Abstract
Haptoglobin (Hp) is an acute-phase protein that is produced by the liver to capture the iron that is present in the blood circulation, thus avoiding its accumulation in the blood. Moreover, Hp has been detected in a wide variety of tissues, in which it performs various functions. In addition, this protein is considered a potential biomarker in many diseases, such as cancer, including ovarian carcinoma; however, its participation in the cancerous processes has not yet been determined. The objective of this work was to demonstrate the expression of Hp and its receptor CCR2 in the ovarian cancer cells and its possible involvement in the process of cell migration through changes in the rearrangement of the actin cytoskeleton using western blot and wound-healing assays and confirming by confocal microscopy. Ovarian cancer cells express both Hp and its receptor CCR2 but only after exposure to ascitic fluid, inducing moderated cell migration. However, when the cells are exposed to exogenous Hp, the expression of CCR2 is induced together with drastic changes in the actin cytoskeleton rearrangement. At the same time, Hp induced cell migration in a much more efficient manner than did ascitic fluid. These effects were blocked when the CCR2 synthetic antagonist RS102895 was used to pretreat the cells. These results suggest that Hp-induced changes in the cell morphology, actin cytoskeleton structure, and migration ability of tumor cells, is possibly “preparing” these cells for the potential induction of the metastatic phenotype.
Keywords: actin cytoskeleton, ascitic fluid, CCR2, cell migration, haptoglobin, ovarian cancer, RS102895
Introduction
Haptoglobin (Hp), an acute-phase protein, is produced mainly in the liver, where it is released into the plasma and is considered the major Hemoglobin (Hb)-binding protein.1,2 The Hp-Hb complex is rapidly removed from the blood through endocytosis by specific receptors that are localized on the liver parenchymal cells, after which the internalized complex is then rapidly degraded, avoiding accumulation in the kidney and producing toxicity.3 Although the liver is the major site of Hp expression, other tissues are involved in its expression, including the lung, skin, spleen, and kidney.4-6
The elevation of Hp occurs in infections, inflammation, and various malignant diseases, including lung and bladder cancers,7 leukemia,8 breast cancer,9 malignant lymphoma,10 urogenital tumors,11 and esophageal squamous cell carcinoma.12 In these tissues, Hp exerts direct angiogenic, anti-inflammatory, and immunomodulatory properties in extravascular tissues and body fluids. Hp is also involved in the epithelial-mesenchymal transition (EMT) in oral cancer cell lines with or without IL-6 stimulation.13 Recent reports have shown that Hp may be a chemoattractant for macrophages in chronic inflammatory processes, such as obesity, demonstrating an association of the Hp with the CCR2 receptor that is present in macrophages.14 The presence of Hp has been reported in tumors, in the ascitic fluid of ovarian cancer15 and even in the serum of patients with ovarian cancer.
Ovarian carcinoma (epithelial ovarian cancer) is the most lethal gynecologic malignancy worldwide.16 Like most other epithelial tumors, ovarian carcinoma spreads initially by direct extension into adjacent organs, especially the fallopian tubes, and uterus, and the rectum, bladder, and pelvic sidewall are occasionally also invaded.17 After direct extension, epithelial ovarian cancer most frequently disseminates via the transcoelomic route, affecting multiple vital organs within the abdomen, including the gastrointestinal and genitourinary systems.18
In previous work in our laboratory, we detected the expression of cytoplasmic Hp within the tumors of patients with ovarian carcinoma, whereas this protein was absent from cancer-free ovarian epithelium.19 With these findings, we wonder what could be the role of a protein that is naturally produced in the liver in the microenvironment of the tumor cells, such as ascitic fluid from ovarian carcinoma.
The results described here demonstrate that tumor cells express the Hp gene. However, the protein is synthesized only when the cells are exposed to ascitic fluid. Moreover, when the cells were exposed to ascitic fluid or to Hp, they were able to express the CCR2 receptor, leading to changes in the actin cytoskeleton rearrangements and inducing processes that are important in the beginning of metastasis, as is the migration of tumoral cells. The participation of CCR2 was confirmed through the use of its natural ligand, the monocyte chemattractant protein-1 (MCP-1) and the specific inhibitor RS-102895.
Based on the results that were obtained in this work, we postulate that Hp may be an important factor in the induction of changes in the cellular morphology through the actin cytoskeleton to prepare the cells to initiate their migration toward the peritoneal cavity.
Results
Several studies have proposed haptoglobin, specifically the glycosylated forms that are produced by fucosylation20-22 as a serum marker for different cancer types, including ovarian cancer.2,9,15,23-26 To date, there are no reports describing the role of Hp in ascitic fluid or exploring its role in the processes that are involved in metastasis, such as changes in the cytoskeleton rearrangements that could participate in the migration of tumor cells.
Our previous studies have demonstrated the presence of a personalized signature of Hp α in the ascitic fluid of Mexican patients; moreover, we also showed that Hp is expressed in the cells from ovarian tumors at different stages.19 Therefore, it was important to establish whether the cancer cell itself is capable of synthesising this protein to further understand its biological function within the tumoral environment.
The cell line SKOV-3 and the 2 primary cultures INCan017 and INCan019 that were recovered from the ascitic fluid of Mexican patients were able to express the Hp gene (Fig. 1A). SKOV-3, INCan017 and INCan019 were unable to express the protein when they were harvested from the medium; however, Hp expression was clearly detected when the cells were exposed for at least 24 h to ascitic fluid (Fig. 1B). It is important to note that the induction of the protein was more accentuated in the INCan017 cells, which correlates with the presence of a higher concentration of transcript (Fig. 1A). These results were confirmed in SKOV-3 cell line by the confocal microscopy detection of Hp with null signal detection in the cells that were exposed to the medium and high signal detection in the cells that were exposed to ascitic fluid (Fig. 1C). A commercial Hp protein that was purified from human serum (Std Hp) was used to confirm the specific recognition of the Hp antibody as a positive control (Fig. 1B). Here, we demonstrate that when cells are exposed to ascitic fluid as in their tumoral microenvironment, there is de novo synthesis of Hp.
Figure 1.
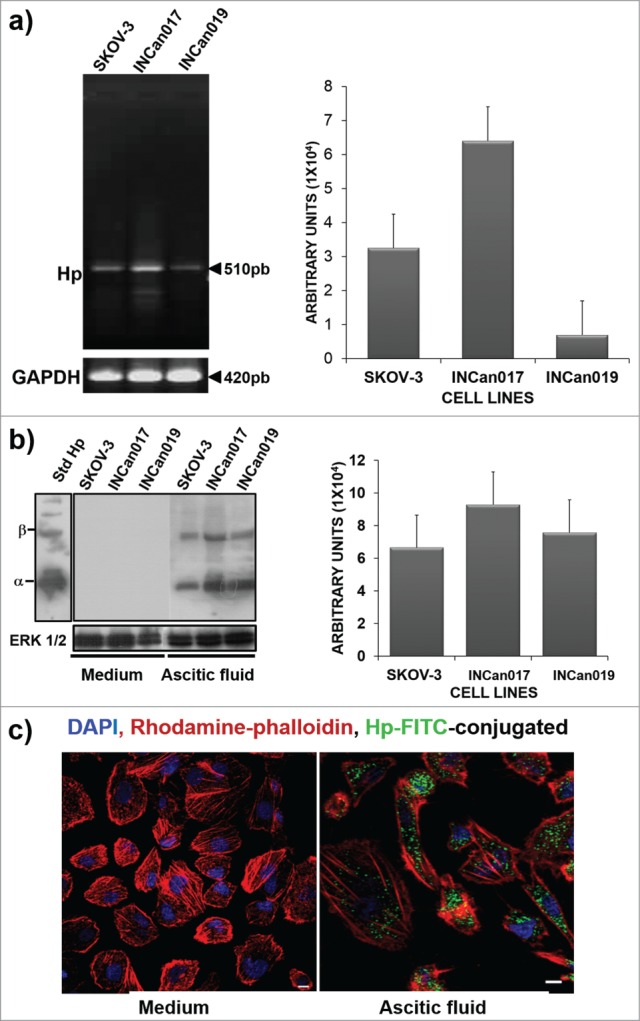
Haptoglobin expression in the SKOV-3 ovarian cancer cell line and primary culture cells (INCan017 and INCan019). (A) RT-PCR assays using specific primers for Hp, showing an amplification product corresponding to 510 bp; (B) Western blot (C) and confocal microscopy (C) analyses using an anti-haptoglobin monoclonal antibody (1:1000, and 1:100, respectively) of cells that were harvested from culture medium or ascitic fluid. The cells in culture medium did not express the protein, whereas these cells, when stimulated with ascitic fluid from an ovarian cancer patient, expressed the haptoglobin protein. Bar scale = 100 μm.
We therefore hypothesize that the tumoral microenvironment that is created by the ascitic fluid induces the expression of both the ligand and its receptor (Hp-CCR2) in a process that is similar to feedback activation. To demonstrate this theory, the total extract from SKOV-3, INCan017 and INCan019 cells that were harvested from the medium was analyzed to detect the expression of CCR2. The results confirmed the absence of this protein under these conditions; these results were confirmed in SKOV-3 cell line by confocal microscopy (Fig. 2A). To demonstrate the de novo expression of CCR2, the same cell lines were incubated with ascitic fluid for 72 h, during which these cells were able to express the CCR2 receptor (Fig. 2B). The presence of Hp in the ascitic fluid and in the cells and of the alternative receptor CCR2 in these cells suggests that if this ligand-receptor combination is present in the ascitic fluid, it could induce cell migration.
Figure 2.
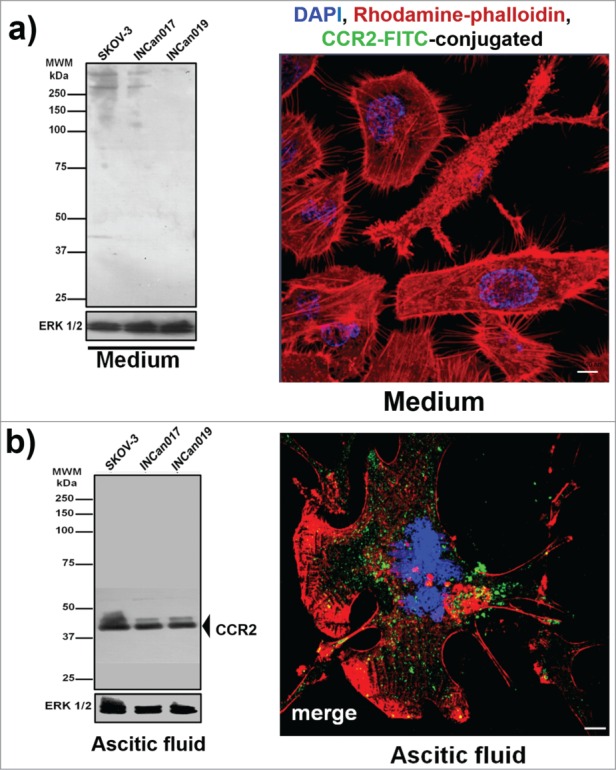
CCR2 (chemokine receptor-2) expression in the ovarian cancer cell line SKOV-3 and primary culture cells (INCan017 and INCan019). (A) Total extracts of tumor cells were separated by electrophoresis and transferred to a nitrocellulose membrane that was incubated with the primary antibodies (α-CCR2, No. Cat. 21667, Abcam; α-ERK 1 (K-23), No. Cat. SC-94, Santa Cruz Biotech, as a loading control). The ovarian cancer cells that were harvested from the culture medium did not express the protein; this result was corroborated by confocal microscopy (105 cells) using an anti-CCR2 antibody (1:100). (B) When the cells were stimulated with ascitic fluid from an ovarian cancer patient, CCR2 protein expression was detected by Western blot and corroborated by confocal microscopy. Bar scale = 100 μm.
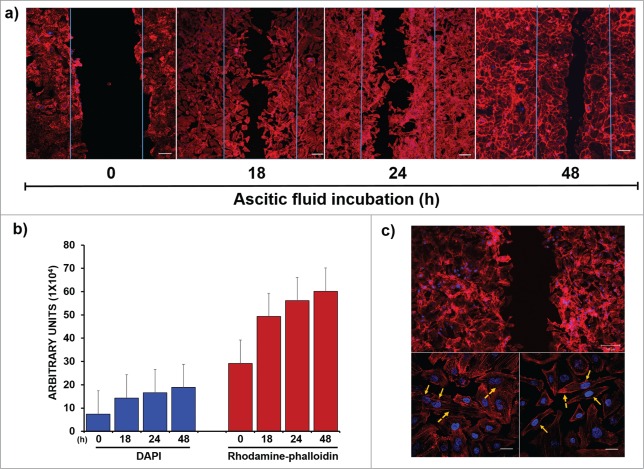
The functional impact of ascitic fluid on the migration of SKOV-3 cells was analyzed in wound and closure assays (Fig. 3A). A wound-healing assay was performed using confocal microscopy to measure changes in actin polymerization and nuclei polarization as 2 important features in the migration processes. The cells that were incubated with ascitic fluid migrated efficiently and almost healed the wound at 48 h. The migration level of each condition was plotted using the Zeiss Software by selecting the area corresponding to the wound and plotting the average relative fluorescence using a histogram under all conditions (Fig. 3B). These results confirmed that ascitic fluid induced cell migration.
Figure 3.
Participation of ascitic fluid in migration of tumor cells. (A) “Wound-Healing Assays” were carried out to measure the capacity of the ascitic fluid as an inducer agent of migration using confocal microscopy: Nuclei were stained with DAPI (blue, 1:50) and structures of polymerized actin by rhodamine-phalloidin (red, 1:25). (B) A quantitative analysis of fluorescence was performed using the Zen 2011 software (Blue edition, Carl Zeiss) considering an average area of 9 × 105 μm2; the program determines the mean fluorescence intensity of each condition, which is plotted in as a histogram. The results were analyzed corresponding to the average of 3 independent experiments, showing a clear increase of fluorescence in both channels due to the migration of cells after 24 h. (C) Morphological changes were analyzed by performing a optical zoom of the cells that were exposed to ascitic fluid (the dashed arrows show the formation of filopodia and extensions of unidirectional membrane protrusions). The nuclear localization in each case is shown with solid arrows. Bar scale = 100 μm
These results were further supported by the analysis of the changes in cell morphology and actin structure during the migration process, where the cells formed protrusions toward the migration front. In the cells that were exposed to ascitic fluid, the formation of lamellipodia with the polarization of actin fibers (dashed arrows) together with the retraction of cell membranes and nuclei polarization opposite to the migration front was visible (solid arrows) (Fig. 3C).
The exposure of tumor cells to ascitic fluid from ovarian cancer patients induced the expression of 2 proteins, Hp and CCR2, which are involved in signal transduction associated with cell migration, an important stage in the beginning of metastasis in cancer. To analyze whether the induction of CCR2 in the SKOV-3 cell line and in the INCan017 and INCan019 primary cultures was a time-dependent process, the kinetics of ascitic fluid exposure were analyzed. The results showed that de novo expression starts after 24 h of ascitic fluid exposition. This expression is maintained longer in INCan017 than in SKOV-3 and INCan019, in which the expression level diminished after this time (Fig. 4A). These results were corroborated by exposing the SKOV-3 cell line to different conditions, medium and ascitic fluid, but adding a new condition in which the cells were also incubated with exogenous Hp (commercially purified from the serum of a healthy human) for 0, 24, and 48 h and analyzed by confocal microscopy. Interestingly, these results not only confirmed the absence of CCR2 expression in the control cells (medium) but also that the expression of the receptor in the cells that were exposed to Hp as the only substrate only occurs after 24 h of incubation (Fig. 4B, green label). These results suggest that Hp is able to induce the expression of its own receptor. This finding is very interesting because it is the first time that a ligand, such as Hp, has been detected in the ascitic fluid, possibly inducing the expression of its putative receptor in a time-dependent manner.
Figure 4.
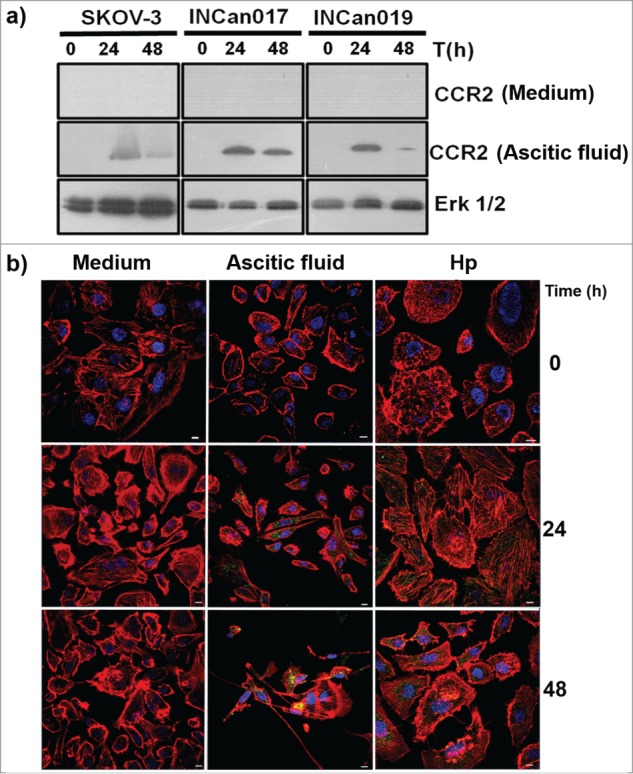
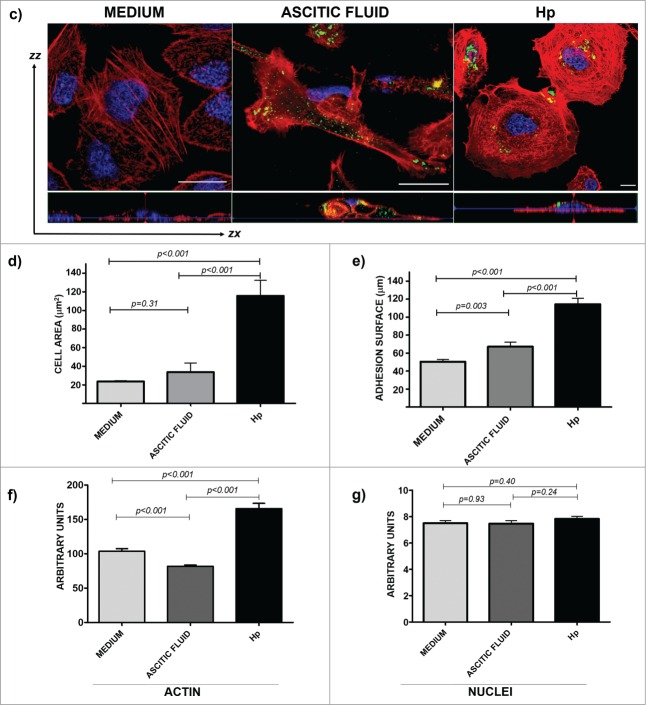
Time-dependent expression of CCR2 in the cells that were exposed to medium, ascitic fluid, and haptoglobin induces changes in actin cytoskeleton. (A) Total extracts of the 3 cell types were processed for protein gel blot assays to analyze the expression level of the CCR2 receptor for 0, 24 and 48 h of exposure in the medium and ascitic fluid. (B) The same kinetics were analyzed by confocal microscopy with a 40 × objective using an LMS 700 confocal microscope; nuclei were stained with DAPI (blue, 1:50), actin structures with rhodamine-phalloidin (red, 1:25), and CCR2 with a primary anti-CCR2 antibody (1:1000 for western blot and 1:100 for confocal microscopy) and a secondary-FITC conjugated antibody (green). The merged zones are shown in yellow. The cells were stimulated with Hp (purified human cancer-free serum). Bar scale = 100 μm. (C) From the cells that were exposed to medium, ascitic fluid or Hp, randomly selected fields to which an optical zoom (crop) was performed were subjected to an ortho analysis to obtain the zx, zy and zz axes from each image. Bar scale = 100 μm. (D-G) A quantitative analysis was performed using the software Zen 2011 (Blue edition, Carl Zeiss). A Shapiro-Wilk analysis was performed to evaluate the data distribution. To determine the differences among the conditions (culture medium, ascitic fluid, and Hp), a Mann-Whitney analysis for non-parametric data was applied. The analyses were performed for at least 30 cells under each condition (P < 0.001).
Figure 4.
Continued
Another interesting feature that was observed in the cells that were exposed to 3 different microenvironments (medium, ascitic fluid, and Hp) was a dramatic change in the rearrangement of the actin cytoskeleton and therefore in the cell morphology in association with CCR2 expression. To analyze and quantify the changes in the actin cytoskeleton in SKOV-3 cells that were exposed to different conditions, random cells were selected, and the fluorescence intensity of the nuclei and polymerized actin was measured through optical orthogonal views (zx, zy and zz, axes for stacks). Under control conditions (medium), polymerized actin forms abundant filaments that are broadly distributed in the cells with a flattened morphology, with lamellipodia and central nuclei, without changes in actin structure throughout the incubation time. Under ascitic fluid condition, the cells were more elongated in shape, with cortical actin filaments with several filopodia and lamellipodia, and most cells touching between them, grouped but with poor actin structure. However, under Hp conditions, the cells exceed 2 or 3 times the size of control cells or of those exposed to ascitic fluid; they were rounded in shape, forming short actin filaments, podosome-like structures, presenting lamellipodia in its membranes, and had plenty of actin points (most likely nucleation centers) and an actin disposition that was very similar to concentric swirls toward the nucleus, with long filaments of actin close to the cell membrane in close contact with the adhesion zone (Fig. 4C).
Figure 4D-G confirms the results described above by a quantitative analysis via confocal microscopy Zen Blue Edition Software (Carl Zeiss). A statistical analysis of several morphological characteristics was performed: the cellular area was manually delimited and measured in μm2 (Fig. 4D), the cellular diameter corresponding to the substrate-contact area was measured in μm (Fig. 4E), arbitrary units of fluorescence intensity were determined for structured actin for every delimited area (Fig. 4F), and of the nuclei (Fig. 4G). For the cellular area, the results indicate a statistically significant increase (P < 0.001) in the cells that were exposed to Hp compared to the cells that were exposed to ascitic fluid and medium, albeit without significant differences between medium and ascitic fluid. When the data from the cellular surface area were analyzed, a statistically significant difference was also found between ascitic fluid and Hp compared to the culture medium (P < 0.001). These changes in the surface area and cell diameter reflect the changes in the actin polymerization; therefore, fluorescence associated with polymerized actin showed significant differences between Hp and ascitic fluid compared to the medium (P < 0.001). Finally, the fluorescent signal from the nuclei in the 3 conditions showed a normal distribution without significant changes (p > 0.001). For measurement information, see supplementary Figure 1.
The invasion of the surrounding tissues and vasculature is an initial step in tumor metastasis that requires cell migration. Hp is a substrate that induces clear changes in tumor cell morphology that are possibly necessary for cell migration and invasion. These processes require the chemotactic migration of cancer cells, steered by the protrusive activity of the cell membrane and its attachment to the extracellular matrix and regulated by elements of the local microenvironment, including the extracellular matrix architecture and other cell types that are found in the tumoral microenvironment.27 These results indicate that Hp is able to change the cell morphology toward a cell migration phenotype and to induce chemotaxis by acting as an alternate ligand for CCR2.
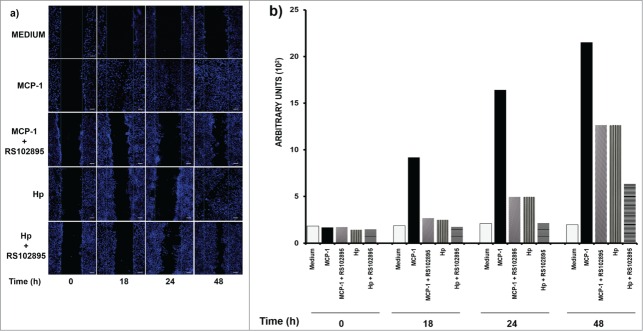
To confirm the role of Hp in cell migration, induced through its interaction with CCR2, we evaluated wound closure by the SKOV-3 cell line when exposed to MCP-1 (250 ng/ml), the CCR2-specific ligand, Hp (250 μg/ml) or BSA (1 mg/ml), herein used as a negative control for chemotaxis (Fig. 5). The data clearly indicate that MCP-1 and Hp were able to induce cancer cell migration but with Hp being less efficient than MCP-1 (Fig. 5A). This event was evaluated using the software for the confocal microscope, showing that the process was more intense when the cells were in contact with MCP-1 and Hp than when the cells were in ascitic fluid (Figs. 3A and 5B). To corroborate migration induction through CCR2 interaction, SKOV-3 cells were pre-incubated for 45 min with the CCR2-specific antagonist RS102895 (5 μM) before wound closure assays. It is important to establish that in this experiment, cell migration was followed only with DAPI staining because, for unknown reasons, RS-102895 modified the signal that was generated by rhodamine-phalloidin, altering the fluorescence emission. The cell responsiveness to MCP1 was reduced by 70%, and a significant reduction of 40% was observed in the capacity of cells to migrate in response to Hp after 18 h of incubation.
Figure 5.
Monocyte chemoattractant protein 1 (MCP1) and haptoglobin (Hp) are affected in their capacity to migrate by CCR2 antagonist (RS1028959 exposition). Wound-healing assays were performed on SKOV-3 cells for 0, 12, 24 and 48 h at the indicated doses of Hp (250 μg) and monocyte chemoattractant protein 1 (MCP1, 250 ng). In addition, the effect of pretreatment with the chemokine (C-C motif) receptor 2 (CCR2) synthetic antagonist RS102895 (5 μM) on the capacity of SKOV-3 cells to migrate toward MCP1 (250 ng/ml) and Hp (0.250 mg/ml) is shown. As a negative control, bovine serum albumin (BSA) (1 mg/ml) was used. Chemotaxis was measured by confocal microscopy; nuclei (blue) were stained with DAPI (1:50). (B) Quantitative analysis of migration by the software Zen 2011 (Blue edition, Carl Zeiss) considering an average area of 9 × 105 μm2. This program determines the mean fluorescence intensity of each condition, which is plotted as a histogram. The results correspond to the average of 3 independent experiments. Bar scale = 100 μm.
The diminishing migration as a response to MCP-1 and Hp after antagonist RS102895 incubation reflects the role of CCR2 signaling in the migration process via interaction with both substrates.
Discussion
Haptoglobin, an acute-phase protein, is generally considered important for the rapid hepatic clearance of hemoglobin from plasma4 by restricting oxidation via the sequestration of free Hb, acting as a powerful antioxidant28,29 inhibiting cathepsin, serving as a chaperone, and assisting in iron recycling.30
The correlation between Hp expression and ovarian carcinoma has been widely reported.31,32 Studies have also shown that both the α- and β-subunits of Hp are significantly increased in early stage ovarian carcinoma patients.33,34 Previous results have shown at least 6 spots of Hp with differences in the protein concentration.35 However, our group has demonstrated differences in both the expression level and the number of Hp α isoforms in ascitic fluid from patients who were diagnosed with different histological grades of ovarian carcinoma.19
The hepatic expression of Hp is strongly induced by inflammatory mediators, such as IL-6 and related cytokines that are released at sites of injury or inflammation28 and whose receptors include CD163 expressed in the monocyte-macrophage system and CD11b (CR3) found on granulocytes, natural killer cells, and small subpopulations of lymphocytes.36 It was recently demonstrated that Hp is a novel chemotactic factor that is able to recruit monocytes, partly mediated by an interaction with the chemokine receptor CCR2 (natural receptor for the monocyte chemotactic protein-1 (MCP-1) in macrophages).37 Hp could by itself induce the migration of tumoral cells to other sites of metastasis, even in the absence of MCP1. These sites of Hp expression could explain the pleiotropic functions of Hp in several diseases and, although not yet fully defined, the function of Hp in other tissues. In the case of ovarian cancer, we believe that although Hp may be initially widespread from the liver to the peritoneal cavity of the patients, as a response to an inflammatory process, at some point the microenvironment of tumor cells present in the ascitic fluid could induce the expression and secretion of Hp, which in turn would induce the expression of its chemoattractant receptor, the CCR2 chemokine receptor. As a result of this interaction via autocrine signaling, cells would be induced to migrate, as a key effect at the beginning of metastasis.
To test this theory, we first analyzed whether the cells that were recovered from the ascitic fluid of patients with ovarian cancer were expressing both the Hp gene and the protein. Surprisingly, we found that although the tumor cells were expressing the Hp RNA when incubated in culture medium, they were unable to synthesize the protein; however, when the cells were exposed to ascitic fluid for at least 24 h, they produced the protein. Hp expression in SKOV-3 has been previously demonstrated, and data from RT-PCR have convincingly demonstrated a strong expression of haptoglobin mRNA in ovarian epithelial cancer cells in culture.38 Therefore, it was possible to use SKOV-3 as a positive control in the Hp gene expression. Interestingly, INCan017 cells showed a higher expression level of both the mRNA and the protein. To confirm the existence of a cell migration signaling pathway that is mediated by Hp in these cells, it was important to determine whether these cells were expressing CCR2. All of the cells expressed the receptor, albeit only when they were exposed to ascitic fluid. The above results confirm that the environment is crucial to inducing changes in tumoral behavior.
The expression of the CCR2 receptor has been demonstrated in a variety of human cell types, including fibroblasts, endothelial cells, smooth muscle cells, epithelial cells and mesangial cells39-41 even by brain endothelial cells42 suggesting that MCP-1 may have functions other than purely driving leukocyte migration during inflammation. Moreover, in prostate cancer, the presence of higher levels of MCP-1 contributes to the development and progression of cancer via 2 major mechanisms: the autocrine effect of MCP-1 as a survival/growth factor for CCR2+ cancer cells and the attraction of tumor associated-macrophages, implicating the key role of this chemokine in supporting tumor survival in an autocrine manner.43 With the previously reported results and the lack of information regarding whether Hp could be part of MCP-1-CCR2 axis in cancer development as an alternative ligand, we emphasize the important role of Hp and MCP-1 as targets for therapy design for cancer.
Confocal microscopy analysis of fluorescence and morphology is becoming the standard tool in cell biology and molecular imaging.44 In this work, confocal microscopy was used as an important tool to measure the changes in actin rearrangements that were involved in the cells that were exposed to different microenvironments. We demonstrated that the exposure to Hp induces extraordinary actin rearrangements, similar to those that have been described during epithelial–mesenchymal transition (EMT) of cells, whose changes start with the dissociation of intercellular junctions as a result of the downregulation of adhesion molecules, such as E-cadherin; therefore, cells adopt a front-back polarity as a result of cytoskeleton reorganization and increase their migratory capacity.45,46 In terms of EMT, no previous studies have provided direct evidence that MCP-1 per se induces EMT in any cell type. However, Soria et al.47 suggested an association between inflammatory mediators and EMT in breast cancer cells, finding that the expression of MCP-1 was related to TNF-α and IL-1β expression and that the continuous stimulation of breast tumor cells by TNF-α and IL-1β led these cells to undergo EMT. Lee et al.,48 demonstrated that the MCP-1/CCR2 system is involved in the process of extracellular matrix accumulation in peritoneal dialysis-related peritoneal fibrosis by inducing peritoneal mesothelial cells to undergo EMT. Numerous previous studies have demonstrated that EMT is involved in a variety of normal physiologic processes, including embryo implantation, embryogenesis, and organ development, as well as pathologic processes, such as cancer metastasis and fibrotic disorders.46 In this work, we noticed a drastic change in cell morphology, suggesting that Hp could possibly induce cellular signaling that would prepare the cells for a new tissue implantation process due to MCP-1 inducing the reorganization of the actin cytoskeleton (stress fiber formation) and the redistribution of the tight junction proteins ZO-1, ZO-2, occludin, and claudin-5 via the RhoA/Rho kinase pathway as a mechanism regulating endothelial permeability.42 Further experiments will be required to demonstrate that Hp is able to induce cell permeability and ultimately EMT.
Thus, an autocrine signaling pathway as a migration mechanism of tumoral cells at the beginning of the dissemination process in ovarian cancer could be a possibility. To demonstrate this theory, confluent wounded cultures were exposed to ascitic fluid or Hp, as well as to MCP-1 and the MCP-1-specific inhibitor RS102895. The effect of Hp on cell migration was even higher than that of the ascitic fluid, albeit lower than the effect of MCP-1. These results not only support a role of Hp as a new chemoattractant but also suggest for the first time that tumoral cell migration could be due to an Hp-mediated signaling pathway in the peritoneal cavity. These findings open the possibility of explaining the initial processes that are generated in the spread of tumor cells into the peritoneal cavity and also open new questions regarding the controversies that are generated around the origin of ovarian cancer.
Methods
Ascitic fluid patient data
This study was conducted with ascitic fluid that was obtained from 2 patients who were diagnosed with ovarian carcinoma and were admitted to the Department of Medical Oncology, the Instituto Nacional de Cancerología de México. These patients were admitted for a first-time diagnosis; the histopathology and tumor grade were assigned by a pathologist according to the International Federation of Gynecology and Obstetrics (FIGO) criteria.20 The study was approved by the Institutional Scientific and Bioethics Committees (protocol numbers INCAN/CC/134/09 and CB/549/09), and written consent was obtained prior to sample collection.
SKOV-3 cell line and primary cell cultures (INCan017 and INCan019)
The SKOV-3 cell line (ATCC, Manassas VA, USA; Cat. No. HTB-77) was originally established from the malignant ascitic fluid of a patient with progressive ovarian carcinoma at Memorial Sloan-Kettering Cancer Center. SKOV-3 cells are capable of generating tumors in the peritoneal cavity of nude mice but are unable to form cell aggregates. Primary cell cultures (INCan017 and INCan019) were obtained from the ascitic fluid from the 2 abovementioned patients (see below).
Culture conditions
All of the cultures included the use of McCoy's 5A medium (ATCC, Manassas, VA, USA; Cat. No. 30-2007) with 10% Fetal Bovine Serum (FBS) (PAA, Piscataway, NJ, USA; Cat. No. A15-701) and 1% penicillin/streptomycin (PAA, Piscataway, NJ, USA; Cat. No. P11-010). Trypsin (Cat. No. T4674) and EDTA (Cat. No. E-4748) both from Sigma, St. Louis, MO, USA, were used for culture propagation.
Purification of the transformed cells from ascitic fluid
Cell selection was performed from 2 malignant ascitic fluids, free of hemolysis (no color) and free of bacteria. The ascitic fluids were placed in culture flasks under sterile conditions and incubated at 37°C with 5% CO2 for at least 3 weeks without any change. Over time, non-tumor cells die (leukocytes, fibroblasts, and erythrocytes), while cancer cells remain adherent and proliferating. After 3 weeks, 10% McCoy's 5A medium that was supplemented with 10% fetal bovine serum was added. At six weeks, the culture medium was removed taking care to not touch attached cells. The adherent cells were gently washed with sterile PBS to remove cellular debris, and McCoy 5A medium that was supplemented with 10% fetal bovine serum was added. The culture flasks were further incubated at 37°C with 5% CO2. Periodically, the cell viability (considering the percentage of confluence and the number of adherent cells) was reviewed. All of the cultures were established with McCoy's 5A medium (ATCC, Cat. No. 30-2007) with 10% Fetal Bovine Serum (FBS) (PAA, Cat. No. A15-701) and 1% penicillin/streptomycin (PAA Cat. No. P11-010). Trypsin (Sigma, Cat. No. T4674) and EDTA (Sigma, Cat. No. E-4748) were used for culture propagation.
CCR2 and Hp detection
For kinetic exposition assays, SKOV-3, INCan017 and INCan019 cells (105) were placed on glass coverslips for 12 h of adaptation and were then starved for 2 h, followed by 3 washes with 1 × PBS before being exposed for different time periods (0, 12, 24, and 48 h) to 3 conditions of stimulation: 2 ml of fresh culture medium (as a negative control), 2 ml of ascitic fluid (as a positive control) and 2 ml of media without SFB, containing 250 μg of Hp protein standard (Abcam, Burlingame, CA USA; Cat. No. ab77872). After the established times, the cells were fixed with 4% paraformaldehyde for 1 h, permeabilized with 0.2% Triton X-100 and blocked with 10% BSA. The following primary antibodies were used: anti-CCR2 (dil 1:100), (Abcam, Burlingame, CA USA; Cat No. ab125686) and anti-Haptoglobin clone 9G10 (dil 1:100) (PIERCE, Rockford, IL USA; Cat. No. HYB 170–06–02). As a secondary antibody, a conjugated goat anti-mouse IgG (H+L) fluorescein (Thermo Scientific, Waltham, MA USA; Cat. No. 31569) was used. Actin was stained with rhodamine-phalloidin (1:25), (Molecular Probes, Gran Island, NY USA; Cat. No. R415) and nuclei with DAPI (1:50), (Molecular Probes, Eugene, OR USA; Cat. No. D1306) for 30 min at 37°C. The coverslips were mounted with VECTASHIELD (Vector Laboratories; Ontario, CA; Cat. No. H-1200) and analyzed by confocal microscopy using a LSM 700 microscope, and the images were analyzed using ZEN 2011 blue edition software (both from Carl Zeiss Group, Oberkochen, Germany).
Wound-healing assays by confocal microscopy
The SKOV-3 ovarian carcinoma cell line (105) was cultured on glass coverslips in the absence of serum for 24 h. After the fasting period, the cells were treated with mitomycin C (0.02 mg/ml, Sigma-Aldrich, St. Louis, MO; Cat. No. M4827) for 2 h to inhibit cell proliferation that could interfere with cell migration. Then, the wounds were carefully made with a 200-μl standard pipette tip in at least 3 different regions across the cell multilayer, so that the surrounding cells were not disturbed. The wounded multilayers were carefully washed twice with 1 × PBS to remove cell debris and to remove mitomycin remnants. Finally, the medium was replaced with fresh complete medium, and the cells were incubated under different conditions: ascitic fluid from an ovarian cancer patient, 250 μg of Hp protein standard (Abcam, Burlingame, CA USA; Cat. No. ab77872) in culture media, 250 ng of monocyte chemoattractant protein 1 (MCP-1) (Abcam, Burlingame, CA USA; Cat. No. A9670) as a positive control, and 1 mg of bovine serum albumin (BSA) (Sigma Aldrich, St. Louis, MO; Cat. No. A9418) as a negative control. At the same time, similar conditions were used for pre-incubation for 45 min at 37°C with CCR2 synthetic antagonist RS102895 (Sigma Aldrich, St. Louis, MO USA; Cat. No. R1903) (5 μM) before the MCP-1 and Hp stimulus to analyze the effect of pretreatment on the ability of SKOV-3 cells to migrate in a volume of 2 ml. The cells were monitored over time by fixing them with 4% paraformaldehyde for 1 h, permeabilized with 0.2% Triton X-100 and blocked with 10% BSA to observe their migratory behavior. The samples were processed by confocal microscopy, and quantitative measurements were made at several culture time points: 0, 24 and 48 h, by determining the distances between the wound-edges. Actin was stained with rhodamine-phalloidin (1:25) (Molecular Probes, Gran Island, NY USA; Cat. No. R415) and nuclei with DAPI (1:50), (Molecular Probes, Eugene, OR, USA; Cat. No. D1306) for 30 min at 37°C. The coverslips were mounted with VECTASHIELD (Vector Laboratories; Ontario, CA, Cat. No. H-1200) and analyzed by a confocal LSM 700 microscope. Cell migration was calculated measuring an identical area for each condition, and the images were analyzed using ZEN 2011 blue edition software (both from Carl Zeiss Group, Oberkochen, Germany).
Western blotting
The total cell extracts (50 μg) were separated by electrophoresis in 12.5% polyacrylamide gels (SDS-PAGE) in duplicate. One sample was stained with the silver stain plus kit (BIO-RAD, Manassas, VA USA; Cat. No. 161-0462 to 64), and the other one was transferred to nitrocellulose membranes (NTCM) for immunodetection. The NTCM was blocked with TBS-T-5% milk for 1 h at room temperature (RT). Subsequently, the filter was incubated overnight at 4°C with the primary antibody [anti-Haptoglobin clone 9G10 (PIERCE, Rockford, IL USA; Cat. No. HYB 170-06-02), anti-CCR2 (Abcam, Burlingame, CA USA; Cat No. ab125686) or anti-ERK1 clone K-23 as a loading control (Santa Cruz Biotech, Dallas, TX USA; Cat. No. sc-94)]. Then, the filters were washed and incubated with a secondary HRP-conjugated antibody (goat anti-mouse IgG, PIERCE, Rockford, IL USA; Cat. No. 31430) in TBS-2.5% milk for 2 h at RT. Finally, the filter was washed, and the signal was revealed by chemiluminescence (SuperSignal West Femto Luminol, Thermo Scientific, Waltham, MA USA; Cat. No. 1856189).
RNA extraction and RT-PCR
SKOV-3, INCan017, and INCan019 cells (105) were cultivated and harvested as described above, centrifuged at 1000 ×g for 5 min and washed with PBS 1X. Purification of total RNA was performed using the Trizol method described by Simms et al.21 The pellet of each cell type was homogenized in an Eppendorf tube with 1 ml of conventional trizol (Life Technologies, Grand Island, NY, Cat. No. 15596-026) and incubated at RT during 15 min, to allow the full decoupling of nucleoproteins. Later, 200 μl of chloroform per ml of trizol were added and homogenized by mixing vigorously for 15 sec; the resulting mixture was incubated at RT for 6 min, centrifuged at 10,000 × g for 15 min at 4 °C. Once the centrifugation was completed, there were an organic red color phase, an interface and a colorless aqueous phase. The aqueous phase was transferred to a new Eppendorf tube and 200 μl of 2-propanol were added and the tubes were incubated at RT for 5 min, and centrifuged at 1000 × g for 15 min at 4 °C. The pellets were washed twice with 0.5 ml of 75% ethanol. The pellets were dried and dissolved in 50 μl DEPC water (di-ethyl pyrocarbonate). The concentration of RNA was measured at an absorbance of 260 nm.
To process the cDNA, first strand cDNA synthesis kit (Thermo scientific, Rockford, IL Cat. No. K1612) was used as indicated by the manufacturer´s protocol [22]. Samples served as a template DNA for 30 rounds of amplification using an iCycler System from BIO-RAD. PCR was performed in a standard 100 μl reaction mixture consisting of 10 μl of 10× Ex taq Buffer, 8 μl of dNTP Mixture, 1 μl of sense and antisense primers, 0.5 μl of Ex taq, 1 μl of cDNA. PCR primers for Hp cDNA were as follow: forward primer, 5'- GGCGTGTGGGT-TATGTTTCT-3'; and anterior primer, 5'- ACCCATCAGCTTCAAACCAC -3'. Amplification was performed at 52.1°C. The amplified PCR products were run on a 1% agarose gel containing 0.005% ethidium bromide. As a loading control for cDNA, amplification of glyceraldehyde-3-phosphate dehydrogenase with the same cDNA was used under identical conditions.
Statistical analysis
A Shapiro-Wilk analysis was performed to evaluate the data distribution. To determine the differences among the conditions (exposed to culture medium, ascitic fluid, and Hp), a Mann-Whitney analysis for non-parametric data was applied. The analyses were performed for at least 30 cells in each condition.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was performed under a General Agreement between the Centro de Investigación y de Estudios Avanzados del IPN (CINVESTAV) and the Instituto Nacional de Cancerología (INCan) and under a Specific Project agreement between the Departamento de Infectómica y Patogénesis Molecular from CINVESTAV and the Departamento de Oncología Médica from INCan.
Authors' Contributions
The work that is presented here was carried out in collaboration between all of the authors. Talamás-Rohana P (TRP) and Garibay-Cerdenares OL (GCOL) were involved in the design of the study. GCOL carried out all of the experiments. TRP, Gallardo-Rincón D (GRD) and Hernández-Ramírez VI (HRVI) were involved in the results interpretation and the manuscript redaction. HRVI, together with GCOL, was involved in confocal image acquisition and analysis. Osorio-Trujillo JC (OTC), together with GCOL, performed tumor cells purification and culture and the propagation of the cell lines. Finally, all of the authors read and approved the final manuscript.
Funding
This work was partially supported by grants 115617 and 233739 from S0008- FONSEC SSA/IMSS/ISSSTE CONACYT, México, and by the GICOM organization. GARIBAY-CERDENARES OL was a recipient of a CONACYT fellowship (210645) and ICyTDF grant B12–007. We thank Herrera-Martínez M, who was involved in the design of Confocal Microscopy analysis, and Belém de-Luna, Maria Elena Cisneros and Irma Miranda for their technical support during this study.
References
- 1.de Kleijn DP, Smeets MB, Kemmeren PP, Lim SK, Van Middelaar BJ, Velema E, Schoneveld A, Pasterkamp G, Borst C. Acute-phase protein haptoglobin is a cell migration factor involved in arterial restructuring. FASEB J 2002; 16:1123-1125; PMID:12039846 [DOI] [PubMed] [Google Scholar]
- 2.Ahmed N, Barker G, Oliva KT, Hoffmann P, Riley C, Reeve S, Smith AI, Kemp BE, Quinn MA, Rice GE. Proteomic-based identification of haptoglobin-1 precursor as a novel circulating biomarker of ovarian cancer. Br J Cancer 2004; 91:129-140; PMID:15199385; http://dx.doi.org/ 10.1038/sj.bjc.6601882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim SK, Kim H, Lim SK, bin Ali A, Lim YK, Wang Y, Chong SM, Costantini F, Baumman H. Increased Susceptibility in Hp Knockout Mice During Acute Hemolysis. Blood 1998; 92(6):1870-1877; PMID:9731043 [PubMed] [Google Scholar]
- 4.D'Armiento J, Dalal SS, Chada K. Tissue, temporal and inducible expression pattern of haptoglobin in mice. Gene 1997; 195(1):19-27; PMID:9300815; http://dx.doi.org/ 10.1016/S0378-1119(97)00123-6 [DOI] [PubMed] [Google Scholar]
- 5.Papp M, Foldi I, Nemes E, Udvardy M, Harsfalvi J, Altorjay I, Mate I, Dinya T, Varvolgyi C, Barta Z, et al.. Haptoglobin polymorphism: a novel genetic risk factor for celiac disease development and its clinical manifestations. Clin Chem 2008; 54(4):697-704; PMID:18258668; http://dx.doi.org/ 10.1373/clinchem.2007.098780 [DOI] [PubMed] [Google Scholar]
- 6.Yang F, Friedrichs WE, Navarijo-Ashbaugh AL, deGraffenried LA, Bowman BH, Coalson JJ. Cell type-specific and inflammatory-induced expression of haptoglobin gene in lung. Lab Invest 1995; 73(3):433-440; PMID:7564277 [PubMed] [Google Scholar]
- 7.Benkmann H, Hanssen HP, Ovenbeck R, Goedde HW. Distribution of alpha-1-antitrypsin and haptoglobin phenotypes in bladder cancer patients. Hum Hered 1987; 37(5):290-293; PMID:3499374; http://dx.doi.org/ 10.1159/000153720 [DOI] [PubMed] [Google Scholar]
- 8.Mitchell R, Carzino R, Janardhana V. Associations between the two serum proteins haptoglobin and transferrin and leukaemia. Hum Hered 1988; 38(3):144-150; PMID:3397067; http://dx.doi.org/ 10.1159/000153775 [DOI] [PubMed] [Google Scholar]
- 9.Awadallah SM, Atoum MF. Haptoglobin polymorphism in breast cancer patients form Jordan. Clin Chim Acta 2004; 341(1):17-21; PMID:14967153; http://dx.doi.org/ 10.1016/j.cccn.2003.10.032 [DOI] [PubMed] [Google Scholar]
- 10.Epelbaum R, Shalitin C, Segal R, Valansi C, Arselan I, Faraggi D, Leviov M, Ben-Shahar M, Haim N. Haptoglobin-related protein as a serum marker in malignant lymphoma. Pathol Oncol Res 1998; 4(4):271-276; PMID:9887357; http://dx.doi.org/ 10.1007/BF02905217 [DOI] [PubMed] [Google Scholar]
- 11.Dunzendorfer U, Jung K, Ohlenschläger G. Transferrin, C3 complement, haptoglobin, plasminogen and alpha 2-microglobulin in patients with urogenital tumors. Eur Urol 1980; 6(4):232-236; PMID:6156074 [DOI] [PubMed] [Google Scholar]
- 12.An JY, Fan ZM, Zhuang ZH, Qin YR, Gao SS, Li JL, Wang LD. Proteomic analysis of blood level of proteins before and after operation in patients with esophageal squamous cell carcinoma at high-incidence area in Henan Province. World J Gastroenterol 2004; 15(10):3365-3368; PMID:15484320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CC, Ho HC, Chien SH, Hsiao SH, Hung SK, Huang TT, Yu CC, Chang SM, Huang HH, Su YC. Association of acute phase protein-haptoglobin, and epithelial-mesenchymal transition in buccal cancer: a preliminary report. Clin Chem Lab Med 2012; 1(13):1-8; PMID:23093274 [DOI] [PubMed] [Google Scholar]
- 14.Maffei M, Funicello M, Vottari T, Gamucci O, Costa M, Lisi S, Viegi A, Ciampi O, Bardi G, Vitti P, et al.. The obesity and inflammatory marker haptoglobin attracts monocytes via interaction with chemokine (C-C motif) receptor 2 (CCR2). BMC Biol 2009; 7:87; PMID:20017911; http://dx.doi.org/ 10.1186/1741-7007-7-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elg S, Carson LF, Fowler JM, Twiggs LB, Moradi MM, Ramakrishnan S. Ascitic fluid levels of haptoglobin in patients with ovarian cancer. Cancer 1993; 71(12):3938-3941; PMID:8389656; http://dx.doi.org/ 10.1002/1097-0142(19930615)71:12%3c3938::AID-CNCR2820711223%3e3.0.CO;2-Q [DOI] [PubMed] [Google Scholar]
- 16.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63(1):11-30; PMID:23335087; http://dx.doi.org/ 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 17.Amadori D, Sansoni E, Amadori A. Ovarian cancer: natural history and metastatic pattern. Front Biosci; 1997; 1(2):56-59; PMID:9159259 [PubMed] [Google Scholar]
- 18.Tan DSP, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol 2006; 7(11):925-934; PMID:17081918; http://dx.doi.org/ 10.1016/S1470-2045(06)70939-1 [DOI] [PubMed] [Google Scholar]
- 19.Garibay-Cerdenares O, Hernández-Ramírez VI, Osorio-Trujillo JC, Hernández-Ortíz M, Gallardo-Rincón D, Cantú de León D, Encarnación-Guevara S, Villegas-Pineda JC, Talamás-Rohana P. Proteomic identification of fucosylated haptoglobin alpha isoforms in ascitic fluids and its localization in ovarian carcinoma tissues from Mexican patients. J Ovarian Res 2014; 7(1):27; PMID:24576319; http://dx.doi.org/ 10.1186/1757-2215-7-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fish RG, Gill TS, Adams M, Kerby I. Serum haptoglobin and α1-acid glycoprotein as indicators of the effectiveness of cis-diamminedichloroplatinum (CDDP) in ovarian cancer patients—a preliminary report. Eu J Cancer Clin Oncol 1984; 20(5):625-630; PMID:6539699; http://dx.doi.org/ 10.1016/0277-5379(84)90007-5 [DOI] [PubMed] [Google Scholar]
- 21.Black JA, Dixon GH. Amino-acid sequence of alpha chains of human haptoglobins. Nature 1968; 218(5143):736-741; PMID:4172407; http://dx.doi.org/ 10.1038/218736a0 [DOI] [PubMed] [Google Scholar]
- 22.Carter K, Worwood M. Haptoglobin: a review of the major allele frequencies worldwide and their association with diseases. Int J Lab Hematol 2007; 29(2):92-110; PMID:17474882; http://dx.doi.org/ 10.1111/j.1751-553X.2007.00898.x [DOI] [PubMed] [Google Scholar]
- 23.Di Girolamo F, Righetti PG. Plasma proteomics for biomarker discovery: A study in blue. Electrophoresis 2011; 32(24):3638-3644; PMID:22180212; http://dx.doi.org/ 10.1002/elps.201100307 [DOI] [PubMed] [Google Scholar]
- 24.Hays JL, Kim G, Giuroiu I, Kohn EC. Proteomics and ovarian cancer: integrating proteomics information into clinical care. J Proteomics 2010; 73(10):1864-1872; PMID:20561909; http://dx.doi.org/ 10.1016/j.jprot.2010.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdul-Rahman PS, Lim BK, Hashim OH. Expression of high-abundance proteins in sera of patients with endometrial and cervical cancers: Analysis using 2-DE with silver staining and lectin detection methods. Electrophoresis 2007; 28:1989-1996; PMID:17503403; http://dx.doi.org/ 10.1002/elps.200600629 [DOI] [PubMed] [Google Scholar]
- 26.Fujimura T, Shinohara Y, Tissot B, Pang PC, Kurogochi M, Saito S, Arai Y, Sadilek M, Murayama K, Dell A, et al.. Glycosylation status of haptoglobin in sera of patients with prostate cancer vs. benign prostate disease or normal subjects. Int J Cancer 2008; 122(1):39-49; PMID:17803183; http://dx.doi.org/ 10.1002/ijc.22958 [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Op Cell Biol 2005; 17(5):559-564; PMID:16098726; http://dx.doi.org/ 10.1016/j.ceb.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 28.Tseng CF, Lin CC, Huang HY, Liu HC, Mao SJ. Antioxidant role of human haptoglobin. Proteomics 2004; 4(8):2221-2228; PMID:15274115; http://dx.doi.org/ 10.1002/pmic.200300787 [DOI] [PubMed] [Google Scholar]
- 29.Friedrichs WE, Navarijo-Ashbaugh AL, Bowman BH, Yang F. Expression and inflammatory regulation of haptoglobin gene in adipocytes. Biochem Biophys Res Commun 1995; 209(1):250-256; PMID:7726843; http://dx.doi.org/ 10.1006/bbrc.1995.1496 [DOI] [PubMed] [Google Scholar]
- 30.Wassell J. Haptoglobin: function and polymorphism. Clin Lab 2000; 46(11–12):547-552; PMID:11109501 [PubMed] [Google Scholar]
- 31.Mueller W, Handschumacher R, Wade M. Serum haptoglobin in patients with ovarian malignancies. Obstet Gynecol 1971; 38(3):427-435; PMID:4999312 [PubMed] [Google Scholar]
- 32.Thompson S, Dargan E, Turner G. Increased fucosylation and other carbohydrate changes in haptoglobin in ovarian cancer. Cancer Lett 1992; 14(66):43-48; PMID:1451094; http://dx.doi.org/ 10.1016/0304-3835(92)90278-4 [DOI] [PubMed] [Google Scholar]
- 33.Ye B, Cramer DW, Skates SJ, Gygi SP, Pratomo V, Fu L, Horick NK, Licklider LJ, Schorge JO, Berkowitz RS, et al.. Haptoglobin-α Subunit as potential serum biomarker in ovarian cancer: identification and characterization using proteomic profiling and mass spectrometry. Clin Cancer Res 2003; 9(8):2904-2911; PMID:12912935 [PubMed] [Google Scholar]
- 34.Ahmed N, Oliva KT, Barker G, Hoffmann P, Reeve S, Smith IA, Quinn MA, Rice GE. Proteomic tracking of serum protein isoforms as screening biomarkers of ovarian cancer. Proteomics 2005; 5(17):4625-4636; PMID:16220531; http://dx.doi.org/ 10.1002/pmic.200401321 [DOI] [PubMed] [Google Scholar]
- 35.Ngounou Wetie AG, Sokolowska I, Woods AG, Wormwood KL, Dao S, Patel S, Clarkson BD, Darie CC. Automated mass spectrometry-based functional assay for the routine analysis of the secretome. JALA 2013; 18(1):19-29; PMID:22853965 [DOI] [PubMed] [Google Scholar]
- 36.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature 2001; 409(6817):198-201; PMID:11196644; http://dx.doi.org/ 10.1038/35051594 [DOI] [PubMed] [Google Scholar]
- 37.Margherita M, Funicello M, Vottari T, Gamucci O, Costa M, Lisi S, Viegi A, Ciampi O, Bardi G, Vitti P, et al.. The obesity and inflammatory marker haptoglobin attracts monocytes via interaction with chemokine (C-C motif) receptor 2 (CCR2). BMC Biology 2009; 7(1):87-87; PMID:20017911; http://dx.doi.org/ 10.1186/1741-7007-7-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao C, Annamalai L, Guo C, Kothandaraman N, Koh SC, Zhang H, Biswas A, Choolani M. Circulating haptoglobin is an independent prognostic factor in the sera of patients with epithelial ovarian cancer. Neoplasia 2007; 9(1):1-7; PMID:17325738; http://dx.doi.org/ 10.1593/neo.06619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elner S, Strieter RM, Elner VM, Rollins BJ, Del Monte MA, Kunkel SL. Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Lab Invest 1991; 64(6):819-825; PMID:2046333 [PubMed] [Google Scholar]
- 40.Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M,Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci 1990; 87(13):5134-5138; PMID:1695010; http://dx.doi.org/ 10.1073/pnas.87.13.5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rovin BH, Yoshiumura T, Tan L. Cytokine-induced production of monocyte chemoattractant protein-1 by cultured human mesangial cells. J Immunol 1992; 148(7):2148-53; PMID:1532001 [PubMed] [Google Scholar]
- 42.Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci 2003; 116(22):4615-4628; PMID:14576355; http://dx.doi.org/ 10.1242/jcs.00755 [DOI] [PubMed] [Google Scholar]
- 43.Izhak L, Wildbaum G, Jung S, Stein A, Shaked Y, Karin N. Dissecting the autocrine and paracrine roles of the CCR2-CCL2 axis in tumor survival and angiogenesis. PLoS One 2012; 7(1):e28305; PMID:22279523; http://dx.doi.org/ 10.1371/journal.pone.0028305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaritsky A, Natan S, Horev J, Hecht I, Wolf L, Ben-Jacob E, Tsarfaty I. A novel segmentation algorithm to quantify multi-celular bright field microscopy images. PLoS One 2011; 6(11):e27593; PMID:22096600; http://dx.doi.org/ 10.1371/journal.pone.0027593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aroeira LS, Aguilera A, Sánchez-Tomero JA, Bajo MA, del Peso G, Jiménez-Heffernan JA, Selgas R, López-Cabrera M. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol 2007; 18(7):2004-2013; PMID:17568021; http://dx.doi.org/ 10.1681/ASN.2006111292 [DOI] [PubMed] [Google Scholar]
- 46.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119(6):1420-1428; PMID:19487818; http://dx.doi.org/ 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soria G, Ofri-Shahak M, Haas I, Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T, Weitzenfeld P, Meshel T, Shabtai E, Gutman M, et al.. Inflammatory mediators in breast cancer: coordinated expression of TNFα & IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition. BMC Cancer 2011; 12(11):130; PMID:21486440; http://dx.doi.org/ 10.1186/1471-2407-11-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee EY, Chung CH, Khoury CC, Yeo TK, Pyagay PE, Wang A, Chen S. The monocyte chemoattractant protein-1/CCR2 loop, inducible by TGF-β, increases podocyte motility and albumin permeability. Am J Phisiol 2009; 297(1):F85-F94; PMID:19420107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.