Abstract
Proper synaptic function requires the seamless integration of the transport, assembly, and regulation of synaptic components and structures. Inasmuch as the synapse is often distant from the neuronal cell body, newly synthesized synaptic proteins, the precursors of synaptic vesicles, active zone compartments, channels and receptors, and mitochondria, must be transported along lengthy neuronal processes to participate in synaptogenesis. Neuronal transport is mediated by motor proteins that associate with their cargoes via adaptors (or receptors) and that travel along the cytoskeleton network within the neuronal processes. Thus, the identity of membranous protein cargoes and the specificity of motor-cargo interactions are critical for correctly targeting cargoes and properly assembling synapses in developing neurons and in remodeling synapses of mature neurons in response to neuronal activity. In this article, the authors review recent progress in characterizing microtubule- and actin-based motor proteins that are involved in delivering synaptic components and discuss potential mechanisms underlying the formation of motor- receptor-cargo complexes that contribute to synaptogenesis and activity-induced synaptic plasticity.
Keywords: synaptogenesis, synaptic plasticity, kinesin, myosin, motor adaptor, axonal transport
Neurons in the CNS are highly specialized cells and consist of three distinct functional and subcellular domains: a cell body (or soma), a long axon with uniform diameter, and thick dendrites with many branches. Whereas the soma and dendrites receive and process information, the axon transfers it by generating an action potential. Essential materials are generally synthesized within the soma, and delivered through the neuronal processes to their final destination, that is, synaptic terminals.
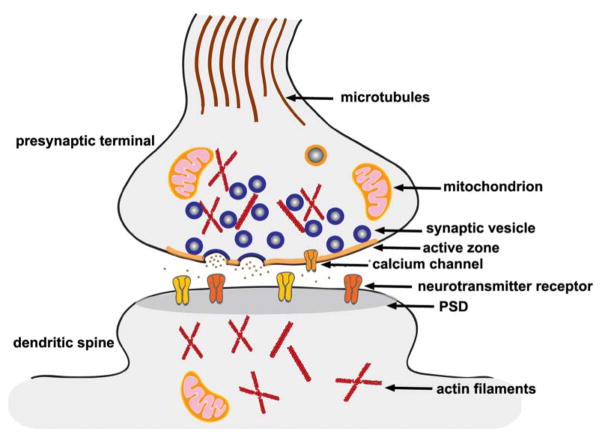
Synapses play a fundamental role in information processing in the CNS by mediating communication between nerve cells. Synaptic junctions are characterized by specialized membrane structures and the accumulation of synaptic vesicles, calcium channels, and neurotransmitter receptors at the presynaptic or postsynaptic sites of cell contact. There are hundreds of neurotransmitter-loaded synaptic vesicles that dock, fuse, and release neurotransmitters at specialized regions of the presynaptic plasma membrane named active zones (AZs). At the AZs, regular arrays of electron-dense proteins are organized and linked together by fine filamentous material known as cytoskeleton matrix (CAZ) or “presynaptic grid.” CAZ is thought to enable the trafficking of synaptic vesicles to their site for fusion with the presynaptic plasma membrane. The two main types of glutamate-receptor channels that open in response to glutamate binding, N-methyl-d-aspartate (NMDA) receptors and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic-acid (AMPA) receptors, are clustered along the postsynaptic density (PSD) characterized as an electron-dense structure at excitatory synapses. Both PSD and CAZ are held together by trans-synaptic adhesion molecules and extracellular matrix proteins (Fig. 1).
Figure 1.
Structural organization of synapses. A synapse is characterized by specialized membrane structures and the accumulation of synaptic vesicles, calcium channels, and neurotransmitter receptors at presynaptic or postsynaptic sites of cell contact. There are hundreds of synaptic vesicles that dock, fuse, and release neurotransmitters at the specialized region of the presynaptic plasma membrane named the active zone. The postsynaptic receptor apparatus is also characterized by an electron-dense thickening referred to as the postsynaptic density.
Synaptogenesis most often occurs as an integration of recognition, adhesion, and protein targeting that begins with axonal guidance to the appropriate brain region and ends with neurotransmitter release opposite the appropriate cluster of neurotransmitter receptors and signaling molecules. Neurons also have the capacity for continued acquisition, attrition, and modification of synapses throughout life. How is the elaborated architecture of the synapse assembled and remodeled? Because the synapse is often distant from the neuronal cell body (up to 1 meter away), newly synthesized synaptic proteins, synaptic vesicle and AZ precursor compartments, channels and receptors, mitochondria, and proteins responsible for the assembly and regulation of vesicle fusion machines must be transported along lengthy neuronal processes to participate in synaptogenesis. Upon arrival at the terminal, cargo-loaded transport vesicles undergo fusion with the plasma membrane to assemble AZs and PSDs and reconstitute synaptic vesicles. Docked and primed synaptic vesicles at AZs are then ready for exocytosis in response to a rise in intracellular [Ca2+] triggered by opening of voltage-gated Ca2+ channels. Proper synaptic function requires the tight coordination of neuronal transport and hardwired assembly. Neuronal transport is mediated by motor proteins that associate with their cargoes via adaptor (or receptor) molecules and that travel along the cytoskeleton network within the neuronal processes. It is certain that stable and dynamic cytoskeletal elements are essential components of synaptic assembly and plasticity. Thus, identity of the membranous protein cargoes and specificity of the motor-cargo interactions are critical for correct targeting of transported cargoes and proper assembly of synapses in developing neurons and remodeling of synapses in mature neurons in responding to neuronal activity.
Organization of Cytoskeleton in Neurons and Its Role in Polarized Transport
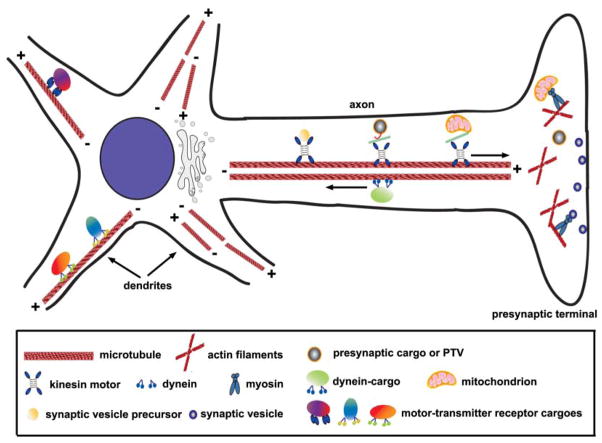
Although microtubules, actin, and intermediate filaments are three major components of the cytoskeleton in neurons, only microtubules and actin filaments play major roles in neuronal transport. Neurons contain an elaborate network of microtubules radiating from the soma into the axonal and dendritic processes. Microtubules are formed from polymerization of heterodimers of α- and β-tubulins, which are oriented with plus and minus ends. In axons, microtubules are uniformly organized with the plus ends facing toward the axonal terminals and the minus ends toward the cell body. However, the organization of microtubules in dendrites is more complex. In the proximal region, there is mixed microtubule polarity, but in the distal dendrites, there is uniform microtubule arrangement similar to that of axons. Microtubular polarity and organization in neurons are critical for the targeted transport of synaptic cargoes and organelles from the soma to synapses by the microtubule-associated motor proteins, which include members of the kinesin superfamily and cytoplasmic dynein. Whereas the kinesin motor proteins are mostly plus end directed, dynein travels toward the minus ends of microtubules. Therefore, the kinesin motors generally mediate anterograde axonal transport of cargoes and dynein drives retrograde axonal transport in neurons (Fig. 2).
Figure 2.
The targeted transport of synaptic cargoes and organelles from the soma to synapses. In axons, microtubules are uniformly organized with the plus (+) ends facing toward the axonal terminals and the minus (−) ends toward the cell body. However, the organization of microtubules in dendrites is more complex. In the proximal region there is mixed microtubule polarity, but in the distal dendrites there is uniform microtubule arrangement similar to that of the axons. Polarity and organization of microtubules in neurons are critical for the targeted transport of synaptic cargoes and organelles by the microtubule-associated motor proteins. Whereas the kinesin motor proteins are mostly plus end directed, dynein travels toward the minus ends of microtubules. Therefore, the kinesin superfamily motors generally mediate anterograde axonal transport of cargoes, and dynein drives retrograde axonal transport in neurons.
In contrast, cellular compartments essential for synaptic function such as presynaptic terminals and dendritic spines have few, if any, microtubules. Thus, actin-associated motors, myosins, are likely to contribute to local or short-distance transport within these compartments. Actin is present in monomeric (G-actin; globular actin) and filamentous (F-actin) forms. F-actin forms bundles and networks that are components of the neuronal actin cytoskeleton. Both presynaptic terminals and dendritic spines contain actin filaments, but their organization in these compartments has not been fully elucidated. Actin filament orientation can directly affect myosin-dependent transport activity (Bridgman 2004).
A “dual transport” model has been proposed in which long-range vesicle transport is microtubule based and short-range movement is actin based (Langford 2002; Bridgman 1999). Microtubule-based motors would ensure long-range transport, whereas myosins could allow local trafficking of organelles on F-actin in areas devoid of microtubules, such as at nerve terminals, growth cones, and subcortical plasma membrane regions. The physical interaction between microtubule- and actin-based motors has been identified, which supports the idea that they could coexist as a motor complex in transporting cargoes or organelles and drive a coordinated movement along both cytoskeletal systems (Langford 2002; Bridgman 2004; Fig. 3).
Figure 3.
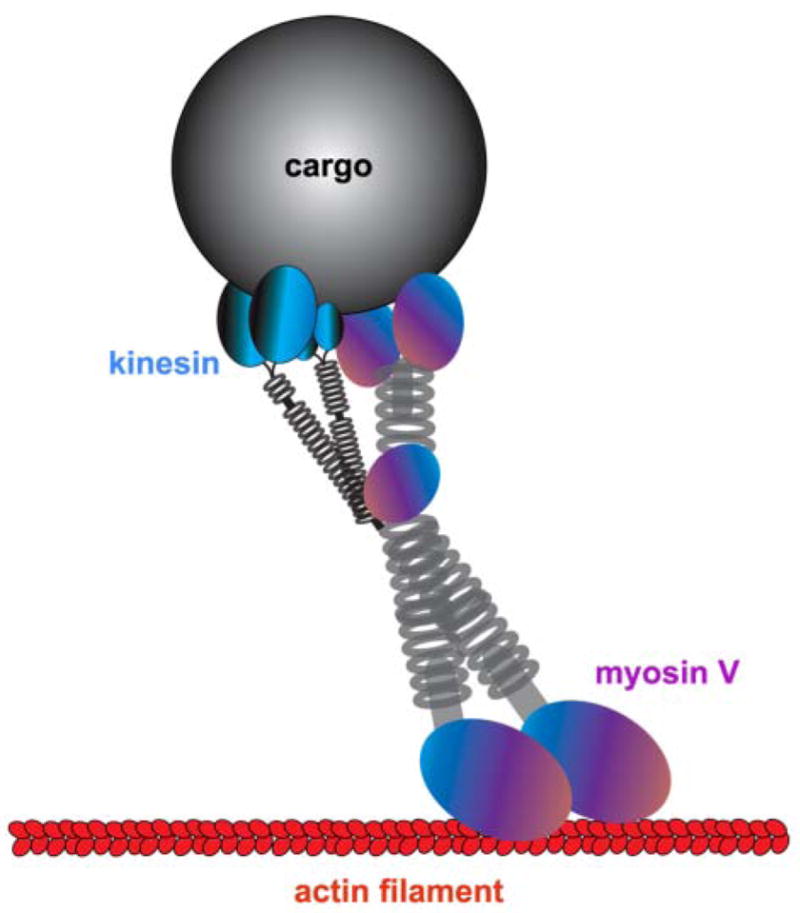
A “dual transport” model for the kinesin/myosin V het-eromotor complex attached to a cargo. Myosin V is shown in an active state with the heads (motor domains) engaging with an actin filament and kinesin is in an inactive state. The microtubule-based motors, kinesins and dynein, drive long distance transport along axons and dendrites, whereas myosins allow local and short-range movement of organelles on F-actin in areas devoid of microtubules, such as at nerve terminals, growth cones, and subcortical plasma membrane regions. The physical interaction of microtubule- and actin-based motors supports the idea that they could exist in multi-functional complexes transporting organelles along both cytoskeletal systems in a coordinated fashion in neurons. Modified from Langford 2002.
Motor Protein Families and Their Transport Modes in Neurons
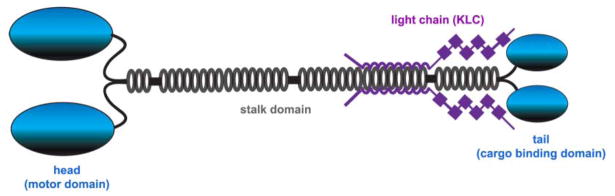
Kinesins are efficient motors that drive the anterograde transport of cargoes a long distance along the axon. Kinesin superfamily genes (kif) in the mammalian and human genomes have been systematically identified, 38 of which are expressed in the brain (Hirokawa and Noda 2008). Kinesin-1, also known as conventional kinesin, was the first identified motor protein (Vale and others 1985; Hirokawa and others 1991). The heavy chain of kinesin-1 consists of three closely related subtypes: KIF5A, KIF5B, and KIF5C. Although KIF5B is ubiquitously expressed, KIF5A and KIF5C are relatively enriched in neurons (Kanai and others 2000). KIF5 motors form homo- or heterodimers among themselves through the coiled-coil region in the stalk domains. Although KIF5 itself possesses the motor function, it also binds to the light chain of kinesin-1 (KLC) through its domain located at the stalk and tail regions (Hirokawa and Noda 2008). Therefore, the specific association of KIF5 with cargoes can be mediated either directly through the specific cargo-binding region in its tail domain, or indirectly via the COOH-terminal domains of KLC, indicating the existence of two forms of KIF5 motor-cargo coupling (Fig. 4).
Figure 4.
KIF5 motors form homodimers among themselves through the coiled-coil region in the stalk domains. Although KIF5 itself possesses the motor function, it also binds to the light chain of kinesin-1 (KLC) through its domain located at the stalk and tail regions. The specific association of KIF5 with cargoes can be mediated either directly through the cargo-binding region in its tail domain, or indirectly via the COOH-terminal domains of KLC, indicating the existence of two forms of KIF5 motor-cargo coupling. Modified from Schnapp 2003.
Cytoplasmic dynein is the major motor driving retrograde transport along microtubules in axons. The accessory or activator complex, dynactin, is required for most dynein functions, such as cargo attachment and progression (King and Schroer 2000; Karki and Holzbaur 1999). Probable candidates for powering short-range movements on the actin filaments are unconventional myosins, such as Va, VI, and VII. Myosin V is a two-headed motor that contains a unique globular tail domain and goes through multiple steps before dissociating from an actin filament (Langford 2002). Myosin V is associated with different nonmembranous cargoes, such as neurofilament NF-L subunit, and membrane-enclosed organelles that include synaptic vesicles, endoplasmic reticulum, secretory granules, and other axonal vesicles. However, it is unclear how much or to what degree synapse-directed transport of organelles or protein complexes is required for myosins or if myosins play unique roles in remodeling synapses in response to neuronal activity or under certain physiological states. With the exception of myosin V, little is known about the localization and function of other unconventional myosins and their roles in neuronal transport (Bridgman 2004).
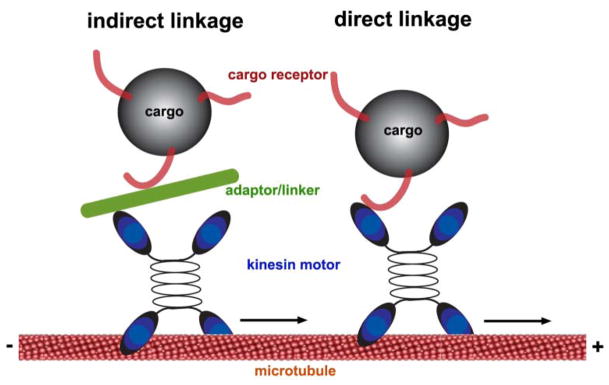
Microtubule-associated motors consist of two functional parts: a motor domain that interacts directly with microtubules and converts the energy of ATP hydrolysis into movement along microtubules; and a tail domain that interacts with intracellular cargoes directly or through accessory light chains. Linkage of cargo vesicles with the appropriate transport motors must occur with a high degree of specificity to preserve organelle identity and the proper targeting and progression of transported vesicles within cells. There are at least two mechanisms through which motors connect with their cargoes: a direct linkage through cargo receptors and an indirect linkage via linker/adaptor molecules. Recent studies indicate that the indirect coupling of motors with transport cargoes through specific adaptors is a significant important mechanism in neurons (Goldstein and Yang 2000; Fig. 5).
Figure 5.
Linkage of cargo vesicles with the appropriate transport motors. There are at least two mechanisms through which motors connect with their cargoes: a direct linkage through cargo receptors and an indirect linkage via linker/adaptor molecules.
There are two main classes of axonal transport observed in neurons: fast (~200–400 mm/d) and slow (~0.2–1 mm/d) (Brown 2003). Fast axonal transport mediates the delivery of many axonal and synaptic components, including synaptic vesicle precursors, AZ precursors known as Piccolo/Bassoon transport vesicles, mitochondria, and APP. Slow axonal transport is thought to move elements of the cytoskeleton, such as subunits of neurofilments, α- and β-tubulin subunits of microtubules, and proteins such as cytosolic enzymes, clathrin, and actin. Because of the narrow width and extreme length of neuronal processes, axonal transport is usually coupled with moving a large volume of different cargoes. Any deleterious event could result in a traffic jam in axons and potentially lead to initiation and development of axonal degeneration.
Kinesin Motors and Their Cargo Adaptors Contribute to Presynaptic Assembly and Remodeling
The formation of new synapses and remodeling of existing synapses requires targeted delivery of synaptic components to the axo-dendritic contact sites, a process that begins with the transport of synaptic carrier vesicles along the secretory pathway (Horton and Ehlers 2004). Presynaptic membrane proteins involved in synaptic vesicle exocytosis and the assembly of AZs are synthesized in the soma of neurons, incorporated into cargo vesicles, and undergo transport along microtubules to the synaptic terminal. Time-lapse imaging studies in cultured neurons have shown that vesicles of variable sizes and shapes accumulate at synapses (Nakata and others 1998). For example, observations of large fluorescent motile puncta in cultured neurons demonstrated a diversity of vesicles and tubulo-vesicular elements containing synaptic vesicle proteins and calcium channels (Ahmari and others 2000). In addition, an 80-nm dense-core granule named Piccolo/ Transport vesicle (PTV), which contains Piccolo, Bassoon, syntaxin, and SNAP-25, is transported to nascent synapses and participates in AZ assembly (Zhai and others 2001). These studies raise questions about which kinesin motor proteins are involved and how different classes of transport vesicles are sorted or linked to their transport motors. Recent identification of the linker/adaptor-motor complexes has advanced our current knowledge of the trafficking of synaptic cargoes and provided clues to elucidate the cellular mechanisms by which synaptic cargo organelles physically associate with their cognate transport motors.
Among the kinesin motor superfamily (KIFs), KIF1A is a brain-specific motor and was first identified as Unc-104 in Caenorhabditis elegans. Its mutation causes a deficiency of synaptic vesicles in axons (Hall and Hedgecock 1991). In kif1A-deficient mice, functional depletion of KIF1A motor leads to severe motor and sensory abnormalities, and a reduced number of synaptic terminals and density of synaptic vesicles at nerve terminals of approximately 50% to 60% of what was seen in wild-type mice (Yonekawa and others 1998). This observation suggests the existence of complementary motor proteins that are also involved in the intracellular transport of synaptic vesicle precursors. One candidate is KIF1Bβ, an isoform generated by alternative splicing of the gene encoding KIF1Bα. Glutathione S-transferase (GST)-pulldown assay and immunoisolation using an anti-KIF1Bβ antibody revealed that KIF1Bβ transports synaptic vesicle precursors containing synaptotagmin, synaptophysin, and SV2 (Zhao and others 2001). kif1B knockout mice, in which neither KIF1Bα nor KIF1Bβ was expressed, exhibit reduced density of synaptic vesicles by approximately 50% to 60%, suggesting a partially overlapping function with that of KIF1A.
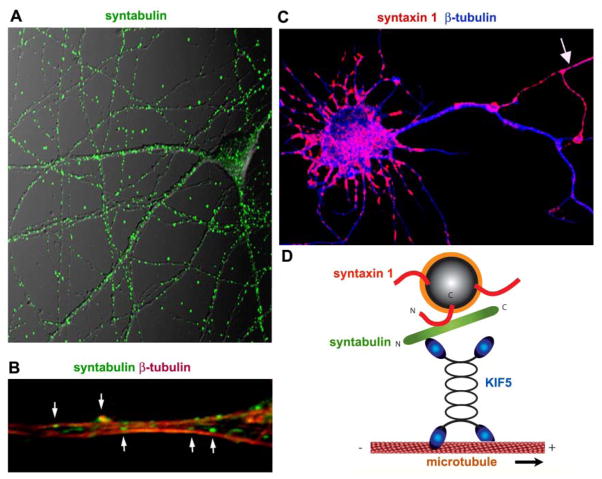
KIF5 proteins play essential roles in axonal transport. Whereas synaptic vesicle precursors can be carried by monomeric motors, such as KIF1A and KIF1Bβ, the neuronal SNARE proteins syntaxin-1 and SNAP-25, which mediate the fusion of synaptic vesicles with the presynaptic plasma membrane, have been shown to be transported by KIF5 motors. SNAP-25 can interact directly with the cargo-binding domain of KIF5, suggesting that this interaction might involve the trafficking of SNAP-25 in neurons (Diefenbach and others 2002). Identifying syntabulin as a syntaxin-1– and KIF5-binding protein opens a new avenue in elucidating the cellular mechanisms that underlie synaptic cargo transport (Su and others 2004). Syntabulin attaches syntaxin-1–containing vesicles to microtubule-based KIF5 for trafficking within hippocampal neuron processes. Conversely, knockdown of syntabulin expression with RNAi or disruption of the syntabulin-syntaxin interaction by expressing the dominant negative binding domain transgene reduces syntaxin-1 distribution in neuronal processes. Syntabulin-mediated syntaxin-1 trafficking is independent of KLC because syntabulin binds directly to the carboxyl terminal cargo-binding domain of KIF5. These findings suggest that syntabulin acts as a linker/adaptor molecule that attaches syntaxin cargoes to the kinesin motors, enabling syntaxin-1 transport to neuronal processes (Fig. 6).
Figure 6.
Syntabulin attaches syntaxin-1–containing vesicles to KIF5 (KHC) for trafficking within the processes of hippocampal neurons. (A) Endogenous syntabulin in cultured hippocampal neurons appears as vesicular shapes and is widely distributed in both the soma and along the developing neuronal processes. (B) By costaining neurons with β-tubulin, vesicular structures of syntabulin are attached to or aligned along the surface of microtubules within neuronal processes. (C) Loss of function of syntabulin in neurons results in accumulation of syntaxin-1 staining puncta in the cell body, and a marked reduction of syntaxin-1 distribution in neuronal processes relative to that from untransfected neurons (pointed by an arrow). (D) Syntabulin is thought to act as a linker/adaptor molecule that attaches syntaxin cargoes to the kinesin motors, enabling transport of syntaxin-1 to neuronal processes.
Both syntaxin-1 and SNAP-25 reside in AZ precursor PTVs, which travel along axons and participate in the assembly of presynaptic terminals. A recent study further demonstra- ted that the syntaxin-1–syntabulin–KIF5 transport complex mediates the anterograde axonal transport of AZ precursor vesicles. Functional depletion of syntabulin leads to reduced axonal trafficking of PTVs, and consequently disrupts presynaptic assembly in developing neurons (Cai and others 2007).
Consistent with the findings from the mammalian system, evidence from Drosophila and C. elegans indicate that synaptic vesicle precursors are transported to nerve terminals by the kinesin-3 family, which includes KIF1A, Unc-104, and the newly characterized immaculate connections (imac; Hall and Hedgecock 1991; Zhao and others 2001). In Drosophila motor neurons lacking imac, few AZs were found at the endings, suggesting that the Imac motor transports essential components required for presynaptic maturation (Pack-Chung and others 2007). A recent study further demonstrated that Unc-104 is a major contributor to the anterograde fast transport of neuropeptide-filled vesicles as well as synaptotagmin-bearing vesicles in Drosophila motor axons (Barkus and others 2008). In Drosophila, kinesin-1 has been proposed as a predominant motor candidate for synaptic vesicle transport (Hurd and others 1996). In khc mutant larvae, various vesicles and organelles, including synaptic vesicle markers, are filled within axonal swellings. In addition, the mutation of Unc-116 in C. elegans, which forms a complex with KLC-2 orthologous to kinesin-1, alters the localization of cargoes containing synaptic vesicle markers. These findings further support a role for kinesin-1 in transporting or localizing synaptic vesicle components.
Whereas KIF1A and Unc-104 appear to be specifically involved in transporting synaptic vesicle precursors, multiple motors were found to be responsible for carrying CAZ components. Mammalian Liprin-α binds to the Unc-104/ KIF1A motor and Drosophila Liprin-α binds to kinesin-1, which was proposed to transport a subset of CAZ proteins. In contrast to the mammalian system, disruption of either motor in the C. elegans and Drosophila nervous systems only mildly perturbs localization of CAZ components, indicating that each motor partially contributes to the transport of Liprin-α and CAZ proteins (Miller and others 2005; Pack-Chung and others 2007). In Drosophila and C. elegans, this dependency of synaptic components on multiple motors suggests the heterogeneous nature of the transport complexes destined for the presynaptic terminals.
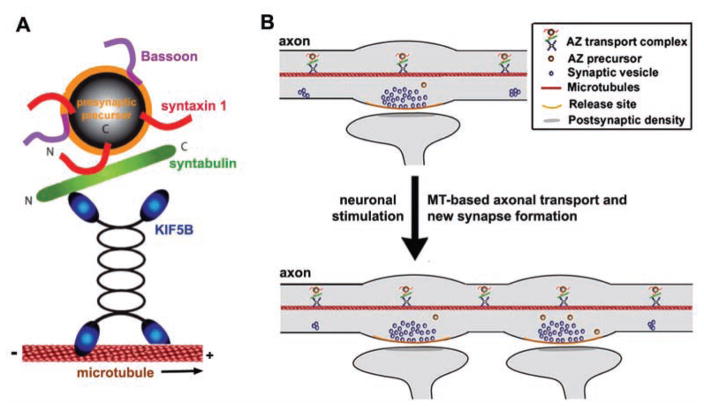
Activity-dependent synaptic plasticity includes subcellular target selection, synapse formation, and modulation of neuronal circuits (Katz and Crowley 2002). Long-term plasticity represents both functional and morphological synaptic changes with contributions from both pre- and postsynaptic mechanisms particularly during neuronal development (Bailey and Kandel 1993). Remodeling preexisting synapses and forming new synapses might play important roles in the various forms of synaptic plasticity of complex neuronal networks. Synaptic reorganization of the local actin network contributes to integrating molecular and morphological changes associated with activity-dependent synaptic plasticity (Zhang and Benson 2001; Shen and others 2006; Yao and others 2006). However, the mechanism of microtubule- based axon transport and the extent to which it contributes to the activity-induced formation of new synapses in developing neurons remain largely unknown. A recent study provided cellular evidence for the first time that syntabulin-KIF5–mediated anterograde transport of PTVs contributes to activity-induced presynaptic plasticity during neuronal development. Disruption of syntabulin-KIF5 coupling reduces both the amplitude of postsynaptic currents and the frequency of asynchronous quantal events, and also abolishes the activity-induced recruitment of new PTVs into the axons and subsequent coclustering with synaptic vesicles. Consequently, syntabulin loss of function blocks formation of new presynaptic boutons during activity-dependent synaptic plasticity in developing neurons. These studies establish that kinesin-mediated anterograde axonal transport is another critical factor for the cellular mechanism underlying activity-dependent presynaptic plasticity (Cai and others 2007; Fig. 7).
Figure 7.
The microtubule (MT)-based axonal transport contributes to the new synapse formation and presynaptic plasticity. (A) The syntaxin-syntabulin-KIF5B complex is MT-based anterograde transport machinery. Syntaxin-1 serves as a receptor on the active zone (AZ) transport vesicles and syntabulin acts as an adaptor capable of conjoining syntaxin-1 and the KIF5B motor, thereby mediating trafficking of the AZ transport carriers. (B) Through this motor-adaptor complex, AZ precursor carriers traverse along the axon to nascent synapses, participate in the formation of AZs, and consequently contribute to the activity-induced presynaptic plasticity in developing neurons. Reprinted from Cai and others 2007 with permission.
Kinesin Motors and Their Cargo Adaptors Contribute to Postsynaptic Assembly and Plasticity
Compared with presynaptic terminal assembly, the mechanisms of assembling the postsynaptic compartments are much less well characterized. Postsynaptic structures contain mainly neurotransmitter receptors, non-ligand-triggered ion channels, transporters, pumps, and associated scaffolding proteins. In addition, Golgi-related structures and different membranous carriers are distributed along dendrites and are present postsynaptically (Sytnyk and others 2002; Horton and Ehlers 2004). The earliest event in organizing the post-synaptic specialization is the appearance of NMDA receptors and PSD95 clusters that can serve as molecular scaffolds opposite the presynaptic specialization (McGee and Bredt 2003). Accumulation of AMPA receptors follows PSD95 cluster formation (Friedman and others 2000).
Kinesins are also important in dendritic transport. Although some motors, such as KIF5, are distributed in both axons and dendrites, KIF17, a member of the kinesin-2 family, is relatively enriched in dendrites. KIF17 is essential in dendritic trafficking of the NMDA receptor 2B (NR2B) through its interaction with a PDZ domain of mLin-10, one component of a multiprotein complex also including mLin-2, mLin7, and NR2B (Setou and others 2000). This interaction was further confirmed by immunoprecipitation and an in vitro reconstitution system (Setou and others 2000). Mutant KIF17 lacking its mLin-10–binding domain retained motor activity but could not transport and deliver NR2B-positive vesicles, indicating that mLin-10 binding is essential for KIF17-dependent vesicle movement (Setou and others 2000). NR2B can be detected in immunoisolated vesicles from the brain with an antibody against KIF17 and moved by KIF17 toward the plus ends of microtubules. In cultured neurons, KIF17 is mainly located in the cell body and dendrites and colocalized with NR2B, but not with PSD95 (Guillaud and others 2003). Suppress- ing KIF17 expression or its function using antisense oligonucleotides or dominant-negative mutants resulted in a reduced density of NR2B clusters, whereas NR2A and NR2C, which do not form complexes with KIF17, were either up-regulated (NR2A) or unaffected (NR2C; Guillaud and others 2003). In the same study, functional blocking of KIF17 decreased the number of NR2B-containing synapses, suggesting that KIF17 plays a crucial and specific role in transporting NR2B to the postsynaptic terminal. KIF17 transgenic mice showed significantly better performance in behavioral tests, such as the Morris water maze tasks for working and spatial memory. The results indicate that enhanced dendritic transport of NR2B may increase synaptic clustering of NR2B, elevate synaptic transmission, and in turn, facilitate learning and memory.
KIF5 was reported to transport the GluR2 subunit of the AMPA receptor in dendrites. Using the cargo-binding domain of KIF5 as bait in yeast two-hybrid assays, the glutamate receptor interacting protein 1 (GRIP1) was identified, which is also known to bind to the GluR2 (Setou and others 2002). The complex of GRIP1, KIF5, and GluR2 was demonstrated by an immunoprecipitation assay. Overexpression of the KIF5 dominant-negative construct lacking the motor domain reduced the density of GluR2 at synapses, suggesting the role of KIF5 in the dendritic trafficking of GluR2. In addition, suppressing GRIP1 expression with siRNA caused a loss of dendrites, a phenotype rescued by overexpressing EphB2, a receptor tyrosine kinase (RTK) implicated in dendritic spine development and synaptic plasticity (Hoogenraad and others 2005). Functional disruption of the GRIP1-KIF5 interaction impaired EphB2 trafficking to dendrites and inhibited dendritic growth supporting the idea that GRIP1 acts as an important kinesin adaptor required for the transport of multiple postsynaptic components and dendritic development (Setou and others 2002; Hoogenraad and others 2005). PSD95 (a PSD scaffolding protein that binds to NMDA receptors) selectively interacts with KIF1Bα, suggesting that the interaction may play a role in trafficking constituents of the postsynapse, although the mechanism remains unclear (Mok and others 2002). KIFC2 is a member of the C-kinesin or kinesin-14 family and localizes to the somatodendritic compartment where it plays a role in retrograde trafficking of multivesicular body-like organelles in dendrites (Saito and others 1997). However, there is still no functional evidence for this motor protein in dendritic transport (Goldstein and Yang 2000).
During synaptogenesis, transport of NR2B-containing NMDA receptors by KIF17 and GluR2/3-containing AMPA receptors by KIF5 through different scaffolding complexes contributes to recruiting these components to postsynaptic sites (Setou and others 2000, 2002). Although NMDA and AMPA receptor transport complexes are independent from those containing PSD95, other data indicate that NMDA receptors may traffic to the synapse with the PSD95 family of proteins (Li and Sheng 2003). Furthermore, AMPA receptors can be recruited from the diffuse plasma membrane pool by lateral migration. Moreover, AMPA receptors are present in a cytoplasmic vesicular pool that participates in the rapid modulation of synaptic AMPA receptor numbers by an exo-endocytic mechanism (Malinow and Malenka 2002). Accumulation of other postsynaptic components, such as calcium calmodulin-dependent protein kinase II (CaMKII), Homer1c, and Shank2/3, occur by regulated trapping of local pools rather than by active vesicle transport (Li and Sheng 2003). Local protein synthesis may also contribute to postsynapse assembly where the mRNA is abundant in dendrites, such as CaMKIIα, Shank, NR1, and GluR1/2 (Steward and Schuman 2001). KIF5 was recently identified as the motor for transporting specific mRNAs in dendrites (Kanai and others 2004). Because local protein synthesis has been implicated as a requirement for synapse formation and maintenance, further investigation of mRNA transport in dendrites is needed to clarify its functional correlation with dendritic development and potential regulatory mechanism in response to neuronal activity.
Actin-Based Myosin Motors in Synaptic Maturation and Remodeling
It is certain that actin plays a critical role in synaptic assembly. Actin is concentrated at developing presynaptic terminals in which the reserve pool of synaptic vesicles is tethered to the F-actin cytoskeleton via synapsin I (Hirokawa and others 1989). This synapsin I–dependent coupling of synaptic vesicles to the actin filaments may contribute to the delay in synaptogenesis observed in synapsin I–deficient mice (Chin and others 1995). Actin is also a major structural component of PSDs, where the actin filaments anchor adhesion molecules, and a population of postsynaptic receptors and signaling molecules opposite presynaptic terminals. It has been shown that synaptogenesis depends on actin dynamics during early development of cultured neurons (Zhang and Benson 2001). Increased synaptic activity can induce the assembly and stabilization of F-actin and synaptic proteins, which consequently facilitates formation of new active presynaptic terminals (Zhang and Benson 2001; Yao and others 2006; Shen and others 2006).
Whereas the microtubule-based motor proteins kinesin and dynein transport synaptic cargoes and organelles quickly through lengthy axons and dendrites, myosins drive short-distance trafficking along the actin filaments in dendritic spines and presynaptic terminals. Myosin V was found to associate with synaptic vesicles in rat cerebrocortical synaptosomes and co-immunoprecipitated with the synaptic vesicle proteins synaptobrevin II and synaptophysin. Inhibition of myosin ATPase activity reduced neurotransmitter release in brain slices (Miller and Sheetz 2000; Bridgman 1999). The association between myosin V and synaptic vesicle proteins also suggests that myosin V may regulate synaptic vesicle trafficking within presynaptic terminals. Myosin V has also been implicated in the transport of other vesicle populations, including those associated with the axonal smooth ER (Langford 2002). Myosin V can interact directly with either a kinesin motor to form a hetero-motor complex or with the 8-kDa light chain of dynein (Naisbitt and others 2000), raising the possibility that the dual motor complexes may facilitate coordination of long-range movement along microtubules and short-range movement on actin filaments (Fig. 3).
Myosin V has also been shown to associate with the PSD protein complex and binds to GKAP through the 8-kDa dynein light chain (Naisbitt and others 2000). GKAP interacts with PSD95, which is required for anchoring glutamate receptors at postsynaptic membranes. In addition, myosin V can transport or anchor smooth ER in dendritic spines of Purkinje cells (Takagishi and others 1996). Thus, myosin V may have a role in regulating long-term changes in postsynaptic responses by modulating the location of proteins or organelles. Myosin VI binds to SAP97, which is a binding partner for the GluR I subunit of the AMPA-type glutamate receptor (Wu and others 2002). The functional significance of this interaction indicates a role in glutamate receptor recycling. SAP97 can also interact with GKAP, suggesting that myosin VI and myosin V may function together to regulate the movement of SAP97-containing cargoes along actin filaments.
Mitochondrial Trafficking and Synapse Formation and Synaptic Plasticity
Mitochondria in the cell bodies of neurons are transported down neuronal processes in response to changes in the local energy state and metabolic demand. Mitochondria accumulate in the vicinity of active growth cones in developing neurons (Morris and Hollenbeck 1993) and are present at synaptic terminals (Shepherd and Harris 1998). Mitochondrial transport and distribution among synapses are directly correlated with axonal growth, spine density, synapse formation, and synaptic function (Morris and Hollenbeck 1993; Li and others 2004; Stowers and others 2002; Lee and Peng 2008; Verstreken and others 2005; Guo and others 2005; Kang and others 2008). Mitochondria are thought to produce more than 90% of cellular ATP, which might specifically support mobilization of synaptic vesicles during intense neuronal activity and the assembly of actin cytoskeleton among synapses (Verstreken and others 2005; Lee and Peng 2008). In addition to the aerobic ATP production, mitochondria have been implicated in certain forms of short-term synaptic plasticity by buffering Ca2+ at synapses (Tang and Zucker 1997). Loss of mitochondria from axonal terminals in Drosophila results in impaired synaptic transmission (Stowers and others 2002; Guo and others 2005; Verstreken and others 2005). Defective transport of axonal mitochondria is also implicated in human neurological disorders and neurodegenerative diseases (Chan 2006).
Whereas cytoplasmic dynein is the driving force behind retrograde movement of mitochondria, KIF5 is the major motor protein for anterograde transport of axonal mitochondria (Stowers and others 2002; Cai and others 2005; Glater and others 2006). Targeted disruption of kif5b resulted in abnormal perinuclear clustering of mitochondria instead of spreading throughout the cytoplasm and toward the cell periphery in undifferentiated extraembryonic cells from mice (Tanaka and others 1998). Using immunocytochemistry and subcellular fractionation assays, KIF1Bα has also been found to colocalize with mitochondria. KIF1Bα can transport purified mitochondria along microtubules in vitro, suggesting that it might be another kinesin motor protein involved in mitochondrial trafficking (Nangaku and others 1994). Although the mutation of kif1B leads to peripheral neuropathies in these animals, a detailed role for KIF1Bα in anterograde mitochondrial transport in neurons still remains unclear.
The Drosophila protein Milton was the first to be identified as the mitochondrial adaptor for KIF5 and is required for axonal transport of mitochondria. The milton mutation in Drosophila results in mitochondrial loss at synaptic terminals and axons (Stowers and others 2002). In addition to its role in linking syntaxin-containing vesicles to KIF5 (Su and others 2004), syntabulin also associates with mitochondria in vivo and links these organelles to KIF5B. This association mediates mitochondrial trafficking along neuronal processes and consequently contributes to proper distribution of mitochondria in neurons (Cai and others 2005). Furthermore, biochemical and genetic evidence demonstrates that kinesin recruitment and mitochondrial transport are independent of kinesin light chain KLC. Although the carboxyl terminal of syntabulin can directly associate with mitochondria (Cai and others 2005), Milton and miro, the mitochondrial Rho-like GTPase, likely form an essential complex that links the KIF5 motor to mitochondria for anterograde axonal transport (Glater and others 2006). Although Milton and syntabulin are crucial for the proper distribution of mitochondria in neurons, it is unlikely that both proteins regulate mitochondrial trafficking via the same mechanism. First, syntabulin directly binds to the cargo-binding domain of KHC (Su and others 2004), but a direct interaction between KHC and Milton has not been observed (Stowers and others 2002). Second, syntabulin is a peripheral membrane-associated protein and associates with mitochondria via its carboxyl-terminal tail, which has the predicted hydrophobic anchor flanked by three positively charged residues at both ends, a similar structure conserved in most outer mitochondrial membrane proteins. However, the question regarding how these adaptor-motor couplings are regulated in response to synapse formation and synaptic activity remains to be addressed. Identification of mitochondrial receptors or adaptor-motor complexes provides targets for further investigation into the molecular and cellular details of how mitochondrial trafficking and distribution are regulated in neurons.
Mitochondria in axons and dendrites display distinct motility patterns and undergo saltatory bidirectional movement, where mitochondria frequently stop, start moving again, and change direction. Although approximately one-third of axonal mitochondria are mobile at instantaneous velocities of 0.3 to 2.0 μm/sec in mature neurons, a large proportion remains stationary. Their net movement is significantly influenced by recruitment to stationary or motile states (Hollenbeck 1996). Such complex mobility patterns suggest that axonal mitochondria might be coupled to two opposing motors (kinesin and dynein) and docking machinery. Efficient control of mitochondrial docking at particular sites of axons and synapses in response to cellular processes and synaptic stimuli is likely essential for neuronal development and synaptic function (Chada and Hollenbeck 2004).
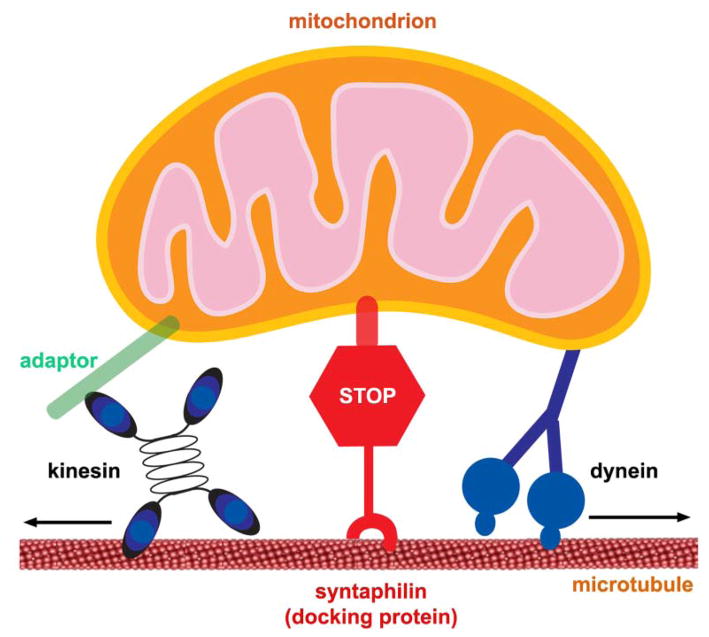
A recent study reveals a role for axon-targeted syntaphilin in mitochondrial docking through its interaction with microtubules. Axonal mitochondria that contain exogenously or endogenously expressed syntaphilin lose mobility. Deletion of the mouse syntaphilin gene results in a substantially higher proportion of axonal mitochondria in the mobile state and reduces the density of mitochondria in axons and at presynaptic boutons. By affecting calcium signaling at presynaptic boutons, the syntaphilin mutant neurons exhibit enhanced short-term facilitation during prolonged stimulation. This phenotype is fully rescued by reintroducing the syntaphilin gene into the mutant neurons. These findings demonstrate a molecular mechanism for controlling mitochondrial docking in axons that has a physiological impact on synaptic function (Kang and others 2008). Presynaptic structure and function are highly plastic and undergo spontaneous and activity-dependent remodeling, thereby changing the demand for mitochondria in axons and at nerve terminals. Mitochondrial balance between the motile and stationary phases is a possible target of regulation by intracellular signals and synaptic activity (Fig. 8). How are motile mitochondria recruited to the syntaphilin-dependent stationary pool in response to neuronal activity and synaptic modification? Identification of syntaphilin as a docking protein provides a molecular target for such regulation. Future studies using the syntaphilin knockout mouse model will provide molecular and cellular details on how syntaphilin regulates mitochondrial motility, and thus, presynaptic formation and function.
Figure 8.
Axonal mitochondria are coupled to two opposing motors (kinesin and dynein) and docking machinery (syntaphilin). Mitochondria in axons display distinct motility patterns and undergo saltatory bidirectional movements where they stop and start moving, frequently changing direction. Efficient control of mitochondrial mobility and their docking at particular sites of axons and synapses is likely essential for neuronal development and synaptic function. Mitochondrial balance between motile and stationary phases is a possible target of regulation by intracel-lular signals and synaptic activity.
Mitochondrial membrane dynamics and movement into postsynaptic spines are also necessary for synaptic plasticity (Li and others 2004). An increased number of mitochondria caused by expressing the mitochondrial fission protein Drp-1 in neurons results in an increased number of dendritic spines and synapses. In contrast, expression of mitochondrial fusion protein OPA1 or the dominant negative version of Drp-1 leads to fewer spines and synapses. In addition, Bcl-2 family members are also involved in stimulating synapse formation through regulating Drp-1 to alter mitochondrial number and function at synapses (Li and others 2008; Liu and Shio 2008). Local ATP supply is essential in the assembly of F-actin cytoskeleton, which involves clustering of synaptic vesicles at the developing presynaptic specialization (Lee and Peng 2008) and plays a prominent role in synapse dynamics. In Drosophila neuromuscular junctions (NMJs), the local ATP supply from mitochondria supports the translocation of synaptic vesicles from the reserved pool (RP) to the readily releasable pool (RRP) via an actin-based motor protein myosin light chain (MLC) along F-actin cytoskeletal tracks (Verstreken and others 2005; Kuromi and Kidokoro 2005). However, further investigation is needed to learn the role of the complex set of molecular motors involved mitochondrial movement along the actin cytoskeleton during synaptogenesis.
Conclusions and Perspectives
Recent emerging evidence provides molecular and genetic clues that microtubule- and actin-dependent organelle/vesicle transport plays a significant role in synapse development and remodeling. It is also clear that microtubules function as tracks for long-distance movement along axons and dendrites whereas actins function as tracks for the local short-range movement and positioning of organelles/vesicles at synapses. Genomics has provided us with a catalog of microtubule-dependent motors and associated components. However, we do not yet fully understand how motor molecules recognize and bind to their specific cargoes, how they determine the transport route and identify the destination for their cargoes, and how they release their cargoes or switch from microtubules to actin filaments at synapses. In addition to the kinesin motor protein superfamily, myosin and dynein motors are also actively involved in neuronal transport, suggesting that multiple motors coordinate transport along microtubule and actin cytoskeletons to bring about the full repertoire of transport and targeting activities in neurons. Formation of synaptic sites is a dynamic and elaborate process that transports and incorporates different synapse-targeted proteins into nascent synapses. It is still unknown how the motor-adaptor complexes modulate the rates of microtubule-based transport and how the transport events contribute to synaptic assembly and in response to synaptic alteration. Despite some progress in characterizing the carriers and associated transport machineries, their subcellular origins remain unclear. Further analyses of the synaptic cargoes is still required to understand the time course of the accumulation of synaptic proteins at nascent synapses as well as the selected delivery of these synaptic proteins to particular types of synapses. It is hoped that these and other questions will be addressed through future research aimed at identifying new cargoes, their linked motor transport complexes, the physiological regulation of their motility and synaptic targeting, and their contribution to synaptic assembly.
Acknowledgments
The authors thank the members of the Sheng lab for helpful discussions, and D. Glazer-Schoenberg and A. Simone for editing. This work was supported by the Intramural Research Program of NINDS, NIH (Z-HS).
References
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–51. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Barkus RV, Klyachko O, Horiuchi D, Dickson BJ, Saxton WM. Identification of an axonal kinesin-3 motor for fast anterograde vesicle transport that facilitates retrograde transport of neuro-peptides. Mol Biol Cell. 2008;19:274–83. doi: 10.1091/mbc.E07-03-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman PC. Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex. J Cell Biol. 1999;146:1045–60. doi: 10.1083/jcb.146.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman PC. Myosin-dependent transport in neurons. J Neurobiol. 2004;58:164–74. doi: 10.1002/neu.10320. [DOI] [PubMed] [Google Scholar]
- Brown A. Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J Cell Biol. 2003;160:817–21. doi: 10.1083/jcb.200212017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Gerwin C, Sheng ZH. Syntabulin-mediated anterograde transport of mitochondria along neuronal processes. J Cell Biol. 2005;170:959–69. doi: 10.1083/jcb.200506042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Pan PY, Sheng ZH. Syntabulin-kinesin-1 family member 5B-mediated axonal transport contributes to activity-dependent presynaptic assembly. J Neurosci. 2007;27:7284–96. doi: 10.1523/JNEUROSCI.0731-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol. 2004;14:1272–76. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–52. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chin LS, Li L, Ferreira A, Kosik KS, Greengard P. Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proc Natl Acad Sci U S A. 1995;92:9230–4. doi: 10.1073/pnas.92.20.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach RJ, Diefenbach E, Douglas MW, Cunningham AL. The heavy chain of conventional kinesin interacts with the SNARE proteins SNAP25 and SNAP23. Biochemistry. 2002;41:14906–15. doi: 10.1021/bi026417u. [DOI] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–57. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LS, Yang Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci. 2000;23:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- Guillaud L, Setou M, Hirokawa N. KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J Neurosci. 2003;23:131–40. doi: 10.1523/JNEUROSCI.23-01-00131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, et al. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–93. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Hall DH, Hedgecock EM. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell. 1991;65:837–47. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Noda Y. Intracellular transport and kinesin superfamily proteins, KIFs: structure, function, and dynamics. Physiol Rev. 2008;88:1089–118. doi: 10.1152/physrev.00023.2007. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Pfister KK, Yorifuji H, Wagner MC, Brady ST, Bloom GS. Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration. Cell. 1989;56:867–78. doi: 10.1016/0092-8674(89)90691-0. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Kobayashi N, Pfister KK, Bloom GS, Brady ST. Kinesin associates with anterogradely transported membranous organelles in vivo. J Cell Biol. 1991;114:295–302. doi: 10.1083/jcb.114.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ. The pattern and mechanism of mitochondrial transport in axons. Front Biosci. 1996;1:91–102. doi: 10.2741/a118. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Milstein AD, Ethell IM, Henkemeyer M, Sheng M. GRIP1 controls dendrite morphogenesis by regulating EphB receptor trafficking. Nat Neurosci. 2005;8:906–15. doi: 10.1038/nn1487. [DOI] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. Secretory trafficking in neuronal den-drites. Nat Cell Biol. 2004;6:585–91. doi: 10.1038/ncb0704-585. [DOI] [PubMed] [Google Scholar]
- Hurd DD, Stern M, Saxton WM. Mutation of the axonal transport motor kinesin enhances paralytic and suppresses Shaker in Drosophila. Genetics. 1996;142:195–204. doi: 10.1093/genetics/142.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–25. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Okada Y, Tanaka Y, Harada A, Terada S, Hirokawa N. KIF5C, a novel neuronal kinesin enriched in motor neurons. J Neurosci. 2000;20:6374–84. doi: 10.1523/JNEUROSCI.20-17-06374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, et al. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–48. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki S, Holzbaur EL. cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr Opin Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- Katz LC, Crowley JC. Development of cortical circuits: lessons from ocular dominance columns. Nat Rev Neurosci. 2002;3:34–42. doi: 10.1038/nrn703. [DOI] [PubMed] [Google Scholar]
- King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–4. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Exocytosis and endocytosis of synaptic vesicles and functional roles of vesicle pools: lessons from the Drosophila neuromuscular junction. Neuroscientist. 2005;11:138–47. doi: 10.1177/1073858404271679. [DOI] [PubMed] [Google Scholar]
- Langford GM. Myosin-V, a versatile motor for short-range vesicle transport. Traffic. 2002;3:859–65. doi: 10.1034/j.1600-0854.2002.31202.x. [DOI] [PubMed] [Google Scholar]
- Lee CW, Peng HB. The function of mitochondria in presynap-tic development at the neuromuscular junction. Mol Biol Cell. 2008;19:150–8. doi: 10.1091/mbc.E07-05-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen Y, Jones AF, Sanger RH, Collis LP, Flannery R, et al. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:2169–74. doi: 10.1073/pnas.0711647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–87. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Li Z, Sheng M. Some assembly required: the development of neuronal synapses. Nat Rev Mol Cell Biol. 2003;4:833–41. doi: 10.1038/nrm1242. [DOI] [PubMed] [Google Scholar]
- Liu QA, Shio H. Mitochondrial morphogenesis, dendrite development, and synapse formation in cerebellum require both Bcl-w and the glutamate receptor delta2. PLoS Genet. 2008;4:e1000097. doi: 10.1371/journal.pgen.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- McGee AW, Bredt DS. Assembly and plasticity of the glutamater-gic postsynaptic specialization. Curr Opin Neurobiol. 2003;13:111–8. doi: 10.1016/s0959-4388(03)00008-4. [DOI] [PubMed] [Google Scholar]
- Miller KE, DeProto J, Kaufmann N, Patel BN, Duckworth A, Van Vactor D. Direct observation demonstrates that Liprin-alpha is required for trafficking of synaptic vesicles. Curr Biol. 2005;15:684–9. doi: 10.1016/j.cub.2005.02.061. [DOI] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Characterization of myosin V binding to brain vesicles. J Biol Chem. 2000;275:2598–606. doi: 10.1074/jbc.275.4.2598. [DOI] [PubMed] [Google Scholar]
- Mok H, Shin H, Kim S, Lee JR, Yoon J, Kim E. Association of the kinesin superfamily motor protein KIF1Balpha with postsynaptic density-95 (PSD-95), synapse-associated protein-97, and synaptic scaffolding molecule PSD-95/discs large/zona occludens-1 proteins. J Neurosci. 2002;22:5253–8. doi: 10.1523/JNEUROSCI.22-13-05253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J Cell Sci. 1993;104:917–27. doi: 10.1242/jcs.104.3.917. [DOI] [PubMed] [Google Scholar]
- Naisbitt S, Valtschanoff J, Allison DW, Sala C, Kim E, Craig AM, et al. Interaction of the postsynaptic density-95/guany-late kinase domain-associated protein complex with a light chain of myosin-V and dynein. J Neurosci. 2000;20:4524–34. doi: 10.1523/JNEUROSCI.20-12-04524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T, Terada S, Hirokawa N. Visualization of the dynamics of synaptic vesicle and plasma membrane proteins in living axons. J Cell Biol. 1998;140:659–74. doi: 10.1083/jcb.140.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–20. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Pack-Chung E, Kurshan PT, Dickman DK, Schwarz TL. A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat Neurosci. 2007;10:980–9. doi: 10.1038/nn1936. [DOI] [PubMed] [Google Scholar]
- Saito N, Okada Y, Noda Y, Kinoshita Y, Kondo S, Hirokawa N. KIFC2 is a novel neuron-specific C-terminal type kinesin superfamily motor for dendritic transport of multivesicular body-like organelles. Neuron. 1997;18:425–38. doi: 10.1016/s0896-6273(00)81243-x. [DOI] [PubMed] [Google Scholar]
- Schnapp BJ. Trafficking of signaling modules by kinesin motors. J Cell Sci. 2003;116:2125–35. doi: 10.1242/jcs.00488. [DOI] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, et al. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–7. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- Shen W, Wu B, Zhang Z, Dou Y, Rao ZR, Chen YR, et al. Activity-induced rapid synaptic maturation mediated by presyn-aptic cdc42 signaling. Neuron. 2006;50:401–14. doi: 10.1016/j.neuron.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Harris KM. Three-dimensional structure and composition of CA3->CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–10. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- Stowers RS, Megeath LJ, Górska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–77. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Su Q, Cai Q, Gerwin C, Smith CL, Sheng ZH. Syntabulin is a microtubule-associated protein implicated in syntaxin transport in neurons. Nat Cell Biol. 2004;6:941–53. doi: 10.1038/ncb1169. [DOI] [PubMed] [Google Scholar]
- Sytnyk V, Leshchyns’ka I, Delling M, Dityateva G, Dityatev A, Schachner M. Neural cell adhesion molecule promotes accumulation of TGN organelles at sites of neuron-to-neuron contacts. J Cell Biol. 2002;159:649–61. doi: 10.1083/jcb.200205098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagishi Y, Oda S, Hayasaka S, Dekker-Ohno K, Shikata T, Inouye M, et al. The dilute-lethal (dl) gene attacks a Ca2+ store in the dendritic spine of Purkinje cells in mice. Neurosci Lett. 1996;215:169–72. doi: 10.1016/0304-3940(96)12967-0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, et al. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–58. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–91. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–78. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wu H, Nash JE, Zamorano P, Garner CC. Interaction of SAP97 with minus-end-directed actin motor myosin VI. Implications for AMPA receptor trafficking. J Biol Chem. 2002;277:30928–34. doi: 10.1074/jbc.M203735200. [DOI] [PubMed] [Google Scholar]
- Yao J, Qi J, Chen G. Actin-dependent activation of presynaptic silent synapses contributes to long-term synaptic plasticity in developing hippocampal neurons. J Neurosci. 2006;26:8137–47. doi: 10.1523/JNEUROSCI.1183-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekawa Y, Harada A, Okada Y, Funakoshi T, Kanai Y, Takei Y, et al. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J Cell Biol. 1998;141:431–41. doi: 10.1083/jcb.141.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger ED, Ziv NE, et al. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron. 2001;29:131–43. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- Zhang W, Benson DL. Stages of synapse development defined by dependence on F-actin. J Neurosci. 2001;21:5169–81. doi: 10.1523/JNEUROSCI.21-14-05169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Takita J, Tanaka Y, Setou M, Nakagawa T, Takeda S, et al. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell. 2001;105:587–97. doi: 10.1016/s0092-8674(01)00363-4. [DOI] [PubMed] [Google Scholar]