Abstract
Aims
The aim of this article is to examine how the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE) recommendations on the classification of diastolic dysfunction (DDF) are interpreted in the scientific community and to explore how variations in the DDF definition affect the reported prevalence.
Methods and results
A systematic review of studies citing the EACVI/ASE consensus document ‘Recommendations for the evaluation of left ventricular diastolic function by echocardiography’ was performed. The definition of DDF used in each study was recorded. Subsequently, several possible interpretations of the EACVI/ASE classification scheme were used to obtain DDF prevalence in a community-based sample (n = 714). In the systematic review, 60 studies were included. In 13 studies, no specification of DDF definition was presented, a one-level classification tree was used in 13, a two-level classification tree in 18, and in the remaining 16 studies, a DDF definition was presented but no grading of DDF was performed. In 17 studies, the DDF definition relied solely on early diastolic tissue velocity and/or left atrial size. In eight of these studies, a single parameter was used, in two studies the logical operator AND was used to combine two or more parameters, and the remaining seven studies used the logical operator OR. The resulting prevalence of DDF in the community-based sample varied from 12 to 84%, depending on the DDF definition used.
Conclusion
A substantial heterogeneity of definitions of DDF was evident among the studies reviewed, and the different definitions had a substantial impact on the reported prevalence of DDF.
Keywords: Echocardiography, Diastolic dysfunction, General population
Introduction
In the guidelines endorsed by the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE), it is recommended that echocardiographic reports should contain information on the presence and grade of left ventricular (LV) diastolic dysfunction (DDF) when the technical quality is adequate and the findings are not equivocal.1,2 An algorithm structured as a two-level decision tree is proposed in which early diastolic myocardial tissue velocities and left atrial (LA) volume indexed by body surface area (LAVi) provide information on the presence or absence of DDF at the first branch point. Subsequently, at a second branch point, more traditional diastolic Doppler variables are used to grade patients with DDF into mild (grade I), intermediate (grade II), or severe (grade III). However, the algorithm, as presented in the EACVI/ASE document, is not unequivocal as no guidance is given on how to handle discordant measurements. The effect of these ambiguities can be seen in less than optimal inter-reader agreement, with kappa values of 0.71–0.76 even when interpreters were given the same pre-measured variables.3,4 Additionally, reports indicate that large differences in DDF definitions exist between studies.5,6 As the concordance of measures of diastolic function has been shown to be poor,7 even small differences in the specific algorithm used can be expected to yield large differences in subject classification and prevalence. To our knowledge, no systematic review has been performed to study how the definitions of DDF varied between studies claiming adherence to the EACVI/ASE recommendations, nor has the effect of such variations on the reported prevalence been described previously.
We aimed to explore how the EACVI/ASE diagnostic scheme has been interpreted in the scientific community by means of a systematic literature review and subsequent analysis of the consequences of using different interpretations of definitions on the prevalence of DDF in a community-based sample.
Methods
Systematic literature review
Studies citing the EACVI/ASE consensus document ‘Recommendations for the evaluation of left ventricular diastolic function by echocardiography’ published in the European Journal of Echocardiography in 2009 were identified through the Thomson Reuters Web of Science Citation Index on 3 December 2014 (n = 498). Articles with titles and/or abstracts containing relevant key phrases (‘diastolic function’, ‘diastolic dysfunction’, ‘ddf’, ‘diastolic left ventricular dysfunction’, ‘diastolic lv dysfunction’, ‘diastolic heart failure’, ‘dhf’, ‘heart failure with preserved/normal ejection fraction’, ‘hfnef’, and ‘hfpef’) were identified and retained (n = 256). The titles and abstracts of these studies were screened, and clinical studies on adult human populations employing echocardiography written in the English language were retained (n = 197). Finally, full-text versions of these articles were reviewed and all studies that (i) classified subjects by the presence or absence of DDF, (ii) specified which variables had been used for the classification, and (iii) cited the EACVI/ASE document as the source of classification were included in the study (n = 60).
Included studies were subsequently analysed and coded independently by two researchers (J.S. and P.H.). Consensus was sought when coding differed. The following criteria were used for coding.
Algorithm for classification of DDF specified—yes or no.
Grading of DDF into grade I, II, or III or similar present—yes or no.
If grading was present, was the classification of DDF and grading carried out by a one-level classification tree (criteria were presented for each grade and DDF was defined as fulfilment of the criteria for any one of these grades) or a two-level classification tree (criteria for DDF were defined and, if fulfilled, subsequent grading took place with additional variables)?
If the variables used for classification had been specified, these variables were recorded. If a two-level classification tree had been used, the variables used for DDF classification and subsequent grading were recorded separately. If multiple parameters had been used within one level of the classification tree, the logical operator used was recorded; in the context of the classification algorithms described, the words ‘and’ and ‘or’ were interpreted as the logical operators AND (all listed criteria had to be fulfilled) and OR (at least one of the listed criteria had to be fulfilled), respectively.
Study population
The participants were recruited from the control group of the Västmanland Myocardial Infarction Study (VaMIS). In the VaMIS study, subjects hospitalized for acute myocardial infarction were included from November 2005 to May 2011. For each included patient, a control subject was recruited from the general population. From the Swedish Population Register in which all Swedish citizens are registered, a subject of the same sex with the nearest date of birth and living in the same municipality as the VaMIS patient was identified and invited to participate. All subjects underwent clinical examination, electrocardiography, echocardiographic examination, and blood sampling. From the control group of the VaMIS study (n = 855), we excluded subjects with a left ventricular ejection fraction (LVEF) <55% (n = 67), non-sinus rhythm (n = 23), valvular disease of moderate grade or more (n = 6), and missing values (n = 45), leaving 714 subjects for further analysis. Subsequently, a low-risk subgroup (n = 129) was created by selecting subjects who were not prescribed cardiovascular or antihypertensive medication and who had no history of diabetes mellitus, hypertension, myocardial infarction, angina pectoris, or transient ischaemic attack (TIA)/stroke. Furthermore, subjects in the low-risk subgroup were required to have normal LV mass on echocardiography,8 no regional wall motion abnormality, blood pressure <140/90 mmHg measured on two separate occasions, body mass index <30 kg/m2, and to be in New York Heart Association class I. In addition, the concentration of N-terminal pro-brain natriuretic peptide (NT-proBNP) was required to be <125 pg/mL.9 A high-risk subgroup (n = 344) was also created with subjects who were prescribed any cardiovascular or antihypertensive medication or who had a history of myocardial infarction, angina pectoris, TIA/stroke, diabetes mellitus, or hypertension.
The study was approved by the Ethics Committee of Uppsala University, Sweden (Dnr 2005:382). All participants gave their written informed consent.
Echocardiography
The methods used for the echocardiographic measurements and calculations have been described in detail elsewhere.10,11 In short, measurements of the linear dimensions of the LV and calculations of the LV mass were performed, according to the EACVI/ASE recommendations.12 The LV mass index (LVMi) was calculated by dividing the LV mass by the body surface area. LV hypertrophy was defined as an LVMi above age- and gender-specific upper reference limits.8 In subjects with adequate image quality, LVEF was obtained by the biplane Simpson's rule,12 and in the remaining cases, LVEF was visually estimated as normal (LVEF > 55%) or mildly to severely depressed. For the assessment of LA volume, the single-plane modified Simpson's rule was used in the apical four-chamber view in the frame immediately preceding mitral valve opening. LAVi was calculated as the LA volume divided by the body surface area. The peak early (E) and late (A) transmitral diastolic flow velocities, the E/A ratio, and the deceleration time of the early filling velocity were obtained at the peak of the mitral leaflets. The peak velocity of the early diastolic wave (E′) was measured using pulsed-wave tissue Doppler with the sample volume close to the mitral valve annulus in the apical four-chamber view in the septal (E′sep) and lateral (E′lat) walls. The E/E′ ratio was calculated on the basis of the transmitral E wave and the average of E′lat and E′sep (E′avg).
Statistical analysis
Continuous data were expressed as mean ± standard deviation (SD) and categorical data as counts and percentages. Skewed continuous data (i.e. NT-proBNP) were expressed as the median and interquartile range. Continuous variables were compared using the Wilcoxon rank-sum test. Categorical variables were compared using the Fisher's exact test. The results were regarded as significant when P < 0.05. STATA version 12.1 (StataCorp LP, College Station, TX, USA) was used for all statistical analyses. Area-proportional ellipse-based Euler diagrams were created using the open-source software eulerAPE v3.13
Results
Systematic literature review
In all included studies (n = 60), a classification of DDF, with or without grading, was presented, and the EACVI/ASE recommendations were cited as the source of this classification. In 13 of these, the variables used for DDF classification were presented but no classification algorithm was specified. In the remaining 47 articles, a classification algorithm was described: 13 studies used a one-level classification tree, 18 studies used a two-level classification tree, and 16 studies only defined the criteria for DDF without any grading. In studies using a one-level classification tree, E′ and LAVi were used in 1 study out of 13, whereas in studies utilizing a two-level classification tree, E′ was used in 16 and LA size measurements in 7 of the 18 studies (Table 1). A summary of how the different variables were combined, ignoring the logical operators used, is displayed in Table 2. The most common combination, studies in which E′ (septal and/or lateral or averaged) and a measurement of LA size were the only parameters used to define DDF, was seen in 17 of the 47 studies (14 of these used a two-level classification tree and in 3 studies no grading was performed). A summary of the detailed DDF definitions used in these 17 studies, including the logical operators, is shown in Table 3. In eight studies, a singular parameter was used (E′sep < 8, E′lat < 10, E′avg < 9, or LAVi > 34). In two studies, the logical operator AND was used to combine two or more parameters, whereas the remaining seven studies used the logical operator OR. LAVi was used in 7 of the 17 studies.
Table 1.
Variables used for DDF classification and grading grouped by different interpretations of the EACVI/ASE 2009 classification algorithm (n = 60)
| Variable | All (n = 60) | Algorithm interpretation specified (n = 47) |
Algorithm interpretation not specified (n = 13) | ||
|---|---|---|---|---|---|
| Classification and grading of DDF by a one-level classification tree (n = 13)S1–S13 | Classification and grading of DDF by a two-level classification tree (n = 18)S14–S31 | Classification of DDF only, no grading (n = 16)S32–S47 | Classification and grading of DDF by unspecified algorithm (n = 13)S48–S60 | ||
| E/A | 44 (73%) | 13 (100%) | 16 (89%) | 3 (19%) | 12 (92%) |
| Deceleration time | 37 (62%) | 9 (69%) | 14 (78%) | 2 (13%) | 12 (92%) |
| IVRT | 7 (12%) | 3 (23%) | 1 (6%) | 1 (6%) | 2 (15%) |
| Any E′ | 28 (47%) | 1 (8%) | 16 (89%) | 3 (19%) | 8 (62%) |
| E′sep | 15 (25%) | 0 (0%) | 11 (61%) | 1 (6%) | 3 (23%) |
| E′lat | 14 (23%) | 1 (8%) | 7 (39%) | 2 (13%) | 4 (31%) |
| E′avg | 6 (10%) | 0 (0%) | 2 (11%) | 1 (6%) | 3 (23%) |
| E′ (location not specified) | 2 (3%) | 0 (0%) | 2 (11%) | 0 (0%) | 0 (0%) |
| Any E/E′ | 40 (67%) | 7 (54%) | 12 (67%) | 12 (75%) | 9 (69%) |
| E/E′sep | 5 (8%) | 1 (8%) | 1 (6%) | 2 (13%) | 1 (8%) |
| E/E′lat | 10 (17%) | 5 (38%) | 2 (11%) | 1 (6%) | 2 (15%) |
| E/E′avg | 21 (35%) | 0 (0%) | 6 (33%) | 10 (63%) | 5 (38%) |
| E/E′ (location not specified) | 5 (8%) | 1 (8%) | 3 (17%) | 0 (0%) | 1 (8%) |
| Pulmonary flow indices | 8 (13%) | 6 (46%) | 0 (0%) | 0 (0%) | 2 (15%) |
| Left atrial size | 19 (32%) | 1 (8%) | 7 (39%) | 6 (38%) | 5 (38%) |
| LAVi | 16 (27%) | 1 (8%) | 6 (33%) | 6 (38%) | 3 (23%) |
| LAD or LAA | 3 (5%) | 0 (0%) | 1 (6%) | 0 (0%) | 2 (15%) |
| Valsalva reversal of E/A | 3 (5%) | 2 (15%) | 0 (0%) | 0 (0%) | 1 (8%) |
DDF, diastolic dysfunction; E, early diastolic inflow velocity; A, late diastolic inflow velocity; E′, early diastolic myocardial tissue velocity; E′sep, E′ of the septal wall; E′lat, E′ of the lateral wall; E′avg, averaged E′; LAVi, left atrial volume index; LAD, left atrial diameter; LAA, left atrial area.
Table 2.
Combinations of variables used for DDF classification and grading grouped by different interpretations of the EACVI/ASE 2009 classification algorithm in studies in which the algorithm interpretation was specified (n = 47)
| Classification and grading of DDF by a one-level classification tree (n = 13)S1–S13 |
Classification and grading of DDF by a two-level classification tree (n = 18)S14–S31 |
Classification of DDF only, no grading (n = 16)S32–S47 |
|---|---|---|
| For the definition of DDF and grading: | For the definition of DDF: | For the definition of DDF: |
| E/A, E/E′, and DT (n = 4)S1,S2,S6,S12 | E′ (n = 8)S14,S16,S17,S20,S26,S27,S29,S30 | E/E′ (n = 6)S35,S40,S42,S45–S47 |
| E/A and PV (n = 3)S3–S5 | E′ and LA size (n = 6)S15,S18,S21,S23,S25,S31 | LA size and E/E′ (n = 3)S36,S41,S43 |
| E/A, E/E′, VSr, DT, and IVRT (n = 1)S10 | E/E′ (n = 2)S22,S24 | DT and IVRT (n = 1)S34 |
| E/A, PV, E/E′, and DT (n = 1)S9 | E′, LA size, and E/E′ (n = 1)S28 | E′ (n = 1)S33 |
| E/A, PV, DT, and IVRT (n = 1)S11 | E′, E/A, and DT (n = 1)S19 | E′, E/E′, and E/A (n = 1)S32 |
| E/A, PV, DT, IVRT, and E′/A′ (n = 1)S7 | LA size (n = 1)S39 | |
| E/A, VSr, and DT (n = 1)S13 | For the subsequent grading of DDF: | LA size, E/E′, and E/A (n = 1)S44 |
| E′, LA size, E/A, and E/E′ (n = 1)S8 | E/A and DT (n = 5)S18,S22,S24,S25,S29 | E/E′, E/A, and DT (n = 1)S38 |
| E/A, E/E′, and DT (n = 4)S21,S26,S27,S30 | E′ and LA size (n = 1)S37 | |
| DT (n = 2)S14,S28 | ||
| E/A (n = 2)S17,S20 | ||
| E/A, E/E′, DT, and IVRT (n = 1)S23 | ||
| E′, E/A, E/E′, and DT (n = 1)S19 | ||
| E′, LA size, E/A, and E/E′ (n = 1)S15 | ||
| Variables used not specified (n = 2)S16,S31 | ||
DDF, diastolic dysfunction; E′, septal, lateral, average, or unspecified early myocardial tissue velocity; E, early transmitral flow velocity; A, late/atrial transmitral flow velocity; LA size, left atrial volume, diameter, or area; DT, deceleration time; IVRT, isovolumetric relaxation time; VSr, Valsalva reversal of E/A; PV, pulmonary venous flow indices.
Table 3.
Detailed definitions of DDF, including used logical operators, in studies in which DDF classification was based on E′ and/or left atrial size only (n = 17)
| E′sep < 8 OR E′lat < 10 OR LAVi > 34 (n = 4)S15,S18,S31,S37 |
| E′sep < 8 (n = 3)S14,S26,S29 |
| E′lat < 10 (n = 2)S17,S33 |
| E′avg < 9 (n = 1)S30 |
| E′sep < 8 AND E′lat < 10 AND LAVi > 34 (n = 1)S23 |
| E′sep < 8 OR E′lat < 10 (n = 1)S27 |
| E′ns < 8 (n = 1)S16 |
| E′sep < 8 OR LAVi > 34 (n = 1)S25 |
| LAVi > 34 (n = 1)S39 |
| E′avg < 9 OR LAVi > 34 (n = 1)S21 |
| E′sep < 8 AND E′lat < 10 (n = 1)S20 |
DDF, diastolic dysfunction; E′, early diastolic myocardial tissue velocity; E′sep, E′ of the septal wall; E′lat, E′ of the lateral wall; E′avg, average E′ of lateral and septal walls; E′ns, E′ of the unspecified wall; LAVi, left atrial volume index.
Prevalence study
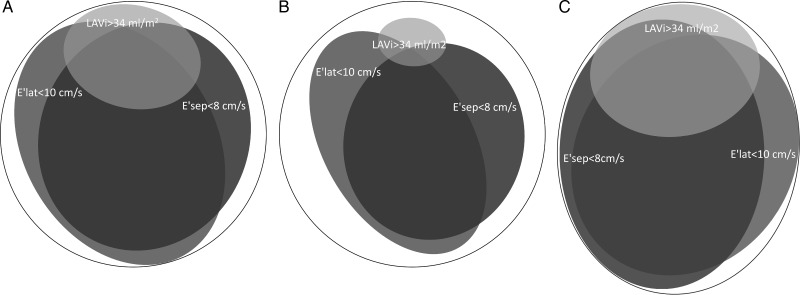
The basic characteristics of the study population and the low- and high-risk subgroups are shown in Table 4. In Table 5, the effect on the prevalence of using several possible interpretations of the EACVI/ASE definition is shown. To further illustrate how the choice of variables and logical operators affected the prevalence, area-proportionate Euler diagrams illustrating the overlap of abnormal E′ and LAVi in our study population are shown in Figure 1.
Table 4.
Basic characteristics of the study population
| All subjects (n = 714) | Low-risk subgroup (n = 129) | High-risk subgroup (n = 344) | P-valuea | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years) | 66.1 (±9.5) | 61.3 (±9.3) | 69.0 (±8.3) | <0.001 |
| Male sex | 493 (71%) | 102 (79%) | 228 (69%) | 0.05 |
| Body mass index (kg/m2) | 26.5 (±3.6) | 24.9 (±2.5) | 27.2 (±3.9) | <0.001 |
| Systolic blood pressure (mmHg) | 144.5 (±20.2) | 126.5 (±8.7) | 147.3 (±19.5) | <0.001 |
| Diastolic blood pressure (mmHg) | 79.4 (±9.5) | 74.2 (±6.7) | 79.0 (±9.5) | <0.001 |
| NT-proBNP (pg/mL) | 75 (40–146) | 51 (29–74) | 101 (57–206) | <0.001 |
| NT-proBNP > 125 (pg/mL) | 204 (29%) | 0 (0%) | 132 (40%) | <0.001 |
| Cardiovascular medication | 321 (46%) | 0 (0%) | 318 (96%) | <0.001 |
| Hypertension | 255 (36%) | 0 (0%) | 253 (76%) | <0.001 |
| Ischaemic heart disease | 48 (7%) | 0 (0%) | 47 (14%) | <0.001 |
| Diabetes mellitus | 55 (8%) | 0 (0%) | 54 (16%) | <0.001 |
| Echocardiography | ||||
| Geometry | ||||
| LV internal diameter (mm) | 48.5 (±4.3) | 48.4 (±4.2) | 48.3 (±4.3) | 0.84 |
| LVMi (g/m2) | 97.1 (±20.1) | 86.7 (±14.6) | 100.5 (±20.6) | <0.001 |
| LV hypertrophy | 151 (22%) | 0 (0%) | 86 (26%) | <0.001 |
| LAVi (mL/m2) | 28.2 (±8.4) | 24.3 (±5.7) | 31.4 (±8.9) | <0.001 |
| Doppler | ||||
| E/A | 1.0 (±0.3) | 1.2 (±0.4) | 1.0 (±0.3) | <0.001 |
| E/E′avg | 7.5 (±2.2) | 6.3 (±1.5) | 8.1 (±2.4) | <0.001 |
| Deceleration time (ms) | 236.2 (±66.3) | 230.4 (±68.2) | 240.0 (±66.7) | 0.06 |
| E′sep (cm/s) | 7.1 (±1.9) | 8.1 (±1.9) | 6.6 (±1.8) | <0.001 |
| E′lat (cm/s) | 9.0 (±2.5) | 10.1 (±2.6) | 8.5 (±2.3) | <0.001 |
Categorical variables presented as counts and percentages and continuous variables as mean ± SD, except for NT-proBNP in which the median and interquartile ranges were used because the distribution was non-normal. LV, left ventricle; LVMi, LV mass index; LAVi, left atrial volume index; E, early mitral inflow velocity; A, late mitral inflow velocity; E′, early myocardial tissue velocity; E′avg, E′ averaged for septal and lateral walls; E′sep, E′ of the septal wall; E′lat, E′ of the lateral wall.
aP-value for differences between the low- and high-risk subgroups.
Table 5.
Effect on the prevalence of DDF by using different interpretations of the EACVI/ASE algorithm for DDF classification
| All subjects (n = 714) | Low-risk subgroup (n = 129) | High-risk subgroup (n = 344) | |
|---|---|---|---|
| (E′sep < 8) OR (E′lat < 10) OR (LAVi > 34) | 84 (82–87) | 67 (59–75) | 94 (90–96) |
| (E′sep < 8) OR (E′lat < 10) | 82 (79–85) | 65 (56–73) | 89 (85–92) |
| (E′avg < 9) OR (LAVi > 34) | 77 (73–80) | 50 (41–59) | 88 (85–92) |
| (E′lat < 10) OR (LAVi > 34) | 76 (73–79) | 54 (45–63) | 86 (82–90) |
| (E′sep < 8) OR (LAVi > 34) | 73 (70–76) | 53 (45–62) | 84 (80–88) |
| (E′avg < 9) | 71 (68–75) | 47 (38–56) | 81 (77–85) |
| (E′lat < 10) | 70 (67–74) | 51 (42–60) | 77 (72–81) |
| (E′sep < 8) | 68 (65–72) | 50 (41–59) | 78 (73–82) |
| (E′sep < 8) AND (E′lat < 10) | 57 (53–61) | 36 (28–45) | 65 (60–70) |
| (LAVi > 34) | 20 (17–23) | 5 (2–10) | 32 (27–37) |
| (E′sep < 8) AND (LAVi > 34) | 16 (13–19) | 2 (0–5) | 26 (21–31) |
| (E′avg < 9) AND (LAVi > 34) | 15 (12–18) | 2 (0–5) | 24 (20–29) |
| (E′lat < 10) AND (LAVi > 34) | 14 (12–17) | 2 (0–5) | 22 (18–27) |
| (E′sep < 8) AND (E′lat < 10) AND (LAVi > 34) | 12 (10–15) | 1 (0–4) | 20 (16–25) |
Values are percentages (95% confidence intervals).
E′, early diastolic myocardial tissue velocity; E′sep, E′ of the septal wall; E′lat, E′ of the lateral wall; E′avg, average of E′lat and E′sep; LAVi, left atrial volume index.
Figure 1.
Euler diagram showing overlapping of LA dilatation (LAVi > 34 mL/m2) with low myocardial tissue velocities in the septal (E′sep < 8 cm/s) and lateral (E′lat < 10 cm/s) walls in all participants (A; n = 714), the low-risk subgroup (B; n = 129), and the high-risk subgroup (C; n = 344), respectively. The central overlapping of all three ellipsoids corresponds to the use of the logical operator AND, whereas the total area of all three ellipsoids corresponds to the use of OR. For combinatorial prevalences, see Table 5.
Discussion
In this study, we have demonstrated that the EACVI/ASE recommendations for the evaluation of LV diastolic function by echocardiography1 with regard to DDF definition have been interpreted differently across different studies. Furthermore, the data show that even among the substantial minority of studies that utilized a two-level classification tree with E′ and/or LAVi at the first branch point—an interpretation that we believe most closely resembles the EACVI/ASE standpoint—there were numerous variants of the actual definition of DDF. Finally, the data show that these differences in interpretation have a huge impact on the obtained prevalence in a community-based sample.
Early attempts to classify diastolic function relied mainly on mitral inflow parameters. Over time, numerous additional parameters have been introduced. Each new parameter has had its inherent shortcomings, and no single parameter could describe the complexities of diastolic function on its own.14 The proposed solution has been to use a multiparametric approach by taking into account several parameters simultaneously.15 However, relatively sparse data exist on how these parameters should be weighted, and although the current guidelines present a list of parameters with suggested cut-offs, the integration of these is left to individual judgement.
We observed substantial differences with respect to the variables and logical operators used, and the overall structure of the classification tree between the studies reviewed. Among studies in which DDF was graded, two distinct strategies could be discerned. First, a one-level decision tree was utilized, in which the criteria for each grade were presented and DDF was defined as the fulfilment of the criteria for any one of these grades. Secondly, a two-level decision tree was utilized in which criteria for DDF was defined, and in a subsequent step, grading of DDF with the aid of additional parameters was performed. Such disparate strategies in studies citing the same recommendations are noteworthy.
A substantial minority of the studies reviewed used only E′ and/or LAVi to define DDF. We believe that this approach most closely resembles the intention of the EACVI/ASE recommendations. However, even among these studies, there was considerable heterogeneity with regard to the specific definition used (Table 3). To demonstrate the effects on the reported prevalence obtained by this heterogeneity, we applied several possible interpretations of the first branch point of the two-level strategy on a community-based sample (Table 5). Several of these interpretations were encountered in the studies reviewed. Using the EACVI/ASE-endorsed fixed cut-offs, the prevalence of DDF calculated for the entire study group varied between 12 and 84% depending on the diagnostic algorithm used and between 1 and 67% in the low-risk subgroup. Because of the exclusion of subjects with exertional dyspnoea, diabetes, hypertension, ischaemic heart disease, obesity, LV hypertrophy, and elevated NT-proBNP from the low-risk subgroup, the prevalence of clinically relevant DDF could reasonably be expected to be very low. If we make the assumption that the true prevalence of DDF was well below 10% in this low-risk subgroup, it was evident that strategies that did not utilize LAVi and strategies that used the logical operator OR severely overestimated the prevalence of DDF (36–67%). Consequently, the specificity of such strategies will be poor. Only algorithms utilizing LAVi in combination with the logical operator AND resulted in what might be considered a reasonable prevalence of DDF in the low-risk subgroup. However, owing to the use of the logical operator AND, such DDF definitions are incompatible with the frequently stated fact that grade I DDF can be observed with normal LA size.1 Furthermore, in these algorithms, DDF classification will depend almost entirely on LAVi, whereas E′ will have only a minor impact. The large influence of LAVi stems from the fact that almost all of the subjects with increased atrial size had E′lat or E′sep below the cut-offs, whereas most subjects with low E′lat or E′sep had normal atrial size, as can be seen in Figure 1A. The large difference in the prevalence of high LAVi and low E′, respectively, as can be observed in Figure 1, is explained by the closeness of the proposed cut-off for LAVi of 34 mL/m2 to the upper normal limit (mean + 2SD) of about 37 mL/m2 in healthy subjects,8 whereas the proposed cut-offs of 8 cm/s for E′sep and 10 cm/s for E′lat are close to the ‘mean’ of healthy middle-aged subjects reported in several studies.16–19 Thus, the prevalence of abnormal LAVi of 5%, E′sep of 50%, and E′lat of 51% found in our low-risk subgroup is very much in line with what can be expected.
The EACVI/ASE recommendations for the evaluation of LV diastolic function provide a comprehensive overview of the full range of echocardiographic methods for evaluating diagnostic function and also provide a validated algorithm for the prediction of elevated filling pressures.20 However, the proposed classification scheme for DDF may benefit from some improvements. The ambiguities of the classification scheme result in a less than optimal interobserver agreement, as identified by others,3,4 and large interstudy differences with regard to the method of classification, as demonstrated in this study. The latter can potentially make interstudy comparison difficult and hazardous because subject classification can vary substantially between studies. Our observation is not unique, as the large variance in DDF definition across studies has also been observed by others.5,6 Furthermore, the sensitivity of the EACVI/ASE algorithm for identifying subjects with heart failure with preserved ejection fraction has been questioned previously,21 and our data indicate that some interpretations of the algorithm most likely lack specificity. We believe that if the EACVI/ASE two-level classification scheme is to be used, it has to be revised and clarified. With the improvement of specificity in mind, one potential solution could be to keep the proposed cut-offs for LAVi, E′sep, and E′lat and stress the use of AND as the logical operator, accepting the fact that the resulting DDF classification will be driven mainly by LA dilatation. Such a strategy resulted in a reasonable DDF prevalence of 1% in our low-risk subgroup. Another potential solution could be to adjust the E′sep, E′lat, and LAVi cut-offs so that they more closely resemble the age-specific reference limits in healthy populations. Age-specific limits, which are more restrictive than the cut-offs endorsed by the EACVI/ASE,16–19 might make the use of OR as the logical operator possible without introducing large proportions of false positives. This would allow for classification of DDF in subjects with normal atrial size. The use of age-specific reference limits, in the context of DDF classification, has been advocated by others.19 However, how the potential alterations outlined in the discussion above would affect the diagnostic accuracy for identifying DDF cannot be discerned from our data owing to the lack of a reference method.
Limitations
There are several limitations to this study. First, a selection bias with regard to reviewed studies is possible because only articles referring to the EACVI/ASE recommendations as published in the European Journal of Echocardiography and not the Journal of the American Society of Echocardiography were included. In addition, inclusion was limited by the presence of defined key phrases in the title/abstract of the screened studies. The full inclusion of all studies citing the EACVI/ASE recommendations might have altered the relative frequency of different interpretations, but it would not have changed the main finding that a multitude of different interpretations exist in the published literature. Secondly, the coding of reviewed studies was sometimes difficult because of vague classification definitions. This was addressed by independent coding by two researchers, where any disagreement was resolved by consensus. However, it is possible that in some cases we interpreted the DDF definitions in a different way from what was intended by the authors. Thirdly, because no gold standard for DDF was available, the diagnostic accuracy of different DDF classification scheme interpretations could not be established, and the discussion of specificity was based on the presumed successful exclusion of subjects with DDF from our low-risk subgroup. Finally, LA volumes were calculated by the monoplane rather than the biplane Simpson’s rule, as recommended by the guidelines. Because the cut-off of 34 mL/m2 is based on biplane measurements, our approach might have resulted in a slight over-22 or underestimation8,19 of the prevalence of atrial dilatation.
Conclusions
In this study, we have demonstrated that the EACVI/ASE recommendations for the evaluation of LV diastolic function by echocardiography have been interpreted differently across studies with regard to the classification of DDF. Furthermore, the findings show that these differences are important and can have a huge impact on subject classification and the obtained prevalence in a community-based sample. Further research that is focused on the development and validation of multiparametric algorithms for the classification of diastolic function is needed.
Supplementary data
Supplementary data are available at European Heart Journal—Cardiovascular Imaging online.
Contributors
Study design and concept: J.S., J.L., and P.H. Analysing and interpretation of the data: J.S., J.L., P.H., and E.H. Draft of the manuscript: J.S. All authors revised the paper.
Funding
This study was supported by grants from Sparbanksstiftelsen Nya, the County of Västmanland, Selanders Stiftelse, and the Swedish Medical Association. Funding to pay the Open Access publication charges for this article was provided by Centre for Clinical Research, Uppsala University, Västmanland County Hospital, Västerås, Sweden.
Acknowledgement
We thank Petra Wahlén, Lena Trollvad, Lolita Backsell, Marja-Leena Ojutkangas, Annika Kärnsund, and Göran Nilsson for their valuable contributions.
Conflict of interest: none declared.
References
- 1.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165–93. [DOI] [PubMed] [Google Scholar]
- 2.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107–33. [DOI] [PubMed] [Google Scholar]
- 3.Chapman CB, Ewer SM, Kelly AF, Jacobson KM, Leal MA, Rahko PS. Classification of left ventricular diastolic function using American Society of Echocardiography Guidelines: agreement among echocardiographers. Echocardiography 2013;30:1022–31. [DOI] [PubMed] [Google Scholar]
- 4.Unzek S, Popovic ZB, Marwick TH, Diastolic Guidelines Concordance I. Effect of recommendations on interobserver consistency of diastolic function evaluation. JACC Cardiovasc Imaging 2011;4:460–7. [DOI] [PubMed] [Google Scholar]
- 5.Kloch-Badelek M, Kuznetsova T, Sakiewicz W, Tikhonoff V, Ryabikov A, González A et al. Prevalence of left ventricular diastolic dysfunction in European populations based on cross-validated diagnostic thresholds. Cardiovasc Ultrasound 2012;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Jaroudi WA, Thomas JD, Rodriguez LL, Jaber WA. Prognostic value of diastolic dysfunction: state of the art review. Cardiol Rev 2013;22:79–90. [DOI] [PubMed] [Google Scholar]
- 7.Petrie MC, Hogg K, Caruana L, McMurray JJ. Poor concordance of commonly used echocardiographic measures of left ventricular diastolic function in patients with suspected heart failure but preserved systolic function: is there a reliable echocardiographic measure of diastolic dysfunction? Heart 2004;90:511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kou S, Caballero L, Dulgheru R, Voilliot D, De Sousa C, Kacharava G et al. Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Hear J Cardiovasc Imaging 2014;15:680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart. Eur Heart J 2012;33:1787–847. [DOI] [PubMed] [Google Scholar]
- 10.Hedberg P, Hammar C, Selmeryd J, Viklund J, Leppert J, Hellberg A et al. Left ventricular systolic dysfunction in outpatients with peripheral atherosclerotic vascular disease: prevalence and association with location of arterial disease. Eur J Heart Fail 2014;16:625–32. [DOI] [PubMed] [Google Scholar]
- 11.Henriksen E, Selmeryd J, Leppert J, Hedberg P. Echocardiographic assessment of maximum and minimum left atrial volumes: a population-based study of middle-aged and older subjects without apparent cardiovascular disease. Int J Cardiovasc Imaging 2014;31:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA et al. Recommendations for chamber quantification. Eur J Echocardiogr 2006;7:79–108. [DOI] [PubMed] [Google Scholar]
- 13.Micallef L, Rodgers P. eulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS ONE 2014;9:e101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitzman DW, Little WC. Left ventricle diastolic dysfunction and prognosis. Circulation 2012;125:743–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh JK, Hatle L, Tajik AJ, Little WC. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol 2006;47:500–6. [DOI] [PubMed] [Google Scholar]
- 16.Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. Normative reference values for the tissue Doppler imaging parameters of left ventricular function: a population-based study. Eur J Echocardiogr 2010;11:51–6. [DOI] [PubMed] [Google Scholar]
- 17.Dalen H, Thorstensen A, Vatten LJ, Aase SA, Stoylen A. Reference values and distribution of conventional echocardiographic Doppler measures and longitudinal tissue Doppler velocities in a population free from cardiovascular disease. Circ Cardiovasc Imaging 2010;3:614–22. [DOI] [PubMed] [Google Scholar]
- 18.Munagala VK, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Association of newer diastolic function parameters with age in healthy subjects: a population-based study. J Am Soc Echocardiogr 2003;16:1049–56. [DOI] [PubMed] [Google Scholar]
- 19.Caballero L, Kou S, Dulgheru R, Gonjilashvili N, Athanassopoulos GD, Barone D et al. Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE Study. Eur Heart J Cardiovasc Imaging 2015;16:1031–41. [DOI] [PubMed] [Google Scholar]
- 20.Dokainish H, Nguyen JS, Bobek J, Goswami R, Lakkis NM. Assessment of the American Society of Echocardiography–European Association of Echocardiography guidelines for diastolic function in patients with depressed ejection fraction: an echocardiographic and invasive haemodynamic study. Eur J Echocardiogr 2011;12:857–64. [DOI] [PubMed] [Google Scholar]
- 21.Shuai X-X, Chen Y-Y, Lu Y-X, Su G-H, Wang Y-H, Zhao H-L et al. Diagnosis of heart failure with preserved ejection fraction: which parameters and diagnostic strategies are more valuable? Eur J Heart Fail 2011;13:737–45. [DOI] [PubMed] [Google Scholar]
- 22.Russo C, Hahn RT, Jin Z, Homma S, Sacco RL, Di Tullio MR. Comparison of echocardiographic single-plane versus biplane method in the assessment of left atrial volume and validation by real time three-dimensional echocardiography. J Am Soc Echocardiogr 2010;23:954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]