Abstract
Terpenes have received a great deal of attention in the scientific literature due to complex, synthetically challenging structures and diverse biological activities associated with this class of natural products. Based on the number of C5 isoprene units they are generated from, terpenes are classified as hemi- (C5), mono- (C10), sesqui- (C15), di- (C20), sester- (C25), tri (C30), and tetraterpenes (C40). Among these, sesterterpenes and their derivatives known as sesterterpenoids, are ubiquitous secondary metabolites in fungi, marine organisms, and plants. Their structural diversity encompasses carbotricyclic ophiobolanes, polycyclic anthracenones, polycyclic furan-2-ones, polycyclic hydroquinones, among many other carbon skeletons. Furthermore, many of them possess promising biological activities including cytotoxicity and the associated potential as anticancer agents. This review discusses the natural sources that produce sesterterpenoids, provides sesterterpenoid names and their chemical structures, biological properties with the focus on anticancer activities and literature references associated with these metabolites. A critical summary of the potential of various sesterterpenoids as anticancer agents concludes the review.
Keywords: Apoptosis resistance, glioma, in vivo, manoalide, multidrug resistance, ophiobolin A, scalarane
1. INTRODUCTION
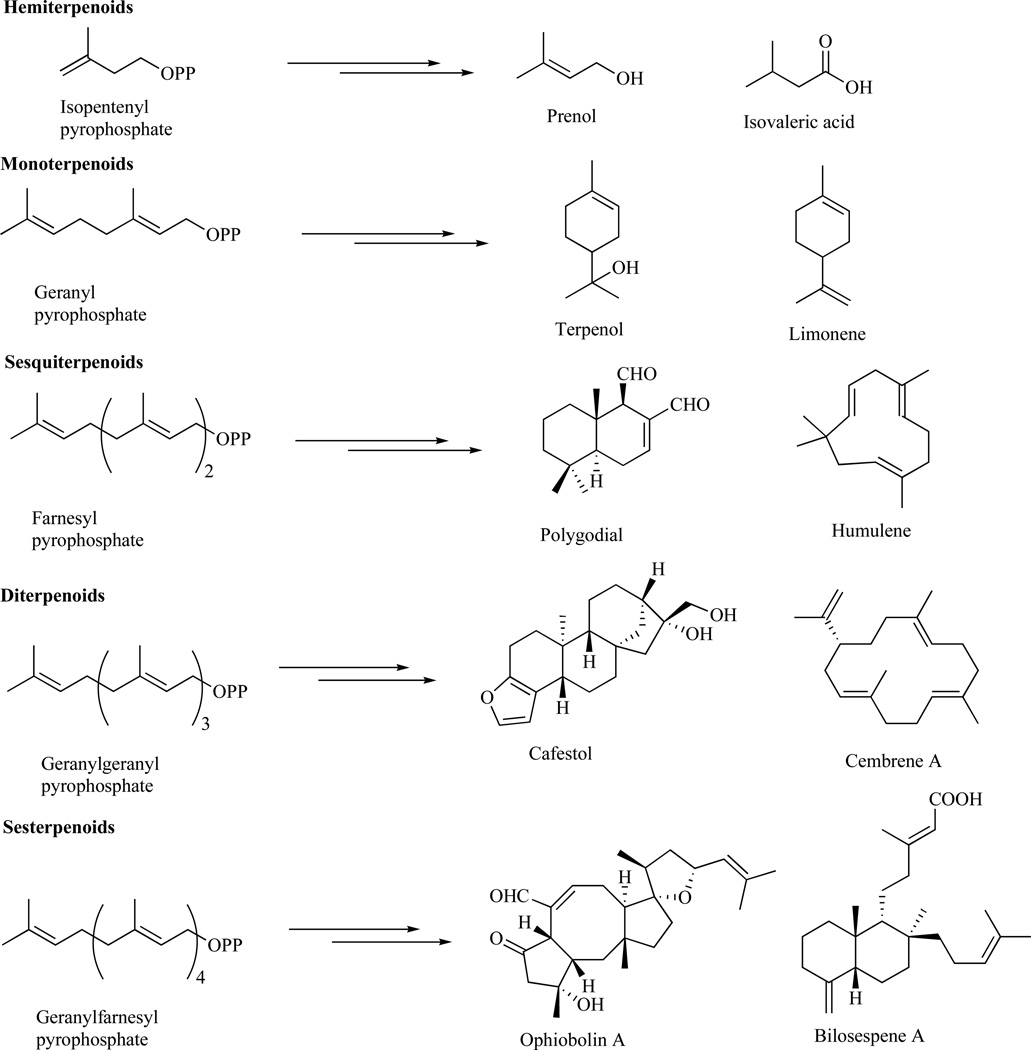
Terpenes are the largest class of structurally diverse natural products isolated from bacteria, plants, marine organisms, and fungi [1–3]. They are generated from C5 isoprene units joined in a head-to-tail fashion and classified as hemi- (C5), mono- (C10), sesqui- (C15), di- (C20), sester-(C25), tri- (C30) and tetraterpenes (C40) (Fig. 1) [1,2].
Fig. 1.
Terpene subgroups.
Hemiterpenes, produced from isopentenyl pyrophosphate, are primarily found in plants as signaling defense molecules and represented by parent isoprene, which is a common volatile substance released from woody trees such as oaks, willows, poplar, and spruce [1,2]. Additional examples of hemiterpenes are fruit and berry metabolite prenol and essential oil constituent isovaleric acid (Fig. 1) [4]. Monoterpenes, generated from geranylpyrophosphate, can be folded into mono-, bi-, and tricyclic structures, which through diverse functionalization of the C10 carbon skeleton give rise to more than 1,000 different known natural products [5]. These play a role in plant defense, pollinator attraction, plant to plant communication, and are used in perfumery, cosmetics, and as insecticides [6]. Examples include flavoring agent terpenol and cosmetic product limonene (Fig. 1). Similarly, sesquiterpenoids and diterpenoids, generated from farnesyl- and geranylgeranylpyrophosphate [7], respectively, are represented by highly diverse carbon skeletons. These play important roles as phytohormones and have numerous applications in pharmacology [1–3]. Some examples are active pepper constituent polygodial, anti-inflammatory agent humulene, coffee bean constituent cafestol and termite pheromone cembrene A (Fig. 1) [8–10].
Sesterterpenoids originate from geranylfarnesylpyrophosphate and are found primarily in fungi and marine organisms [1–3,11]. Examples of specific metabolites produced through various diverse cyclizations of the linear C25 precursor include cytotoxic agents ophiobolin A [12] and bilosespene A [13], fungal and marine metabolites, respectively (Fig. 1). Sesterterpenoids have been a subject of a lot of recent investigations aimed at structural, chemical, and biological characterizations and were found to show a broad spectrum of biological activities against bacteria, fungi, and nematodes [3,11,14]. Many of them exhibit strong anticancer activity via novel mode(s) of action. Description of these studies with an emphasis on anticancer activity represents the aim of the present review.
1.1. Sesterterpenoids from Bacteria
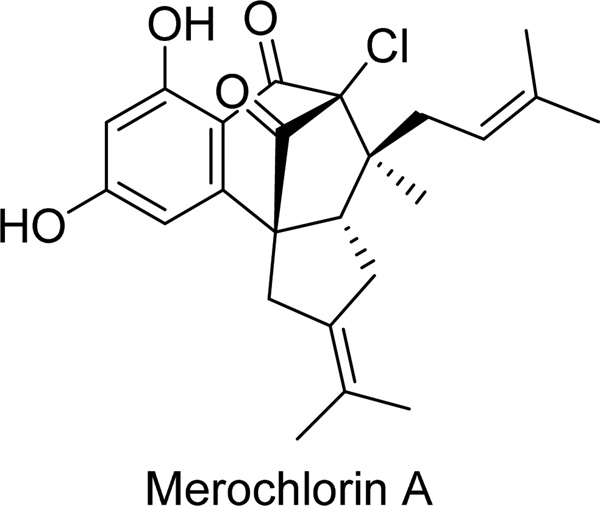
Merochlorin A (Fig. 2, Table 1) is the first complex chlorinated resorcinol-containing metabolite isolated from a marine-derived actinomycete strain CNH189 through a screening effort against clinically resistant bacteria [15]. Merochlorin A was identified as an agent active against a number of Gram-positive strains, including methicillin-resistant Staphylococcus aureus (MRSA, bacterium), which is a leading cause of hospital-acquired and community-associated bacterial infections [15]. However, the activity was lost in the presence of 20% serum and it was inactive against Gram-negative bacteria [15]. Cytotoxicity was assayed in tissue culture assays at 24 and 72 hours against human HeLa cervical and L929 mouse sarcoma cell lines. Cytotoxicity, defined as the 50% reduction in absorbance at 490 nm due to the reduced MTS ((3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) reagent in live cells, was observed at 150 µM and 4 µM in HeLa cells at 24 and 72 hours, respectively, and at 80 µM and 9 µM in L929 cells at 24 and 72 hours, respectively [15].
Fig. 2.
Table 1.
| Chemical Name | Chemical Structure | Source | References |
|---|---|---|---|
| Merochlorin A | Fig. 2 | Streptomyces sp. | [15] |
| Ophiobolin A | Fig. 3 | Drechslera gigantea | [12,25,29,31,32] |
| Ophiobolin B | Fig. 3 | Bipolaris oryzae | [12,19,21,39] |
| Ophiobolin C | Fig. 3 | Aspergillus section Usti | [39] |
| 6-Epi-ophiobolin G | Fig. 3 | Ulocladium sp. | [37] |
| Ophiobolin K | Fig. 2 | Aspergillus section Usti | [39] |
| Ophiobolin T | Fig. 3 | Ulocladium sp. | [37] |
| Ophiobolin O | Fig. 3 | Aspergillus ustus | [35] |
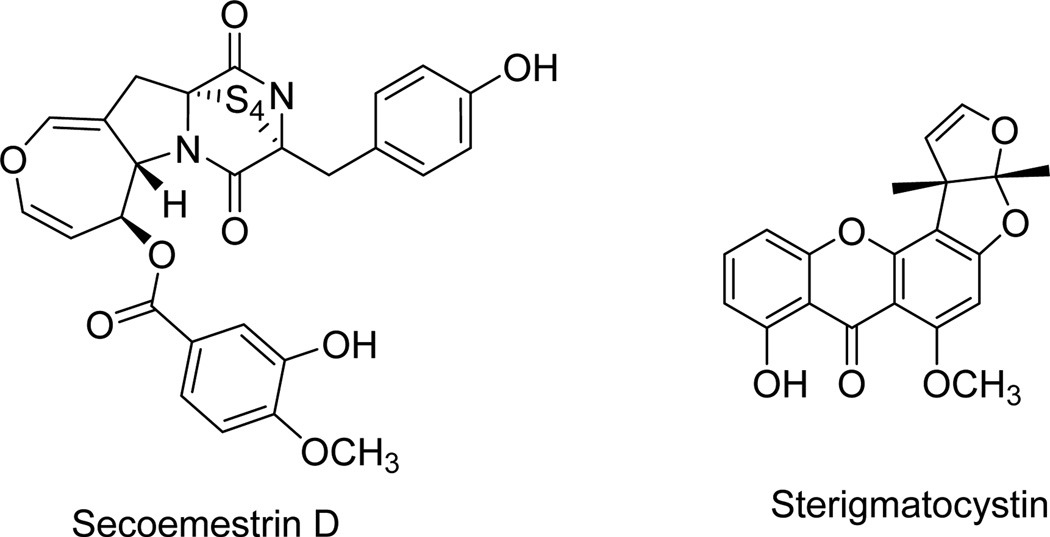
| Secoemestrin D | Fig. 4 | Emericella sp. AST0036 | [41] |
| Sterigmatocystin | Fig. 4 | Emericella sp. AST0036 | [41] |
| Manoalide | Fig. 5 | Luffariella variabilis | [45] |
| Luffariellolide | Fig. 5 | Acanthodendrilla sp. | [49] |
| 25-O-Methyl analog of luffariellolide | Fig. 5 | Acanthodendrilla sp. | [49] |
| Acantholide E | Fig. 5 | Acanthodendrilla sp. | [49] |
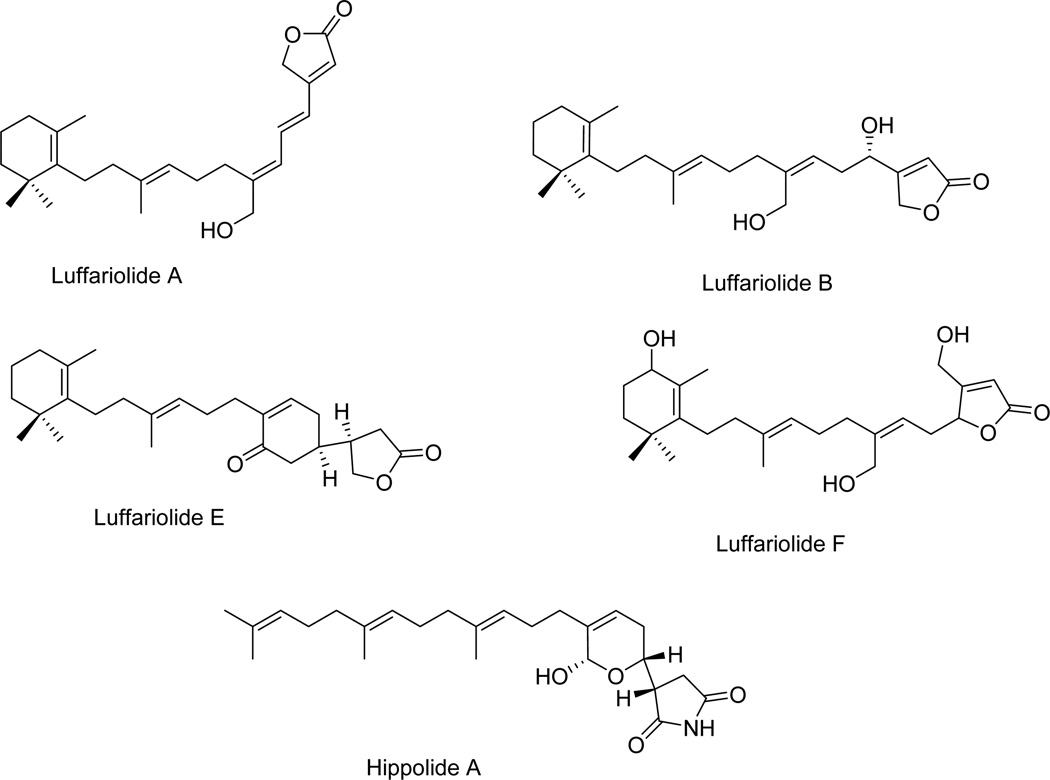
| Luffariolide A | Fig. 6 | Luffariella sp. | [50] |
| Luffariolide B | Fig. 6 | Luffariella sp. | [50] |
| Luffariolide E | Fig. 6 | Luffariella sp. | [50] |
| Luffariolide F | Fig. 6 | Luffariella sp. | [51] |
| Hippolide A | Fig. 6 | Hippospongia lachne | [52] |
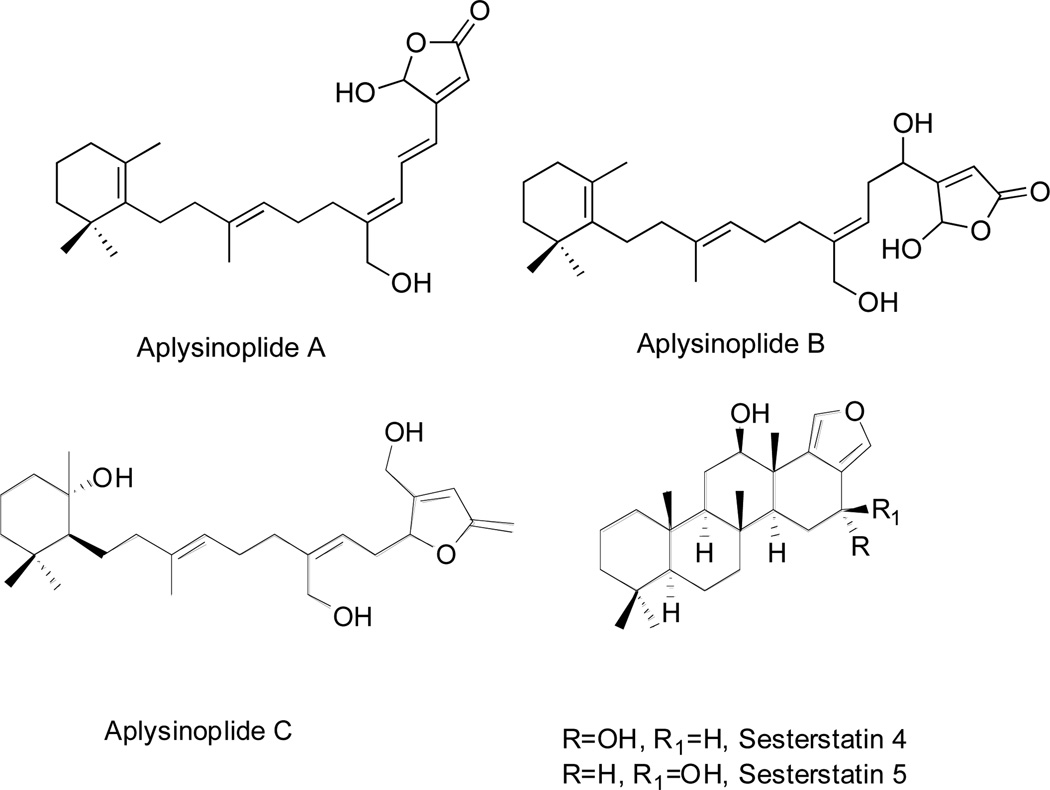
| Aplysinoplide A | Fig. 7 | Aplysinopsis digitata | [54] |
| Aplysinoplide B | Fig. 7 | Aplysinopsis digitata | [54] |
| Aplysinoplide C | Fig. 7 | Aplysinopsis digitata | [54] |
| Sesterstatin 4 | Fig. 7 | Hyrtios erecta | [55] |
| Sesterstatin 5 | Fig. 7 | Hyrtios erecta | [55] |
| Irciformonin I | Fig. 8 | Ircinia formosana | [56] |
| 15-Acetylirciformonin B | Fig. 8 | Ircinia sp. | [57] |
| 10-Acetylirciformonin B | Fig. 8 | Ircinia sp. | [57] |
| Compound 1–7 | Fig. 8 | Sarcotragus sp. | [58] |
| Furospinosulin-1 | Fig. 8 | the source was not found | [59,60] |
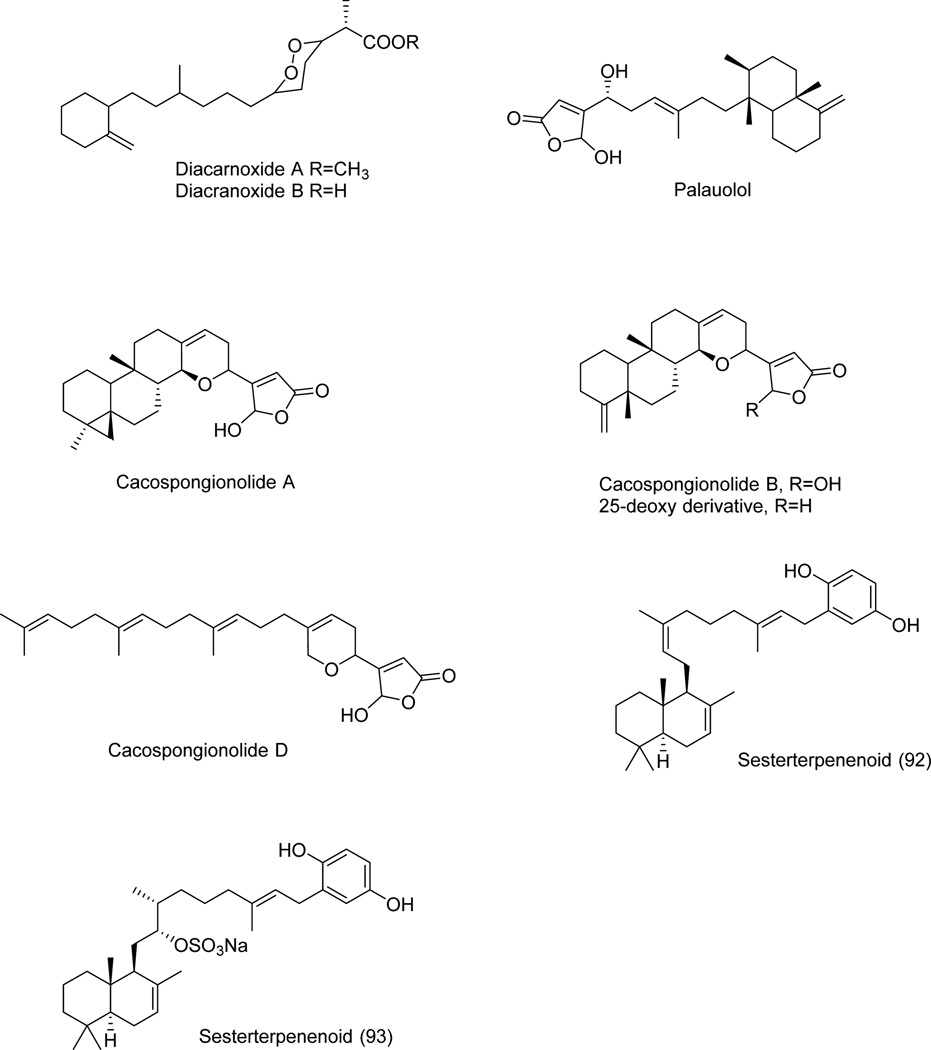
| Diacarnoxides A | Fig. 9 | Diacarnus levii | [61] |
| Diacarnoxides B | Fig. 9 | Diacarnus levii | [61] |
| Palauolol | Fig. 9 | Thorectandra sp. | [62] |
| Cacospongionolide A | Fig. 9 | Fasciospongia cavernosa | [63,64] |
| Cacospongionolide B | Fig. 9 | Fasciospongia cavernosa | [63,64] |
| Cacospongionolide D | Fig. 9 | Fasciospongia cavernosa | [63,64] |
| Sesterterpenoid 92 | Fig. 9 | Coscinoderma sp. | [66] |
| Sesterterpenoid 93 | Fig. 9 | Coscinoderma sp. | [66] |
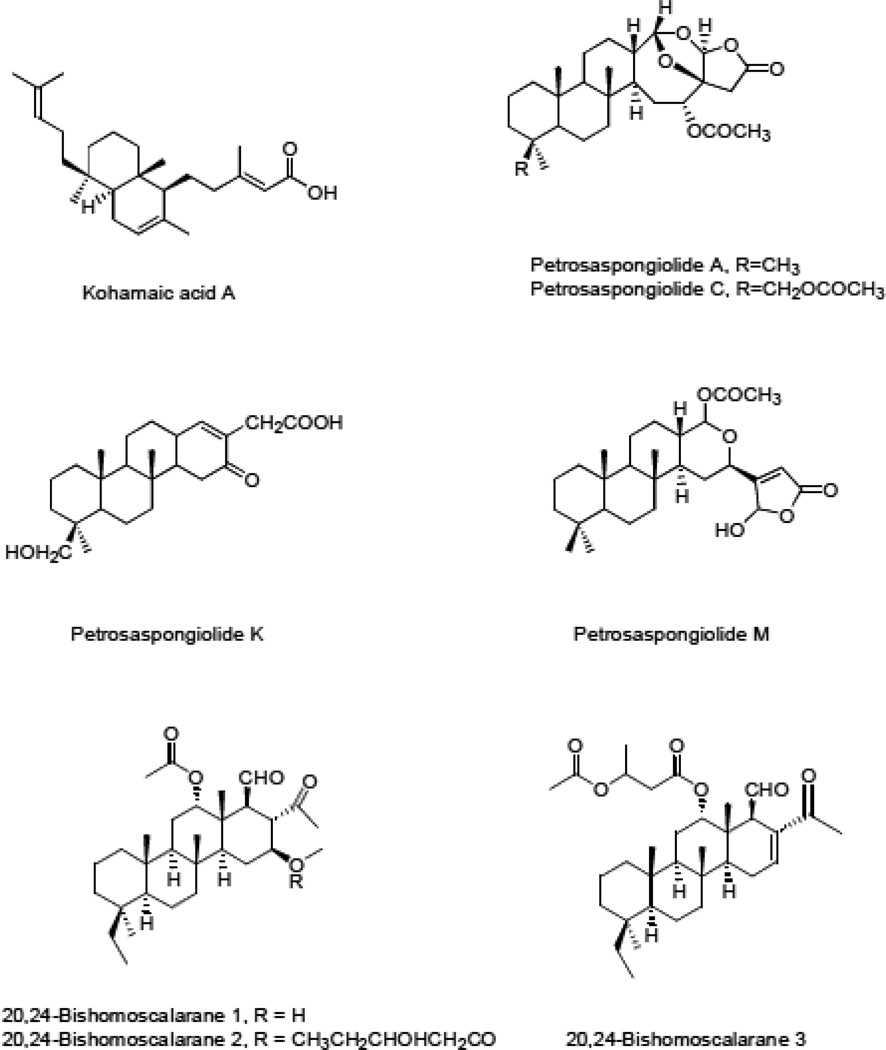
| Kohamic acid A | Fig. 10 | Ircinia sp. | [71] |
| Petrosaspongiolide A | Fig. 10 | Petrosaspongia nigra | [72] |
| Petrosaspongiolide C | Fig. 10 | Petrosaspongia nigra | [72] |
| Petrosaspongiolide K | Fig. 10 | Petrosaspongia nigra | [72] |
| Petrosaspongiolide M | Fig. 10 | Petrosaspongia nigra | [73,74] |
| 20-24 Bishomoscalarane 1 | Fig. 10 | Strepsichorabia lendenfeldi | [77] |
| 20-24 Bishomoscalarane 2 | Fig. 10 | Strepsichorabia lendenfeldi | [77] |
| 20-24 Bishomoscalarane 3 | Fig. 10 | Strepsichorabia lendenfeldi | [77] |
| 16-O-Deacetyl-16-epi-scalarolbutenolide | Fig. 12 | Hyrtios cf. erectus | [78] |
| 12-O-Acetyl-16-O-deacetyl-16-epi-scalarolbutenolide | Fig. 12 | Hyrtios cf. erectus | [78] |
| 12-Deacetoxy-21-acetoxyscalarin | Fig. 12 | Hyrtios cf. erectus | [78] |
| Lintenolide G | Fig. 12 | Cacospongia cf. linteiformis | [79] |
| Lintenolide E | Fig. 12 | Cacospongia cf. linteiformis | [79] |
| Lintenolide A | Fig. 12 | Cacospongia cf. linteiformis | [79] |
| Mooloolabene A | Fig. 12 | Hyatella intestinalis | [80] |
| Mooloolabene B | Fig. 12 | Hyatella intestinalis | [80] |
| Scalarane-type 1 | Fig. 13 | Hyrtios erecta | [81] |
| Scalarane-type 2 | Fig. 13 | Hyrtios erecta | [81] |
| Compound 8 and 10 | Fig. 13 | Hyatella sp. | [82] |
| Phyllophenone D | Fig. 13 | Phyllospongia foliascens | [83] |
| Phyllophenone E | Fig. 13 | Phyllospongia foliascens | [83] |
| Hyppospongide B | Fig. 13 | Hyppospongia sp. | [84] |
| Heteronemin | Fig. 13 | Hyrtius sp. | [85] |
| Bilosespenes A | Fig. 14 | Dysidea cinerea | [86] |
| Bilosespenes B | Fig. 14 | Dysidea cinerea | [86] |
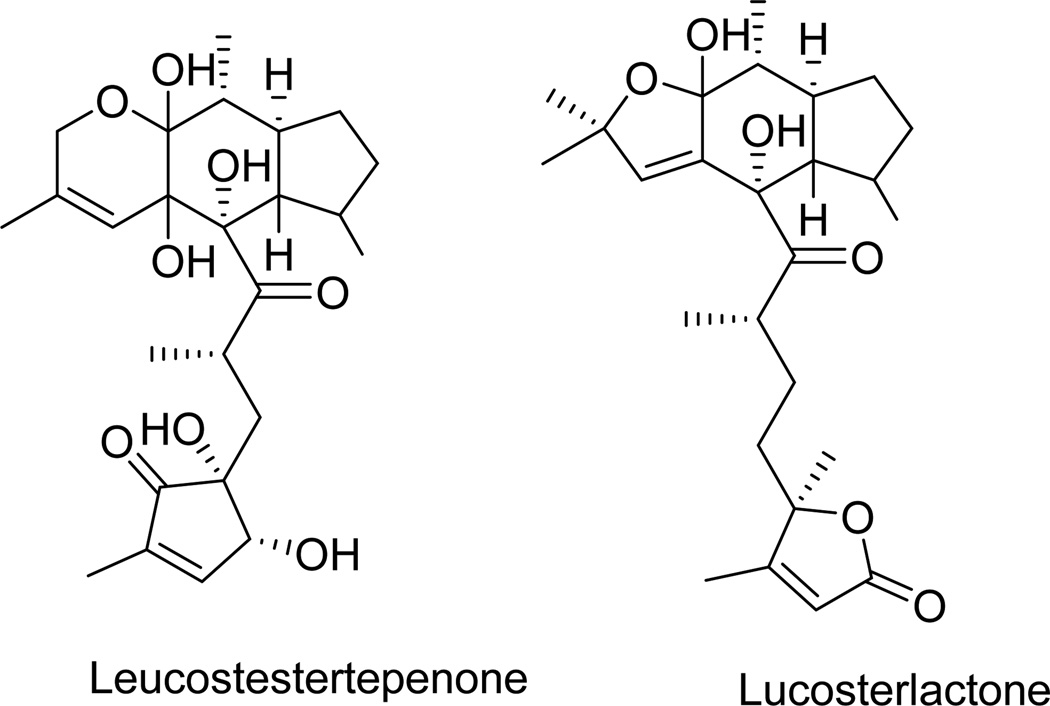
| Leucosesterterpenone | Fig. 15 | Leusceptrum canum | [87] |
| Leucosterlactone | Fig. 15 | Leusceptrum canum | [87] |
| Twenty-four sesterterpenoids tricyclic furan-2-ones | Fig. 16 | Salvia dominica | [88] |
1.2. Sesterterpenoids from Fungi
The ophiobolins are a group of secondary phytotoxic metabolites produced by pathogenic fungi attacking agricultural crops [12]. These sestertepenoids share the same 5-8-5 carbotricyclic skeleton with fusicoccins and cotylenins, two groups of diterpenoids produced by Fusicoccum amygdali (fungus) [16] and by Cladiosporum sp. 501-7W (fungus) [17,18]. Ophiobolin A (Fig. 3, Table 1) was the first member of the group independently isolated from Bipolaris oryzae (fungus) by Canonica et al. [19] and Nozoe et al. [20]. In addition to ophiobolin A, several additional analogues were isolated in the late sixties. These include ophiobolin B (Fig. 3, Table 1) from B. oryzae [21], ophiobolin C (Fig. 3, Table 1) from Bipolaris zizanie (fungus) [20], ophiobolin D from Cephalosporium caerulens (fungus) [22,23], and ophiobolin F from Bipolaris maydis (fungus) [24]. Since then, numerous additional members of this family of natural products have been reported. Of interest, studies have found that the types of ophiobolins produced by a particular fungal strain vary with culture conditions. For example, Drechslera gigantea (fungus), a potential mycoherbicide of grass weeds proposed for the biocontrol of crabgrass (Digitaria sanguinalis), was found to produce ophiobolin A, 6-epi- and 3-anhydro-6-epi-ophiobolin A, and ophiobolin I in liquid culture [25]. However, the same fungus grown in solid culture produced new ophiobolins E and epi-ophiobolin J together with known ophiobolins B and J [26]. Numerous literature reports have appeared describing the biological activities of activities of ophiobolins [12]. The following paragraphs describe the specific members of this family with reported anticancer activities.
Fig. 3.
Original investigations revealed ophiobolin A’s potent phytotoxic activity through the induction of ion leakage, blockade of hexose transport in higher plants and inhibition of calmodulin activity [12,27]. Ophiobolin A was also found to inhibit proton extrusion in plant cells due to its effect on the permeability of the plasma membrane to potassium [28]. A more recent investigation of ophiobolin A’s phytotoxicity revealed that it induced cell death in Nicotiana tabacum L. cv. Bright Yellow 2 (TBY-2) plant cells at concentrations ≥10 µM, with the TBY-2 cells exhibiting typical features of an apoptosis-like cell death [29].
Earlier studies revealed that this sesterterpenoid also had potent antiproliferative effects in human cancer cell lines [12] and induced apoptosis in mouse leukemia cells [30]. However, a detailed investigation of its anticancer activity was described only recently [29,31,32]. When tested on eight cancer cell lines at concentrations of less than 1 µM, ophiobolin A inhibited cancer cell growth by 50% after 3 days of culture irrespective of whether the cells displayed a multidrug resistance (MDR) phenotype or were resistant to pro-apoptotic stimuli [29]. Ophiobolin A was also found to display moderate in vivo antitumor activity by extending the survival of the mice bearing B16F10 mouse melanoma cells with lung pseudometastases when assayed at 10 mg/kg [29].
The mechanism of cell death induced by ophiobolin A was studied in glioblastoma (GBM) cells and found to involve, at least partly, the induction of paraptosis through the disruption of internal potassium ion homeostasis [31]. Because GBM cells are resistant to the induction of apoptosis [33], and thus to most of the current chemotherapeutic agents [34], these results are significant. In this study, ophiobolin A was also found to interfere with the F-actin cytoskeleton and to inhibit growth and migration of GBM cells, possibly by targeting the big conductance Ca2+-activated K+ channel (BKCa) [31]. The paraptosis-like cell death induced by ophiobolin A was consistent with intracellular vacuolization, possibly possibly arising from the mitochondrial and/or the endoplasmic reticulum swelling [31]. Correspondingly, ophiobolin A-induced cell death in GBM cells did not involve the activation of caspases, a common event in apoptotic cell death [31].
In another recent development, ophiobolin A was investigated as a potential treatment of rhabdomyosarcoma (RD), the most common soft tissue sarcoma in children, through its incorporation into chemoembolization particles [32]. The multimodal chemoembolization particles were generated by inserting mesoporous silica nanoparticles, prepared by the sol-gel method, onto the surface of polystyrene microspheres, obtained by suspension copolymerization. The chemoembolization particles were subsequently loaded with ophiobolin A and demonstrated to exhibit good efficacy against RD cells in culture [32].
Ophiobolin O (Fig. 3, Table 1) was isolated from Aspergillus ustus 094102 (fungus) and differs from ophiobolin A by the absence of the tetrahydrofuran ring D with the corresponding modifications in the hydrocarbon tail C15-C19 (Fig. 3). Furthermore, the reactive dicarbonyl functionality at C5 and C21 is masked with a bis-methyl acetal, which may or may not undergo unmasking under physiological conditions (Fig. 3). Thus, further work is required to elucidate whether ophiobolin O’s anticancer properties are due to the intact bis-acetal form or whether it can be considered a pseudo-prodrug of ophiobolin A, whose mode of action relies on the intracellular hydrolysis of the bis-acetal moiety. Ophiobolin O induced cell cycle arrest in the G0/G1 phase in MCF-7 human breast cancer cells and reduced the viability of these cells in a time- and dose-dependent manner through the induction of apoptosis, as was established using the flow cytometric Annexin V/PI assay [35]. Mechanistic studies revealed that ophiobolin O activated JNK (c-Jun NH2-terminal kinase), p38 MAPK (mitogen activated protein kinase) and ERK (extracellular signal-regulated kinase), and inhibited Bcl-2 phosphorylation [35]. Recent studies also demonstrated that ophiobolin O reverses the MCF-7/ADR cell resistance to adriamycin by downregulating the resistance genes, triggering the G2/M cell cycle arrest and increasing the adriamycin-induced apoptosis in these cells [35]. Furthermore, the co-administration of ophiobolin O and adriamycin in tumor growth inhibition, indicating the capability of ophiobolin O to reverse chemotherapy resistance in cancer cells [36].
Ophiobolins P, Q, R, S, T, 6-epi-21-O-dihydroophiobolin G, 6-epi-ophiobolin G and 6-epi-ophiobolin K, were purified from the acetone extract of the endolichenic fungus Ulocladium sp. isolated from the lichen Everniastrum sp. [37]. Endolichenic fungi live in the thalli of lichens and are similar to the plant endophytic fungi that inhabit in the intercellular spaces of the hosts [38]. These ophiobolins also differ from ophiobolin A by the absence of the tetrahydrofuran ring D with the corresponding modifications in the hydrocarbon tail C15-C19 [37]. In addition, ophiobolins Q, R, S, T, 6-epi-21-O-dihydro-ophiobolin G, 6-epi-ophiobolin G and 6-epi-ophiobolin K all contain the epimerized C6 position and ophiobolins Q, R and T are also dehydrated at C3-C4 [37]. Furthermore, 6-epi-21-O-dihydro-ophiobolin G incorporates the reduced C21 aldehyde, while in ophiobolin P the same aldehyde is oxidized to the corresponding carboxylic group, which in turn is lactonized with the hemiacetalized ketone at C4 [37]. All these ophiobolins were tested for cytotoxic effects against KB (human cervical carcinoma) and HepG2 (human hepatocarcinoma) cell lines [37]. Ophiobolin T and 6-epi-ophiobolin G (Fig. 3, Table 1) exhibited the most potent cytotoxic activity with IC50 inhibitory concentrations against HepG2 of ~0.2 and ~0.4 µM, respectively [37].
New ophiobolin U was isolated together with the known ophiobolins C, H, K (Fig. 3, Table 1) as well as 6-epi-ophiobolins G, K and N from three fungal strains in the Aspergillus section Usti [39]. This ophiobolin differs from ophiobolin A by the absence of the tetrahydrofuran ring D with the corresponding modifications in the hydrocarbon tail C15-C19, also present in ophiobolins P, Q, R, S and T as mentioned above. Additionally, ketone C5 is reduced to the secondary β-hydroxyl group. In this study, ophiobolins A, B (Fig. 3), C and K displayed antiproliferative activity towards leukemia cells with the induction of apoptosis at nanomolar concentrations [39]. The remaining ophiobolins were mainly inactive or only moderately active at micromolar concentrations [39]. Interestingly, 6-epi-ophiobolins G and N isolated previously from the fungus Emericella variecolor had been reported to be active at concentrations of 1–3 µM against the neuroblastoma cell line Neuro 2A, inducing cell shrinkage and chromatin condensation in these cells [40]. An analysis of the anticancer structure-activity relationships resulting from all above-mentioned studies highlights the importance of the C5,C21-dicarbonyl functionality, intact C3-hydroxy and the A/B-cis ring structure. In contrast, rings C and D can be modified without a significant impact on activity.
Secoemestrin D (Fig. 4, Table 1), a new epitetrathiodioxopiperizine, and five sesterterpenoids having a new carbon skeleton and named emericellenes A–E were produced by the endophytic fungal strain Emericella sp. AST0036 isolated from a healthy leaf tissue of Astragalus lentiginosus [41]. The new metabolites were isolated together with the previously known fungal metabolites: a tetrasubstituted anthracenone sterigmatocystin (Fig. 4, Table 1), arugosin C and epi-isoshamixanthone [41]. Emericella species are the perfect sexual states of the fungus Aspergillus [42], and both of these fungal strains produce carcinogenic mycotoxins [43]. These metabolites compounds were evaluated in vitro in a panel of six tumor cell lines and normal human fibroblast cells using the resazurin-based colorimetric assay [44]. Only secoemestrin D and sterigmatocystin displayed cytotoxic activity, with the former metabolite being more potent registering IC50 values as low as 0.06 to 0.2 µM and exhibiting moderate selectivity toward SF-268 human glioma and MDA-MB-231 metastatic breast adenocarcinoma cell lines [41].
Fig. 4.
1.3. Sesterterpenoids from Marine Organisms
Manoalide (Fig. 5, Table 1) was firstly isolated from the marine sponge Luffariella variabilis native to Palau (Oceania) and reported to be a strong inhibitor of phospholipase A2 (PLA2), an enzyme catalyzing the hydrolysis of phospholipids leading to the release of proinflammatory mediator eicosanoids [45]. A structure-activity relationships study revealed that the γ-hydroxybutenolide is involved in the initial interaction with PLA2 with hemiacetal in the α-hydroxydihydropyran playing a crucial role in the irreversible binding [46]. The hydrophobic trimethylcyclohexenyl ring system facilitates non-bonded interactions with the enzyme enhancing the potency [46,47]. This natural product is commercially available as a biochemical standard to block the action of PLA2 [48]. Although not investigated for anticancer activities, its congeners display prominent antitumor properties as detailed below.
Fig. 5.
Luffariellolide (Fig. 5, Table 1) is another 4,5-disubstituted furanone related to manoalide isolated from Luffariella sp. (sponge). Luffarielloide is less potent against PLA2 than manoalide and it showed a partial reversible inhibition of this enzyme [47]. Luffariellolide, its 25-O-methyl analog (Fig. 5, Table 1) and another closely related sesterterpenoid acantholide E (Fig. 5, Table 1) were also isolated from the Indonesian sponge Acanthodendrilla sp. and displayed cytotoxicity against the L5178Y mouse lymphoma cell line with the IC50 values of ~9, ~2 and ~17 µM, respectively [49]. The presence of the 25-O-methyl group in luffarielloide and the stereochemistry of the 1-acetylcyclopentan-5-ol portion of the molecule in acantholide E are important structural features for the activity [49].
Luffariolides A, B, E and F (Fig. 6, Table 1) are other manoalide analogues isolated from different collections of the marine sponge Luffariella sp. found in Okinawa. Luffariolide A, B and F differ from luffariellolide by the aliphatic bridge joining the 5-hydroxy-furan-2-one and the trimethylcyclohexene rings [50,51]. Another structural difference is in the functionalization of the furan-2-one ring: the 5-hydroxy group of luffariellolide is absent in luffariolide A and B, while luffariolide F contains a hydroxymethyl group at C4 and the aliphatic bridge is attached at C5 instead of C4 as in the other analogues [50,51]. Luffariolides A, B, E and F displayed cytotoxicity against the L1210 murine lymphoma cell line with the IC50 inhibitory concentrations ranging between ~3 and ~4 µM [50,51].
Fig. 6.
Hippolide A (Fig. 6, Table 1) is a manoalide-related sesterterpene isolated from the sea sponge Hippospongia lachne collected in South China [52]. It differs from manoalide by the absence of the trimethylcyclohexene and the presence of 4,8,9-trimethyl-dodeca-3,7,11-trienyl chain attached to the hemiacetalic dihydropyranyl and succinimidyl linked ring systems [52]. It exhibited cytotoxic activity against A549 non-small cell lung cancer (NSCLC; IC50 of ~50 nM), HeLa cervical carcinoma (IC50 of ~50 nM) and HCT-116 colon carcinoma (IC50 of ~10 µM) cell lines [52]. It is of note that the A549 NSCLC cells display various levels of resistance to pro-apoptotic stimuli [53].
Aplysinoplides A-C (Fig 7, Table 1), were isolated from Aplysinopsis digitata (sponge) collected in Oshima-shinsone, Japan [54]. Aplysinoplides A and B are closely related to luffariellolide due to the aliphatic bridge joining the 5-hydroxyfuran-2-one residue with the trimethylcyclohexene moiety [54]. Aplysinoplide C is similar to luffariolide with the different functionalization of trimethylcyclohexene residue [54]. Aplysinoplides A-C displayed cytotoxicity against P388 mouse leukemia cells with the IC50 values of ~1, ~1 and ~26 µM, respectively [54]. Aplysinoplides A and B did not inhibit bovine pancreatic PLA2 at concentrations up to of 100 µM, in comparison with manoalide that exhibited potent activity in a parallel experiment [46]. These results confirmed the important role of the C24 aldehyde for the inhibition of PLA2 [46].
Fig. 7.
Sesterstatins 4 and 5 (Fig 7, Table 1), are new pentacyclic furanosesterterpenes belonging to the scalarane subgroup isolated from the marine black sponge Hyrtios erecta incorporating a benzoperhydrophenanthrene ring system joined to a furan ring [55]. These natural products are epimers at C4. Sesterstatins 4 and 5 inhibited in vitro the growth of various cancer cell lines, including murine P338 leukemia and human BXPC-3 pancreas, RPMI-7951 melanoma, U251 central nervous system (CNS), KAT-4 and SW1736 thyroid, NCI-H460 NSCLC, FADU pharynx and DU-145 prostate cancer models with the IC50 growth inhibitory concentrations ranging between 4–13 and 5–26 µM, respectively [55].
Irciformonin I (Fig. 8, Table 1), a nor-sesterterpenoid, containing a furanone and furan rings joined by means of an unsaturated and methoxylated bridge, was isolated from Ircinia formosana (sponge) collected in Taiwan [56]. This compound appeared quite toxic as it displayed the inhibition of peripheral blood mononuclear cell proliferation [57]. 15-O-acetylirciformonin B and 10-O-acetylirciformonin B (Fig. 8, Table 1) are C22 furanosesterterpenoids, which differ from irciformonin I by the functionalization of the bridge linking the furanone and furan rings. They were isolated from Ircinia spp. and displayed cytotoxic activity against K562 human leukemia, DLD-1 human colon carcinoma, HepG2 human hepatocarcinoma and Hep3B human hepatocarcinoma cancer cell lines [57]. 15-Acetylirciformonin B was the most potent compound against these four cancer cell lines with the IC50 growth inhibitory concentrations ranging between 0.03 and ~5 µM [57].
Fig. 8.
Five new and two known furanosesterterpene tetronic acids (1–7) (Fig. 8, Table 1) were isolated from the marine sponge Sarcotragus sp. and showed cytotoxicity against a panel of five human tumor cell lines: A549 NSCLC, SK-OV-3 human ovarian cancer, SK-Mel-2 human skin cancer, XF498 human CNS cancer and HCT 15 human colon cancer. The ED50 values were in the range 9–75 µM. However, compounds 1–7 were generally unstable, especially the unconjugated tetronic acid furanosesterterpenes (1–3) that easily decomposed even at low temperature when kept in the solid state [58].
Furospinosulin-1 (Fig. 8, Table 1), incorporating a 3-substituted furan ring and closely related to irciformonins, exhibited in vitro antiproliferative activity against DU145 human prostate cancer cells under hypoxic conditions (IC50 range 1–100 µM) and in vivo antitumor activity (10–50 mg/kg) in mice inoculated with sarcoma S180 cells after oral administration. It was also shown to suppress the transcription of the insulin-like growth factor-2 (IGF-2) gene [59]. Its C4-desmethyl analogue also showed significant selective activity under hypoxic conditions and antitumor activity in vivo after an oral administration [60].
Diacarnoxides B and A (Fig. 9, Table 1) are sesterterpenoid C2-derivatives of propanoic acid and its methyl ester, which were isolated from Diacarnus levii (sponge) collected in Papa Nueva Guinea [61]. They exhibited cytotoxic properties and weak selective activity under hypoxic conditions in a panel of five human cancer cell lines including two prostate (DU145 and PC-3) and three breast (MCF-7, MDA-MB-231 and T47D) cancer models. Diacarnoxide B inhibited cancer cell proliferation under normoxic versus hypoxic conditions with IC50 ratios of ~7/5 (DU145), ~17/13 (PC-3), ~14/7 (MCF-7), ~14/6 (MDA-MB-231) and ~6/6 (T47D) µM [61]. Diacarnoxide A, the methyl ester of diacarnoxides B, was far less active highlighting the importance of the free acid for the inhibitory activity [61].
Fig. 9.
Palauolol (Fig. 9, Table 1) is a 5-hydroxy-4-substituted sesterterpenoid furan-2-one isolated from the marine sponge Thorectandra sp. collected in Palau (Oceania) together with closely related sesterterpenoids thorectandrols A-E [62]. Palauolol showed antiproliferative activity against MCF-7 breast, SNB-19 CNS, COLO-205 and KM12 colon, MOLT-4 leukemia, H460 NSCLC, LOX and MALME-3M melanoma, OVCAR-3 and IGROV1 ovarian, and 786-0 renal human tumor cell lines with the IC50 growth inhibitory concentrations of ~1–2 µM [62]. Thorectandrols A-E displayed only weak in vitro growth inhibitory activities [62]. It was shown that the presence of the 16-hydroxyl and the hemiacetal lactone functionalities in palauolol played an important role for these in vitro growth inhibitory effects on cancer cells [62].
Cacospongionolides A, B and D (Fig. 9, Table 1), other 5-hydroxy-4-substituted sesterterpenoid furan-2-ones closely related to luffarins and thorectandrols, were isolated from marine sponge Fasciospongia cavernosa (aka Cacospongia mollior) collected from the Mediterranean sea [63,64]. Although in this original study antitumor activity was not demonstrated with these natural products, cacospongionolides A, showed strong cytotoxicity (IC50 = 0.25 µM) in the brine shrimp assay and high levels of inhibition (75%) in the crown-gall potato disc assay. Furthermore, cacospongionolide A proved to be very toxic in a fish toxicity bioassay [63]. More recently, cacospongionolide was found to induce apoptosis in the A431 human epidermoid carcinoma, T47D human breast cancer, HeLa human cervical carncer and HCT116 human colon cancer cells [65]. Incubation of HCT116 and HeLa cells with cacospongionolide for 24 h caused an increased expression of pro-apoptotic proteins and a concentration-dependent loss of mitochondrial trans-membrane potential. These effects paralleled those achieved with p50 and p65, NF-κB subunits at the nuclear level [65].
Two sesterterpenoid hydroquinones (Fig. 9, Table 1) were isolated from Coscinoderma sp. (sponge) collected in Micronesia and named sesterterpenoids 92 and 93, respectively [66]. Sesterterpenoid 92 is the 12E-isomer of coscinoquinol, a closely related sesterterpenoid hydroquinone isolated from the same sponge. It showed cytotoxic activity of higher potency than doxorubicin against K562 cells with the IC50 growth inhibitory concentration of 8 µM [66]. Sesterterpenoid 93 inhibits Na+/K+-ATPase activity with the IC50 inhibitory concentration of 5 µM [66]. Inhibiting the Na+/K+-ATPase activity in cancer cells can represent an innovative approach to kill apoptosis-resistant and/or multidrug resistant cancer cells [67–69].
Kohamaic acid A (Fig. 10, Table 1), a C5-decalin-substituted sesterterpenoid 3-metylpent-2-enoic acid, was isolated from the sponge Ircinia spp. collected in Okinawa [70]. It was found to be a powerful DNA polymerase inhibitor [71], although it displayed only moderate cytotoxicity towards cancer cells [70].
Fig. 10.
Petrosaspongiolides A-L are steroid-like sesterterpenoids, isolated from Petrosaspongia nigra, a sponge collected in Caledonia [72]. Petrosaspongialide A and C (Fig. 10, Table 1) incorporate C4-disubstituted-4,5-dihydro-furan-2-one and tetramethylperhydrophenanthrene rings joined to 7,9-dioxabicyclo[4.2.1]nonane ring and differ by the substituent at C4 [72]. Petrosaspongiolide K lacks the lactone but contains a similar perhydrophenathrene moiety (Fig. 10). Petrosaspongiolides C and K (Fig. 10, Table 1) showed the best (lowest) IC50 growth inhibitory concentrations (~1 and ~4 µM) against NSCLC-N6 human bronchopulmonary carcinoma cells [72]. Petrosaspongiolide A was tested in an in vivo model involving immunosuppressed rats with the NSCLC-N6 tumors and displayed inhibition of tumor growth at 20 mg/kg without apparent toxic effects [72].
Petrosaspongiolide M (Fig. 10, Table 1) is also a steroid-like 5-hydroxy-4-substituted furan-2-one. It reacts selectively with the Lys-67 residue of sPLA2-IIA and form a covalent bond through imine formation [14,73]. Studies carried out on the target protein indicated its involvement in the NF-kappa B inflammatory response by the inhibition of the ubiquitin proteasome system [74]. Petrosaspongiolide M acts therefore as a proteasome inhibitor [74] and the inhibition of proteasome activity in some cancer types represent a promising approach to combat cancers associated with dismal prognoses [75,76].
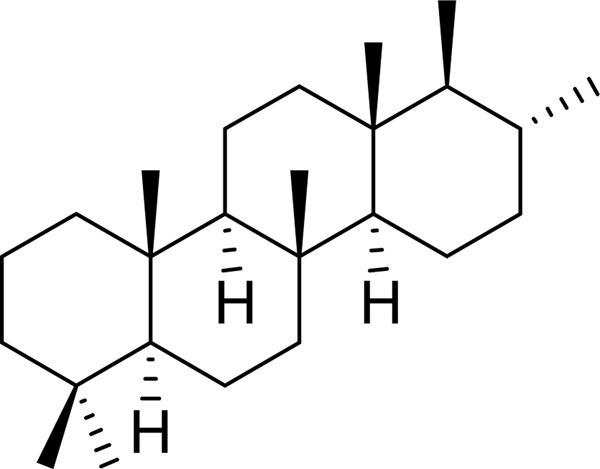
Scalaranes are sesterterpenoids characterized by the typical tetracyclic carbon skeleton, which are exclusively isolated from sponges and shell molluscs (nudibranchs) [11]. Most of these natural products are involved in chemical defense of producer marine organisms and are of interest due to their potential application in medicine and as a molecular marker in the chemotaxonomic classification of sponges [11]. The first scalarane was isolated from a Mediterranean sponge in 1980 [11]. In the last two decades several new members of this family were isolated and characterized by different research groups [11]. In agreement with the IUPAC recommendation, the saturated hydrocarbon ring system shown in Fig. 11 was named “scalarane”
Fig. 11.
Nine sesterterpenes belonging to 20,24-bishomoscalaranes were isolated from the Thorectide sponge Strepsichorabia lendenfeldi Bergquist (Dictyoceratida) obtained from the Great Barrier Reef, Australia [77]. All of these exhibited moderate cytotoxicity against murine P-388 leukemia and human A549 NSCLC cells with the IC50 values of ~0.2–2.0 µM in both cell lines. 20,24-Bishomoscalaranes 1, 2, and 3 (Fig. 10, Table 1) differ in the functionalization of the scalarane carbon skeleton [14]. All three contain the ethanoyl and methanoyl group at C17 and C18, while 20,24-bishomoscalaranes 1 and 2 also incorporate the acetoxy group at C12 and the 3-hydroxypentanoyl functionality at C16 (Fig. 10) [77]. 20,24-Bishomoscalarane 3 on the other hand contains the 3-acetoxylbutanoyloxy group at C12 [77]. All three 20,24-bishomoscalaranes were evaluated against HT-29 human colon carcinoma, CV-1 monkey kidney fibroblast, P-388 murine leukemia and A549 NSCLC cell lines registering IC50 values of < 3 µM [77].
Scalarane sesterterpenes are common metabolites of Dictyoceratida sponges. Indeed, three new cytotoxic sesterterpenes 16-O-deacetyl-16-epi-scalarolbutenolide, 12-O-acetyl-16-O-deacetyl-16-epi-scalarolbutenolide, 12-deacetoxy-21-acetoxyscalarin (Fig. 12, Table 1), were successively isolated from the marine sponge Hyrtios cf. erectus collected in southern Japan [78]. These pentacyclic sesterterpenes contain a furan-2-one (butenolide) ring joined to the D ring of the tetracyclic scalarane ring system and differ in the functionalities at C12, C16 and C19 (Fig. 12). They exhibited cytotoxicity against P-388 murine leukemia cells with the IC50 growth inhibitory concentrations ranging between 0.9 and 4.9 µM [78].
Fig. 12.
Lintenolides A–E new pentacyclic sesterterpenes, were isolated from the Caribbean sponge Cacospongia cf. linteiformis [79]. Lintenolides A and G (Fig. 12, Table 1), contain a perhydrophenanthrene ring system fused with a furanyl moiety and differ by the methoxycarbonyl versus hydroxyl group at C13. In contrast, in lintenolide E the perhydrophenanthrene ring system is fused with a pyranyl moiety (Fig. 12, Table 1). These compounds exhibited in vitro cytotoxicity against WEHI 164 murine fibrosarcoma, J774 murine monocyte/macrophage, P388 murine leukemia, and GM7373 bovine endothelial cell lines with the IC50 values in the range of 0.2 to 4 µM [79].
Mooloolabenes A-E, isolated from Hyatella intestinalis collected in Australia, are scalarane norsesterterpenoids that showed cytotoxic activity against P388 mouse leukemia cell line [80]. Of these, mooloolabenes A and B (Fig. 12, Table 1), epimers at C18, were most potent with the IC50 growth inhibitory concentrations of ~2 and ~3 µM, respectively [80].
Scalarane sesterterpenoids (types 1 and 2 in Fig. 13, Table 1), structurally related to 24-β-methoxyscalaroide, were isolated from Hyrtios erecta (sponge) collected in Papua New Guinea [81]. The scalarane type 1 contains a butenolide joined to the D ring of the scalarane system and two methoxycarbonyls at C12 and C16, while scalarane type 2 has a 5-methoxybutanolide joined to the D ring and a hydroxyl group at C12. They showed weak cytotoxicity against MCF-7 breast cancer cells with the IC50 of 125 µM [81].
Fig. 13.
Five new sesterterpenes, along with six known compounds, isolated from the Korean sponge Hyatella sp., exhibited moderate to weak cytotoxicity. In particular, two of them, compounds 8 and 10 (Fig. 13, Table 1), showed activity comparable (IC50 of ~20 and ~15 µM, respectively) to doxorubicin (IC50 of ~10 µM) against K562 cell line [82].
Phyllophenones D and E (Fig. 13, Table 1), two other scalarane sesterterpenoids, were isolated from the sponge Phyllospongia foliascens collected in South China [83]. The first contains a cyclopent-2-enone ring joined to the D ring of the scalarane ring system, hydroxyl group at C12 and a trisubstituted double bond between C16 and C17, while the second incorporates the same functionalities in the scalarane skeleton as well as two hydroxyl and the ethanoyl groups at C12, C-18, and C17, respectively [83]. Phyllofenone D has a rare α,β-unsaturated ketone ring, while phyllofenone E possesses also a relatively rare 25-norscalarane framework. Phyllofenone D showed cytotoxic activity against P388 leukemia cell line with the IC50 growth inhibitory concentration of 17 µM. In contrast, phyllofenone E was inactive in this assay [83].
Hyppospongide B (Fig. 13, Table 1), a scalarane sesterterpenoid isolated from the sponge Hipponspongia sp. collected in Taiwan, differs from scalarane type 2 by the absence of the methoxy group at C5 of the butanolide ring [84]. It exhibited weak, if any, cytotoxic activity against DLD-1 and HCT-116 human colon, T-47D human breast, and K562 human leukemia cancer cell lines with the IC50 growth inhibitory concentrations of >10 µM [84]. A closely related heteronemin (Fig. 13, Table 1), displayed significant cytotoxic activity with the IC50 values of ~0.001 µM against the same cell lines [84]. This scalarane was isolated from the sponge Hyrtius sp [85]. It contains a 4,5-dihydrofuran ring joined to the D ring of the scalarane ring system and two methoxycarbonyl groups at C16 and C5. Heteronemin induced apoptotic cell death and inhibited TNF α-induced NF-kappa B activation through proteasome inhibition in chronic myelogenous leukemia cells [14,85].
Bilosespenes A and B (Fig. 14, Table 1), were isolated from the Red sea sponge Dysidea cinerea collected in the Dahlak archipelago, Eritrea [86]. These metabolites, isolated as an inseparable mixture, exhibited low micromolar antiproliferative effects against P-388 mouse lymphoma, A-549 human lung cancer, HT-29 human colon cancer and MEL-28 human melanoma [86].
Fig. 14.
1.4. Sesterterpenoids from Plants
Leucosesterterpenone and leucosterlactone (Fig. 15, Table 1) are two tetracyclic sesterterpenoids isolated from Himalayan plant Leusceptrum canum [87]. Leucosesterterpenone inhibited fibroblast growth factor-2-dependent proliferation, migration, chemotaxis and tube formation with small and large vessel endothelial cells, whereas leucosterlactone was completely inactive. These two compounds formed a complex with the fibroblast growth factor-2 receptor-1 with the IC50 inhibitory concentrations of ~1 and 132 µM, respectively, with simultaneous down-regulation of ERK1/2 phosphorylation [87]. Leucosesterterpenone is less hydrophobic than leucosterlactone, which may contribute to its increased activity and thus potential application as an anti-angiogenic agent [87].
Fig. 15.
Salvia is a very important genus comprising about 900 species in the plant family Lamiaceae [88]. They have high diversity in their secondary metabolites with numerous pharmacological effects. The main small molecule constituents of Salvia spp. are polyphenols, flavonoids, and terpenoids. Twenty-four tricyclic sesterterpenoids (Fig. 16, Table 1) were isolated from the aerial parts of Salvia dominica [88]. All of them contain a differentially functionalized decalin and furan-2-one rings joined with a substituted pentane side chain [88]. These natural products were evaluated for biological activity by means of a panel of chemical and biological approaches, including chemical proteomics, surface plasmon resonance (SPR) measurements, and various biochemical assays. They were also tested for cytotoxic activity against three cell lines, MCF-7 human breast adenocarcinoma, J774 murine monocytes/macrophages and HEK293 human epithelial kidney, but did not show significant activity at the doses evaluated (>100 µM) [88].
Fig. 16.
CONCLUSIONS
Sesterterpenoids are ubiquitous secondary metabolites in fungi, marine organisms and plants. Their structural diversity encompasses carbotricyclic ophiobolanes, polycyclic anthracenones, polycyclic furan-2-ones, polycyclic hydroquinones, among many other carbon skeletons. The sesterterpenoids’ names, references to figures depicting their structures, natural sources and literature are all organized in Table 1. As is evident from the above discussion, many of these natural products possess cytotoxicity against cancer cells. However, when assayed in vitro, a compound that kills cancer cells is not a potential anticancer agent - it is just a toxic compound. To become a potential anticancer agent, a cytotoxic compound must be selective for killing cancer cells over the normal ones [89]. Thus, once a compound displays in vitro cytotoxic activity against cancer cells, it must be further evaluated against normal cells to determine its level of selectivity. Once such selective action is found, it must be further assayed against cancer cell lines expressing a multidrug-resistant phenotype (MDR) [89]. Indeed, the intracellular concentration of cytotoxic compounds in chemoresistant cancer cells is often reduced through the overexpression of efflux pumps [89]. If the compound of interest is not active against MDR cancer cells or toxic to normal cancer cell lines, it cannot be considered as a potential anticancer agent. Finally, compounds active against apoptosis-sensitive cancer cells by inducing apoptosis are actually of diminished interest for reasons explained hereafter.
Human MCF-7 breast and HeLa cervical carcinoma cell lines are sensitive to pro-apoptotic stimuli. As of April 2015, the Scopus database contained ~6,600 publications including the keywords “MCF-7 AND apoptosis.” The Scopus database also contained ~9,900 publications with the keywords “HeLa AND apoptosis.”
The mouse P388 and L1210 leukemia cell lines are also extremely chemosensitive [90]. In sharp contrast, clinical cancers, which are associated with dismal prognoses, are highly resistant to pro-apoptotic stimuli and thus to compounds inducing apoptotic cancer cell death [34,91–97]. Thus, MCF-7, HeLa, P388 and L1210 cells are poor models of clinical drug-resistant cancer, and compounds inducing apoptosis in these cells are not regarded as promising anticancer agents.
Therefore, compounds active against cancer cells that resist pro-apoptotic insults or display the MDR phenotype should be privileged [89]. Of all the sesterterpenes reviewed here, ophiobolins appear to be most promising if the above reasoning is applied to prioritize these compounds and identify agents for further development as anticancer therapies. Although less potent against cancer cell cultures, sterigmatocystin and its congener methoxy-sterigmatocystin, evaluated in vitro and in vivo at the National Institutes of Health (NIH; Table 2), showed significant therapeutic benefit in a melanoma mouse model. These compounds also warrant further evaluation against resistant cancers and perhaps the involvement of synthetic chemists to derive structure-activity relationship data. The other sesterterpenoids that have been tested in vivo by the NIH are described in Table 2.
Table 2.
National Cancer Institute (Bethesda, USA) informations on anti-cancer activity of sesterterpenes. In vitro data presented are those from the last 60 cancer cell line screening (NCI Cancer Screen; 60 cell/ 5 dose) available to date. All the in vivo experiments found in the database are presented. Therapeutic benefit was considered only if i) survival was >120% or tumor weight reduced by 20% and ii) all animals of the group were surviving at the toxicological evaluation day (usually day 5). When anti-cancer effects were observed according to those criteria in several groups of an experiment, we selected the one with the best response rate. Model used are presented as follows: cancer type, name of the cell line, route of graft. Therapeutic scheme are presented as in the database: QnnDXppp or QnnHXppp: Qnn=treatment interval (Q=every “nn”); D or H=interval units (day or week); Xppp= “ppp” times (total number of injections). Example: Q01DX009: one administration every day during 9 days.
| Chemical Name | In vitro Data (µM) | In vivo Data | ||||||
|---|---|---|---|---|---|---|---|---|
| GI50 | LC50 | Model | Therapeutic Scheme |
Formulation | Dose Range (mg/kg/dose) |
End Point | Best Re- sults % (Dose) |
|
| Ophiobolin A | 0.07 | 0.6 | Leukemia L1210 ip | Q01DX001 ip | saline | 4 → 400 | survival | No benefit |
| Leukemia L1210 ip | Q01DX009 ip | ST | 2.5 → 75 | survival | No benefit | |||
| sterigmatocystin | 8 | 110 | Melanoma B16 ip | Q01DX009 ip | HPC | 3.75 → 60 | survival | 146% (30) |
| ST | 4 → 128 | survival | 145% (64) | |||||
| Colon carcinoma 38 sc | Q07DX002 ip | ST | 1.25 → 200 | Tumor weight |
No benefit | |||
| Ependymoblastoma IC | Q01DX005 ip | ST | 16 → 256 | survival | No benefit | |||
| Leukemia L1210 ip | Q01DX009 ip | saline | 4 → 128 | survival | 200% (64) | |||
| ST | 3.75 → 60 | survival | 165% (60) | |||||
| Lewing Lung carcino- ma sc |
Q01DX009 ip | HPC | 3.75 → 60 | survival | No benefit | |||
| ST | 4 → 128 | survival | 128% (64) | |||||
| Sarcoma M5076 sc | Q02DX011 ip | HPC | 50 → 200 | Tumor weight |
−74% (100) | |||
| Lymphoma P388 ip | Q01DX005 ip | ST | 7.5 → 120 | survival | 196% (60) | |||
| Q01DX005 iv | ST | 7.5 → 120 | survival | No benefit | ||||
| Q01DX009 ip | saline | 0.5 → 32 | survival | 172% (32) | ||||
| Q04DX003 ip | ST | 50 → 400 | survival | 192% (100) | ||||
| Leukemia L1210 ip | Q01DX001 ip | saline | 50 → 200 | survival | 130% (200) | |||
| Methoxy- sterigmatocystin |
4 | 91 | Melanoma B16 ip | Q01DX009 ip | HPC | 4 → 256 | survival | 134% (16-32) |
| ST | 4 → 64 | survival | 152% (16) | |||||
| Leukemia L1210 ip | Q03HX008 ip | saline | 4 → 64 | survival | 143% (32) | |||
| HPC | 2 → 32 | survival | 155% (16) | |||||
| Q03HX016 ip | saline | 4 → 64 | survival | 158% (32) | ||||
| HPC | 4 → 64 | survival | 196% (8) | |||||
| Q01DX001 ip | saline | 32 → 512 | survival | 147% (256) | ||||
| HPC | 32 → 512 | survival | 158% (128) | |||||
| Q01DX009 ip | ST | 25 → 100 | survival | 162% (50) | ||||
| Lewis Lung carci- noma sc |
Q01DX009 ip | ST | 6.25 → 100 | survival | 132% (25) | |||
| Lymphoma P388 ip | Q01DX009 ip | ST | 6.25 → 100 | survival | 245% (50) | |||
| Q01DX009 ip | saline | 3.13 → 100 | survival | 202% (50) | ||||
| Q04DX003 ip | ST | 25 → 100 | survival | 162% (50) | ||||
| Acantholide | NA | NA | Melanoma B16 ip | Q01DX009 ip | ST | 1 → 16 | survival | No effect |
| Leukemia L1210 ip | Q01DX009 ip | ST | 1 → 16 | survival | No benefit * | |||
| Lymphoma P388 ip | Q01DX009 ip | Saline + alco- hol |
8 → 32 | survival | No benefit | |||
| Manoalide | NA | NA | Melanoma B16 ip | Q01DX009 ip | HPC | 3 → 12 | survival | No benefit |
| Lymphoma P388 ip | Q01DX009 ip | HPC | 1 → 16 | survival | No benefit | |||
Abbreviations: GI50 : in vitro growth inhibitory concentration 50%; LC50: in vitro lethal concentration 50%; NA: not available; ip: intraperitoneal; iv: intravenous; IC: intracranial; HPC: hydroxypropylcellulose; ST: saline + tween 80;
two similar experiments were performed.
Acknowledgments
RK is a director of research with the Fonds National de la Recherche Scientifique (FRS-FNRS, Belgium). AK thanks the Welch Foundation (Grant No. AI-0045), National Institutes of Health (1R15-CA186046-01A1) and National Science Foundation (grant 0946998) for financial support.
AE thanks Dipartimento di Scienze Chimiche, Università di Napoli Federico II, Napoli, Italy for financial support.
Biography

Robert Kiss
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Dewick PM. Medicinal Natural Products – A Biosynthetic Approach. Chichester: John Wiley & Sons Ltd; 2009. pp. 197–306. [Google Scholar]
- 2.Osbourn AE, Lanzotti V. Plant-derived Natural Products – Synthesis, Function and Application. Dortdrecht: Springer; 2009. pp. 3–50. [Google Scholar]
- 3.Cimmino A, Andolfi A, Evidente A. Phytotoxic terpenes produced by phytopathogenic fungi and allelopathic plants. Nat. Prod. Comm. 2014;9:401–408. [PubMed] [Google Scholar]
- 4.Schnitzler J-P, Louis S, Behnke K, Loivamäki M. Poplar volatiles - biosynthesis, regulation (eco)physiology of isoprene and stress-induced isoprenoids. Plant Biol. 2012;12:302–316. doi: 10.1111/j.1438-8677.2009.00284.x. [DOI] [PubMed] [Google Scholar]
- 5.Banthorpe DV, Charlwood BV, Francis MJO. The Biosynthesis of monoterpenes. Chem. Rev. 1972;72:115–119. doi: 10.1021/cr60276a002. [DOI] [PubMed] [Google Scholar]
- 6.Mohamoud SS, Croteau RB. Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci. 2002;7:366–373. doi: 10.1016/s1360-1385(02)02303-8. [DOI] [PubMed] [Google Scholar]
- 7.Cordell GA. Biosynthesis of sesquiterpenes. Chem Rev. 1976;76:425–460. [Google Scholar]
- 8.Barnes CS, Loder JW. Structure of Polygodial - a new sesquiterpene dialdehyde from Polygonum hydropiper L. Aust. J. Chem. 1962;15:322–327. [Google Scholar]
- 9.Vanderah DJ, Rutledge N, Schmitz FJ, Ciereszko LS. Marine natural products: cembrene-A and cembrene-C from a soft coral, Nephthea species. J. Org. Chem. 1978;43:1614–1616. [Google Scholar]
- 10.Lam LK, Sparnins VL, Wattenberg LW. Isolation and identification of kahweol palmitate and cafestol palmitate as active constituents of green coffee beans that enhance glutathione S-transferase activity in the mouse. Cancer Res. 1982;42:1193–1198. [PubMed] [Google Scholar]
- 11.González MA. Scalarane sesterterpenoids. Curr. Bioact. Comp. 2010;6:178–210. [Google Scholar]
- 12.Au TK, Chick PC, Leung PC. The biology of ophiobolins. Life Sci. 2000;67:733–742. doi: 10.1016/s0024-3205(00)00668-8. [DOI] [PubMed] [Google Scholar]
- 13.Rudi A, Yesief T, Schleyer M, Kashman Y. Bilosespens A and B: two novel cytotoxic sesterpenes from the marine sponge Dysidea cinerea. Org. Lett. 1999;1:471–472. doi: 10.1021/ol990058m. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Yang B, Lin X-P, Zhou X-F, Liu Y. Sesterterpenoids. Nat Prod. Rep. 2013;30:455–473. doi: 10.1039/c3np20089b. [DOI] [PubMed] [Google Scholar]
- 15.Sakoulas G, Nam S-J, Loesgen S, Fenuical W, Gensen PR, Nizet V, Hensler M. Novel bacterial metabolite merhochlorin A demonstrates in vitro activities against multi-drug resistant methicillin-resistant Staphylococcus aureus. PLoS One. 2012;7:e29439. doi: 10.1371/journal.pone.0029439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballio A, Graniti A. Phytotoxins and their involvment in plant disease. Experientia. 1981;47:751–864. [Google Scholar]
- 17.Sassa T. Cotylenines leaf growth substances produced by fungus. Part I. Isolation and characterization of cotylenins. Agric. Biol. Chem. 1971;35:1415–1418. [Google Scholar]
- 18.Sassa T, Neguro T, Ueki H. Production and characterization of a new fungal metabolite, cotylenol. Agric. Biol. Chem. 1972;36:2281–2285. [Google Scholar]
- 19.Canonica L, Fiecchi A, Galli Kienle M, Scala A. The constitution of cochliobolin. Tetrahedron Lett. 1966;11:1211–1218. [Google Scholar]
- 20.Nozoe S, Hirai K, Tsuda K. The structure of zizanin-A and -B, C25-terpenoids isolated from Helminthosporium zizaniae. Tetraedron Lett. 1966;20:2211–2216. [Google Scholar]
- 21.Canonica L, Fiecchi A, Galli Kienle M, Scala A. Isolation and constitution of cochliobolin. Tetrahedron Lett. 1966;13:1329–1333. [Google Scholar]
- 22.Itai A, Nozoe S, Tsuda K, Okuda S, Iitaka Y, Makayama Y. Structure of cephalonic acid, a pentaprenyl terpenoid. Tetraedron Lett. 1967;42:4111–4112. doi: 10.1016/s0040-4039(01)89701-x. [DOI] [PubMed] [Google Scholar]
- 23.Nozoe S, Itai A, Tsuda K, Okuda S. Chemical trasformation of cephalonic acid. Tetraedron Lett. 1967;42:4113–4117. doi: 10.1016/s0040-4039(01)89702-1. [DOI] [PubMed] [Google Scholar]
- 24.Nozoe S, Morisaki M, Fukushima K, Okuda S. The isolation of an acyclic C25-isoprenoid alcohol, geranylnerolidol, and a new ophiobolin. Tetraedron Lett. 1968;42:4457–4458. [Google Scholar]
- 25.Evidente A, Andolfi A, Cimmino A, Vurro M, Fracchiolla M, Charudattan R. Herbicidal potential of ophiobolins produced by Drechslera gigante. J. Agic. Food Chem. 2006;54:1779–1783. doi: 10.1021/jf052843l. [DOI] [PubMed] [Google Scholar]
- 26.Evidente A, Andolfi A, Cimmino A, Vurro M, Fracchiolla M, Charudattan R, Motta A. Ophiobolin E and 8-epi-ophiobolin J produced by Drechslera gigantea, a potential mycoherbicide of weedy grasses. Phytochemistry. 2006;67:2281–2287. doi: 10.1016/j.phytochem.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Tipton CL, Paulsen PV, Betts RE. Effects of ophiobolin A on ion leakage and hexose uptake by maize roots. Plant Physiol. 1977;59:907–910. doi: 10.1104/pp.59.5.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cocucci SH, Morgutti S, Cocucci M, Ginani L. Effects of ophiobolin A on potassium permeability, transmembrane electrical potential and proton extrusion in maize root. Plant Sci. 1983;32:9–16. [Google Scholar]
- 29.Bury M, Novo-Uzal E, Andolfi A, Cimini S, Wauthoz N, Heffeter P, Lallemand B, Avolio F, Delporte C, Cimmino A, Dubois J, Van Antwerpen P, Zonno MC, Vurro M, Poumay Y, Berger W, Evidente A, De Gara L, Kiss R, Locato V. Ophiobolin A, a sesterterpenoid fungal phytotoxin, displays higher in vitro growth-inhibitory effects in mammalian than in plant cells and displays in vivo antitumor activity. Int. J. Oncol. 2013;38:227–232. doi: 10.3892/ijo.2013.1979. [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara H, Matsunaga K, Kumagai H, Ishizuka M, Ohizumi Y. Ophiobolin A, a novel apoptosis-inducing agent from fungus strain f-7438. Pharm. Pharmacol. Commun. 2000;6:427–431. [Google Scholar]
- 31.Bury M, Girault A, Megalizzil V, Spiegl-Kreinecker S, Mathieu V, Berger W, Evidente A, Kornienko A, Gailly P, Vandier C, Kiss R. Ophiobolin A induces paraptosis-like cell death in human glioblastoma cells by decreasing BKCa channel activity. Cell Death Dis. 2013;4:e561. doi: 10.1038/cddis.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison R, Gardiner C, Evidente A, Kiss R, Townley H. Incorporation of ophiobolin A into novel chemoembolization particles for cancer cell treatment. Pharm. Res. 2014;31:290–2917. doi: 10.1007/s11095-014-1386-3. [DOI] [PubMed] [Google Scholar]
- 33.Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas, with a special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J. Clin. Oncol. 2005;23:2411–2422. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 34.Lefranc F, Sadeghi N, Camby I, Metens T, Dewitte O, Kiss R. Present and potential future issues in glioblastoma treatment. Expert Rev. Anticancer Ther. 2006;6:719–732. doi: 10.1586/14737140.6.5.719. [DOI] [PubMed] [Google Scholar]
- 35.Yang T, Lu Z, Meng L, Wei S, Hong K, Zhu W, Huang C. The novel agent ophiobolin O induces apoptosis and cell cycle arrest of MCF-7 cells through activation of MAPK signalling pathways. Bioorg. Med. Chem. Lett. 2012;22:579–585. doi: 10.1016/j.bmcl.2011.10.079. [DOI] [PubMed] [Google Scholar]
- 36.Sun W, Lv C, Zhu T, Yang X, Wei S, Sun J, Hong K, Zhu W, Huang C. Ophiobolin-O reverses adriamycin resistance via cell cycle arrest and apoptosis sensitization in adriamycin-resistant human breast carcinoma (MCF-7/ADR) cells. Mar. Drugs. 2013;11:4570–4584. doi: 10.3390/md11114570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q-X, Bao L, Yang X-L, Liu D-L, Guo H, Dai H-Q, Song F-H, Zhang L-X, Guo L-D, Li S-J, Liu HW. Ophiobolins P-T, five new cytotoxic and antibacterial sesterterpenes from the endolichenic fungus Ulocladium sp. Fitoterapia. 2013;90:220–227. doi: 10.1016/j.fitote.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Elizabeth AA. Understanding the diversity of foliar endophytic fungi progress, challenges, and frontiers. Fungal Biol. Rev. 2007;21:51–66. [Google Scholar]
- 39.Thorskov Bladt T, Dürr C, Boldsen Knudsen PB, Kildgaard S, Frisvad JC, Held Gotfredsen C, Seiffert M, Ostenfeld Larsen T. Bio-activity and dereplication-based discovery of ophiobolins and other fungal secondary metabolites targeting leukemia cells. Molecules. 2013;18:14629–14650. doi: 10.3390/molecules181214629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei H, Itoh T, Kinoshita M, Nakai Y, Kurotaki M, Kobayashi M. Cytotoxic sesterterpenes, 6-epi-ophiobolin G and 6-epi-ophiobolin N, from marine derived fungus Emericella variecolor GF10. Tetrahedron. 2004;60:6015–6019. [Google Scholar]
- 41.Xu Y-M, Espinosa-Artiles P, Liu MX, Arnold AE, Gunatilaka AAL. Secoemestrin D, a cytotoxic epitetrathiodioxopiperizine emericellenes A-E, five sesterterpenoids from Emericella sp. AST0036, a fungal endophyte of Astragalus lentiginosus. J. Nat. Prod. 2013;76:2330–2336. doi: 10.1021/np400762k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hawksworth DL. Naming Aspergillus species: progress towards one name for each species. Med. Mycol. 2011;49:S70–S76. doi: 10.3109/13693786.2010.504753. [DOI] [PubMed] [Google Scholar]
- 43.Rank C, Nielsena KF, Larsena TO, Vargab J, Samsonc RA, Frisvad JC. Distribution of sterigmatocystin in filamentous fungi. Fungal Biol. 2011;115:406–420. doi: 10.1016/j.funbio.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Vijeratne FMK, Bashyal BP, Liu MX, Rocha DD, Gunaherath GMKB, U’Ren JM, Gunatilaka MK, Arnold AE, Whitsell L, Gunatilaka AAL. Geopyxins A-E, ent-kaurane diterpenoids from endolichenic fungal strains Geopyxis aff. majalis and Geopyxis sp. AZ0066: structure-activity relationships of geopyxins and their analogues. J. Nat. Prod. 2012;75:361–369. doi: 10.1021/np200769q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potts BCM, Faulker DJ, Jacobs RS. Phospholipase A2 inhibitors from marine organisms. J. Nat. Prod. 1992;55:1701–1717. doi: 10.1021/np50090a001. [DOI] [PubMed] [Google Scholar]
- 46.Glaser KB, de Carvalho MS, Jacobs RS, Kernan MR, Faulkner DJ. Manoalide: structure-activity studies and definition of the pharmacophore for phospholipase A2 inactivation. Mol Pharmacol. 1989;36:782–788. [PubMed] [Google Scholar]
- 47.Ebada SS, Lin WH, Proksch P. Bioactive sesterterpenes and triterpenes from marine sponge: occurrence and pharmacological significance. Mar. Drugs. 2010;8:313–346. doi: 10.3390/md8020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gross H, König GM. Terpenoids from marine organisms: unique structures and their pharmacological potential. Phytochem. Rev. 2006;5:115–141. [Google Scholar]
- 49.Elkhayat E, Edrada RA, Ebel R, Wray V, van Soest R, Wiryowidagdo S, Mohamed MH, Muller WEG, Proksch P. New luffariellolide derivatives from the Indonesian sponge Acanthodendrilla sp. J. Nat Prod. 2004;67:1809–1817. doi: 10.1021/np040118j. [DOI] [PubMed] [Google Scholar]
- 50.Tsuda M, Shigemori H, Ishibashi M, Sasaki T. Luffariolides A-E, new cytotoxic sesterpenes from the Okinawan marine sponge Luffariella sp. J. Org. Chem. 1992;57:3503–3507. [Google Scholar]
- 51.Kobayashi J, Zeng CM, Ishibashi M, Sasaki T. Luffariolides F and G, new manoalide derivatives from the Okinawan marine sponge Luffariella sp. J. Nat. Prod. 1993;56:436–439. doi: 10.1021/np50093a020. [DOI] [PubMed] [Google Scholar]
- 52.Piao S-J, Zhang H-J, Lu H-I, Yang F, Jiao W-H, Yi Y-H, Chen W-S, Lin H-W. Hippolides A-H, acyclic manoalide derivatives from the marine sponge Hippospongia lachne. J. Nat. Prod. 2011;74:1248–1254. doi: 10.1021/np200227s. [DOI] [PubMed] [Google Scholar]
- 53.Mathieu A, Remmelink M, D’Haene N, Penant S, Gaussin JF, Van Ginckel R, Darro F, Kiss R, Salmon I. Development of a chemoresistant orthotopic human nonsmall cell lung carcinoma model in nude mice: Analyses of tumor heterogeneity in relation to the immunohistochemical levels of expression of cyclooxygenase-2, ornithine decarboxylase, lung-related resistance protein, prostaglandin E synthetase, and glutathione-S-transferase-alpha (GST)-alpha-, GST-mu, and GST-pi. Cancer. 2004;101:1908–1918. doi: 10.1002/cncr.20571. [DOI] [PubMed] [Google Scholar]
- 54.Ueoka R, Nakao Y, Fujii S, van Soest WM, Matsunaga S. Aplysinoplides A-C, cytotoxic sesterterpenes from the marine sponge Aplysinopsis digitata. J. Nat. Prod. 2008;71:1089–1091. doi: 10.1021/np8001207. [DOI] [PubMed] [Google Scholar]
- 55.Pettit GR, Tan R, Melody N, Cichacz ZA, Herald DL, Hoard MS, Pettit RK, Chapuis JC. Antineoplastic agents 397: isolation and structure of sesterstatins 4 and 5 from Hyrtios erecta (The Republic of Maldive) Bioorg. Med. Chem. Lett. 1998;8:2093–2098. doi: 10.1016/s0960-894x(98)00373-4. [DOI] [PubMed] [Google Scholar]
- 56.Shen Y-C, Lo K-L, Lin Y-C, Khalil AT, Kuo Y-K, Shih P-S. Novel linear C22-sesterterpenoids from sponge Ircinia formosana. Tetrahedron Lett. 2006;47:4007–4010. [Google Scholar]
- 57.Su J-H, Tseng S-W, Lu M-C, Liu L-L, Chou Y, Sung P-J. Cytotoxic C21 and C22 terpenoid-derived metabolites from the sponge Ircinia sp. J. Nat. Prod. 2011;74:2005–2009. doi: 10.1021/np2004209. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, Bae BH, Alam N, Ong J, Sim CJ, Lee CO, Im KS, Jung JH. New cytotoxic sesterterpenes from the sponge Sarcotragus species. J. Nat. Prod. 2001;64:1301–1304. doi: 10.1021/np0101494. [DOI] [PubMed] [Google Scholar]
- 59.Arai M, Kawachi T, Seitawan A, Kobayashi M. Hypoxia-selective growth inhibition of cancer cells by furospinosulin-1, a furanosesterpene isolated from an Indonesian marine sponge. Chem. Med. Chem. 2010;5:1919–1926. doi: 10.1002/cmdc.201000302. [DOI] [PubMed] [Google Scholar]
- 60.Kotoku N, Fujioka S, Nakata C, Yamada M, Sumii Y, Kawachi T, Arai M, Kobayashi M. Concise synthesis and structure-activity relationship of furospinosulin-1 a hypoxia-selective growth inhibitor from marine sponge. Tetrahedron. 2011;67:6673–6678. [Google Scholar]
- 61.Dai J, Liu Y, Zhou Y-D, Nagle DG. Hypoxia-selective antitumor agents: norsesteterpene peroxides from the marine sponge Diacarnus levii preferentially suppress the growth of tumor cells under hypoxia conditions. J. Nat. Prod. 2007;70:648–651. doi: 10.1021/np0604883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Charan RD, McKee TC, Boyd MR. Thorectandrols C, D and E, new sesterterpenes from the marine sponge Thorectandra sp. J. Nat. Prod. 2002;65:492–495. doi: 10.1021/np010439k. [DOI] [PubMed] [Google Scholar]
- 63.De Rosa S, De Stefano S, Zavodnik N. Cacospongionolide: a new antitumoral sesterpene, from the marine sponge Cacospongia mollior. J. Org. Chem. 1988;53:5020–5023. [Google Scholar]
- 64.De Rosa S, De Giulio A, Crispino A, Iodice C, Tommonaro G. Further bioactive sesterterpenes from the Tyrrhenian sponge Fasciospongia cavernosa. Nat. Prod. Res. 1997;10:267–274. [Google Scholar]
- 65.de Stefano D, Tommonaro G, Malik SA, Iodice C, de Rosa S, Maiuri MC, Carnuccio R. Cacospongionolide and scalaradial, two marine sesterterpenoids as potent apoptosis-inducing factors in human carcinoma cell lines. Plos One. 2007;4:e33031. doi: 10.1371/journal.pone.0033031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bae J, Jeon JE, Lee YJ, Lee SH, Sim CJ, Oh KB, Shin J. Sesterterpenes from the tropical sponge Coscinoderma sp. J. Nat. Prod. 2011;74:1805–1811. doi: 10.1021/np200492k. [DOI] [PubMed] [Google Scholar]
- 67.Lefranc F, Kiss R. The sodium pump alpha-1 subunit as a potential target to combat apoptosis-resistant glioblastomas. Neoplasia. 2008;10:198–206. doi: 10.1593/neo.07928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mathieu V, Pirker C, Martin de Lassalle E, Vernier M, Mijatovic T, DeNeve N, Gaussin JF, Dehoux M, Lefranc F, Berger W, Kiss R. The sodium pump alpha-1 subunit: a disease progression-related target for metastatic melanoma treatment. J. Cell. Mol. Med. 2009;13:3960–3972. doi: 10.1111/j.1582-4934.2009.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mijatovic T, Jungwirth U, Heffeter P, Reza Hoda MA, Dornetshuber R, Kiss R, Berger W. The Na+/K+-ATPase is the Achilles Heel of multidrug resistant cancer cells. Cancer Lett. 2009;282:30–34. doi: 10.1016/j.canlet.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 70.Kokubo S, Yogi K, Uddin MJ, Inuzuka T, Suenaga K, Ueda K, Uemura D. Kohamaic acids A and B, novel cytotoxic sesterterpenic acids, from the marine sponge Ircinia sp. Chem. Lett. 2001;2:176–177. [Google Scholar]
- 71.Mizushina Y, Manita D, Takeuchi T, Sugawara F, Kumamoto-Yonezawa Y, Matsui Y, Takemura M, Sasaki M, Yoshida H, Takikawa H. The inhibitory action of kohamaic acid A derivatives on mammalian DNA polymerase β. Molecules. 2009;14:102–121. doi: 10.3390/molecules14010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomez-Paloma L, Randazzo A, Minale L, Debitus C, Roussakis C. New cytotoxic sesterterpenes from the new Caledonian marine sponge Petrosaspongia nigra (Bergquist) Tetrahedron. 1997;53:10451–10458. [Google Scholar]
- 73.Monti MC, Casapullo A, Cavasotto CN, Tosco A, Dal Piaz F, Ziemys A, Margarucci L, Riccio R. The binding mode of petrosaspongiolide M to the human group IIA phospholipase A(2): exploring the role of covalent and noncovalent interactions in the inhibition process. Chem. Eur. J. 2009;15:1155–1163. doi: 10.1002/chem.200801512. [DOI] [PubMed] [Google Scholar]
- 74.Margarucci L, Monti MC, Tosco A, Riccio R, Casapullo A. Chemical proteomics discloses petrosapongiolide M, an antiinflammatory marine sesterterpene, as a proteasome inhibitor. Angew. Chem. – Int. Ed. 2010;49:3960–3963. doi: 10.1002/anie.200907153. [DOI] [PubMed] [Google Scholar]
- 75.Pal A, Young MA, Donato NJ. Emerging potential of therapeutic targeting of ubiquitin-specific proteases in the treatment of cancer. Cancer Res. 2014;74:4955–4966. doi: 10.1158/0008-5472.CAN-14-1211. [DOI] [PubMed] [Google Scholar]
- 76.Johnson DE. The ubiquitin-proteasome system: opportunities for therapeutic intervention in solid tumors. Endocr. Relat. Cancer. 2015;22:T1–T17. doi: 10.1530/ERC-14-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bowden BF, Coll JC, Li H, Cambie RC, Chernan MR, Bergquist PR. New cytotoxic scalarane sesterterpenes from the Dictyoceratida sponge Strepsichorabia lendenfeldi. J. Nat. Prod. 1992;55:1234–1240. doi: 10.1021/np50087a009. [DOI] [PubMed] [Google Scholar]
- 78.Ryu G, Matsunaga S, Fusetani N. Three new cytotoxic sesterterpenes from the marine sponge Hyrtios cf erectus. J. Nat. Prod. 1996;59:515–517. doi: 10.1021/np960130e. [DOI] [PubMed] [Google Scholar]
- 79.Carotenuto A, Fattorusso E, Lanzotti V, Magno S, Carnuccio R, Iuvone T. Antiproliferative sesterterpenes from the caribbean sponge Cacospongia cf. linteiformis. Comp. Biochem. Physiol. 1998;119C:119–123. doi: 10.1016/s0742-8413(97)00197-7. [DOI] [PubMed] [Google Scholar]
- 80.Somerville MJ, Hooper JNA, Garson MJ. Mooloolabenes A-E, norsesterterpenes from the Australian sponge Hyattella intestinalis. J. Nat. Prod. 2006;69:1587–1590. doi: 10.1021/np060244i. [DOI] [PubMed] [Google Scholar]
- 81.Diaz-Marrero AR, Matainaho T, van Soest R, Roberge M, Andersen RJ. Scalarane-based metabolites isolated from the antimitotic extract of the marine sponge Hyrtios erectus. Nat. Prod. Res. 2008;22:1304–1309. doi: 10.1080/14786410701824023. [DOI] [PubMed] [Google Scholar]
- 82.Jeon JE, Bae J, Lii KJ, Oh KB, Shen J. Scalarane sesterterpenes from the sponge Hyatella sp. J. Nat. Prod. 2011;74:847–851. doi: 10.1021/np1006873. [DOI] [PubMed] [Google Scholar]
- 83.Zhang H-J, Fang H-F, Yi Y-H, Lin H-W. Scalarane sesterpenes from the Chinese spongia Phyllospongia foliascens. Helv. Chim. Acta. 2009;92:762–767. [Google Scholar]
- 84.Chang Y-C, Tseng S-W, Liu L-L, Chou Y, Ho Y-S, Lu M-C, Su J-H. Cytotoxic sesterterpenoids from a sponge Hyppospongia sp. Mar. Drugs. 2012;10:987–997. doi: 10.3390/md10050987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schumacher M, Cerella C, Eifes S, Chateuvieux S, Morceau F, Jaspars M, Dicato M, Diederich M. Heteronemin, a spongean sesterterpene, inhibits TNF alpha-induced NF-kB activation through proteasome inhibition and induces apoptotic cell death. Biochem. Pharmacol. 2010;79:610–622. doi: 10.1016/j.bcp.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 86.Rudi A, Yosief T, Schleyer M, Kashman Y. Bilosespens A and B: Two novel cytotoxic sesterpenes from the marine sponge Dysidea cinerea. Org. Lett. 1999;1:471–472. doi: 10.1021/ol990058m. [DOI] [PubMed] [Google Scholar]
- 87.Hussain S, Slevin M, Matou S, Ahmed N, Iqbal Choudhary M, Ranjit R, West D, Gaffney J. Anti-angiogenic activity of sesterterpenes; natural product inhibitors of FGF-2-induced angiogenesis. Angiogenesis. 2008;11:245–256. doi: 10.1007/s10456-008-9108-2. [DOI] [PubMed] [Google Scholar]
- 88.Dal Piaz F, Vassallo A, Lepore L, Tosco A, Bader A, De Tommasi N. Sesterterpenes as tubulin tyrosine ligase inhibitors. First insight of structure-activity relationships and discovery of new lead. J. Med. Chem. 2009;52:3814–3828. doi: 10.1021/jm801637f. [DOI] [PubMed] [Google Scholar]
- 89.Kornienko A, Mathieu V, Rastogi SK, Lefranc F, Kiss R. Therapeutic agents triggering nonapoptotic cancer cell death. J. Med. Chem. 2013;56:4823–4839. doi: 10.1021/jm400136m. [DOI] [PubMed] [Google Scholar]
- 90.Darro F, Decaestecker C, Gaussin JF, Mortier S, Van Ginckel R, Kiss R. Are syngeneic mouse tumor models still valuable experimental models in the field of anti-cancer drug discovery? Int. J. Oncol. 2005;27:607–616. [PubMed] [Google Scholar]
- 91.Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138–3151. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 92.Mathieu V, Mijatovic T, Van Damme M, Kiss R. Gastrin exerts pleitropic effects on human melanoma cell biology. Neoplasia. 2005;7:930–943. doi: 10.1593/neo.05379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chiarugi P, Giannoni E. Anoikis: A necessary death program for anchorage-dependent cells. Biochem. Pharmacol. 2008;76:1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 94.Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272:177–185. doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 95.Wong HH, Lemoine NR. Pancreatic cancer: molecular pathogenesis and new therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2009;6:412–422. doi: 10.1038/nrgastro.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bussink J, van Herpen C, Kaanders J, Oyen W. PET-CT for response assessment and treatment adaptation in head and neck cancer. Lancet Oncol. 2010;11:661–669. doi: 10.1016/S1470-2045(09)70353-5. [DOI] [PubMed] [Google Scholar]
- 97.Kennedy B, Gargoum F, Bystricky BR, Curran DM, O’Connor T. Novel agents in the management of lung cancer. Curr. Med. Chem. 2010;17:4291–4325. doi: 10.2174/092986710793361289. [DOI] [PubMed] [Google Scholar]
- 98.Xu Y, Espinosa-Artiles P, Liu MX, Arnold AE, Gunatilaka AAL. Secoemestrin D, a Cytotoxic Epitetrathiodioxopiperizine Emericellenes AE, Five Sesterterpenoids from Emericella sp AST0036, a Fungal Endophyte of Astragalus lentiginosus. J. Nat. Prod. 2013;76:2330–2336. doi: 10.1021/np400762k. [DOI] [PMC free article] [PubMed] [Google Scholar]