW) to the same degree as the mkc1Δ mutant (Fig. 4A). The cell wall sensitivity was mediated by the PTS region of the protein, because the ED1Δ and ED1/2Δ mutants showed sensitivity whereas other mutants did not (Fig. 4B). ED2Δ was not sensitive which distinguished its role in cell-wall integrity from its role in regulating survival at 42°C (Fig. 2B).
Fig. 4. Msb2 regulates the PKC pathway.
A. Growth of strains in YPD, YPD + CFW (100 µg/ml) and YPD + CR (100 µg/ml). Serial dilutions were spotted, and plates were incubated for 48 h at 37°C.
B. Growth of Msb2 deletion mutants was examined as in panel 4A.
C. Activity of the CEK and PKC pathways in response to different stimuli. Mid-log phase cells in YPD were incubated in YPD media alone, YPD + CFW (100 µg/ml) and YPD + CR (100 µg/ml) for 3 h at the indicated temperatures. Total protein (20 µg) was examined by immunoblot analysis with α-phospho p42/44 MAPK rabbit monoclonal antibodies, α-Cek1 and α-Act1 as a control for total protein levels.
D. Cells were grown to mid-log phase in YNB + 2% Glucose for 3 h at 37°C and 42°C and observed by microscopy at 20X magnification. Arrows, examples of hyphae at 42°C. Bar, 10 microns.
We next tested whether Msb2 was required to activate the PKC pathway by temperature or cell wall stresses by examining phosphorylation of Mkc1 (P~Mkc1). The msb2Δ mutant was defective for phosphorylation of P~Mkc1 (and P~Cek1; P~Mkc1 and P~Cek1 are recognized by the same p42/p44 P~ERK antibodies) at all temperatures tested and in response to CR and CFW (Fig. 4C). Optimal phosphorylation of Mkc1 was seen at 37°C (for YPD and CFW) or 30°C (for CR). These results demonstrate a role for Msb2 in regulating the PKC pathway in C. albicans. Furthermore, the results show that the extracellular domain of Msb2 (but not the CYT domain) regulates the PKC pathway. The mkc1Δ mutant was defective for hyphae production at 42°C (Fig. 4D, Supporting Information Figs. S1 and S2A). Therefore, two MAP kinase pathways controlled by Msb2 (CEK and PKC) are involved in regulating the response to high temperature stress. Because the cek1Δ and mkc1Δ mutants show intermediate temperature sensitivity compared to the msb2Δ mutant (Supporting Information Fig. S3), Msb2 may regulate survival at 42°C by a mechanism that involves combinatorial action of the CEK and PKC pathways. Based on phospho-MAPK analysis, the MKC pathway is not activated at 42°C, and although the CEK pathway is activated at 37°C, it is not further induced at 42°C. It is possible that the two pathways (CEK and MKC), which are redundant for growth at 42°C, buffer each other’s activities at 42°C Alternatively, growth at 42°C may also involve a third pathway.
Msb2 is required to survive UPR stress and for induction of genes encoding UPR regulators and targets
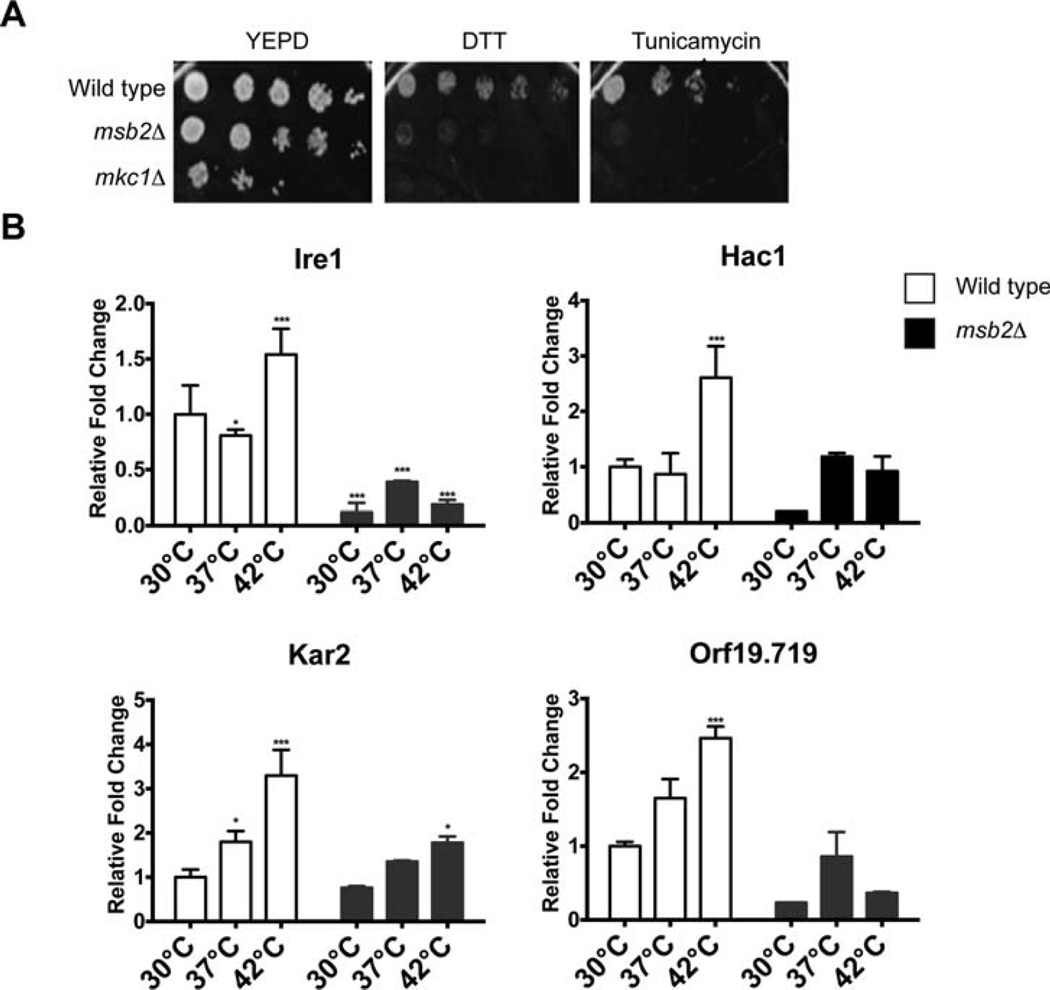
Among the pathways that the PKC pathway regulates is the UPR (Patil and Walter, 2001; Wimalasena et al., 2008; Scrimale et al., 2009; Gardner et al., 2013). The UPR is a QC pathway that regulates the folding and degradation of proteins in the endoplasmic reticulum in response to high temperature and other stresses. The UPR is regulated by the sensor kinase Ire1, which functions in the endoplasmic reticulum (Cox et al., 1993; Mori et al., 2000). The ire1Δ mutant had a growth defect that resembled the cek1Δ and mkc1Δ mutants (Supporting Information Fig. S3), which indicates that Ire1 is required for growth at 42°C In S. cerevisiae, Msb2 and the Kss1 MAP kinase pathway (Cek1 equivalent pathway) regulate the UPR (Adhikari et al., 2015). We therefore examined the possible connection between Msb2 and the UPR in C. albicans. The msb2Δ mutant was sensitive to chemical inducers of the UPR including the reducing agent dithiothreitol (DTT) and tunicamycin (Fig. 5A). In response to these compounds, the msb2Δ mutant had the same phenotype as the mkc1Δ mutant (Fig. 5A).
Fig. 5. Msb2 regulates the response to ER stress and expression of regulators of the UPR and other QC pathways in the endoplasmic reticulum.
A. Growth of the indicated strains on YPD, YPD + DTT (10mM) and YPD + Tunicamycin (2 µg/ml). Equal concentrations of cells were spotted in serial dilutions onto the indicated medium. Plates were incubated for 48 h at 37°C.
B. Expression of genes encoding UPR regulators by quantitative RT-PCR analysis. Cells were grown for 3 h at 30°C, 37°C and 42°C. Transcript levels were normalized to the ACT1 gene. Levels are expressed relative to wild type, which was set to 1. Data are the mean and SD of three independent biological replicates. Error bars refer to the standard deviation between trials. The *** indicates P value < 0.001, ** indicates P < 0.01 and * indicates P < 0.5.
Ire1 is the sensor kinase for the UPR pathway, and Hac1 is the major transcription factor for the UPR (Cox and Walter, 1996). Among the transcriptional targets of the UPR is the heat shock protein Kar2 (de Keyzer et al., 2009; Hale et al., 2010). Proteins that become mis-folded in the endoplasmic reticulum can be degraded in the cytoplasm by ERAD, which requires the ubiquitin ligase Hrd1 [(Scrimale et al., 2009; Kanehara et al., 2010; Miller et al., 2010) Orf19.719 in C. albicans]. We examined whether Msb2 played a role in regulating expression of genes encoding UPR pathway regulators, targets, or ERAD regulators. In wild-type cells, the expression of IRE1, HAC1, HRD1 and ORF19.719 were found to be induced by growth at elevated temperatures by a factor of >1.5-fold (Fig. 5B, white). The expression of these genes was reduced in the msb2Δ mutant by a factor of at least twofold at 42°C (Fig. 5B, black). These results connect Msb2 to the regulation of QC pathways that operate in the endoplasmic reticulum in C. albicans. It is possible that the connection is mediated by Msb2 regulation of the PKC pathway. In addition, the CEK pathway may be involved, which is supported by the intriguing observation that the temperature-dependent depletion of Cek1 is seemingly Msb2 dependent (Fig. 4C).
Ssa1 is required for survival at 42°C and regulates the CEK and PKC pathways
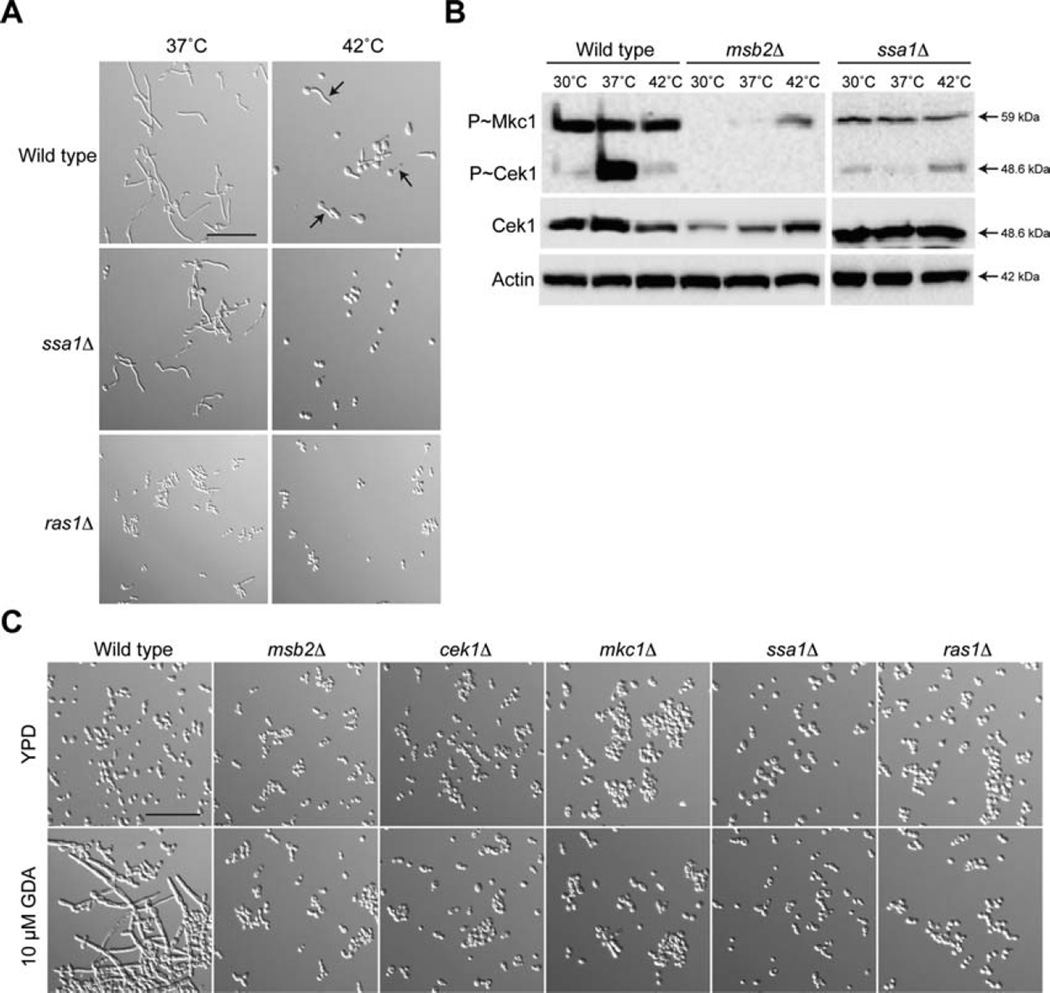
A third protein that was required for normal growth at 42°C was the heat-shock regulator and abundant cell-wall protein Ssa1 (Sun et al., 2010). Among ~40 mutants tested, the growth defect of the ssa1Δ and msb2Δ mutants were the most severe (Supporting Information Fig. S3). The ssa1Δ mutant was defective for hyphae formation at 42°C (Fig. 6A, Supporting Information Figs. S1 and S2A). The ssa1Δ mutant was also required for activation of the CEK and PKC pathways (Fig. 6B). P~MAPK levels showed some differences depending on growth conditions. Thus, Ssa1 may be part of the sensing/signalling mechanism that allows cells to respond to high-temperature stress.
Fig. 6. Role of Ssa1 in hyphae formation and regulation of the CEK and PKC pathways.
A. Cells were grown in YNB + 2% Glu for 3 h at 37°C or 42°C. Cells were examined by microscopy at 20X magnification. Arrows refer to examples of hyphae at 42°C. Bar, 10 microns.
B. Cells were grown to mid-log phase and incubated in YNB + GlcNac media pre-warmed to 37°C for 3 h at the indicated temperatures. Total cellular protein (20 µg) from cell lysates was examined by immunoblot analysis with α-phospho p42/44 MAPK, α-Cek1 and α-Act1 antibodies (as a control for protein loading).
C Wild type cells and the indicated mutants were incubated in YPD or YPD with 10 µM GDA at 30°C for 6 h. Cells were examined by microscopy at 20X magnification. Bar, 10 microns.
Growth at 37°C induces, among other responses, the formation of hyphae. Inhibiting the function of Hsp90 mimics the heat-shock response at 30°C (Dai et al., 2012; Diezmann et al., 2012; Leach et al., 2012b). We used geldanamycin (GDA), a inhibitor of Hsp90 (Wehrli and Staehelin, 1971), to induce hyphae formation at 30°C. When added to wild-type cells, GDA induced hyphae at 30°C (Fig. 6C). The msb2Δ, cek1Δ, mkc1Δ, and ssa1Δ mutants did not (Fig. 6C). The RAS pathway also regulates the response to high temperature (Shapiro et al., 2009; Huang et al., 2010; Langford et al., 2013; Rao et al., 2013). The ras1Δ mutant was required for hyphae production at 42°C (Fig. 6A) and GDA-dependent hyphae formation at 30°C (Fig. 6C). This experiment provides validation of the above findings. Therefore, the morphogenetic response to high temperature stress involves the action of the CEK, PKC, UPR and RAS pathways, in part through the action of the signalling pathway regulators Msb2 and Ssa1.
Discussion
Temperature is a common inducer of fungal dimorphism. Although many signalling pathways that respond to temperature stress have been identified, the sensors have not been well characterized. We show here that the signalling mucin-like glycoprotein Msb2 is a regulator of thermal stress in the fungal pathogen C. albicans. Msb2 was required for a variety of responses that occur at higher temperatures, including optimal growth (at 37°C), survival (at 42°C), and hyphae formation (at 42°C). The identification of a regulatory protein that functions at the plasma membrane and is required for the response to thermal stress is an important step in understanding how changes in temperature are detected by fungal cells.
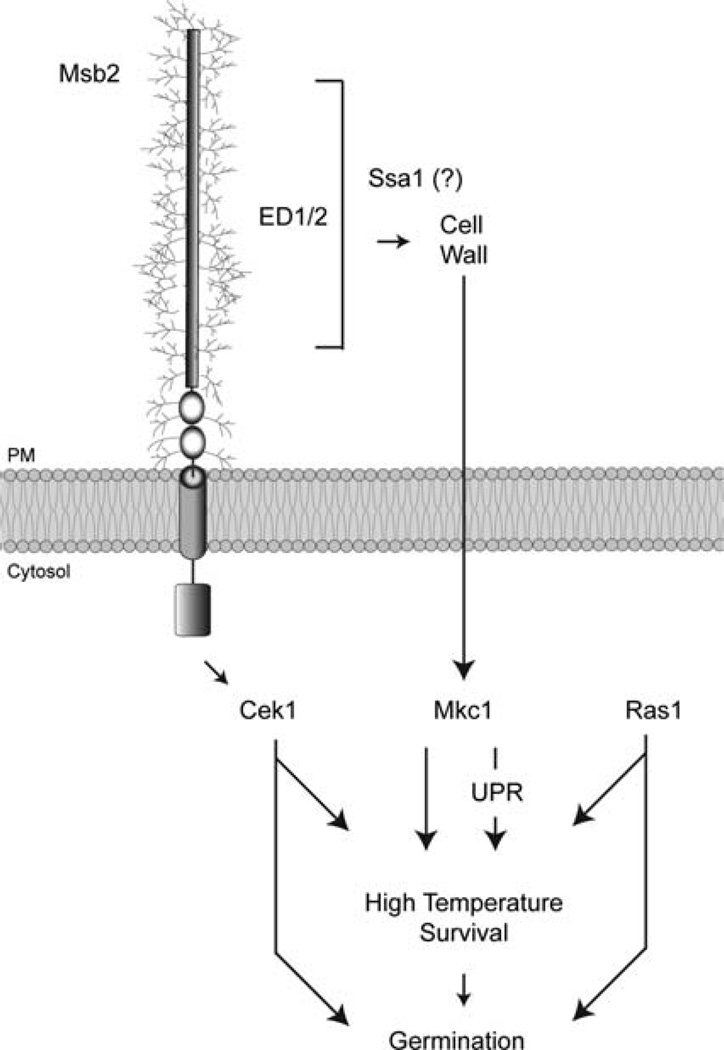
Msb2 controls the response to high temperature by regulating at least three different signalling pathways (Fig. 7). Msb2 is an established regulator of the CEK pathway (Puri et al., 2012; Szafranski-Schneider et al., 2012; Perez-Nadales and Di Pietro, 2014; Swidergall et al., 2015) and was required for activation of the CEK pathway during growth at high temperatures. The extracellular and CYT domains of Msb2 were required for this response. It is reasonable to speculate that Msb2 regulates the CEK pathway by binding to and regulating cytosolic components of that pathway (Fig. 7). Msb2 also regulates thermo-tolerance through the PKC pathway. The PKC pathway is an established regulator of the response to high temperatures (Levin, 2005; LaFayette et al., 2010; Diezmann et al., 2012), but Msb2 has not been previously shown to regulate the PKC pathway. Interestingly, the extracellular domain of Msb2 - but not the cytosolic domain - was required to regulate the PKC pathway (Fig. 7). Although it is not clear how the extracellular domain connects to the PKC pathway, one possibility is that the highly glycosylated extracellular domain of Msb2 impacts the integrity or stability of proteins or carbohydrates in the cell wall. A second possibility is that Msb2 regulates one or more of the sensors that regulate the PKC pathway. The third pathway that Msb2 regulates is the UPR. The role of Msb2 in regulating the UPR may be mediated by its regulation of the PKC pathway (Fig. 7). The cek1Δ, mkc1Δ, and ire1Δ mutants show temperature sensitivity at 42°C but not to the same degree as the msb2Δ mutant. Thus, Msb2 may function as a ‘master regulator’ that coordinates the combinatorial response of these pathways to growth at high temperatures. In humans, signalling mucins similarly coordinate the action of many different pathways (Regimbald et al., 1996; Kam et al., 1998; Rahn et al., 2004; Wei et al., 2005; Singh and Hollingsworth, 2006; Wei et al., 2006).
Fig. 7. The role of Msb2 and Ssa1 in regulating pathways that control the high temperature response.
The signalling mucin Msb2 plays two roles in regulating the response to high temperature. Through its extracellular and cytosolic domains, Msb2 regulates the activity of the CEK pathway (Cek1). Through its extracellular (but not cytosolic domain), Msb2 regulates the PCK pathway (Mkc1). Msb2 also regulates the UPR, potentially through the PKC pathway. Together with the RAS pathway (Ras1), the CEK (Cek1) and PKC (Mkc1) pathways regulate survival and hyphae formation at high temperatures. Ssa1 regulates the CEK and PKC pathways, potentially at the level of the cell wall, through a mechanism that has yet to be elucidated, as indicated by the question mark.
We also show that Ssa1 plays a critical role in thermoregulation in C. albicans. Ssa1 is putative heat shock protein that is present on the cell surface and in the cytosol (Sun et al., 2010). Given that Ssa1 is a cell wall protein, Ssa1 may interact with Msb2 or the cell wall to detect and regulate adaptation to thermal stress. Alternatively, Ssa1 may regulate MAPK signalling as a heat shock protein in the cytosol. These possibilities are not mutually exclusive.
Given that multiple pathways regulate the response to thermal stress, it may not be surprising that different phenotypes emerge by examining different mutants. For example, Msb2 controls dimorphism by regulating the CEK and PKC pathways; however, the msb2Δ mutant forms hyphae at 37°C Furthermore, cells lacking Msb2 retain the ability to mount one type of thermotolerance response (as evinced by the induction of cytosolic HSP encoding genes), but not another (based on the diminished capacity of UPR targets and regulators). Thus, Msb2 may play a particular role in the cell’s overall heat shock response. In the future, it will be important to understand how Msb2 function may connect to Hsp90, the key regulator of temperature-dependent morphogenesis (Shapiro et al., 2009; Shapiro and Cowen, 2010; Senn et al., 2012; Shapiro et al., 2012b). Hsp90 controls temperature sensing through many pathways, including the RAS pathway (Shapiro et al., 2009), and it may be interesting to know whether Msb2 function impacts the RAS pathway. Like Ssa1, Hsp90 also localizes on the cell surface as well as in the cytoplasm (Urban et al., 2003; Pitarch et al., 2004), so it will be instructive to define from where different HSPs regulate the response.
Intriguingly, several connections between signalling mucins and the heat shock response have been made in other systems. In humans, HSP90 targets MUC1 to the mitochondria (Ren et al., 2006). A related HSP, HSP70, regulates MUC1 secretion (Fang et al., 2013). It will be interesting to learn more about how heat shock proteins regulate signalling mucin function and vise versa.
In summary, we have identified a new role for Msb2 (and Ssa1) in regulating survival and dimorphism at high temperatures in C. albicans. Msb2 regulates multiple pathways to regulate the response. Given that temperature is critical for dimorphism and virulence in C. albicans and other fungal species, the identification of new regulators may provide insight into the molecular basis of fungal pathogenesis.
Experimental procedures
Strains, media and growth conditions
C. albicans strains used in this study are listed in Table 1. CAI4 strain was the wild type control for all experiments. Strains were grown in YPD (1% yeast extract, 2% peptone, 2% glucose, 2% agar) or YNB (2% glucose, 0.17% yeast nitrogen base, 0.5% ammonium sulphate, 2% agar). For growth sensitivity assays, cells were grown to saturation for 16 h, and cells were diluted to OD600 0.1 for spotting serial dilutions onto media. For immunoblot analysis, cells were grown in YPD, YNB and YNB with 1.25% N-Acetyl-D Glucosamine (GlcNac) for 3 h.
Table 1.
C. albicans strains used in the study.
| Strain | Genotype | Reference |
|---|---|---|
| CAI4 | Δura3::imm434/Δura3::imm434 | (Fonzi and Irwin, 1993) |
| Msb2-HA/msb2Δ | Δura3::imm434/Δura3::imm434 Msb2/msb2Δ::FRT/Msb2-HA | (Puri et al., 2012) |
| Msb2/msb2Δ | Δura3::imm434/Δura3::imm434 Msb2/msb2Δ::FRT/Msb2 | (Puri et al., 2012) |
| msb2Δ | Δura3::imm434/Δura3::imm434 Δ/Δmsb2::URA | (Puri et al., 2012) |
| ED1Δ | Δura3::imm434/Δura3::imm434 Msb2/msb2Δ::FRT/Msb2-HA 100–450Δ | This study |
| ED2Δ | Δura3::imm434/Δura3::imm434 Msb2/msb2Δ::FRT/Msb2-HA 450–900Δ | This study |
| ED1/2Δ | Δura3::imm434/Δura3::imm434 Msb2/msb2Δ::FRT/Msb2-HA 100–900Δ | This study |
| ED3/4Δ | Δura3::imm434/Δura3::imm434 Msb2/msb2Δ::FRT/Msb2-HA 900–1250Δ | This study |
| ED4Δ | Δura3::imm434/Δura3::imm434 Msb2/msb2Δ::FRT/Msb2-HA 1050–1250Δ | This study |
| TM-CYTΔ | Δura3::imm434/Δura3::imm434 Msb2/msb2Δ::FRT/Msb2-HA 1250–1409Δ | This study |
| cek1Δ | ura3/ura3 cek1Δ::hísG/cek1Δ::hisG | (Csank et al., 1998) |
| mkc1Δ | CAI-4, mkc1Δ::hisG/mkc1Δ::hisG mkc1 ::pCK70 (URA3) | (Kumamoto, 2005) |
| íre1Δ | Aaron Mitchell kinase collection | (Blankenship et al., 2010) |
| hac1Δ |
ura3::λimm434/ura3::λimm434,his1::hisG/his1::hisG,arg4::hisG/arg4::hisG, hac1::loxP/hac1::loxP Clp30 (URA3 HIS1 ARG4) |
(Wimalasena et al., 2008) |
| ssa1Δ | Δura3::imm434 Δssa1::FRT/Δura3::imm434Δssa1::FRT | (Sun et al., 2010) |
| ssa2Δ | Δura3::imm434 Δssa2::FRT/Δura3::imm434Δssa2::FRT | (Sun et al., 2010) |
| muc1Δ |
his1Δ/his1Δ leu2Δ/leu2Δ arg4Δ/arg4Δ::C.dubliniensis ARG4 URA3/ura3Δ:: imm434,IRO1/iro1Δ::imm434orf19.4183Δ::C.dubliniensisHIS1/orf19.4183Δ::: C.maltosaLEU2 |
(Nobile et al., 2003) |
| sgt1Δ |
his1Δ/his1Δ leu2Δ/leu2Δ arg4Δ/arg4Δ::C.dubliniensisARG4 URA3/ura3Δ::imm434 IRO1/iro1Δ::imm434orf19.4183Δ::C.dubliniensisHIS1/orf19.4089Δ:::C.maltosaLEU2 |
(Nobile et al., 2003) |
| cpp1Δ | ura3/ura3 cpp1Δ::hisG/cpp1Δ::hisG | (Csank et al., 1998) |
| cph1Δ | cph1 Δ::hisG/cph1 Δ::hisG-URA3-hisG, ura3 Δ/ura3 Δ | (Fonzi and Irwin, 1993) |
| sap1/2/3Δ | sap1Δ::hisG/sap1Δ::hisG sap2Δ::hisG/sap2Δ::hisG sap3Δ::hisG sap3Δ::hisG | (Kretschmar et al., 2002) |
| sap4/5/6Δ | sap6Δ::hisG/sap6Δ::hisG sap4Δ::hisG/sap4Δ::hisG sap5Δ::hisG/sap5Δ::hisG | (Sanglard et al., 1997) |
| sap6Δ | sap6::hisG/sap6::hisG-URA3-hisG | (Kumar et al., 2015) |
| sap8Δ | Δsap8::hisG/Δsap8::hisG-URA3-hisG | (Puri et al., 2012) |
| sap9/10Δ | Δsap8::hisG/Δsap8::hisG-URA3-hisG/SAP8 | (Albrecht et al., 2006) |
| ssk1Δ | Δura3::imm434/Δura3::imm434 Δssk1::hisG/Δssk1::hisG-URA3-hisG | (Chauhan et al., 2003) |
| ssk2Δ | ssk2::loxP-ARG4-loxP/ssk2::loxP-HIS 1 -loxP | (Cheetham et al., 2007) |
| hog1Δ |
ura3:: λimm434/ura3:: λimm434, his1:: hisG/his1:: hisG, hog1:: LoxP-ura3-LoxP, hog1:: LoxP-HIS1-LoxP Clp20 (URA3, HIS1) |
(Smith et al., 2004) |
| ras1Δ | ura3Δ::λimm434/ura3Δ::λimm434 ras1::hisG/ras1::hisG::RAS1G13V-URA3 | (Piispanen et al., 2011) |
| cyr1Δ | ura3Δ::λimm434/ura3Δ::λimm434 cyr1Δ::hisG::cyr1Δ::hisG | (Lindsay et al., 2012) |
| tpk1Δ |
ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisGarg4::hisG/arg4::hisG tpk1::ARG4/tpk1::URA3 |
(Piispanen et al., 2011) |
| tpk2Δ |
ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisGarg4::hisG/arg4::hisG tpk2::ARG4/tpk2::URA3 |
(Piispanen et al., 2011) |
| efg1Δ |
ura3::λimm434/ura3::λimm434 efg1::hisG/efg1::hisG-URA3-hisG |
(Lo et al., 1997) |
| sho1Δ | Δura3::imm434/Δura3::imm434, Δ/Δsho1::URA | Dr. Edgerton lab strain |
| sln1Δ | Δura3::imm434 Δsln1::FRT/Δura3::imm434Δsln1::FRT | Dr. Edgerton lab strain |
| wsc1Δ | Aaron Mitchell kinase collection | (Blankenship et al., 2010) |
| wsc2Δ | Aaron Mitchell kinase collection | (Blankenship et al., 2010) |
| wsc4Δ | Aaron Mitchell kinase collection | (Blankenship et al., 2010) |
| bck1Δ | Aaron Mitchell kinase collection | (Blankenship et al., 2010) |
| mkk2Δ | Aaron Mitchell kinase collection | (Blankenship et al., 2010) |
| hwp1Δ | Aaron Mitchell kinase collection | (Blankenship et al., 2010) |
| hwp2Δ | Aaron Mitchell kinase collection | (Blankenship et al., 2010) |
| mp65Δ | Aaron Mitchell kinase collection | (Blankenship et al., 2010) |
| cdc28Δ | Aaron Mitchell kinase collection | (Blankenship et al., 2010) |
| ste11Δ | Aaron Mitchell kinase collection | (Blankenship et al., 2010) |
| hst7Δ | Aaron Mitchell kinase collection | Mitchell collection |
| cst20Δ | Aaron Mitchell kinase collection | Mitchell collection |
| cek2Δ | Aaron Mitchell kinase collection | Mitchell collection |
Msb2 deletion derivative strains were constructed by a PCR-based approach (Wilson et al., 2000) using the URA-Blaster technique (Fonzi and Irwin, 1993). PCR primers were designed to amplify the 3HA-URA3-3HA cassette in plasmid pCaMPY-3XHA (Liu et al., 2007), tailed with an additional 80 nucleotides of sequence flanking the open reading frame (ORF) of the region to be disrupted. PCR products were verified by gel electrophoresis. The purified PCR product (10 µg) was transformed into the MSB2/msb2Δ strain with Frozen-EZ Yeast Transformation II Kit (Zymo Research, CA, USA). Uracil-deficient agar (YNB-URA) media was used for selection of URA positive colonies. PCR-based analysis of transformants was performed with primer pairs internal to the wild-type locus and the URA cassette. Homozygous transformants were reverted to the URA negative phenotype by selection on 5-fluororotic acid (5-FOA). Domain deletion knockouts were verified by immunoblot analysis. Hence, all deletions represent the sole copy of MSB2 expressed at the MSB2 locus from its endogenous promoter.
Protein and immunoblot Analysis
Anti-phospho p42/44 MAPK ERK1/2 Thr202/Tyr204 rabbit monoclonal antibody was used to detect P~Cek1 and P~Mkc1 (Signalling Technology). Anti-HA antibodies were used to detect HA-Msb2 (Abcam, ab75640). To detect actin, anti-Act1 antibody was used (Santa Cruz Biotechnology, sc47778). Goat anti-rabbit IgG-HRP (Jackson ImmunoResearch Laboratories, Inc.) was used as the secondary antibody.
For protein extraction, cell lysis was performed as described (Puri et al., 2012). Cells were grown to mid-log phase and harvested by centrifugation. Cell pellets were resuspended in 300 µl of 10% TCA Buffer (10 mM Tris-HCl pH 8.0, 10% trichloroacetic acid, 25 mM NH4OAc, 1 mM sodium EDTA). Cells were lysed using acid washed glass beads by vortexing for 40 s × 10 cycles using Fast PrepH-24 Instrument (MP Biomedicals LLC). Cell debris and glass beads were separated by centrifugation. Cell lysates were then centrifuged at 4°C for 10 min at 13,000 rpm. 150 µl of resuspension buffer (0.1 M Tris-HCl pH 11.0, 3% SDS) was added to the pellets. For immunoblotting, normalized protein content (20 µg) was separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked in 5% Milk or Bovine Serum albumin (BSA) in TBS containing 0.1% Tween-20 at (TBST) at room temperature for 1 h. Blots were incubated in primary antibody for 16 h at 4°C. Membranes were washed twice with TBST and probed with a secondary antibody for 1 h at 25°C. Secondary antibodies were detected using SuperSignal West Pico detection kit (Thermo Scientific).
For quantifying Msb2 shedding in liquid culture media, overnight cultures were diluted to OD600 0.3 and allowed to grow for 3 h under different conditions. Culture supernatants were collected, filter sterilized and precipitated with acetone. Precipitated proteins were quantified using BCA protein quantification kit (Thermo Scientific) and normalized protein content (20 µg) was examined by slot blot with anti-HA antibody.
Sensitivity to drugs and temperature
Aliquots of overnight cultures were washed two times with phosphate buffer saline. Drop tests were performed by spotting 5 µl of serial 10-fold dilutions of overnight culture of an optical density OD600 of 0.1 on YPD plates, YPD plates were supplemented with CR (100 µg/ml), CFW (100 µg/ml), DTT (30 mM) and tunicamycin (2 µg/ml) at the indicated concentrations. For temperature sensitivity, plates were incubated at 30°C, 37°C, 42°C for 48 h and photographed. Growth curves for each strains were recorded by measuring their OD600 at 6 h interval for up to 40 h using a spectrophotometer (Lambda 25, UV/VS Spectrophotometer, Perkin Elmer).
Germination assay
Overnight YPD cultures were diluted in YNB+2%Glucose, YNB+1.25%GlcNAc, Spider and YPD+10% Serum media and incubated for 3 h with shaking at 37°C, 42°C and visualized at 20X magnification using differential interference contrast (DIC) microscopy using a Zeiss Axio Fluorescence microscope. For Hsp90 compromise mediated germination, diluted cultures were grown for 6 h in YPD and YPD media supplemented with 10 µM GDA at 30°C. The percentage of hyphae or yeast form cells was determined by the number of yeast/hyphal cells divided by total number of cells by microscopy (500 cells were counted/strain). The germinated cells were screened for survival at various temperatures by determining Colony Forming Units (CFU). Cells grown at the indicated temperatures and plated on YPD agar media and compared to control cells to determine percent survival.
Quantitative real time PCR (qRT-PCR)
Primers for qPCR used in the study are listed in Table 2. To monitor expression levels of heat shock genes and unfolded protein response genes at different temperatures, overnight cultures of wild-type cells and the msb2Δ mutant were inoculated in YPD media to 5ml final volume and incubated for 3 h with shaking at 30°C, 37°C and 42°C. Cultures were harvested and cell pellets were resuspended in 1 ml of TRIzol® Reagent (Life Technologies) and vortexed (4 cycle, 6m/s) with acid washed glass beads using a Fast Prep instrument (MP Biomedicals). Chloroform (200 µl) was added in the lysed cells and mixed vigorously for 15 s and kept at room temperature for 2 min. Cell lysates were centrifuged at 11,500 × g for 10 min at 4°C. RNA containing upper aqueous layer was mixed with 0.5 volume of 100% ethanol to precipitate total RNA. Total precipitated RNA was purified using an RNeasy kit from Qiagen according to manufacturer’s instructions. Following isolation, RNA purity and concentrations were determined using gel electrophoresis and Nano-drop 1000 (Thermo Scientific). Total cDNA was synthesized for each sample using iScript™ cDNA Synthesis Kit (Bio-Rad) following the manufacturer’s instruction with equal amounts of RNA (1 µg in 20 µl reaction).
Table 2.
Primers for qPCR used in the study.
| SFL1 F |
| ACAACAGCAACAGCAACAGC |
| SFL1R |
| GTGGAATTGGTCCGCTAAAA |
| HSP21F |
| TGCAGAAATTGGTGAGCAAG |
| HSP21 R |
| TTGCAGCTGCTTTGGAAATA |
| HSP 90 F |
| AGTTGAAACCGATGGAGCTG |
| HSP 90 R |
| ATGGTTCGTCCAAGGTGAAA |
| HSP 70 F |
| AACCTACTGCTGCTGCCATT |
| HSP 70 R |
| AAGTACCACCACCCAAATCG |
| IRE1 F |
| TGCCCCATCCTTTGAAAGTG |
| IRE1 R |
| TCTGAAACTCATAGCCACCCA |
| HAC1 F |
| GAGGATGAACACCAAGAAGAAGG |
| HAC1 R |
| AGATGGTGGTGTAGACGTCA |
| KAR2 F |
| CAATGAACCTACTGCTGCCG |
| KAR2 R |
| AGCCAAGACTTCGAAAACACC |
| Orf19.719 F |
| ACACTGTTAAGAGGATGGCAAG |
| Orf19.719 R |
| TCCACTTGCCCAGACTCATT |
| ACTIN1-F |
| TCGGTGACGAAGCTCAATCCAAGA |
| ACTIN1-R |
| CAATGGATGGACCACTTTCGTCGT |
| TDH3/GAPDH-F |
| AAGAGTTGCTTTGGGCAGAA |
| TDH3/GAPDH-R |
| GTCGTCACCAGAAGCAGTGA |
To quantify the transcript levels of heat shock genes and UPR regulators, PCR primers were designed to amplify 100 to 150 bps of the target gene. Synthesized cDNA (1 µl) was used to amplify transcripts of selected genes. Amplification and detection were carried out in 96-well plates on an iCycler iQ real-time detection system (Bio-Rad). All samples contained 10 µl iQ SYBR Green supermix (2× concentration), 1 µl forward primer, 1 µl reverse primer, 1 µl template (cDNA) and 17 µl nuclease-free water. Fluorescent data were collected and analyzed with iCycler iQ software. Threshold value (ΔCT) was obtained by difference between CT values of the target gene and the control genes (ACT1 and GAPDH2, which gave the same results and were used interchangeably). Results represent the mean of at least three independent biological replicates. Statistical analysis was determined by Student’s t-test using Expert qPCR Analysis software.
Acknowledgments
Thanks to A. Mitchell for encouragement and generously providing strains. Thanks to lab members for discussions and help with the manuscript. The work was supported by a grant from the NIDCR (DE022720).
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Adhikari H, Vadaie N, Chow J, Caccamise LM, Chavel CA, Li B, et al. Role of the unfolded protein response in regulating the mucin-dependent filamentous growth MAPK pathway. Mol Cell Biol. 2015;35(8):1414–1432. doi: 10.1128/MCB.01501-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht A, Felk A, Pichova I, Naglik JR, Schaller M, de Groot P, et al. Glycosylphosphatidylinositol-anchored proteases of Candida albicans target proteins necessary for both cellular processes and host-pathogen interactions. J Biol Chem. 2006;281:688–694. doi: 10.1074/jbc.M509297200. [DOI] [PubMed] [Google Scholar]
- Alonso-Monge R, Navarro-Garcia F, Molero G, Diez-Orejas R, Gustin M, Pla J, et al. Role of the mitogen-activated protein kinase Hoglp in morphogenesis and virulence of Candida albicans. J Bacteriol. 1999;181:3058–3068. doi: 10.1128/jb.181.10.3058-3068.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29:2893–2904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchenough GM, Johansson ME, Gustafsson JK, Bergstrom JH, Hansson GC. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015;8(4):712–719. doi: 10.1038/mi.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev. 2007;71:348–376. doi: 10.1128/MMBR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship JR, Fanning S, Hamaker JJ, Mitchell AP. An extensive circuitry for cell wall regulation in Candida albicans. PLoS Pathog. 2010;6:e1000752. doi: 10.1371/journal.ppat.1000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Leach MD, Nicholls S. The relevance of heat shock regulation in fungal pathogens of humans. Virulence. 2010;1:330–332. doi: 10.4161/viru.1.4.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan N, Inglis D, Roman E, Pla J, Li D, Calera JA, Calderone R. Candida albicans response regulator gene SSK1 regulates a subset of genes whose functions are associated with cell wall biosynthesis and adaptation to oxidative stress. Eukaryot Cell. 2003;2:1018–1024. doi: 10.1128/EC.2.5.1018-1024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham J, Smith DA, da Silva Dantas A, Doris KS, Patterson MJ, Bruce CR, Quinn J. A single MAPKKK regulates the Hog1 MAPK pathway in the pathogenic fungus Candida albicans. Mol Biol Cell. 2007;18:4603–4614. doi: 10.1091/mbc.E07-06-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletta A, Pinney JW, Solis DY, Marsh J, Pettifer SR, Attwood TK. Low-complexity regions within protein sequences have position-dependent roles. BMC Syst Biol. 2010;4:43. doi: 10.1186/1752-0509-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996;87:391–404. doi: 10.1016/s0092-8674(00)81360-4. [DOI] [PubMed] [Google Scholar]
- Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- Csank C, Schroppel K, Leberer E, Harcus D, Mohamed O, Meloche S, et al. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun. 1998;66:2713–2721. doi: 10.1128/iai.66.6.2713-2721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ. Post-translational regulation of signaling mucins. Curr Opin Struct Biol. 2011;21:590–596. doi: 10.1016/j.sbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ, Sabbagh W, Jr, Graham E, Irick MM, van Olden EK, Neal C, et al. A signaling mucin at the head of the Cdc42- and MAPK-dependent filamentous growth pathway in yeast. Genes Dev. 2004;18:1695–1708. doi: 10.1101/gad.1178604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai B, Wang Y, Li D, Xu RY, Liang L, Zhao Y, et al. Hsp90 is involved in apoptosis of Candida albicans by regulating the calcineurin-caspase apoptotic pathway. PloS One. 2012;7:e45109. doi: 10.1371/journal.pone.0045109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desseyn JL, Tetaert D, Gouyer V. Architecture of the large membrane-bound mucins. Gene. 2008;410:215–222. doi: 10.1016/j.gene.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Diezmann S, Michaut M, Shapiro RS, Bader GD, Cowen LE. Mapping the Hsp90 genetic interaction network in Candida albicans reveals environmental contingency and rewired circuitry. PLoS Genet. 2012;8:e1002562. doi: 10.1371/journal.pgen.1002562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Enfert C. Hidden killers: persistence of opportunistic fungal pathogens in the human host. Curr Opin Microbiol. 2009;12:358–364. doi: 10.1016/j.mib.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Fang S, Crews AL, Chen W, Park J, Yin Q, Ren XR, Adler KB. MARCKS and HSP70 interactions regulate mucin secretion by human airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2013;304:L511–L518. doi: 10.1152/ajplung.00337.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5:a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U, Heitman J. Origins of eukaryotic sexual reproduction. Cold Spring Harb Perspect Biol. 2014;6:1–21. doi: 10.1101/cshperspect.a016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale SJ, Lovell SC, de Keyzer J, Stirling CJ. Interactions between Kar2p and its nucleotide exchange factors Sil1p and Lhs1p are mechanistically distinct. J Biolo Chem. 2010;285:21600–21606. doi: 10.1074/jbc.M110.111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman J. Cell biology. A fungal Achilles’ heel. Science. 2005;309:2175–2176. doi: 10.1126/science.1119321. [DOI] [PubMed] [Google Scholar]
- Huang G, Yi S, Sahni N, Daniels KJ, Srikantha T, Soil DR. N-acetylglucosamine induces white to opaque switching, a mating prerequisite in Candida albicans. PLoS Pathog. 2010;6:e1000806. doi: 10.1371/journal.ppat.1000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam JL, Regimbald LH, Hilgers JH, Hoffman P, Krantz MJ, Longenecker BM, Hugh JC. MUC1 synthetic peptide inhibition of intercellular adhesion molecule-1 and MUC1 binding requires six tandem repeats. Cancer Res. 1998;58:5577–5581. [PubMed] [Google Scholar]
- Kanehara K, Xie W, Ng DT. Modularity of the Hrd1 ERAD complex underlies its diverse client range. J Cell Biol. 2010;188:707–716. doi: 10.1083/jcb.200907055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Keyzer J, Steel GJ, Hale SJ, Humphries D, Stirling CJ. Nucleotide binding by Lhs1p is essential for its nucleotide exchange activity and for function in vivo. J Biol Chem. 2009;284:31564–31571. doi: 10.1074/jbc.M109.055160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert B, Narberhaus F. Microbial thermosensors. Cell Mol Life Sci. 2009;66:2661–2676. doi: 10.1007/s00018-009-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmar M, Felk A, Staib P, Schaller M, Hess D, Callapina M, et al. Individual acid aspartic proteinases (Saps) 1–6 of Candida albicans are not essential for invasion and colonization of the gastrointestinal tract in mice. Microb Pathog. 2002;32:61–70. doi: 10.1006/mpat.2001.0478. [DOI] [PubMed] [Google Scholar]
- Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufe DW. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene. 2013;32:1073–1081. doi: 10.1038/onc.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto CA. A contact-activated kinase signals Candida albicans invasive growth and biofilm development. Proc Natl Acad Sci USA. 2005;102:5576–5581. doi: 10.1073/pnas.0407097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto CA, Vinces MD. Alternative Candida albicans lifestyles: growth on surfaces. Annu Rev Microbiol. 2005;59:113–133. doi: 10.1146/annurev.micro.59.030804.121034. [DOI] [PubMed] [Google Scholar]
- Kumar R, Saraswat D, Tati S, Edgerton M. Novel aggregation properties of Candida albicans secreted aspartyl proteinase Sap6 mediates virulence in oral candidiasis. Infect immun. 2015;83:2614–2626. doi: 10.1128/IAI.00282-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFayette SL, Collins O, Zaas AK, Schell WA, Betancourt-Quiroz M, Gunatilaka AA, et al. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog. 2010;6:e1001069. doi: 10.1371/journal.ppat.1001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford ML, Hargarten JC, Patefield KD, Marta E, Blankenship JR, Fanning S, et al. Candida albicans Czf1 and Efg1 coordinate the response to farnesol during quorum sensing, white-opaque thermal dimorphism, and cell death. Eukaryot Cell. 2013;12:1281–1292. doi: 10.1128/EC.00311-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latge JP. Tasting the fungal cell wall. Cell Microbiol. 2010;12:863–872. doi: 10.1111/j.1462-5822.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- Leach MD, Brown AJ. Posttranslational modifications of proteins in the pathobiology of medically relevant fungi. Eukaryot Cell. 2012;11:98–108. doi: 10.1128/EC.05238-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach MD, Cowen LE. Surviving the heat of the moment: a fungal pathogens perspective. PLoS Pathog. 2013;9:e1003163. doi: 10.1371/journal.ppat.1003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach MD, Cowen LE. Membrane fluidity and temperature sensing are coupled via circuitry comprised of Ole1, Rsp5, and Hsf1 in Candida albicans. Eukaryot Cell. 2014a;13:1077–1084. doi: 10.1128/EC.00138-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach MD, Cowen LE. To sense or die: mechanisms of temperature sensing in fungal pathogens. Curr Fungal Infect Rep. 2014b;8:185–191. [Google Scholar]
- Leach MD, Budge S, Walker L, Munro C, Cowen LE, Brown AJ. Hsp90 orchestrates transcriptional regulation by Hsf1 and cell wall remodelling by MAPK signalling during thermal adaptation in a pathogenic yeast. PLoS Pathog. 2012a;8:e1003069. doi: 10.1371/journal.ppat.1003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach MD, Tyc KM, Brown AJ, Klipp E. Modelling the regulation of thermal adaptation in Candida albicans, a major fungal pathogen of humans. PloS One. 2012b;7:e32467. doi: 10.1371/journal.pone.0032467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E, Harcus D, Broadbent ID, Clark KL, Dignard D, Ziegelbauer K, et al. Signal transduction through homologs of the Ste20p and Ste7p protein kinases can trigger hyphal formation in the pathogenic fungus Candida albicans. Proc Natl Acad Sci USA. 1996;93:13217–13222. doi: 10.1073/pnas.93.23.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler KB, Davidson RC, D’Souza C, Harashima T, Shen WC, Wang P, et al. Signal transduction cascades regulating fungal development and virulence. Microbiol Mol Biol Rev. 2000;64:746–785. doi: 10.1128/mmbr.64.4.746-785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. Varying patterns of protein synthesis in Drosophila during heat shock: implications for regulation. Dev Biol. 1980;77:463–479. doi: 10.1016/0012-1606(80)90488-1. [DOI] [PubMed] [Google Scholar]
- Lindsay AK, Deveau A, Piispanen AE, Hogan DA. Farnesol and cyclic AMP signaling effects on the hypha-to-yeast transition in Candida albicans. Eukaryot Cell. 2012;11:1219–1225. doi: 10.1128/EC.00144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Znaidi S, Barker KS, Xu L, Homayouni R, Saidane S, et al. Genome-wide expression and location analyses of the Candida albicans Tac1p regulon. Eukaryot Cell. 2007;6:2122–2138. doi: 10.1128/EC.00327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chang A. Heat shock response relieves ER stress. EMBO J. 2008;27:1049–1059. doi: 10.1038/emboj.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Madhani HD, Fink GR. The control of filamentous differentiation and virulence in fungi. Trends Cell Biol. 1998;8:348–353. doi: 10.1016/s0962-8924(98)01298-7. [DOI] [PubMed] [Google Scholar]
- Mayer FL, Wilson D, Jacobsen ID, Miramon P, Slesiona S, Bohovych IM, et al. Small but crucial: the novel small heat shock protein Hsp21 mediates stress adaptation and virulence in Candida albicans. PloS One. 2012;7:e38584. doi: 10.1371/journal.pone.0038584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KA, DiDone L, Krysan DJ. Extracellular secretion of overexpressed glycosylphosphatidylinositol-linked cell wall protein Utr2/Crh2p as a novel protein quality control mechanism in Saccharomyces cerevisiae. Eukaryot Cell. 2010;9:1669–1679. doi: 10.1128/EC.00191-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran GR, Coleman DC, Sullivan DJ. Comparative genomics and the evolution of pathogenicity in human pathogenic fungi. Eukaryot Cell. 2011;10:34–42. doi: 10.1128/EC.00242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Ogawa N, Kawahara T, Yanagi H, Yura T. mRNA splicing-mediated C-terminal replacement of transcription factor Hac1p is required for efficient activation of the unfolded protein response. Proc Natl Acad Sci USA. 2000;97:4660–4665. doi: 10.1073/pnas.050010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Nather K, Munro CA. Generating cell surface diversity in Candida albicans and other fungal pathogens. FEMS Microbiol Lett. 2008;285:137–145. doi: 10.1111/j.1574-6968.2008.01263.x. [DOI] [PubMed] [Google Scholar]
- Navarro-Garcia F, Alonso-Monge R, Rico H, Pla J, Sentandreu R, Nombela C. A role for the MAP kinase gene MKC1 in cell wall construction and morphological transitions in Candida albicans. Microbiology. 1998;144(Pt 2):411–424. doi: 10.1099/00221287-144-2-411. [DOI] [PubMed] [Google Scholar]
- Nemecek JC, Wuthrich M, Klein BS. Global control of dimorphism and virulence in fungi. Science. 2006;312:583–588. doi: 10.1126/science.1124105. [DOI] [PubMed] [Google Scholar]
- Nobile CJ, Mitchell AP. Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol. 2006;8:1382–1391. doi: 10.1111/j.1462-5822.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- Nobile CJ, Bruno VM, Richard ML, Davis DA, Mitchell AR. Genetic control of chlamydospore formation in Candida albicans . Microbiology. 2003;149:3629–3637. doi: 10.1099/mic.0.26640-0. [DOI] [PubMed] [Google Scholar]
- O’Meara TR, Cowen LE. Hsp90-dependent regulatory circuitry controlling temperature-dependent fungal development and virulence. Cell Microbiol. 2014;16:473–481. doi: 10.1111/cmi.12266. [DOI] [PubMed] [Google Scholar]
- Patil C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol. 2001;13:349–355. doi: 10.1016/s0955-0674(00)00219-2. [DOI] [PubMed] [Google Scholar]
- Perez-Nadales E, Di Pietro A. The transmembrane protein Sho1 cooperates with the mucin Msb2 to regulate invasive growth and plant infection in Fusarium oxysporum. Mol Plant Pathol. 2014;16(6):593–603. doi: 10.1111/mpp.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piispanen AE, Bonnefoi O, Carden S, Deveau A, Bassilana M, Hogan DA. Roles of Ras1 membrane localization during Candida albicans hyphal growth and farnesol response. Eukaryot cell. 2011;10:1473–1484. doi: 10.1128/EC.05153-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitarch A, Abian J, Carrascal M, Sanchez M, Nombela C, ad Gil C. Proteomics-based identification of novel Candida albicans antigens for diagnosis of systemic candidiasis in patients with underlying hematological malignancies. Proteomics. 2004;4:3084–3106. doi: 10.1002/pmic.200400903. [DOI] [PubMed] [Google Scholar]
- Polvi EJ, Li X, O’Meara TR, Leach MD, Cowen LE. Opportunistic yeast pathogens: reservoirs, virulence mechanisms, and therapeutic strategies. Cell Mol Life Sci. 2015;72(12):2261–2287. doi: 10.1007/s00018-015-1860-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Kumar R, Chadha S, Tati S, Conti HR, Hube B, Cullen PJ, Edgerton M. Secreted aspartic protease cleavage of Candida albicans Msb2 activates Cek1 MAPK signaling affecting biofilm formation and oropharyngeal candidiasis. PloS One. 2012;7:e46020. doi: 10.1371/journal.pone.0046020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn JJ, Shen Q, Mah BK, Hugh JC. MUC1 initiates a calcium signal after ligation by intercellular adhesion molecule-1. J Biol Chem. 2004;279:29386–29390. doi: 10.1074/jbc.C400010200. [DOI] [PubMed] [Google Scholar]
- Rao KH, Ghosh S, Natarajan K, Datta A. N-acetylglucosamine kinase, HXK1 is involved in morphogenetic transition and metabolic gene expression in Candida albicans. PloS One. 2013;8:e53638. doi: 10.1371/journal.pone.0053638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regimbald LH, Pilarski LM, Longenecker BM, Reddish MA, Zimmermann G, Hugh JC. The breast mucin MUCI as a novel adhesion ligand for endothelial intercellular adhesion molecule 1 in breast cancer. Cancer Res. 1996;56:4244–4249. [PubMed] [Google Scholar]
- Ren J, Bharti A, Raina D, Chen W, Ahmad R, Kufe D. MUC1 oncoprotein is targeted to mitochondria by heregulin-induced activation of c-Src and the molecular chaperone HSP90. Oncogene. 2006;25:20–31. doi: 10.1038/sj.onc.1209012. [DOI] [PubMed] [Google Scholar]
- Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Rispail N, Soanes DM, Ant C, Czajkowski R, Grunler A, Huguet R, et al. Comparative genomics of MAP kinase and calcium-calcineurin signalling components in plant and human pathogenic fungi. Fungal Genet Biol. 2009;46:287–298. doi: 10.1016/j.fgb.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Roman E, Cottier E, Ernst JF, Pla J. Msb2 signaling mucin controls activation of Cek1 mitogen-activated protein kinase in Candida albicans. Eukaryot Cell. 2009;8:1235–1249. doi: 10.1128/EC.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H. Regulation of cross-talk in yeast MAPK signaling pathways. Current Opinion in Microbiology. 2010;13(6):677–683. doi: 10.1016/j.mib.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Sanglard D, Hube B, Monod M, Odds FC, Gow NA. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect Immun. 1997;65:3539–3546. doi: 10.1128/iai.65.9.3539-3546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimale T, Didone L, de Mesy Bentley KL, Krysan DJ. The unfolded protein response is induced by the cell wall integrity mitogen-activated protein kinase signaling cascade and is required for cell wall integrity in Saccharomyces cerevisiae. Mol Biol Cell. 2009;20:164–175. doi: 10.1091/mbc.E08-08-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully C, el-Kabir M, Samaranayake LP. Candida and oral candidosis: a review. Crit Rev oral Biol Med. 1994;5:125–157. doi: 10.1177/10454411940050020101. [DOI] [PubMed] [Google Scholar]
- Selmecki A, Forche A, Berman J. Genomic plasticity of the human fungal pathogen Candida albicans. Eukaryot cell. 2010;9:991–1008. doi: 10.1128/EC.00060-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn H, Shapiro RS, Cowen LE. Cdc28 provides a molecular link between Hsp90, morphogenesis, and cell cycle progression in Candida albicans. Mol Biol Cell. 2012;23:268–283. doi: 10.1091/mbc.E11-08-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RS, Cowen L. Coupling temperature sensing and development: Hsp90 regulates morphogenetic signalling in Candida albicans. Virulence. 2010;1:45–48. doi: 10.4161/viru.1.1.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RS, Cowen LE. Thermal control of microbial development and virulence: molecular mechanisms of microbial temperature sensing. mBio. 2012a;3:1–6. doi: 10.1128/mBio.00238-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RS, Cowen LE. Uncovering cellular circuitry controlling temperature-dependent fungal morphogenesis. Virulence. 2012b;3:400–404. doi: 10.4161/viru.20979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RS, Uppuluri P, Zaas AK, Collins C, Senn H, Perfect JR, et al. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr Biol. 2009;19:621–629. doi: 10.1016/j.cub.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RS, Sellam A, Tebbji E, Whiteway M, Nantel A, Cowen LE. Pho85, Pcl1, and Hms1 signaling governs Candida albicans morphogenesis induced by high temperature or Hsp90 compromise. Curr Biol. 2012a;22:461–470. doi: 10.1016/j.cub.2012.01.062. [DOI] [PubMed] [Google Scholar]
- Shapiro RS, Zaas AK, Betancourt-Quiroz M, Perfect JR, Cowen LE. The Hsp90 co-chaperone Sgt1 governs Candida albicans morphogenesis and drug resistance. PloS One. 2012b;7:e44734. doi: 10.1371/journal.pone.0044734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–476. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Smith DA, Nicholls S, Morgan BA, Brown AJ, Quinn J. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol Biol Cell. 2004;15:4179–4190. doi: 10.1091/mbc.E04-03-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004;12:317–324. doi: 10.1016/j.tim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Sun JN, Solis NV, Phan QT, Bajwa JS, Kashleva H, Thompson A, et al. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 2010;6:e1001181. doi: 10.1371/journal.ppat.1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidergall M, van Wijlick L, Ernst JF. Signaling domains of mucin Msb2 in Candida albicans. Eukaryot cell. 2015;14:359–370. doi: 10.1128/EC.00264-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafranski-Schneider E, Swidergall M, Cottier E, Tielker D, Roman E, Pla J, Ernst JF. Msb2 shedding protects Candida albicans against antimicrobial peptides. PLoS Pathog. 2012;8:e1002501. doi: 10.1371/journal.ppat.1002501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarp MA, Clausen H. Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim Biophys Acta. 2008;1780:546–563. doi: 10.1016/j.bbagen.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Tian E, Ten Hagen KG. Recent insights into the biological roles of mucin-type O-glycosylation. Glycoconj J. 2009;26:325–334. doi: 10.1007/s10719-008-9162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban O, Sohn K, Lottspeich E, Brunner H, Rupp S. Identification of cell surface determinants in Candida albicans reveals Tsa1p, a protein differentially localized in the cell. FEBS Lett. 2003;544:228–235. doi: 10.1016/s0014-5793(03)00455-1. [DOI] [PubMed] [Google Scholar]
- Vadaie N, et al. Cleavage of the signaling mucin Msb2 by the aspartyl protease Yps1 is required for MAPK activation in yeast. The Journal of Cell Biology. 2008;181(7):1073–1081. doi: 10.1083/jcb.200704079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W, Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971;35:290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Xu H, Kufe D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer cell. 2005;7:167–178. doi: 10.1016/j.ccr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Wei X, Xu H, Kufe D. MUC1 oncoprotein stabilizes and activates estrogen receptor alpha. Mol Cell. 2006;21:295–305. doi: 10.1016/j.molcel.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Wilson RB, Davis D, Enloe BM, Mitchell AR. A recyclable Candida albicans URA3 cassette for PCR product-directed gene disruptions. Yeast. 2000;16:65–70. doi: 10.1002/(SICI)1097-0061(20000115)16:1<65::AID-YEA508>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Wimalasena TT, Enjalbert B, Guillemette T, Plumridge A, Budge S, Yin ZA, et al. Impact of the unfolded protein response upon genome-wide expression patterns, and the role of Hac1 in the polarized growth, of Candida albicans. Fungal Genet Biol. 2008;45:1235–1247. doi: 10.1016/j.fgb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Yost HJ, Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986;45:185–193. doi: 10.1016/0092-8674(86)90382-x. [DOI] [PubMed] [Google Scholar]
- Zeuthen E. Synchrony in Tetrahymena by heat shocks spaced a normal cell generation apart. Exp Cell Res. 1971;68:49–60. doi: 10.1016/0014-4827(71)90585-4. [DOI] [PubMed] [Google Scholar]
- Zhao X, Mehrabi R, Xu JR. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot cell. 2007;6:1701–1714. doi: 10.1128/EC.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znaidi S, Nesseir A, Chauvel M, Rossignol T, d’Enfert C. A comprehensive functional portrait of two heat shock factor-type transcriptional regulators involved in Candida albicans morphogenesis and virulence. PLoS Pathog. 2013;9:e1003519. doi: 10.1371/journal.ppat.1003519. [DOI] [PMC free article] [PubMed] [Google Scholar]