Abstract
Cholesterol is considered indispensible for the recruitment and functioning of integrins in focal adhesions for cell migration. However, the physiological cholesterol pools that control integrin trafficking and focal adhesion assembly remain unclear. Using Niemann Pick Type C1 (NPC) mutant cells, which accumulate Low Density lipoprotein (LDL)-derived cholesterol in late endosomes (LE), several recent studies indicate that LDL-cholesterol has multiple roles in regulating focal adhesion dynamics. Firstly, targeting of endocytosed LDL-cholesterol from LE to focal adhesions controls their formation at the leading edge of migrating cells. Other newly emerging literature suggests that this may be coupled to vesicular transport of integrins, Src kinase and metalloproteases from the LE compartment to focal adhesions. Secondly, our recent work identified LDL-cholesterol as a key factor that determines the distribution and ability of several Soluble NSF Attachment Protein (SNAP) Receptor (SNARE) proteins, key players in vesicle transport, to control integrin trafficking to the cell surface and extracellular matrix (ECM) secretion. Collectively, dietary, genetic and pathological changes in cholesterol metabolism may link with efficiency and speed of integrin and ECM cell surface delivery in metastatic cancer cells. This commentary will summarize how direct and indirect pathways enable LDL-cholesterol to modulate cell motility.
Keywords: cholesterol, cell migration, integrin trafficking, low density lipoprotein, late endosomes, niemann pick type C, SNARE proteins, trans-Golgi-network
Abbreviations
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- HDL
High Density Lipoprotein
- LDL
Low Density Lipoprotein
- NPC1
Niemann Pick Type C1
- LE
late endosomes
- SNARE
Soluble NSF Attachment Protein (SNAP) Receptor
- SNAP23
soluble N-ethylmaleimide-sensitive fusion protein 23
- Stx
syntaxin
- TGN
trans-Golgi network.
Introduction
At the cell surface, heterodimeric integrin receptors composed of α and β subunits bind to ECM, enabling cells to adhere and migrate.1 For cells to move forward, the continuous assembly and disassembly of focal adhesions at the leading edge is critical. Cell surface integrins are central to focal adhesion structure and function, and for cell migration, are constantly internalized and recycled for redistribution at the leading edge.1 Integrin trafficking is regulated by a complex set of proteins, including Rabs, ARFs, protein kinase Cα and p120GAP and coupled to the recruitment and spatiotemporal signaling of numerous signaling proteins including focal adhesion kinase (FAK), Src, Rho GTPases, and growth factor receptors.1 Most relevant to this commentary, cholesterol is essential for focal adhesion function and the ability of cholesterol to stabilize and establish specialized membrane microdomains at the leading edge, provides the compartmentalized platforms for tight coordination of multiple signaling and trafficking events in a temporal and spatial manner relevant for cell migration and crucial to tumor initiation, progression and metastasis.1-3
Role of cholesterol in integrin-dependent cell migration and invasion
Cholesterol is closely connected to integrin function and regulates αvβ3 integrin signal complex formation, β1 integrin recycling and overall cell adhesion and migration.1,4 The underlying mechanisms potentially involve the dynamic recruitment of integrins to specialized, cholesterol-containing microdomains, including focal adhesions and caveolae, a subtype of microdomains that comprises many receptors and signaling proteins driving cell migration.1-3 Indeed, integrin recycling targets cholesterol-containing focal adhesions1 and disrupting the integrity of microdomains inhibits the dynamics of focal adhesion assembly and disassembly in migrating cancer cells.2,5 Furthermore, integrin-mediated adhesion regulates the trafficking and endocytosis of microdomains, including caveolae, but also membrane order at focal adhesions.2,3 Altogether, cholesterol is an important factor for focal adhesion dynamics and integrity. This widely accepted concept is predominantly based on studies employing unphysiological and vigorous cholesterol depletion, using methyl-β-cyclodextrin, at the plasma membrane. As it remains difficult to trace cellular movement of endogenously synthesized or lipoprotein-derived cholesterol, these observations still lack physiological context. Based on recent findings from our laboratory and others, using NPC1 mutant cells as models,6,7 we will discuss direct and indirect pathways of cholesterol pools derived from LDL or High Density lipoprotein (HDL) that can differentially control key events in cell motility.
Cholesterol from the late endosomal compartment contributes to cell migration
Besides de novo synthesis, cells acquire cholesterol by LDL endocytosis. The lack of LDL receptor function in familial hypercholesterolemia or the loss of NPC1/2 proteins causing cholesterol accumulation in LE/lysosomes exemplify its physiological importance.8 Inhibition of cholesterol egress from LE in NPC mutant cells strongly reduces cholesterol levels in other compartments such as the Golgi and plasma membrane.6-9 Importantly, loss of NPC function is associated with enlargement and reduced motility and segregation of LE vesicles, emphasizing dysfunctional membrane trafficking from LE to other sites.7,9-11
Newly emerging literature suggests that blocked LDL-cholesterol export from LE also affects cell migration. In particular, Ikonen and coworkers examined the LDL-cholesterol transport from LE to the plasma membrane in live human A431 squamous carcinoma cells, using BODIPY cholesteryl linoleate-labeled LDL (LDL-BC).7 Interestingly, LDL-BC containing vesicles emanating from LE/lysosomes were CD63-positive and predominantly targeted into the proximity of focal adhesions. In contrast, in NPC1-depleted cells, segregation, motility and arrival of LDL-BC containing vesicles at the plasma membrane as well as formation of focal adhesions at the leading edge of migrating cells, was compromised.7 Focal adhesions only represent a minor fraction of the plasma membrane, indicating a substantial enrichment of LDL-cholesterol from LE in these highly specialized membrane domains. As integrin recycling targets focal adhesions in a cholesterol-dependent manner,1-3 coupling transport of LE-cholesterol with both integrins and CD63 might be essential, possibly enabling CD63 to interact and ensure delivery of integrins to focal adhesions.12 Although NPC1/2 deficiency may also cause accumulation of endogenous cholesterol in LE/lysosomes in some cell types, this could indicate that migrating cells have an increased demand to acquire cholesterol by LDL endocytosis. However, experimental evidence for this hypothesis is still lacking and as outlined below, HDL-derived cholesterol can also promote cell migration.
Several other observations further implicate LDL-cholesterol transport from LE to the cell surface being intimately linked with protein delivery required for cell migration.13-15 Src kinase, which promotes disassembly and turnover of cell-ECM adhesions for cell migration, translocates from LE to focal adhesions.14,15 Remarkably, total and phosphorylated Src protein in liver homogenates from heterozygous and homozygous NPC1 KO-mice are highly upregulated.16 Src protein levels were not increased in Triton-X insoluble fractions, indicating that the majority of total and activated Src from NPC1-deficient hepatocytes is not enriched in cholesterol-rich focal adhesions, but remains in LE and other intracellular sites. Similarly, we observed upregulated total and phosphorylated Src in NPC1 mutant Chinese Hamster Ovary (CHO) cell lines (Hoque and Grewal, unpublished data). Likewise, U18666A-induced LE-cholesterol accumulation similar to the NPC1 mutation, was associated with elevated Src activation in the kidney proximal tubule cell line LLC-PK1.17 Further connecting LDL-cholesterol transport routes with Src-induced tumorigenesis, loss of Endosomal Sorting Complexes Required for Transport (ESCRT) and Rab7 function, 2 regulators of LE membrane transport, not only induce an NPC1-like phenotype,10,11 but are critical for Src translocation from LE to focal adhesions in primary mouse fibroblasts and HeLa cells14,18 Although intracellular Src is mostly inactive, large amounts of oncogenic Src are found in perinuclear structures resembling LE.14,15 Thus, dietary, genetic or pathological fluctuations of LDL levels may be associated with aberrant Src signaling.
Integrins are normally rapidly endocytosed and recycled, while only small amounts are targeted for lysosomal degradation. Interestingly, the Norman laboratory identified that in various invasive cancers, transport of α5β1 integrin and membrane type 1 metalloprotease (MT1-MMP) from LE to the cell surface was critical in maintaining persistency during invasion.13,19-22
LDL-cholesterol regulates cell migration through modulation of secretory pathways emanating from the Golgi apparatus
As outlined above, LDL-cholesterol from LE is transported through exocytic pathways to reach the cell surface. However, multiple exocytic LDL-cholesterol trafficking routes seem to exist. Depending on the cell type and cellular environment, LDL-cholesterol transport from LE to the plasma membrane is direct, or through recycling endosomes and/or TGN compartments.7,8 Several studies from our laboratories strongly suggest that LDL-cholesterol trafficking through TGN and recycling endosomes indirectly controls several important regulators of membrane transport, all of which involved in integrin trafficking and ECM secretion.
Utilizing pharmacological LE-cholesterol accumulation or Annexin A6 overexpression as NPC1 mutant models in CHO and A431 squamous carcinoma, we initially examined caveolin transport, which traffics from the TGN to the plasma membrane in a cholesterol-sensitive manner.9 Inhibition of LE-cholesterol export reduced cholesterol at the Golgi and plasma membrane, and interfered with cholesterol-dependent caveolin-1 transport from the Golgi to the cell surface, thereby reducing numbers of caveolae.9 Hence, LDL-cholesterol delivery to the TGN may be one of the yet unknown balancing factors that determines the ability of caveolin-1 and caveolae to promote integrin internalization, an important step in the transition from cell adhesion to migration.3 Follow-up studies revealed that decreased cholesterol availability in the Golgi of NPC1 mutant-like CHO cells perturbed Golgi recruitment of cytosolic phospholipase A2, which is required to drive cholesterol-dependent Golgi vesiculation, including caveolin transport.23 Interestingly, caveolin-1 is upregulated in NPC1-deficient human and mouse tissues and besides its cholesterol- and integrin-binding properties,2,3 recent studies in human fibroblasts even suggest a direct interaction of caveolin-1 with NPC1 to facilitate cholesterol export from LE.24
Besides caveolin, SNARE proteins are key players in the regulation of secretory pathways emanating from the Golgi apparatus. Therefore, we recently extended our analysis and examined SNARE family members that facilitate targeting of secretory vesicles to the plasma membrane. We first focused on the widely expressed t-SNAREs syntaxin 4 (Stx4) and soluble N-ethylmaleimide-sensitive fusion protein 23 (SNAP23), which cluster in cholesterol-rich cell surface domains25 to promote ECM (fibronectin) secretion and integrin recycling.26 Indeed, cell surface clustering of Stx4 and SNAP23 of NPC1 mutant models in CHO and A431 cells was strongly reduced, with Stx4 and SNAP23 accumulating in Golgi membranes.27 Consequently, Stx4/SNAP23-dependent secretion of fibronectin was strongly inhibited in NPC1 mutant models. Thus, other Stx4/SNAP23-dependent migration events, including α5 integrin, Src and FAK trafficking and activation at focal adhesions, and MT1-MMP delivery to invadopodia in MDA-MB-231 breast cancer cells, may also be affected by LDL-cholesterol egress from LE.28,29
Further insight comes from Stx6, which participates in LE-cholesterol delivery to the ER, and regulates lipid and protein transport, including caveolin, required for caveolae endocytosis.30 In HeLa and other cancer cell models, Stx6 depletion reduces caveolae numbers, impedes α3β1 and α5β1 integrin recycling and delivery of FAK to focal adhesions.6,26,30 Stx6 predominantly interacts with VAMP4 in the TGN, but can shuttle to recycling endosomes and assemble with VAMP3.6,26,30 Most strikingly, cholesterol diminution in the Golgi of NPC1 mutant CHO cells triggered increased Stx6/VAMP3 assembly in recycling endosomes.6 Replenishment of Golgi cholesterol using LDL in NPC mutant cells induced Stx6 recruitment back to the TGN. In contrast, incubation of CHO wild-type cells with High Density lipoproteins (HDL) caused Stx6 dispersion from the TGN to recycling endosomes (Fig. 1).6 HDL binds to the scavenger receptor class B type I (SR-BI) at the cell surface, which facilitates rapid delivery of HDL-cholesterol first to the plasma membrane and then to recycling endosomes,31,32 which could favor Stx6/VAMP3 assembly in the recycling compartment.
Figure 1.
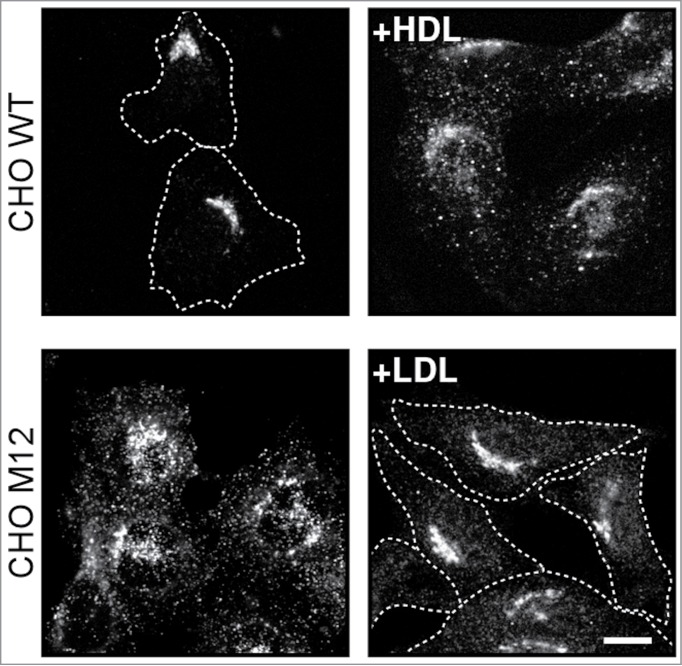
Low and High Density lipoproteins differentially regulate Stx6 localization. (A) Chinese Hamster Ovary (CHO) wild-type (wt) and Niemann Pick Type C (NPC1) mutant CHO M12 cells were incubated ± High Density lipoproteins (HDL; 0.1 mg/ml) for 30 min or Low Density lipoproteins (LDL; 0.05 mg/ml) for 24 hours as indicated. Cells were fixed and immunolabeled with anti-Stx6. Dotted lines indicate cell shape. Bar is 10 μm. Upper panels: Control CHOwt cells show compact staining of Stx6 in the Golgi. HDL induces partially scattered Stx6 staining, resembling recycling endosomes. Lower panels: NPC1 mutant CHO M12 cells show dispersed, endosomal Stx6 staining. Prolonged LDL loading re-establishes compact Stx6 staining in the Golgi.6
Stx6 mislocalization in NPC1 mutant CHO cells and NPC1 patient fibroblasts correlated with diminished αVβ3 and α5β1 integrin surface expression (Fig. 2), reduced migration and invasion in 2- and 3-dimensional environments (Fig. 3).6 This may also involve Rab11, which modulates cholesterol trafficking through recycling endosomes and facilitates β1 integrin recycling.1,33 Taken together, the different transport routes of LDL- and HDL-cholesterol may differentially regulate integrin trafficking events requiring caveolin-1, Stx4/SNAP23 or Stx6, making these regulatory circuits a valuable target sensitive to alterations in cholesterol homeostasis (see working model in Fig. 4).
Figure 2.
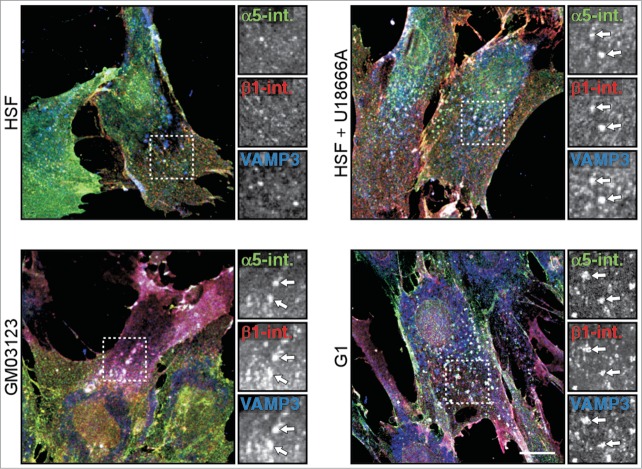
Reduced cell surface expression of integrins in NPC1 mutant cells. Human skin fibroblasts (HSF) from control (± U18666A) and NPC1 patients (G03123, G1) were analyzed for the localization of recycling endosome marker VAMP3 (blue), α5 (green) and β1 integrins (red). α5 and β1 integrins were highly enriched at the cell surface of HSF control fibroblasts, but accumulated and co-localized with VAMP3-positive recycling endosomes in GM03123, G1 and U18666A-treated HSF (see arrows in enlarged inlets). Bar is 10 μm. Figure reproduced from original highlighted manuscript (6 courtesy of Cell Reports).
Figure 3.
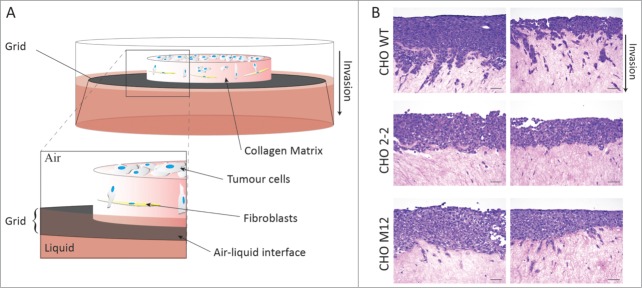
(A) Schematic of an organotypic invasion assay for the 3-dimensional assessment of tumor cell behavior. Primary human fibroblasts are embedded in 3D collagen-I matrix prepared from rat tails by acid extraction. Detached, polymerized matrix is allowed to contract for 5–6 d in media until embedded fibroblasts have contracted the matrix to ∼1.5 cm diameter to form a matrix with high in vivo fidelity. Then tumor cells are plated on top of the matrix in complete media and allowed to grow to confluence for ∼3–5 d The matrix is then mounted on a metal grid and raised to the air/liquid interface, resulting in the matrix being fed from the media below. Media is changed every 1–2 days, and after 8–21 days, cultures can be fixed, processed (e.g. hematoxylin and eosin staining) and analyzed.6,22,46 (B) Representative images of CHO wild-type (CHO WT) and NPC1 mutant cell lines (CHO 2–2, CHO M12) seeded on 3D-matrices and allowed to invade over an air-liquid interface for 21 days, prior to fixation and hematoxylin and eosin staining. Scale bars represent 50 μm. Invasion of NPC1 mutant cells into the organotypic matrix is strongly reduced compared to the control cells.
Figure 4.
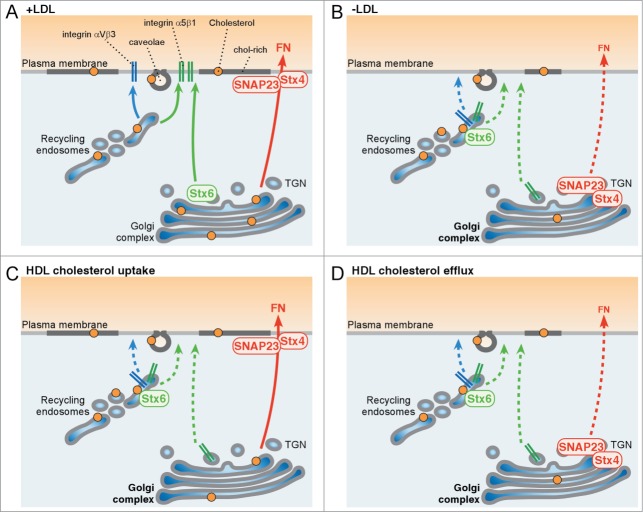
Working model of LDL- and HDL-cholesterol regulating Stx4/SNAP23-mediated fibronectin secretion and Stx6–dependent α5β1 and αvβ3 integrin recycling. (A) + LDL: LDL delivers cholesterol to the plasma membrane, TGN and recycling endosomes. (i) SNAP23 and Stx4 cluster at the plasma membrane to promote fibronectin (FN) secretion. (ii) Stx6 is located in the Golgi to promote α5β1 and αvβ3 recycling through recycling endosomes and the trans-Golgi-network (TGN). (B) – LDL: Inhibition of NPC1-dependent LDL-cholesterol export from late endosomes strongly reduces cholesterol levels at the plasma membrane and TGN. This causes (iii) Stx4/SNAP23 accumulation in the Golgi, which interferes with FN secretion and (iv) Stx6 mislocalization in recycling endosomes, which inhibits α5β1 and αvβ3 recycling. (C) HDL-cholesterol uptake increases cholesterol at the plasma membrane and recycling endosomes. (v) Stx4/SNAP23-dependent FN secretion is not affected, but (vi) Stx6 accumulates in recycling endosomes, which inhibits α5β1 and αvβ3 recycling. (D) HDL-induced cholesterol efflux depletes cellular cholesterol. (iii) This disrupts plasma membrane localization of Stx4/SNAP23 to reduce FN secretion and (iv) blocks the ability of Stx6 to promote α5β1 and αvβ3 recycling.
Intriguingly, recent proteomics identified several cholesterol-binding SNAREs, including Stx4, SNAP23, Stx6, Stx12, VAMP3 and VAMP2.34,35 Besides Stx4/SNAP23, Stx6 and VAMP3 (see above), VAMP2 also participates in transport and recycling of α5, α3, β1 integrin and FAK,26 while Stx12 together with SNAP23 is required for Src and β1 integrin transport to cholesterol-rich invadopodia for cell invasion.29 These regulatory circuits seem to exist in various cancer cell lines, and it is tempting to speculate that all these SNAREs in a large number of cells may respond to temporal and local changes in LDL- or HDL-cholesterol delivery. The molecular basis enabling cholesterol to control integrin trafficking via SNAREs remains to be determined. Fluctuation of cholesterol levels may alter membrane fluidity and SNARE clustering. Alternatively, targeting cholesterol into the vicinity of SNARE complexes or SNARE tethering factors, possibly via vesicular or non-vesicular cholesterol carriers, may allow SNARE-cholesterol interactions.
Is there a link between lipoprotein profiles and metastasis?
LDL- or HDL-derived cholesterol, via caveolin, SNAREs and integrins, could substantially impact on the ability of cancer cells to move and spread. But is there clinical or experimental evidence that possibly link dietary, genetic or pathological changes in cholesterol metabolism with ECM and integrin cell surface delivery in cancer cell migration?
Increased energy requirements are now well recognized as an additional hallmark of cancer, and commonly include an increased demand for cholesterol.36 However, initial retrospective studies addressing the anticancer potential of cholesterol-lowering statins, which effectively inhibit cholesterol synthesis and reduce LDL-cholesterol, were inconclusive. But over the years, statins have been shown to exhibit anti-proliferative and pro-apoptotic effects in numerous clinical and experimental settings.36 By inhibiting the generation of isoprenoids in the cholesterol synthesis pathway, statins effectively block protein prenylation and consequently, appropriate membrane targeting and localization of members of the Ras superfamily of GTPases, with established roles in cell proliferation, survival, migration and invasion.36,37
Lipoprotein receptors and cancer cell migration and invasion
Finally, accumulation of cholesterol and its metabolites appears to contribute to cancer growth and progression. Many cancer cells show elevated LDL receptor levels and increased LDL uptake,36,38 which would favor a scenario enabling caveolin, Stx6, Stx4 and SNAP23 to promote integrin and ECM delivery to the surface. Furthermore, SR-BI is often overexpressed in tumors, and considered to contribute to increase HDL-cholesterol uptake in cancer cells.36,38 Overall, the increased cellular demand for cholesterol may explain consistently low plasma HDL levels and hypocholesterolemia in many cancer patients. Hence, LDL receptor and SR-BI overexpression, together with LDL and HDL particles exhibiting a long residence time in the circulation, showing high-drug payload and small size (<30 nm), enabling deep penetration of tumors, have made LDL and HDL pathways attractive candidates for anticancer drug delivery.36,38 LDL endocytosis may provide options to target players relevant for cell migration in the LE compartment, while SR-BI mediated drug delivery using reconstituted HDL particles may facilitate rapid and localized delivery of Src or FAK kinase or integrin inhibitors to focal adhesions, as substantial amounts of SR-BI are found in cholesterol-rich membrane domains.31,32
Despite the numerous epidemiological studies, it is still unclear if lipoprotein profiles in blood can predict risk of cancer metastasis.38 Insights from cell and animal models seem more conclusive. In MDA-MB-231 breast cancer cells, a classical model for aggressive cell behavior, LDL receptor is upregulated, and LDL stimulates cell migration.40 In A431 epidermoid carcinoma, invasion and efficient integrin recycling are associated with elevated LDL receptor and LDL uptake.21,41 In mice, deficiency of proprotein convertase subtilisin/kexin type 9, which increases hepatic clearance of LDL, reduces melanoma metastasis in liver.42 In MDA-MB-231 cells, knockdown of SR-BI inhibits migration in cell culture and tumor growth in vivo.43 In MMTV-PyMT mice, a well-established mouse model for the development of mammary tumors, a high-fat/high cholesterol diet more rapidly induced aggressive tumors and more pulmonary metastasis than controls. SR-BI was upregulated in these tumors.44 Furthermore, a high-fat and -cholesterol diet in apoE-KO mice, a model that develops hypercholesterolemia, increased mammary metastasis.45 Alikhani and coworkers proposed that the hypercholesterolemic environment may increase formation of cholesterol-rich invadopodia, which as outlined above, have prominent roles for integrins in migration and invasion.45
β1 integrins, in particular α5β1, and αvβ3 integrins, are most promising therapeutic targets in melanoma, breast and prostate cancers,46 all cancers with recognized links to cholesterol metabolism. In the liver, the central organ for lipoprotein metabolism with high LDL receptor and SR-BI levels, α5β1 and αvβ3 integrins are downregulated during development of hepatocellular carcinoma, while other β1 containing integrins increase.47 Clarifying how dietary or pathological changes in LDL or HDL metabolism impact on the biology of integrins may contribute to the development of new biomarkers for clinically stratifying the potential efficacy of integrin-targeting therapies, such as cilengitide or volociximab, or clinically relevant non-receptor tyrosine kinase inhibitors, such as dasatinib or PF00562271, which target focal adhesion-based cross-talk signaling nodes in cancer, such as Src and FAK.48-50
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
We would like to thank all members of our laboratories, past and present, for their invaluable contributions and apologize to all those researchers whose work could not be discussed owing to space limitations.
Funding
T.G. acknowledges support from The University of Sydney, Australia (U1758, U7007). C.E is supported by BFU2012–36272 and CSD2009–00016 from Ministerio de Economía y Competitividad, Spain (MINECO) and PI042182 from Fundació Marató TV3 (Spain). C.R. is thankful to CONSOLIDER-INGENIO (MEC) research program for post-doctoral fellowship. R.M. acknowledges support through the NHMRC Fellowship (457247). P.T. acknowledges support from the NHMRC, Australian Research Council and Cancer Institute New South Wales (CINSW).
References
- 1.Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 2009; 10:843-53; PMID:19904298; http://dx.doi.org/ 10.1038/nrm2799 [DOI] [PubMed] [Google Scholar]
- 2.Gaus K, Le Lay S, Balasubramanian N, Schwartz MA. Integrin-mediated adhesion regulates membrane order. J Cell Biol 2006; 174:725-34; PMID:16943184; http://dx.doi.org/ 10.1083/jcb.200603034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Echarri A, Del Pozo MA. Caveolae internalization regulates integrin-dependent signaling pathways. Cell Cycle 2006; 5:2179-82; PMID:16969102; http://dx.doi.org/ 10.4161/cc.5.19.3264 [DOI] [PubMed] [Google Scholar]
- 4.Ramprasad OG, Srinivas G, Rao KS, Joshi P, Thiery JP, Dufour S, Pande G. Changes in cholesterol levels in the plasma membrane modulate cell signaling and regulate cell adhesion and migration on fibronectin. Cell Motil Cytoskeleton 2007; 64:199-216; PMID:17238130; http://dx.doi.org/ 10.1002/cm.20176 [DOI] [PubMed] [Google Scholar]
- 5.Wang R, Bi J, Ampah KK, Ba X, Liu W, Zeng X. Lipid rafts control human melanoma cell migration by regulating focal adhesion assembly. Biochim Biophys Acta 2013; 1833:3195-205; PMID:24055995; http://dx.doi.org/ 10.1016/j.bbamcr.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 6.Reverter M, Rentero C, Garcia-Melero A, Hoque M, Vilà de Muga S, Alvarez-Guaita A, Conway JR, Wood P, Cairns R, Lykopoulou L, et al.. Cholesterol regulates Syntaxin 6 trafficking at trans-Golgi network endosomal boundaries. Cell Rep 2014; 7:883-97; PMID:24746815; http://dx.doi.org/ 10.1016/j.celrep.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 7.Kanerva K, Uronen RL, Blom T, Li S, Bittman R, Lappalainen P, Peranen J, Raposo G, Ikonen E. LDL cholesterol recycles to the plasma membrane via a Rab8a-Myosin5b-actin-dependent membrane transport route. Dev Cell 2013; 27:249-62; PMID:24209575; http://dx.doi.org/ 10.1016/j.devcel.2013.09.016 [DOI] [PubMed] [Google Scholar]
- 8.Maxfield FR, van Meer G. Cholesterol, the central lipid of mammalian cells. Curr Opin Cell Biol 2010; 22:422-9; PMID:20627678; http://dx.doi.org/ 10.1016/j.ceb.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cubells L, Vilà de Muga S, Tebar F, Wood P, Evans R, Ingelmo-Torres M, Calvo M, Gaus K, Pol A, Grewal T, et al.. Annexin A6-induced alterations in cholesterol transport and caveolin export from the Golgi complex. Traffic 2007; 8:1568-89; PMID:17822395; http://dx.doi.org/ 10.1111/j.1600-0854.2007.00640.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebrand C, Corti M, Goodson H, Cosson P, Cavalli V, Mayran N, Fauré J, Gruenberg J. Late endosome motility depends on lipids via the small GTPase rab7. EMBO J 2002; 21:1289-300; PMID:11889035; http://dx.doi.org/ 10.1093/emboj/21.6.1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du X, Kazim AS, Brown AJ, Yang H. An essential role of Hrs/Vps27 in endosomal cholesterol trafficking. Cell Rep 2012; 1:29-35; PMID:22832105; http://dx.doi.org/ 10.1016/j.celrep.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 12.Pols MS, Klumperman J. Trafficking and function of the tetraspanin CD63. Exp Cell Res 2009; 315:1584-92; PMID:18930046; http://dx.doi.org/ 10.1016/j.yexcr.2008.09.020 [DOI] [PubMed] [Google Scholar]
- 13.Rainero E, Norman JC. Late endosomal and lysosomal trafficking during integrin-mediated cell migration and invasion: cell matrix receptors are trafficked through the late endosomal pathway in a way that dictates how cells migrate. Bioessays 2013; 35:523-32; PMID:23605698; http://dx.doi.org/ 10.1002/bies.201200160 [DOI] [PubMed] [Google Scholar]
- 14.Tu C, Ortega-Cava CF, Winograd P, Stanton MJ, Reddi AL, Dodge I, Arya R, Dimri M, Clubb RJ, Naramura M, et al.. Endosomal-sorting complexes required for transport (ESCRT) pathway-dependent endosomal traffic regulates the localization of active Src at focal adhesions. Proc Natl Acad Sci U S A 2010; 107:16107-12; PMID:20805499; http://dx.doi.org/ 10.1073/pnas.1009471107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timpson P, Jones GE, Frame MC, Brunton VG. Coordination of cell polarization and migration by the Rho family GTPases requires Src kinase activity. Curr Biol 2001; 11:1836-46; PMID:11728306; http://dx.doi.org/ 10.1016/S0960-9822(01)00583-8 [DOI] [PubMed] [Google Scholar]
- 16.Garver WS, Hossain GS, Winscott MM, Heidenreich RA. The Npc1 mutation causes an altered expression of caveolin-1, annexin II and protein kinases and phosphorylation of caveolin-1 and annexin II in murine livers. Biochim Biophys Acta 1999; 1453:193-206; PMID:10036317; http://dx.doi.org/ 10.1016/S0925-4439(98)00101-X [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Li X, Ye Q, Tian J, Jing R, Xie Z. Regulation of alpha1 Na/K-ATPase expression by cholesterol. J Biol Chem 2011; 286:15517-24; PMID:21362623; http://dx.doi.org/ 10.1074/jbc.M110.204396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borg M, Bakke O, Progida C. A novel interaction between Rab7b and actomyosin reveals a dual role in intracellular transport and cell migration. J Cell Sci 2014; 127:4927-39; PMID:25217632; http://dx.doi.org/ 10.1242/jcs.155861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macpherson IR, Rainero E, Mitchell LE, van den Berghe PV, Speirs C, Dozynkiewicz MA, Chaudhary S, Kalna G, Edwards J, Timpson P, et al.. CLIC3 controls recycling of late endosomal MT1-MMP and dictates invasion and metastasis in breast cancer. J Cell Sci 2014; 127:3893-901; PMID:25015290; http://dx.doi.org/ 10.1242/jcs.135947 [DOI] [PubMed] [Google Scholar]
- 20.Yu X, Zech T, McDonald L, Gonzalez EG, Li A, Macpherson I, Schwarz JP, Spence H, Futo K, Timpson P, et al.. N-WASP coordinates the delivery and F-actin-mediated capture of MT1-MMP at invasive pseudopods. J Cell Biol 2012; 199:527-44; PMID:23091069; http://dx.doi.org/ 10.1083/jcb.201203025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL, Gosselin P, et al.. Mutant p53 drives invasion by promoting integrin recycling. Cell 2009; 139:1327-41; PMID:20064378; http://dx.doi.org/ 10.1016/j.cell.2009.11.026 [DOI] [PubMed] [Google Scholar]
- 22.Muller PA, Trinidad AG, Timpson P, Morton JP, Zanivan S, van den Berghe PV, Nixon C, Karim SA, Caswell PT, Noll JE, et al.. Mutant p53 enhances MET trafficking andf signaling to drive scattering and invasion. Oncogene 2013; 32:1252-65; PMID:22580601; http://dx.doi.org/ 10.1038/onc.2012.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cubells L, Vilà de Muga S, Tebar F, Bonventre JV, Balsinde J, Pol A, Grewal T, Enrich C. Annexin A6-induced inhibition of cytoplasmic phospholipase A2 is linked to caveolin-1 export from the Golgi. J Biol Chem 2008; 283:10174-83; PMID:18245088; http://dx.doi.org/ 10.1074/jbc.M706618200 [DOI] [PubMed] [Google Scholar]
- 24.Jelinek D, Heidenreich RA, Orlando RA, Garver WS. The Niemann-Pick C1 and caveolin-1 proteins interact to modulate efflux of low density lipoprotein-derived cholesterol from late endocytic compartments. J Mol Biochem 2014; 3:14-26; PMID:25285302 [PMC free article] [PubMed] [Google Scholar]
- 25.Lang T. SNARE proteins and ‘membrane rafts’. J Physiol 2007; 585:693-8; PMID:17478530; http://dx.doi.org/ 10.1113/jphysiol.2007.134346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riggs KA, Hasan N, Humphrey D, Raleigh C, Nevitt C, Corbin D, Hu C. Regulation of integrin endocytic recycling and chemotactic cell migration by syntaxin 6 and VAMP3 interaction. J Cell Sci 2012; 125:3827-39; PMID:22573826; http://dx.doi.org/ 10.1242/jcs.102566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reverter M, Rentero C, Vilà de Muga S, Alvarez-Guaita A, Mulay V, Cairns R, Wood P, Monastyrskaya K, Pol A, Tebar F, et al.. Cholesterol transport from late endosomes to the Golgi regulates t-SNARE trafficking, assembly, and function. Mol Biol Cell 2011; 22:4108-23; PMID:22039070; http://dx.doi.org/ 10.1091/mbc.E11-04-0332R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams KC, McNeilly RE, Coppolino MG. SNAP23, Syntaxin4, and vesicle-associated membrane protein 7 (VAMP7) mediate trafficking of membrane type 1-matrix metalloproteinase (MT1-MMP) during invadopodium formation and tumor cell invasion. Mol Biol Cell 2014; 25:2061-70; PMID:24807903; http://dx.doi.org/ 10.1091/mbc.E13-10-0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams KC, Coppolino MG. SNARE-dependent interaction of Src, EGFR and beta1 integrin regulates invadopodia formation and tumor cell invasion. J Cell Sci 2014; 127:1712-25; PMID:24496451; http://dx.doi.org/ 10.1242/jcs.134734 [DOI] [PubMed] [Google Scholar]
- 30.Jung JJ, Inamdar SM, Tiwari A, Choudhury A. Regulation of intracellular membrane trafficking and cell dynamics by syntaxin-6. Biosci Rep 2012; 32:383-91; PMID:22489884; http://dx.doi.org/ 10.1042/BSR20120006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grewal T, De Diego I, Kirchhoff MF, Tebar F, Heeren J, Rinninger F, Enrich C. High density lipoprotein-induced signaling of the MAPK Pathway involves scavenger receptor type BI-mediated activation of Ras. J Biol Chem 2003; 278:16478-81; PMID:12637559; http://dx.doi.org/ 10.1074/jbc.C300085200 [DOI] [PubMed] [Google Scholar]
- 32.Heeren J, Beisiegel U, Grewal T. Apolipoprotein E recycling: implications for dyslipidemia and atherosclerosis. Arterioscler Thromb Vasc Biol 2006; 26:442-8; PMID:16373604; http://dx.doi.org/ 10.1161/01.ATV.0000201282.64751.47 [DOI] [PubMed] [Google Scholar]
- 33.Holtta-Vuori M, Tanhuanpaa K, Mobius W, Somerharju P, Ikonen E. Modulation of cellular cholesterol transport and homeostasis by Rab11. Mol Biol Cell 2002; 13:3107-22; PMID:12221119; http://dx.doi.org/ 10.1091/mbc.E02-01-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hulce JJ, Cognetta AB, Niphakis MJ, Tully SE, Cravatt BF. Proteome-wide mapping of cholesterol-interacting proteins in mammalian cells. Nat Methods 2013; 10:259-64; PMID:23396283; http://dx.doi.org/ 10.1038/nmeth.2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enrich C, Rentero C, Hierro A, Grewal T.. Role of cholesterol in SNARE-mediated trafficking on intracellular membranes. J Cell Sci 2015; 128:1071-81; PMID:25653390; doi: 10.1242/jcs.164459 [DOI] [PubMed] [Google Scholar]
- 36.Cruz PM, Mo H, McConathy WJ, Sabnis N, Lacko AG. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: a review of scientific findings, relevant to future cancer therapeutics. Front Pharmacol 2013; 4:119; PMID:24093019; http://dx.doi.org/ 10.3389/fphar.2013.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osmak M. Statins and cancer: current and future prospects. Cancer Lett 2012; 324:1-12; PMID:22542807; http://dx.doi.org/ 10.1016/j.canlet.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 38.Ng KK, Lovell JF, Zheng G. Lipoprotein-inspired nanoparticles for Cancer theranostics. Acc Chem Res 2011; 44:1105-13; PMID:21557543; http://dx.doi.org/ 10.1021/ar200017e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silvente-Poirot S, Poirot M. Cholesterol and cancer, in the balance. Science 2014; 343:1445-6; PMID:24675946; http://dx.doi.org/ 10.1126/science.1252787 [DOI] [PubMed] [Google Scholar]
- 40.Antalis CJ, Uchida A, Buhman KK, Siddiqui RA. Migration of MDA-MB-231 breast cancer cells depends on the availability of exogenous lipids and cholesterol esterification. Clin Exp Metastasis 2011; 28:733-41; PMID:21744083; http://dx.doi.org/ 10.1007/s10585-011-9405-9 [DOI] [PubMed] [Google Scholar]
- 41.Freed-Pastor WA, Mizuno H, Zhao X, Langerod A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li W, Polotskaia A, et al.. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 2012; 148:244-58; PMID:22265415; http://dx.doi.org/ 10.1016/j.cell.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X, Essalmani R, Day R, Khatib AM, Seidah NG, Prat A. Proprotein Convertase Subtilisin/Kexin Type 9 deficiency reduces melanoma metastasis in liver. Neoplasia 2012; 14:1122-31; PMID:23308045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danilo C, Gutierrez-Pajares JL, Mainieri MA, Mercier I, Lisanti MP, Frank PG. Scavenger receptor class B type I regulates cellular cholesterol metabolism and cell signaling associated with breast cancer development. Breast Cancer Res 2013; 15:R87; PMID:24060386; http://dx.doi.org/ 10.1186/bcr3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danilo C, Frank PG. Cholesterol and breast cancer development. Curr Opin Pharmacol 2012; 12:677-82; PMID:22867847; http://dx.doi.org/ 10.1016/j.coph.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 45.Alikhani N, Ferguson RD, Novosyadlyy R, Gallagher EJ, Scheinman EJ, Yakar S, LeRoith D. Mammary tumor growth and pulmonary metastasis are enhanced in a hyperlipidemic mouse model. Oncogene 2013; 32:961-7; PMID:22469977; http://dx.doi.org/ 10.1038/onc.2012.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 2010; 10:9-22; PMID:20029421; http://dx.doi.org/ 10.1038/nrc2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y, Qiao X, Qiao S, Yu L. Targeting integrins in hepatocellular carcinoma. Expert Opin Ther Targets 2011; 15:421-37; PMID:21332366 [DOI] [PubMed] [Google Scholar]
- 48.Nobis M, McGhee EJ, Morton JP, Schwarz JP, Karim SA, Quinn J, Edward M, Campbell AD, McGarry LC, Evans TR, et al.. Intravital FLIM-FRET imaging reveals dasatinib-induced spatial control of src in pancreatic cancer. Cancer Res 2013; 73:4674-86; PMID:23749641; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-4545 [DOI] [PubMed] [Google Scholar]
- 49.Morton JP, Karim SA, Graham K, Timpson P, Jamieson N, Athineos D, Doyle B, McKay C, Heung MY, Oien KA, et al.. Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 2010; 139:292-303; PMID:20303350; http://dx.doi.org/ 10.1053/j.gastro.2010.03.034 [DOI] [PubMed] [Google Scholar]
- 50.Lee BY, Timpson P, Horvath LG, Daly RJ. FAK signaling in human cancer as a target for therapeutics. Pharmacol Ther 2014. [DOI] [PubMed] [Google Scholar]