Abstract
Herein we report the synthesis and characterization of a novel series of N-phenylsulfonyl-1H-pyrrole picolinamides as novel positive allosteric modulators of mGlu4. We detail our work towards finding phenyl replacements for the core scaffold of previously reported phenyl sulfonamides and phenyl sulfone compounds. Our efforts culminated in the identification of N-(1-((3,4-dimethylphenyl)sulfonyl)-1H-pyrrol-3-yl)picolinamide as a potent PAM of mGlu4.
Keywords: Metabotropic glutamate receptor 4, Positive allosteric modulator (PAM), Pyrrole sulfonamide, Phenyl replacement, PK
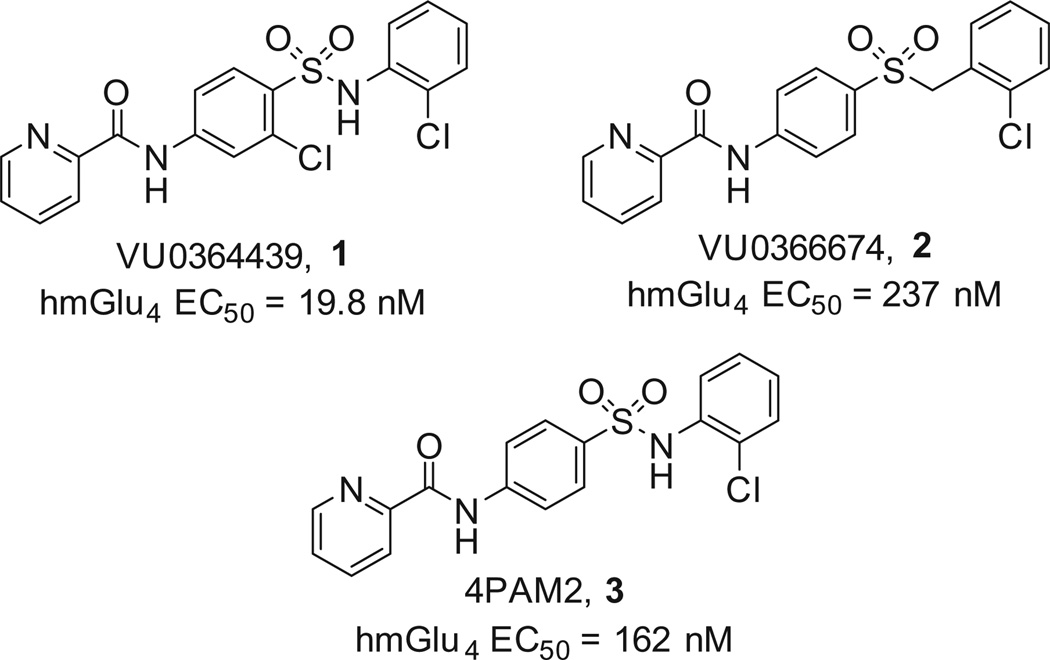
The metabotropic glutamate receptors are Class C G-protein coupled receptors (GPCRs) and are further separated into three family classes based on their receptor structure, G-protein coupling and ligand selectivity (Group I: mGlu1 and mGlu5; Group II: mGlu2 and mGlu3; Group III: mGlu4, mGlu6, mGlu7 and mGlu8).1 This family of GPCRs has received significant attention over the past 10 years as potential therapeutic targets for a number of CNS disorders, such as schizophrenia,2–4 Fragile X,5,6 generalized anxiety disorder7,8 and Parkinson’s disease.9–14 Until recently, the Group III family had received less attention than the Group I and Group II family receptors; however, many tool compounds for the mGlu4 subtype have been disclosed recently from our laboratories,15–17 and others.18–20 A common motif in many of the disclosed compounds is the presence of an N-phenyl picolinamide core scaffold.15,16,21,22 There have been reports of efforts aimed at discovering amide replacements23; however, there have not been disclosures around replacement of the central phenyl ring system. Herein, we report our efforts towards phenyl ring replacements culminating in the discovery of a unique series of N-pyrrolesulfonamides as novel PAMs of mGlu4.
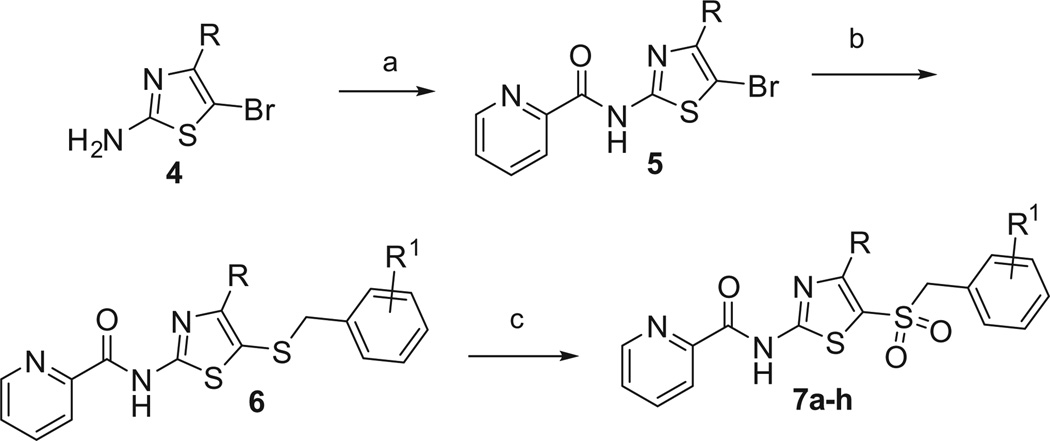
The starting point for our exploration centered around a series of phenylsulfonamides and phenylsulfones that were previously disclosed (Fig. 1).24,25 Our first attempts at replacing the phenyl group centered on a thiazole ring and utilized the sulfone moiety as in 2 in order to limit the number of H-bond donors in the molecule.26 The synthesis started with the commercially available 5-bromo-thiazol-2-amine (4-Me, Et, or H) which was acylated to yield the picolinamide 5 (picolinyl chloride, DiPEA, CH2Cl2) (Scheme 1). Next, palladium catalyzed cross-coupling of the bromothiazole, 5, with an appropriately substituted benzylthiol (Pd2(dba)3, XantPhos, DIEA, 1,4-dioxane, 100 °C) yielded the N-(5-(benzylthio)-thiazol-2-yl)picolinamide, 6.27 Lastly, oxidation of the sulfide to the sulfone (m-CPBA, CH2Cl2) yielded the desired compounds, 7a–h.
Figure 1.
Previously disclosed phenylsulfonamide and phenylsulfone mGlu4 PAMs.
Scheme 1.
Reagents and conditions. (a) Picolinyl chloride·HCl, diisopropylethylamine, CH2Cl2, 24–39%; (b) Pd2(dba)3, XantPhos, HSCH2Ar, diisopropylethylamine, 1,4-dioxane, 100 °C, 51–87%; (c) m-CPBA, CH2Cl2, 38–87%.
The SAR analysis of the thiazole sulfone analogs is summarized in Table 1. Gratifyingly, the thiazole is a tolerated replacement for the internal phenyl ring as the thiazole derivative, 7a, is equipotent with the phenyl derivative, 2 (7a, EC50 = 189 nM vs 2, EC50 = 237 nM). In addition to R1 = H, the methyl and ethyl substituents are also tolerated in the 4-position (7b, EC50 = 448 nM; 7e, EC50 = 217 nM). Overall, the replacement of the phenyl group with a thiazole was a productive change in terms of potency with 7c being one of the most potent compounds discovered to date (EC50 = 83 nM). The sulfide intermediates, 6, were tested and were significantly less potent (EC50’s = 5000–8000 nM); the sulfoxides were not tested as the oxidation procedure led directly to the sulfones. Unfortunately, these compounds suffered from significant pharmacokinetic liabilities (poor brain penetration, metabolic instability) limiting the utility of these compounds to in vitro tool compounds.
Table 1.
SAR of the sulfonylthiazoles, 7a–h
 | |||||
|---|---|---|---|---|---|
| Compd | R1 | R2 | mGlu4 EC50
a (nM) |
pEC50 ± SEMa | %GluMaxb |
| 7a | H |  |
189 | 6.72 ± 0.13 | 30.6 ± 0.5 |
| 7b | Me |  |
448 | 6.35 ± 0.06 | 94.8 ± 3.6 |
| 7c | H |  |
83 | 7.08 ± 0.14 | 35.1 ± 1.2 |
| 7d | Me |  |
4137 | 5.38 ± 0.06 | 112.5 ± 1.6 |
| 7e | Et |  |
217 | 6.66 ± 0.09 | 119.9 ± 1.3 |
| 7f | Me |  |
547 | 6.26 ± 0.05 | 93.5 ± 1.8 |
| 7g | Me |  |
295 | 6.53 ± 0.09 | 99.3 ± 1.7 |
| 7h | Et |  |
150 | 6.82 ± 0.12 | 109.0 ± 1.4 |
Calcium mobilization human mGlu4 assay; values are the average of n = 3.
Amplitude of response in the presence of 30 µM test compound, normalized to a standard compound, PHCCC, and represented as %GluMax.
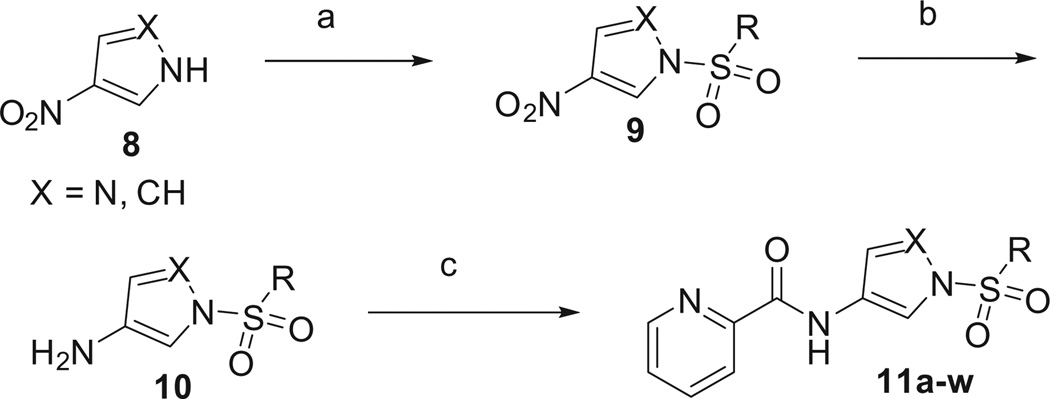
Next, we turned our attention to other replacement groups, namely, pyrrole and pyrazole.28,29 The synthesis of these groups is detailed in Scheme 2. The nitro pyrrole (or pyrazole) was converted to the sulfonamide (DBU, RSO2Cl) and then the nitro was reduced to the amino compound using Raney Nickel, 10 (EtOH, Ra–Ni, 40 psi H2). The final compounds were completed via acylation of the amino group with the acid chloride (picolinyl chloride, DIEA) yielding the desired compounds, 11a–w.30,31
Scheme 2.
Reagents and conditions. (a) DBU, ArSO2Cl or BnSO2Cl, CH2Cl2, 50–96%; (b) EtOH, Ra–Ni, 40 psi H2, 2 h; 93–97%; (c) diisopropylethylamine, picolinyl chloride, CH2Cl2, 39–43%.
The initial set of compounds evaluated were the benzyl sulfonamides, comparators to the thiazole benzylsulfones. Similar to the thiazole core compounds, the pyrrole compounds were well tolerated, with the 2-chlorobenzyl sulfonamide being equipotent to the thiazole (7a) and phenyl (2) derivatives (11g, EC50 = 174 nM). As seen previously, most of the benzyl compounds that we evaluated were active as mGlu4 PAMs with most EC50’s < 500 nM. Although these compounds were active as PAMs, they suffered from the same PK liabilities as the thiazole compounds, namely, metabolic stability issues. It was determined that oxidation of the benzylic CH2 group was the major metabolic liability and efforts were undertaken to block this site. Thus, compounds 11i–k were synthesized. Unfortunately, these compounds were significantly less potent as mGlu4 PAMs, with the mono-fluoro compound being the most active (11j, EC50 = 1024 nM). In addition, blocking the presumed site of metabolism did not inherently improve the metabolic stability of these compounds, presumably due to a shift of the site of instability to other areas of the molecule (Table 2).
Table 2.
SAR of the pyrrole benzylsulfonamides, 11a–k
 | ||||||
|---|---|---|---|---|---|---|
| Compd | R1 | R2 | R3 | mGlu4 EC50 a (nM) |
pEC50 ± SEMa | %GluMaxb |
| 11a |  |
H | H | 572 | 6.24 ± 0.04 | 98.6 ± 2.6 |
| 11b | 188 | 6.72 ± 0.04 | 88.7 ± 4.4 | |||
| 11c |  |
4179 | 5.38 ± 0.16 | 112.3 ± 5.3 | ||
| 11d |  |
84 | 7.07 ± 0.04 | 122.4 ± 3.7 | ||
| 11e | 398 | 6.40 ± 0.06 | 140.3 ± 1.7 | |||
| 11f |  |
251 | 6.60 ± 0.07 | 117.2 ± 7.1 | ||
| 11g |  |
174 | 6.76 ± 0.02 | 119.0 ± 3.5 | ||
| 11h |  |
190 | 6.72 ± 0.04 | 108.9 ± 5.2 | ||
| 11i | Me | H | 2970 | 5.53 ± 0.26 | 59.6 ± 12.7 | |
| 11j |  |
F | H | 1021 | 5.99 ± 0.02 | 71.0 ± 2.6 |
| 11k |  |
F | F | >30,000 | <4.5 | 33.5 ± 4.9 |
Calcium mobilization human mGlu4 assay; values are the average of n = 3.
Amplitude of response in the presence of 30 µM test compound, normalized to a standard compound, PHCCC, and represented as %GluMax.
Next, we turned our efforts to eliminating the benzylic site altogether by evaluating a series of phenyl sulfonamides. Much like the benzyl derivatives, the phenylsulfonamides were well tolerated as a substituent. Alkyl substituted pyrrole sulfonamides were the most potent of the series (11l, EC50 = 122 nM; 11m, EC50 = 104 nM; 11r, EC50 = 62 nM), with the halogen substituted analogs being less potent (e.g., 11n, EC50 = 740 nM; 11o, EC50 = 1238 nM). Some potency could be recaptured by the addition of an alkyl group as in 11t (EC50 = 163 nM) and 11u (EC50 = 268 nM). The pyrazole moiety was also tolerated as a phenyl replacement (Table 3).
Table 3.
SAR of the pyrrole phenylsulfonamides, 11l–w
 | |||||
|---|---|---|---|---|---|
| Compd | X | R1 | mGlu4 EC50
a (nM) |
pEC50 ± SEMa | %GluMaxb |
| 11l | CH | 122 | 6.91 ± 0.03 | 88.8 ± 3.4 | |
| 11m | CH | 104 | 6.98 ± 0.04 | 84.8 ± 5.3 | |
| 11n | CH | 740 | 6.13 ± 0.03 | 78.8 ± 3.5 | |
| 11o | CH | 1238 | 5.91 ± 0.18 | 102.8 ± 6.2 | |
| 11p | CH | 440 | 6.36 ± 0.10 | 72.9 ± 2.5 | |
| 11q | CH | 344 | 6.46 ± 0.10 | 83.0 ± 0.3 | |
| 11r | CH | 62 | 7.21 ± 0.01 | 80.3 ± 4.3 | |
| 11s | N | 148 | 6.83 ± 0.04 | 92.9 ± 1.9 | |
| 11t | CH | 163 | 6.79 ± 0.05 | 72.2 ± 1.7 | |
| 11u | CH | 268 | 6.57 ± 0.06 | 98.9 ± 2.3 | |
| 11v | N | 242 | 6.62 ± 0.06 | 35.3 ± 3.0 | |
| 11w | CH | 739 | 6.13 ± 0.07 | 35.6 ± 3.7 | |
Calcium mobilization human mGlu4 assay; values are the average of n = 3.
Amplitude of response in the presence of 30 µM test compound, normalized to a standard compound, PHCCC, and represented as %GluMax.
Having established the pyrrole scaffold as a novel phenyl replacement as mGlu4 PAMs, we next evaluated the compounds in our battery of Tier 1 in vitro PK assays (Table 4). The intrinsic clearance (CLINT) was assessed in rat hepatic microsomes and the subsequent predicted hepatic clearance (CLHEP) was calculated.23,32 Many of the compounds displayed high intrinsic and predicted hepatic clearance, except for the 3,4-dimethylphenyl analog, 11r, which had moderate predicted hepatic clearance (CLHEP = 38.3 mL/min/kg). Utilizing an equilibrium dialysis approach, the protein binding of the compounds was evaluated in rat plasma. The fraction unbound (Fu) of the analogs tested was very low, except, again, in the case of 11r which showed slightly better unbound fraction (Fu = 0.012).
Table 4.
In vitro pharmacokinetic data for selected N-phenylsulfonamide pyrroles
| Compd | CL (mL/min/kg) | PPB (Fu) | |
|---|---|---|---|
| Rat CLINT | Rat CLHEP | Rat | |
| 11l | 2704 | 68.2 | 0.009 |
| 11m | 4035.1 | 68.8 | 0.001 |
| 11o | 1126.4 | 65.9 | 0.004 |
| 11r | 293 | 38.3 | 0.012 |
| 11s | 3804 | 68.7 | 0.005 |
| 11t | 3445 | 68.6 | 0.004 |
Lastly, we evaluated two analogs in an in vivo IV clearance experiment in order to assess whether the in vitro data would be predictive of in vivo PK (in vitro:in vivo correlation, IV/IVC). The two compounds chosen were 11r and 11t, one predicted to be moderately cleared and the other highly cleared. Both compounds were dosed IV (1 mg/kg) and the in vivo plasma clearance closely resembles the in vitro predicted hepatic clearance suggesting the major route of clearance to be hepatic oxidative metabolism. In order to more fully establish this route of metabolism, we co-dosed 11t with 1-aminobenzotriazole (ABT), a pan-irreversible inhibitor of the cytochrome P450s. After PO dosing, a significant amount of 11t was detected in the HPV (hepatic portal vein); however, only ~1% of that amount was detected in the plasma, indicating a significant amount of oxidative metabolism. The brain:plasma ratio was ~1:1, although the total amount in the brain was very low, which is in contrast to the phenylsulfones which displayed low brain:-plasma ratios. When co-dosed with ABT, the concentrations of 11t in the HPV were nearly identical with the non-ABT dosed animals; however, the concentrations detected in the plasma were significantly higher (198.5 ng/mL vs 7.4 ng/mL). Again, while the brain:plasma ratio was ~1:1; as with the total plasma concentrations, the total amount in the brain was higher when co-dosed with ABT (Table 5).
Table 5.
In vivo Rat PK IV clearance (1 mg/kg)
| 11r | 11t | ||
|---|---|---|---|
| CL (mL/min/kg) | 37.2 | 86.8 | |
| T1/2 (min) | 252 | 167 | |
| MRT (min) | 149 | 84.7 | |
| Vss (L/kg) | 5.5 | 7.4 | |
| AUC (h ng/mL) | 447.5 | 191.7 | |
| po dosing of 11t with and without ABT, 1 h | |||
| Dose (mg/kg) | Concentration (ng/mg or g) | ||
| Plasma systemic | Plasma HPV | Brain | |
| 10/Veh | 7.4 | 731 | 8.1 |
| 10/ABT | 198.5 | 764.5 | 175.5 |
In conclusion, we report the discovery and characterization of unique thiazolesulfone and pyrrolesulfonamide core scaffolds as mGlu4 PAMs. These series were synthesized as a replacement for the previously disclosed phenylsulfonamide/phenylsulfone compounds. The thiazolesulfone analogs were potent mGlu4 PAMs (EC50’s < 500 nM), but suffered from metabolic instability. The pyrrolesulfonamide scaffold also was a well-tolerated change with many compounds exhibiting EC50’s < 500 nM. Compounds from this scaffold displayed an IV/IVC with regards to clearance (CLp) following IV adminstration; however, the compounds were not orally bioavailable and displayed poor exposure following PO dosing (systemic:HPV ratio <0.01). Co-dosing in vivo with the pan-irreversible P450 inhibitor, ABT, blocked extensive P450-mediated metabolism and significantly improved the systemic plasma and brain concentrations (plasma systemic:HPV ratio 0.26).
Acknowledgments
The authors would like to thank the Michael J. Fox Foundation for Parkinson’s Research for their support.
References and notes
- 1.Niswender CM, Conn PJ. Annu. Rev. Pharmacol. Toxicol. 2010;50:295. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conn PJ, Lindsley CW, Jones CK. Trends Pharmcol. Sci. 2009;30:25. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matosin N, Newell KA. Neurosci. Biobehav. Rev. 2013;37:256. doi: 10.1016/j.neubiorev.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Matosin N, Fernandez-Enright F, Frank E, Deng C, Wong J, Huang XF, Newell KA. J. Psychiatry Neurosci. 2014;39:407. doi: 10.1503/jpn.130242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bear MF, Huber KM, Warren ST. Trends Neurosci. 2004;2004:370. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Pop AS, Gomez-Mancilla B, Neri G, Willemsen R, Gasparini F. Psychopharmacology. 2014;231:1217. doi: 10.1007/s00213-013-3330-3. [DOI] [PubMed] [Google Scholar]
- 7.Swanson CJ, Bures M, Johnson MP, Linden A-M, Monn JA, Schoepp DD. Nat. Rev. Drug Disc. 2005;4:131. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- 8.Gravius A, Pietraszek M, Dekundy A, Danysz W. Curr. Top. Med. Chem. 2010;10:187. doi: 10.2174/156802610790411018. [DOI] [PubMed] [Google Scholar]
- 9.Lopez S, Turle-Lorenzo N, Acher F, De Leonibus E, Mele A, Amalric M. J. Neurosci. 2007;27 doi: 10.1523/JNEUROSCI.0299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Nat. Rev. Neurosci. 2005;6:787. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- 11.Valenti O, Marino MJ, Wittmann M, Lis E, DiLella AG, Kinney GG, Conn PJ. J. Neurosci. 2003;23:7218. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopkins CR, Lindsley CW, Niswender CM. Future Med. Chem. 2009;1:501. doi: 10.4155/fmc.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindsley CW, Niswender CM, Engers DW, Hopkins CR. Curr. Top. Med. Chem. 2009;9:949. [PubMed] [Google Scholar]
- 14.Robichaud AJ, Engers DW, Lindsley CW, Hopkins CR. ACS Chem. Neurosci. 2011;2:433. doi: 10.1021/cn200043e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engers DW, Field JR, Le U, Zhou Y, Bolinger JD, Zamorano R, Blobaum AL, Jones CK, Jadhav S, Weaver CD, Conn PJ, Lindsley CW, Niswender CM, Hopkins CR. J. Med. Chem. 2011;54:1106. doi: 10.1021/jm101271s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones CK, Engers DW, Thompson AD, Field JR, Blobaum AL, Lindsley SR, Zhou Y, Gogliotti RD, Jadhav S, Zamorano R, Bogenpohl J, Smith Y, Morrison R, Daniels JS, Weaver CD, Conn PJ, Lindsley CW, Niswender CM, Hopkins CR. J. Med. Chem. 2011;54:7639. doi: 10.1021/jm200956q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones CK, Bubser M, Thompson AD, Dickerson JW, Turle-Lorenzo N, Amalric M, Blobaum AL, Bridges TM, Morrison RD, Jadhav S, Engers DW, Italiano K, Bode J, Daniels JS, Lindsley CW, Hopkins CR, Conn PJ, Niswender CM. J. Pharmacol. Exp. Ther. 2012;340:404. doi: 10.1124/jpet.111.187443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Poul E, Boléa C, Girard F, Poli S, Charvin D, Campo B, Bortoli J, Bessif A, Luo B, Koser AJ, Hodge LM, Smith KM, DiLella AG, Liverton N, Hess F, Browne SE, Reynolds IJ. J. Pharmacol. Exp. Ther. 2012;343:167. doi: 10.1124/jpet.112.196063. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez HN, Liu KG, Hong S-P, Reitman MS, Uberti MA, Bacolod MD, Cajina M, Nattini M, Sabio M, Doller D. Bioorg. Med. Chem. Lett. 2012;22:3235. doi: 10.1016/j.bmcl.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Bennouar K-E, Uberti MA, Melon C, Bacolod MD, Jimenez HN, Cajina M, Kierkerian-Le Goff L, Doller D, Gubellini P. Neuropharmacology. 2013;66:158. doi: 10.1016/j.neuropharm.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Engers DW, Niswender CM, Weaver CD, Jadhav S, Menon UN, Zamorano R, Conn PJ, Lindsley CW, Hopkins CR. J. Med. Chem. 2009;52:4115. doi: 10.1021/jm9005065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung Y-Y, Zamorano R, Blobaum AL, Weaver CD, Conn PJ, Lindsley CW, Niswender CM, Hopkins CR. ACS Comb. Sci. 2011;13:159. doi: 10.1021/co1000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engers DW, Blobaum AL, Gogliotti RD, Cheung Y-Y, Salovich JM, Garcia-Barrantes PM, Daniels JS, Morrison RD, Jones CK, Soars MG, Zhuo X, Hurley J, Macor JE, Bronson JJ, Conn PJ, Lindsley CW, Niswender CM, Hopkins CR. ACS Chem. Neurosci. 2016 doi: 10.1021/acschemneuro.6b00035. http://dx.doi.org/10.1021/acschemneuro.6b00035. Just accepted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds IJ. Metabotropic glutamate receptors as therapeutic targets in Parkinson’s disease. 6th International Meeting on Metabotropic Glutamate Receptors 2008; Taormina, Sicily, Italy. 2008. [Google Scholar]
- 25.Engers DW, Gentry PR, Williams R, Bolinger JD, Weaver CD, Menon UN, Conn PJ, Lindsley CW, Niswender CM, Hopkins CR. Bioorg. Med. Chem. Lett. 2010;20 doi: 10.1016/j.bmcl.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conn PJ, Lindsley CW, Hopkins CR, Weaver CD, Niswender CM, Gogliotti RD, Cheung YY, Salovich JM, Engers DW. WO 2011/143466. 2011
- 27.Mispeleare-Canivet C, Spindler J-F, Perrio S, Beslin P. Tetrahedron Lett. 2005;61:5253. [Google Scholar]
- 28.Conn PJ, Lindsley CW, Hopkins CR, Niswender CM, Gogliotti RD. WO 2011/050305. 2011
- 29.Conn PJ, Lindsley CW, Hopkins CR, Niswender CM, Gogliotti RD, Salovich JM. WO 2011/050316. 2011
- 30.All final compounds were purified by high-throughput HPLC and characterized by LCMS and/or 1H NMR and found to be in agreement with their structures (>95% purity).
- 31.1-((3,4-Dimethylphenyl)sulfonyl)-3-nitro-1H-pyrrole (9): To a stirred solution of 3-nitro-1H-pyrrole (200 mg; 1.78 mmol) in CH2Cl2 (8 mL) was added 1,8-diazabicycloundec-7-ene, DBU, (347 µL; 2.32 mmol) followed by 3,4-dimethylbenzenesulfonyl chloride (438 mg; 2.14 mmol). The reaction was stirred at rt overnight and then was diluted with CH2Cl2 and washed with 2 N HCl, satd NaHCO3, dried (MgSO4), filtered and concentrated under vacuum. Material was purified by CombiFlash (Isco—40 g column) eluting with 0–30% EtOAc/hexanes to give the final product (329 mg; 85%). LCMS: RT = 2.41 min, >98% at 215 nm, m/z = 219.1 [M+H]+.N-(1-((3,4-Dimethylphenyl)sulfonyl)-1H-pyrrol-3-yl)picolinamide (11r): To reaction vessel was charged the 9 (180 mg; 0.826 mmol), EtOH (10 mL) and Raney Nickel (cat.) and the mixture was subjected to 40 psi H2 in a Parr shaker for 2 h. The reaction was worked up by adding a magnetic stir bar to bind the Ra–Ni and the solution was transferred by pipette and filtered through a Whatman filter disc. The filtrate was concentrated to give the crude amine which was taken through without further purification. To the crude amine was added CH2Cl2 (10 mL), diisopropylethylamine (374 µL; 2.15 mmol) and picolinyl chloride·HCl (152 mg; 0.860 mmol) and the mixture was stirred at rt overnight. The reaction mixture was diluted with CH2Cl2, washed with H2O, dried (MgSO4) filtered and concentrated. The material was purified by CombiFlash (Isco, 12 g) eluting with 0–40% EtOAc/hexanes to give the final product as a yellow solid (136 mg; 54%). LCMS: RT = 1.078 min, >98% at 215 and 254 nm, m/z = 356 [M+H]+. 1H NMR (DMSO-d6) 400 MHz δ 10.9 (s, 1H), 8.70 (d, 1H, J = 4.6 Hz), 8.11–8.03 (m, 2H), 7.74 (d, 2H, J = 7.5 Hz), 7.66 (d, 2H, J = 8.0 Hz), 7.40 (d, 1H, J = 8.0 Hz), 7.29 (s, 1H), 6.71 (d, 1H, J = 2.3 Hz), 2.29, 2.27 (2 s, 6H).
- 32.Lin JH, Chiba M, Balani SK, Chen I-W, Kwei GY-S, Vastag KJ, Nishime JA. Drug. Metab. Dispos. 1996;24:1111. [PubMed] [Google Scholar]