Abstract
Recent developments in computed tomography (CT) technology have fulfilled the prerequisites for the clinical application of myocardial CT perfusion (CTP) imaging. The evaluation of myocardial perfusion by CT can be achieved by static or dynamic scan acquisitions. Although both approaches have proved clinically feasible, substantial barriers need to be overcome before its routine clinical application. The current review provides an outline of the current status of CTP imaging and also focuses on disparities between static and dynamic CTPs for the evaluation of myocardial blood flow.
Keywords: myocardial perfusion imaging, computed tomography, stress CT perfusion, static, dynamic
Introduction
There is mounting evidence favouring the functional relevance of coronary stenoses over the angiographic severity of CAD with regard to clinical decision-making and future outcomes.1,2 Additionally, it has been shown that the functional significance of coronary artery disease (CAD) is not unavoidably reflected by the angiographic appearance of CAD as estimated by coronary computed tomography angiography (CCTA) and invasive coronary angiography (ICA).3 Although it enables the rule out of obstructive CAD with near to absolute certainty, the morphologic information provided by CCTA remains insufficient to determine the downstream functional consequences of a given coronary lesion.4,5 However, recent developments in computed tomography (CT) technology have fulfilled the technical prerequisites for the application of stress CT myocardial perfusion for the evaluation of CAD. As such, CT provides not only anatomical information,6 but it became also capable of determining the functional relevance of coronary stenosis,7 rendering it a potential ‘one stop shop’ procedure for the diagnosis and management of CAD.
The aim of the present review is to discuss the background of CT perfusion (CTP), to address the different approaches of CTP imaging and their concomitant limitations, and to provide an overview of the clinical value of CTP for the non-invasive detection of myocardial ischaemia.
Cardiac CT approaches for the evaluation of myocardial perfusion: static CT imaging
Static imaging of myocardial attenuation during first-pass perfusion provides a snapshot of myocardial iodine distribution at one time point. The assessment of defects is qualitative and hypo-enhanced regions are compared with normal remote myocardial segments or normalized to the attenuation of the left ventricular cavity. Analogous to nuclear MPI, perfusion defects on the stress images are evaluated against baseline perfusion to determine reversibility and hence myocardial ischaemia, whereas an irreversible defect is indicative of dead tissue (e.g. scar tissue). An important caveat is that such a qualitative assessment may disguise globally reduced myocardial perfusion, since it highly relies on the presence of a normally perfused area to act as a reference. Nevertheless, the presence of severe CAD on CCTA may unmask balanced ischaemia undetected by static CTP imaging. In addition, the high spatial resolution of CT facilitates the detection of subendocardial ischaemia as a sign of three-vessel disease. All in all, studies thus far have showed no inferiority of static CTP compared with a quantitative evaluation. However, one of the drawbacks of static CTP lies in the acquirement of only one sample of data and mistiming of the contrast bolus results in poor contrast-to-tissue ratios by missing the peak attenuation.8 Cardiac output and flow rate of the contrast material may affect bolus timing. In addition, the acquisition of data from sequential heartbeats affects the attenuation gradient and may result in a heterogeneous iodine distribution, mimicking perfusion defects. However, with the introduction of 320-detector row CT devices, the coverage of the entire heart in one gantry rotation is accomplished ensuring temporal uniformity.
Cardiac CT approaches for the evaluation of myocardial perfusion: dynamic CT imaging
Another approach to obtain CTP data is by dynamic imaging. In contrast to static CTP, a high temporal resolution and detectors that allow for entire myocardial coverage are required in order to obtain multiple consecutive images at high heart rates.9 Although dynamic CTP is feasible with narrow detector CT, the shuttle mode that allows (sub)total coverage of the left ventricular myocardium is a source of motion affecting the construction of the time-attenuation curves (TACs). With the advent of cutting edge scanners with 320 detector rows, coverage of the entire heart is possible with one gantry rotation. As such, temporal uniformity is ensured without a concomitant decrease in temporal resolution. Quantitative CTP images can be acquired by considering the time course of myocardial iodine distribution by serial temporal sampling at different time points after injection to create TACs (Figure 1). Mathematic modelling of these TACs allow for the quantification of myocardial perfusion in absolute terms.10 Notably, the first-pass extraction into the myocardium of iodinated contrast is low and is dependent on flow rate. As such, the relationship between iodinated contrast retention and myocardial perfusion is not linear, resulting in the systemic underestimation of perfusion as determined by microspheres by dynamic CTP.11,12 Flow-dependent correction factors are needed to account for this phenomenon and subsequently to correct for the underestimation of perfusion at the higher flow range. However, if extraction is rather low such as for iodinated contrast agents, correction models will also multiply potential scatter leading to more noise especially at the higher flow range. However, quantitation of myocardial perfusion might be useful for the uncovering of balanced ischaemia. The anticipated improved detection of balanced ischaemia by quantitative CTP imaging is explained by the fact that a homogeneously reduced left ventricle perfusion may be disguised by qualitative approaches, since these rely on a normally perfused area to act as a reference. As a consequence, only the most impaired region is considered pathological with qualitative imaging. It is therefore not argued that triple vessel or left main disease always goes undetected with static imaging, and its presence may be detected if heterogeneity in flow exists. Furthermore, quantitative imaging has opened new opportunities and has extended the scope from detection of haemodynamic significant epicardial lesions towards an emphasis on microvascular health. In the absence of obstructive CAD, abnormal myocardial blood flow (MBF) is indicative of coronary microvascular dysfunction,13 which is considered the functional counterpart of traditional risk factors. Several studies demonstrated the injurious impact of cardiovascular risk factors on coronary microvascular function and have encouraged stringent therapeutic strategies for cardiovascular risk factor modifications. The incremental prognostic value of quantitative MBF imaging has been established by studies showing adverse outcomes in patients with normal regional perfusion images in whom quantification revealed abnormal MBF values.14,15 Paradoxically, the apparent advantage of quantitative analysis to reveal low perfusion in the context of microvascular dysfunction, a condition that does not yield false-positive results with qualitative analysis, is at the same time the Achilles' Heel of quantitative MPI. It remains rather challenging to distinguish between microvascular dysfunction and epicardial disease based on quantitative results alone. An important advantage of CTP, however, lies in the simultaneous visualization of coronary anatomy, which allows one to differentiate between these two conditions. Additionally, recent quantitative PET and CTP studies reported relative flow reserve (i.e. MBF in comparison with healthy remote myocardium) to provide a more accurate discrimination of haemodynamic significant CAD compared with absolute MBF, arguably by mitigating the impact of microvascular resistance on perfusion values.16–18
Figure 1.
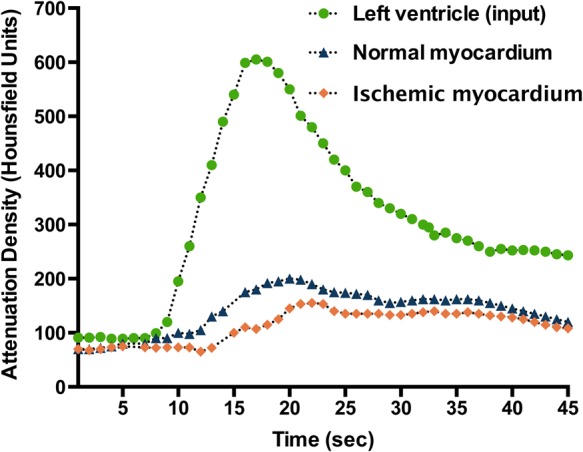
Schematic illustration of typical TACs as acquired by dynamic myocardial CTP imaging. The green curve reflects the TAC of the ascending aorta, while the blue curve in the graph shows the TAC of normally perfused myocardium and the orange curve that of an ischaemic myocardium.
Important considerations when interpreting quantitative CT myocardial blood flow images
Although dynamic stress CTP has some distinct advantages over the static technique, substantial barriers need to be confronted before its routine clinical application. Firstly, determining an optimal cut-off value of hyperaemic MBF with high diagnostic accuracy may prove difficult in clinical practice. Table 1 summarizes the literature on absolute flow by dynamic myocardial CTP for various definitions of ischaemia-causing lesions. There is a wide range of cut-off values, which is probably attributable to study design, sample sizes, coronary risk profile, prevalence of CAD, and the applied reference standard. Interestingly, studies in patients without obstructive CAD have reported a broad range in myocardial perfusion values due to a large physiological variation of minimal microvascular resistance.19–21 Age, gender, and traditional cardiovascular risk factors have been proved to govern MBF irrespective of CAD status.21,22 This is consistent with recent findings reporting large heterogeneity in hyperaemic perfusion values as determined by CTP in a low-risk population and healthy volunteers.19,20 In line with prior results obtained from quantitative PET imaging,21,23 Kim et al. also found physiological differences in perfusion with regard to gender as assessed by quantitative CTP.20 It seems that women have in general a lower microvascular resistance compared with their male counterparts, which may necessitate the implementation of gender-specific cut-off values. Altogether, this will hamper the discriminatory power of a single threshold with high diagnostic accuracy to distinguish between normal and pathological MBFs. Therefore, large databases on normal perfusion values are warranted to enable accurate interpretation of quantitative perfusion values.
Table 1.
Ischaemic cut-off values of stress MBF as assessed by dynamic CTP
| First author | Citation | N | Reference standard | Stress MBF |
|
|---|---|---|---|---|---|
| Cut-off | AUC | ||||
| Bamberg et al. | Radiology 2011;260(3):689–98 | 33 | FFR ≤ 0.75 | 75 mL/100 mL/min | 0.71 |
| Greif et al. | Heart 2013;99(14):1004–11 | 65 | FFR < 0.80 | 75 mL/100 mL/min | 0.71 |
| Huber et al. | Radiology 2013;269(2):378–86. | 32 | FFR ≤ 0.75 + ICA > 75% | 1.64 mL/g/min | 0.86 |
| Rossi et al. | Eur Heart J Cardiovasc Imaging 2014;15(1):85–94 | 80 | FFR ≤ 0.75 | 78 mL/100 mL/min | 0.95 |
| Meinel et al. | AJR 2014;203(2):W174–80 | 146 | Defect visual CTP + CCTA > 50% DS | 105 mL/100 mL/min | 0.96 |
| Kono et al. | Invest Radiol 2014;49(12):801–7 | 42 | FFR ≤ 0.80 | 103.1 mL/100 mL/min | 0.75 |
| Bamberg et al. | JACC Cardiovasc Imaging 2014;267–77 | 31 | MRI | 88 mL/100 mL/min | 0.84 |
| Wichmann et al. | AJR 2015;205(1):W67–72 | 137 | CCTA ≥ 50% DS | 103 mL/100 mL/min | 0.88 |
AUC, area under the curve; CCTA, coronary computed tomography angiography; CTP, computed tomography perfusion; DS, diameter stenosis; FFR, fractional flow reserve; ICA, invasive coronary angiography; MBF, myocardial blood flow.
Disparity in rest/stress vs. stress/rest scan protocols
The sequence of scan acquisition is still arbitrary and both rest–stress and stress–rest sequences are used in clinical practice. However, these sequences are not interchangeable, and the applied scan sequence has some important implications. There has been variability of when to perform the ‘rest’ portion vs. the ‘stress’ portion, with many contending that vasodilator stress is important to perform first to reduce the chance of residual contrast that may confound perfusion defects. In contrast, others have argued for a rest-first protocol, which allows one to optimally benefit from the information provided by CCTA. By harnessing the ability of CCTA to rule out obstructive CAD with near to absolute certainty, one may only proceed to stress CTP in the presence of anatomically defined obstructive CAD. This approach will obviate the need of further downstream testing in a significant portion of patients with concomitant reduction of contrast and radiation dose. Nevertheless, such an approach relies highly on the image quality of CCTA, which necessitates the use of sublingual nitrates and beta-blockers to optimize its quality. However, beta-blockers are known to disguise myocardial ischaemia by an increase in the diastolic perfusion time.24 Altogether, a rest–stress sequence comes at the expense of cross-contamination of contrast in the stress phase and the use of beta-blockers, which both may impede the sensitivity of stress CTP for detection of myocardial ischaemia. However, one may argue to start with a stress phase to optimize the stress perfusion study in patients who are expected to benefit from a stress perfusion study, such as individuals with a high pre-test likelihood of CAD or with a previous cardiac history yielding their CCTA likely not interpretable due to severe coronary calcifications. In this specific subgroup, the question is not whether they have CAD, but rather whether there is ischaemia and how to guide subsequent revascularizations.
Diagnostic performance of CT myocardial perfusion imaging
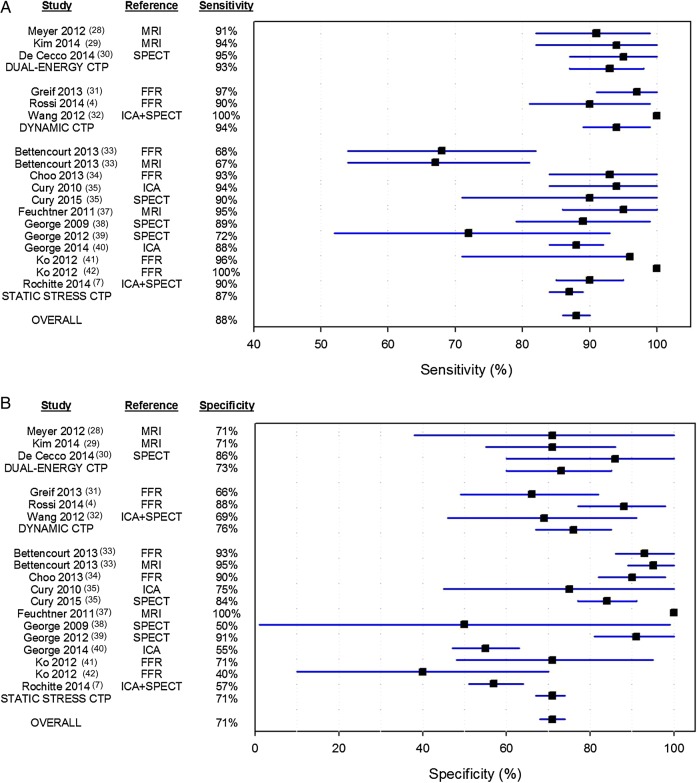
The assessment of myocardial ischaemia is of upmost importance to initiate an adequate therapeutic strategy. Stress myocardial CTP has been well validated in preclinical studies demonstrating CT-derived perfusion values to correlate favourably with microsphere derived MBF data. Nowadays, a rapidly expanding body of literature shows CTP to exhibit high accuracy for the detection of myocardial perfusion defects attributable to flow-limiting stenosis,4,7,25–39 comparable with SPECT and magnetic resonance imaging (MRI) myocardial perfusion imaging (Figure 2). Pooled analysis of the current literature demonstrates a sensitivity and specificity on a per-patient level of 88 and 71%, respectively. Of note, it seems that quantitative and dual-energy CTP tends to have a higher sensitivity than static CTP imaging (Figure 2). This might be attributable to a higher detection of subtle perfusion defects that cannot be visually appreciated by static CTP. A preclinical study by Bamberg et al. showed the ability of dynamic CTP to detect subtler perfusion defects than a qualitative approach. Moreover, the exploitation of two different energy levels by dual-energy CT (DECT) increases the conspicuity of hypoperfused regions leading to an improved accuracy. With regard to specificity, the number of patients with false-positive findings seems to be equally divided amongst the different CTP imaging techniques (Figure 2). However, the diagnostic performance of CTP, albeit very high, has mainly been determined against SPECT, MRI, and conventional angiography, despite the fact that FFR is nowadays considered the gold standard for the functional assessment of CAD severity. In this regard, a recently published meta-analysis by Takx et al. revealed that CTP permits accurate assessment of flow-limiting coronary stenoses, as indicated by FFR, with a sensitivity and specificity of 88 and 80%, respectively.40 Surprisingly, they also found that CTP imaging yielded less false-negative findings than SPECT as reflected by a higher sensitivity of 88 vs. 74%, respectively. These findings challenge the utility of SPECT, which is consistent with the findings of a prospective multicentre study by George et al., who showed in a head-to-head fashion myocardial CTP imaging to be more accurate than SPECT in the diagnosis of a ≥50% stenosis as defined with ICA.37 Furthermore, in a recently published multicentre trial by Cury et al., wherein 110 patients were randomized to rest/regadenoson-stress SPECT on day 1 followed by a stress/rest CTP protocol on day 2 or vice versa, CTP displayed a sensitivity and specificity of 90 and 84%, respectively, for the detection of myocardial ischaemia as defined by a reversible perfusion defect on SPECT.33 Interestingly, due to the multicentre setting of both studies, the results will be highly generalizable to the clinical population, rendering CTP a potential alternative to SPECT MPI. The diagnostic differences between CTP imaging and SPECT may be attributable to the higher spatial resolution of CT and the use of iodinated contrast as a perfusion tracer. In contrast to SPECT, the higher spatial resolution of CT allows perfusion defects to be more conspicuous and therefore easily appreciated.
Figure 2.
Pooled analysis (on a per-patient basis) of studies on the diagnostic performance of myocardial CTP imaging for the assessment of haemodynamic significant CAD. CTP, computed tomography myocardial perfusion; FFR, fractional flow reserve; ICA, invasive coronary angiography; MRI, magnetic resonance imaging; SPECT, single photon emission computed tomography
Hybrid cardiac imaging by cardiac CT
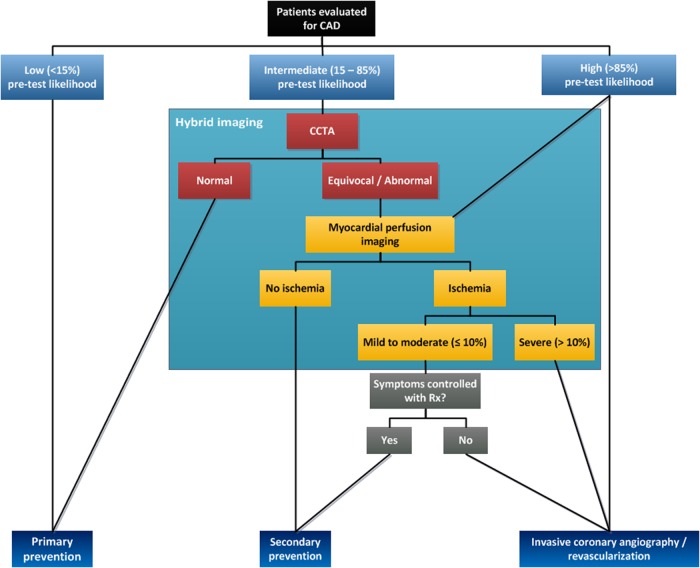
A large body of evidence has established CCTA as an imaging modality with high diagnostic accuracy for the detection of CAD.41 Although all studies unambiguously portray the same picture of a high sensitivity and negative predictive value (NPV),41 CCTA is characterized by a systemic overestimation of the degree of stenosis as reflected by high a modest specificity and positive predictive value, indicating high rates of false-positive findings prompting unnecessary referrals of patients to the catheterization laboratory. These concerns have proved to be real problems as indicated by the findings of Shreibati et al., who found that patients who underwent CCTA were twice as likely to be referred for ICA and underwent significantly more coronary interventions compared with those who underwent SPECT imaging as an initial diagnostic test, while outcome was similar amongst these two groups.42 Although the focus of cardiac CT has been on the non-invasive evaluation of coronary anatomy, its unique characteristics also enable the assessment of myocardial perfusion. As such, CT provides complementary information and may become a hybrid tool on its own right. Supplementary data online, Table S1 lists the clinical studies that have evaluated the incremental diagnostic value of CTP as an adjunct to CCTA. Of note, the majority of these studies were hampered by a single-centre setting, questioning the generalizability of their results. Nevertheless, a recently published multicentre study by Cury et al. demonstrated that the addition of myocardial CTP imaging to CCTA resulted in an improvement of accuracy from 69 to 85%, which was primarily driven by a reduction in the rate of false-positive CCTA scans.33 Furthermore, the CORE320 study, a prospective multicentre study, which included 381 patients, has clearly demonstrated the benefit of CTP as an adjunct to CCTA for the detection of >50% stenosis on ICA with concomitant perfusion abnormalities on SPECT.7 The combined CCTA-CTP approach yielded a significantly higher accuracy (AUC = 0.87) compared with CCTA alone (0.84; P = 0.02). A case example showing the incremental value of CTP is provided in Supplementary data online, Figure S1. In contrast, the limited benefit of hybrid imaging in patients with a previous cardiac history was another interesting observation of the CORE320 trial.7 This finding is in line with the results of a previous study by Greif et al. in high-risk patients, who described no diagnostic benefit of a hybrid approach in this specific population.28 Functional imaging alone probably suffices in patients with a previous cardiac history, as the exclusion of haemodynamic significant CAD will not alter the treatment regime since coronary atherosclerosis already exists, rendering the role of CCTA limited in these specific populations. As such, valid arguments have been made whether the routine use of hybrid imaging is justified, especially considering the current financial constraints and the high radiation dose of a hybrid CCTA-CTP protocol. Subjects with a low-to-intermediate risk will likely benefit from a CCTA first strategy, since roughly half of these patients will not display obstructive CAD at CCTA. The added value of CTP is therefore limited in these cases, considering the high NPV of CCTA, which obviates the need of further testing in patients without angiographically obstructive stenoses. The importance of tailored imaging protocols was already recognized at the very early stages of hybrid devices by Berman et al., who therefore proposed a diagnostic algorithm43 (Figure 3). The findings by Greif et al.,28 which were confirmed by the CORE320 study,7 emphasize the importance of staged CTP protocols to gain the highest benefit in terms of accuracy and costs along with a reduction in contrast and radiation dose.
Figure 3.
Diagnostic algorithm for detecting CAD using hybrid imaging. By harnessing the high sensitivity of CCTA, one may only proceed to CTP imaging if CCTA reveals an angiographically obstructive coronary stenosis. As such, the rate of false-positive findings by CCTA will be reduced with a concomitant increase in diagnostic accuracy, while obviating the need for additional imaging and subsequent radiation exposure to patients if the CCTA shows no (obstructive) CAD. Adapted from Danad I et al. J Nucl Cardiol. 2013 Oct.;20(5):874–90 with permission of the publisher.
Image artefacts
For CTP, there are a few artefacts that may impede accurate evaluation of myocardial perfusion by CT, namely motion and beam-hardening (BH) artefacts. Both artefacts may lead to the appearance of hypoperfused areas that resemble myocardial ischaemia resulting in false-positive interpretations. Motion artefacts arise by patient movement during the scan procedure or by cardiac motion due to high or irregular heart rates. A way to limit the impact of motion artefacts on interpretation of CTP scans is to examine different cardiac phases. A motion artefact lasts only for a few cardiac phases, while true perfusion defects persist in all phase of the cardiac cycle. BH, on the other hand, arises from the polychromatic nature of X-rays. The high volume of high-density iodinated contrast in the left ventricle is a potential source of BH artefacts. High-density structures preferable attenuate low-energy photons more than their high-energy counterpart, thereby giving rise to a shift towards a high-energy X-ray beam. Consequently, this may result in hypo-enhanced areas in the myocardium resembling myocardial ischaemia, hampering accurate assessment of myocardial perfusion. Hence, images acquired during systole when the lowest amount of contrast is present in the left ventricle are less susceptible to BH artefacts. The correction of BH is a challenging task, and post-reconstruction BH algorithms have been applied to minimize the effect of BH on myocardial perfusion images. In addition, DECT has been proposed as a more effective CT technique for the correction of BH. The unique features of DECT (i.e. the exploitation of two different X-ray energy levels) allow for the generation of virtual monochromatic images, which depict a scanned object at one X-ray energy level. Therefore, since BH arises from the polychromatic nature of X-rays, images acquired with a monochromatic X-ray beam are in theory free of BH.
Radiation dose aspects
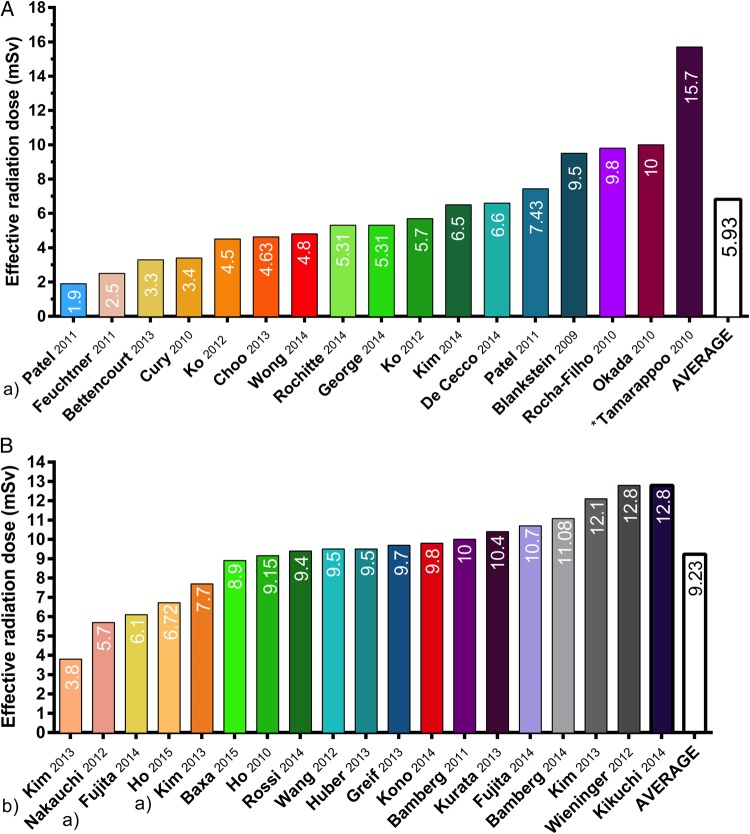
Rapid growth in CT procedures has sparked concerns about potential deleterious effects from cardiac CT and the high radiation doses associated with stress CTP imaging. The natural lifetime risk of dying from cancer is ∼21% for the US population, and a cardiac CT scan with an effective radiation dose of 10 mSv could add 0.05% to that risk in absolute terms, corresponding with a relative increase of 0.2%,44 yielding the natural risk of cancer to be much greater than the radiation-induced occurrence of malignancies. It is important to realize, however, that cardiac patients are likely to undergo multiple imaging thereby increasing their lifetime radiation exposure. As such, the high radiation exposure during stress CTP acquisitions remains a concern. In particular, dynamic CTP protocols are associated with high radiation doses since they require multiple acquisitions for the generation of TACs. The average radiation exposure of static CTP varies between 1.9 and 15.7 mSv with an average value of 5.93 mSv (Figure 4). Effective dose values between 3.8 and 12.8 mSv have been reported for dynamic CTP imaging, yielding an average radiation dose of 9.23 mSv (Figure 4), which is comparable with nuclear MPI exams.45 Nevertheless, applications of radiation dose reducing techniques seem to be promising. For instance, low-tube-voltage settings enable dynamic CTP imaging in patients with normal body mass indices with a 40% reduced radiation dose with preserved image quality and MBF assessment.46 Similarly, Kim et al. demonstrated a 36% dose reduction of dynamic CT protocols with automated tube current modulation.47 In this study, an additional dose reduction of 32% was achieved by halving scan acquisition times; however, quantification of MBF was not possible due to the lack of a pre-contrast scan at the initiation of contrast injection.47 All in all, despite these promising results, dynamic myocardial perfusion imaging by CT still requires relatively high radiation exposure (Figure 4). In this light, tailoring of CTP imaging protocols is mandatory to avoid unnecessary radiation exposure and procedure-related risks.
Figure 4.
Estimated effective radiation dose (mSv) for published studies of static (A) and dynamic (B) CT myocardial perfusion imaging. (a) Low radiation dose achieved by low-tube-voltage imaging. (b) Combination of reducing scan acquisition time and low-tube-voltage imaging resulted in an effective radiation dose of 3.8 mSv. *Inclusion of coronary artery bypass graft patients required greater scan lengths for visualization of both the native arteries as well as the grafts, which resulted in a higher radiation dose.
Conclusions
Cardiac CT has extended beyond coronary anatomy due to novel developments in CT technology that have fulfilled the prerequisites for the clinical implementation of myocardial CTP imaging. In line with MRI and PET imaging, CTP may provide qualitative or quantitative perfusion data. However, the technique is still in its infancy, and substantial work is needed before (dynamic) CTP is clinically embraced. Notably, promising results are seen with regard to the diagnostic value of CTP for the detection of myocardial ischaemia even when compared against a background of FFR. In addition, CTP provides incremental value over CCTA mainly by reducing the number of false-positive findings allowing a more accurate detection of CAD.
Funding
This study was supported in part by grants from the National Institutes of Health (R01HL111141 and R01HL118019). This study was also funded, in part, by a generous gift from the Dalio Institute of Cardiovascular Imaging and the Michael Wolk Foundation.
Supplementary material
Supplementary material is available at European Heart Journal–Cardiovascular Imaging online.
Conflict of interest: J.K.M. serves as a consultant to HeartFlow and GE Healthcare and receives research funding from GE Healthcare.
References
- 1.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–24. [DOI] [PubMed] [Google Scholar]
- 2.Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 2010;55:2816–21. [DOI] [PubMed] [Google Scholar]
- 3.Gaemperli O, Schepis T, Valenta I, Koepfli P, Husmann L, Scheffel H et al. Functionally relevant coronary artery disease: comparison of 64-section CT angiography with myocardial perfusion SPECT. Radiology 2008;248:414–23. [DOI] [PubMed] [Google Scholar]
- 4.Rossi A, Papadopoulou SL, Pugliese F, Russo B, Dharampal AS, Dedic A et al. Quantitative computed tomographic coronary angiography: does it predict functionally significant coronary stenoses? Circ Cardiovasc Imaging 2014;7:43–51. [DOI] [PubMed] [Google Scholar]
- 5.Meijboom WB, Van Mieghem CA, van Pelt N, Weustink A, Pugliese F, Mollet NR et al. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol 2008;52:636–43. [DOI] [PubMed] [Google Scholar]
- 6.Techasith T, Cury RC. Stress myocardial CT perfusion: an update and future perspective. JACC Cardiovasc Imaging 2011;4:905–16. [DOI] [PubMed] [Google Scholar]
- 7.Rochitte CE, George RT, Chen MY, Arbab-Zadeh A, Dewey M, Miller JM et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J 2014;35:1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bischoff B, Bamberg F, Marcus R, Schwarz F, Becker HC, Becker A et al. Optimal timing for first-pass stress CT myocardial perfusion imaging. Int J Cardiovasc Imaging 2013;29:435–42. [DOI] [PubMed] [Google Scholar]
- 9.Schuleri KH, George RT, Lardo AC. Applications of cardiac multidetector CT beyond coronary angiography. Nat Rev Cardiol 2009;6:699–710. [DOI] [PubMed] [Google Scholar]
- 10.Valdiviezo C, Ambrose M, Mehra V, Lardo AC, Lima JA, George RT. Quantitative and qualitative analysis and interpretation of CT perfusion imaging. J Nucl Cardiol 2010;17:1091–100. [DOI] [PubMed] [Google Scholar]
- 11.Bamberg F, Hinkel R, Schwarz F, Sandner TA, Baloch E, Marcus R et al. Accuracy of dynamic computed tomography adenosine stress myocardial perfusion imaging in estimating myocardial blood flow at various degrees of coronary artery stenosis using a porcine animal model. Invest Radiol 2012;47:71–7. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz F, Hinkel R, Baloch E, Marcus RP, Hildebrandt K, Sandner TA et al. Myocardial CT perfusion imaging in a large animal model: comparison of dynamic versus single-phase acquisitions. JACC Cardiovasc Imaging 2013;6:1229–38. [DOI] [PubMed] [Google Scholar]
- 13.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830–40. [DOI] [PubMed] [Google Scholar]
- 14.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol 2009;54:150–6. [DOI] [PubMed] [Google Scholar]
- 15.Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740–8. [DOI] [PubMed] [Google Scholar]
- 16.Kono AK, Coenen A, Lubbers M, Kurata A, Rossi A, Dharampal A et al. Relative myocardial blood flow by dynamic computed tomographic perfusion imaging predicts hemodynamic significance of coronary stenosis better than absolute blood flow. Invest Radiol 2014;49:801–7. [DOI] [PubMed] [Google Scholar]
- 17.Wichmann JL, Meinel FG, Schoepf UJ, Lo GG, Choe YH, Wang Y et al. Absolute Versus Relative Myocardial Blood Flow by Dynamic CT Myocardial Perfusion Imaging in Patients With Anatomic Coronary Artery Disease. AJR Am J Roentgenol 2015;205:W67–72. [DOI] [PubMed] [Google Scholar]
- 18.Stuijfzand WJ, Uusitalo V, Kero T, Danad I, Rijnierse MT, Saraste A et al. Relative flow reserve derived from quantitative perfusion imaging may not outperform stress myocardial blood flow for identification of hemodynamically significant coronary artery disease. Circ Cardiovasc Imaging 2015;8. [DOI] [PubMed] [Google Scholar]
- 19.Ho KT, Ong HY, Tan G, Yong QW. Dynamic CT myocardial perfusion measurements of resting and hyperaemic blood flow in low-risk subjects with 128-slice dual-source CT. Eur Heart J Cardiovasc Imaging 2015;16:300–6. [DOI] [PubMed] [Google Scholar]
- 20.Kim EY, Chung WJ, Sung YM, Byun SS, Park JH, Kim JH et al. Normal range and regional heterogeneity of myocardial perfusion in healthy human myocardium: assessment on dynamic perfusion CT using 128-slice dual-source CT. Int J Cardiovasc Imaging 2014;30 Suppl. 1:33–40. [DOI] [PubMed] [Google Scholar]
- 21.Danad I, Raijmakers PG, Appelman YE, Harms HJ, de Haan S, van den Oever ML et al. Coronary risk factors and myocardial blood flow in patients evaluated for coronary artery disease: a quantitative [15O]H2O PET/CT study. Eur J Nucl Med Mol Imaging 2012;39:102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufmann PA, Camici PG. Myocardial blood flow measurement by PET: technical aspects and clinical applications. J Nucl Med 2005;46:75–88. [PubMed] [Google Scholar]
- 23.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoghbi GJ, Dorfman TA, Iskandrian AE. The effects of medications on myocardial perfusion. J Am Coll Cardiol 2008;52:401–16. [DOI] [PubMed] [Google Scholar]
- 25.Meyer M, Nance JW Jr, Schoepf UJ, Moscariello A, Weininger M, Rowe GW et al. Cost-effectiveness of substituting dual-energy CT for SPECT in the assessment of myocardial perfusion for the workup of coronary artery disease. Eur J Radiol 2012;81:3719–25. [DOI] [PubMed] [Google Scholar]
- 26.Kim SM, Chang SA, Shin W, Choe YH. Dual-energy CT perfusion during pharmacologic stress for the assessment of myocardial perfusion defects using a second-generation dual-source CT: a comparison with cardiac magnetic resonance imaging. J Comput Assist Tomogr 2014;38:44–52. [DOI] [PubMed] [Google Scholar]
- 27.De Cecco CN, Harris BS, Schoepf UJ, Silverman JR, McWhite CB, Krazinski AW et al. Incremental value of pharmacological stress cardiac dual-energy CT over coronary CT angiography alone for the assessment of coronary artery disease in a high-risk population. AJR Am J Roentgenol 2014;203:W70–7. [DOI] [PubMed] [Google Scholar]
- 28.Greif M, von Ziegler F, Bamberg F, Tittus J, Schwarz F, D'Anastasi M et al. CT stress perfusion imaging for detection of haemodynamically relevant coronary stenosis as defined by FFR. Heart 2013;99:1004–11. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Qin L, Shi X, Zeng Y, Jing H, Schoepf UJ et al. Adenosine-stress dynamic myocardial perfusion imaging with second-generation dual-source CT: comparison with conventional catheter coronary angiography and SPECT nuclear myocardial perfusion imaging. AJR Am J Roentgenol 2012;198:521–9. [DOI] [PubMed] [Google Scholar]
- 30.Bettencourt N, Chiribiri A, Schuster A, Ferreira N, Sampaio F, Pires-Morais G et al. Direct comparison of cardiac magnetic resonance and multidetector computed tomography stress-rest perfusion imaging for detection of coronary artery disease. J Am Coll Cardiol 2013;61:1099–107. [DOI] [PubMed] [Google Scholar]
- 31.Choo KS, Hwangbo L, Kim JH, Park YH, Kim JS, Kim J et al. Adenosine-stress low-dose single-scan CT myocardial perfusion imaging using a 128-slice dual-source CT: a comparison with fractional flow reserve. Acta Radiol 2013;54:389–95. [DOI] [PubMed] [Google Scholar]
- 32.Cury RC, Magalhaes TA, Borges AC, Shiozaki AA, Lemos PA, Junior JS et al. Dipyridamole stress and rest myocardial perfusion by 64-detector row computed tomography in patients with suspected coronary artery disease. Am J Cardiol 2010;106:310–5. [DOI] [PubMed] [Google Scholar]
- 33.Cury RC, Kitt TM, Feaheny K, Blankstein R, Ghoshhajra BB, Budoff MJ et al. A randomized, multicenter, multivendor study of myocardial perfusion imaging with regadenoson CT perfusion vs single photon emission CT. J Cardiovasc Comput Tomogr 2015;9:103–12 e2. [DOI] [PubMed] [Google Scholar]
- 34.Feuchtner G, Goetti R, Plass A, Wieser M, Scheffel H, Wyss C et al. Adenosine stress high-pitch 128-slice dual-source myocardial computed tomography perfusion for imaging of reversible myocardial ischemia: comparison with magnetic resonance imaging. Circ Cardiovasc Imaging 2011;4:540–9. [DOI] [PubMed] [Google Scholar]
- 35.George RT, Arbab-Zadeh A, Miller JM, Kitagawa K, Chang HJ, Bluemke DA et al. Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging: a pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ Cardiovasc Imaging 2009;2:174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George RT, Arbab-Zadeh A, Miller JM, Vavere AL, Bengel FM, Lardo AC et al. Computed tomography myocardial perfusion imaging with 320-row detector computed tomography accurately detects myocardial ischemia in patients with obstructive coronary artery disease. Circ Cardiovasc Imaging 2012;5:333–40. [DOI] [PubMed] [Google Scholar]
- 37.George RT, Mehra VC, Chen MY, Kitagawa K, Arbab-Zadeh A, Miller JM et al. Myocardial CT perfusion imaging and SPECT for the diagnosis of coronary artery disease: a head-to-head comparison from the CORE320 multicenter diagnostic performance study. Radiology 2014;272:407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko BS, Cameron JD, Meredith IT, Leung M, Antonis PR, Nasis A et al. Computed tomography stress myocardial perfusion imaging in patients considered for revascularization: a comparison with fractional flow reserve. Eur Heart J 2012;33:67–77. [DOI] [PubMed] [Google Scholar]
- 39.Ko BS, Cameron JD, Leung M, Meredith IT, Leong DP, Antonis PR et al. Combined CT coronary angiography and stress myocardial perfusion imaging for hemodynamically significant stenoses in patients with suspected coronary artery disease: a comparison with fractional flow reserve. JACC Cardiovasc Imaging 2012;5:1097–111. [DOI] [PubMed] [Google Scholar]
- 40.Takx RA, Blomberg BA, El Aidi H, Habets J, de Jong PA, Nagel E et al. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging 2015;8. [DOI] [PubMed] [Google Scholar]
- 41.Ollendorf DA, Kuba M, Pearson SD. The diagnostic performance of multi-slice coronary computed tomographic angiography: a systematic review. J Gen Intern Med 2011;26:307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shreibati JB, Baker LC, Hlatky MA. Association of coronary CT angiography or stress testing with subsequent utilization and spending among Medicare beneficiaries. JAMA 2011;306:2128–36. [DOI] [PubMed] [Google Scholar]
- 43.Berman DS, Hachamovitch R, Shaw LJ, Friedman JD, Hayes SW, Thomson LE et al. Roles of nuclear cardiology, cardiac computed tomography, and cardiac magnetic resonance: noninvasive risk stratification and a conceptual framework for the selection of noninvasive imaging tests in patients with known or suspected coronary artery disease. J Nucl Med 2006;47:1107–18. [PubMed] [Google Scholar]
- 44.Muirhead CR, O'Hagan JA, Haylock RG, Phillipson MA, Willcock T, Berridge GL et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer 2009;100:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation 2007;116:1290–305. [DOI] [PubMed] [Google Scholar]
- 46.Fujita M, Kitagawa K, Ito T, Shiraishi Y, Kurobe Y, Nagata M et al. Dose reduction in dynamic CT stress myocardial perfusion imaging: comparison of 80-kV/370-mAs and 100-kV/300-mAs protocols. Eur Radiol 2014;24:748–55. [DOI] [PubMed] [Google Scholar]
- 47.Kim SM, Kim YN, Choe YH. Adenosine-stress dynamic myocardial perfusion imaging using 128-slice dual-source CT: optimization of the CT protocol to reduce the radiation dose. Int J Cardiovasc Imaging 2013;29:875–84. [DOI] [PubMed] [Google Scholar]