Abstract
6-Oxopurine derivatives containing a northern (N) methanocarba modification (i.e., fused cyclopropane and cyclopentane rings in place of the ribose) were synthesized and the adenosine receptor affinity measured. Guanine or hypoxanthine was coupled at the 7-position, or 1,3-dibutylxanthine was coupled at the 9-position. The pseudoribose ring was also substituted at the 5′-position with an N-methyluronamide or with phosphate groups.
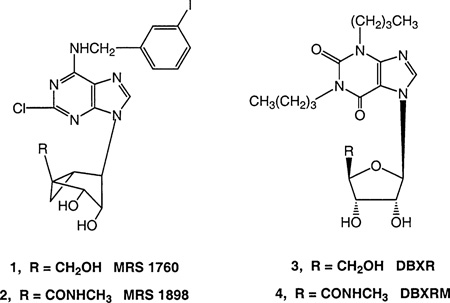
We recently examined conformational requirements of the ribose moiety in adenosine derivatives acting as agonists or partial agonists at P2 receptors and at subtypes of adenosine receptors (A1, A2A, and A3 ARs).1–3 Using a methanocarba modification originally introduced by Marquez and co-workers,4 the ribose-like moiety of these derivatives was constrained to either a northern [(N), 2′-exo] or southern [(S), 2′-endo] conformation through fused cyclopropane and cyclopentane rings. Such analogues helped to define the role of ribofuranosyl puckering in stabilizing the active AR-bound conformation, and thereby allowed identification of the (N)-methanocarba conformation as the isomer which preserved affinity at the A3 and to a lesser extent, A1 AR. Thus, the (N)-methanocarba analogues, 1 and 2, were highly potent as full agonist (2)3 or partial agonist (1) at the human A3 AR.2 These analogues contained substituent groups known to enhance A3 affinity in the ribose series [i.e., N6-(3-iodobenzyl) and 2-chloro]. The affinity of adenosine methanocarba analogues at the A2A AR was greatly decreased for the (N)-methanocarba isomer and nearly absent for the (S)-methanocarba isomer.
In this study, we combined the (N)-methanocarba modification with known purine receptor ligands containing a 6-oxo-substitution. Among the nucleoside analogues synthesized were derivatives of hypoxanthine, guanosine, and xanthine. For inosine5,6 and xanthine-7-riboside7 analogues, a 5′-methyluronamide modification (nucleoside numbering) known to enhance the A3 AR affinity was included.3,5 In comparison, xanthine-7-riboside derivatives 3 and 4 bound to and activated the rat A3 AR with partial or full efficacy, respectively,7,8 and were used as reference compounds. In other analogues, phosphate groups were introduced at the 5′-position with the aim of targeting P2 receptors,9 G proteins (in the case of a guanosine 5′-triphosphate analogue)10 or enzymes involved in purine metabolism (such as IMP dehydrogenase).11
The structures of (N)-methanocarba purine analogues (5–12) synthesized for testing in binding and other biochemical assays are shown in Table 1. We introduced the (N)-methanocarba modification of known potent adenosine agonists having equivalents of either 5′-hydroxymethyl (5, 8, and 10) or 5′-uronamide groups (6 and 11). These 6-oxopurine bases were coupled at either the 7-(10–12) or 9-position (5–9) to the pseudoribose moiety, according to well-characterized alkylation patterns of purine bases.7,12 A 5′-monophosphate derivative, 7a, and several 5′-triphosphate derivatives were included (9 and 12).
Table 1.
Affinities of (N)-methanocarba analogues of 6-oxpurine ribosides in radioligand binding assays at rat A1,a rat A2A,b and human A3 receptors,c unless noted, and in other biochemical assay systemsd
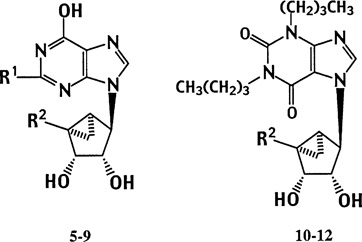 | |||||
|---|---|---|---|---|---|
| Compound | R1 | R2 | Ki (µM) or % displacement at 100 µM | ||
| rA1a | rA2Ab | hA3c | |||
| 5 MRS 1957 | H | CH2OH | < 10% | < 10% | 30 ± 10% |
| 6 MRS 1997 | H | CONHCH3 | < 10% | < 10% | 28.8 ± 5.2 |
| 7a MRS 2397d | H | CH2O–phosphate | 11 ± 1% | ||
| 7b MRS 2398d | H | CH2O–phosphate]2 dimer | 21.6 ± 0.4 | ||
| 8 MRS 1941 | NH2 | CH2OH | < 10% | < 10% | 20.5 ± 2.1 |
| 9 MRS 2351d | NH2 | CH2O–triphosphate | < 10% | ||
| 10 MRS 1971 | — | CH2OH | 2.97 ± 0.15 | < 10% | 4.35 ± 0.40 |
| 11 MRS 1998 | — | CONHCH3 | 24.4 ± 4.8 | < 10% | 1.86 ± 0.23 |
| 12 MRS 2396 | — | CH2O–triphosphate | < 10% | ||
Displacement of specific [3H]R-PIA binding to A1 receptors in rat brain membranes, expressed as Ki ± SEM or % (n = 3–5).
Displacement of specific [3H]CGS 21680 binding to A2A receptors in rat striatal membranes, expressed as Ki ± SEM or % (n = 3–6).
Displacement of specific [125I]AB-MECA binding at human A3 receptors expressed in HEK-293 cells, in membranes, expressed as Ki ± SEM or % (n = 3–5).
Selected compounds were examined in HL-60 cells for effects on calcium mobilization. Compounds 7a and 7b (100 µM) were inactive in raising intracellular calcium, while compound 9 (100 µM) caused a rise in intracellular calcium equivalent to 53 ± 18% of the effect of 1 µM UTP (n = 3).
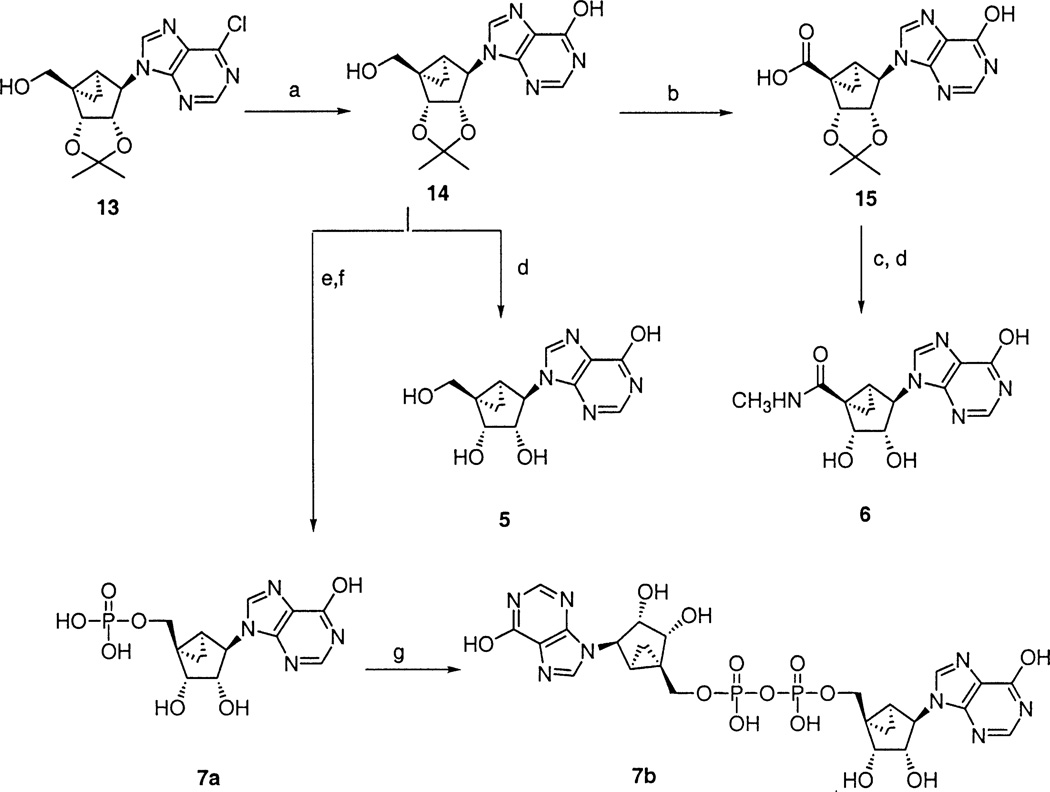
The synthetic methods used to prepare the new analogues are outlined in Schemes 1–3.13–15 6-Monosubstituted derivatives were prepared from the 6-chloropurine derivative3 as a common intermediate. Refluxing 13 in the presence of 2-mercaptoethanol and NaOMe in MeOH gave the protected inosine derivative, 14, which was converted into triol 5 in acidic conditions or to the 5′-uronamide, 6, via a 5′-carboxylic acid, 15. Treatment of 14 with RuCl3, NaIO4, followed by C18 column chromatography gave 15, which condensed with MeNH2 in the presence of BOP-Cl [N,N-bis(2-oxo-3-oxazolidinyl)-phosphinic chloride] to yield 6. Use of RuCl3 instead of RuO2 produced a cleaner reaction. The 5′-monophosphate derivative 7a was prepared by reacting 14 with dit-butyl-N,N-diethylphosphoramidite in the presence of tetrazole and then with mCPBA at −78 °C, followed by the treatment with Dowex resin. Attempted synthesis of the corresponding triphosphate by treating with 1,1′-carbonyldiimidazole followed by tetrabutylammonium pyrophosphate failed, and instead a dimer, 7b, was obtained.
Scheme 1.
(a) HSCH2CH2OH, NaOMe, MeOH, reflux; (b) RuCl3, NaIO4, CH3CN/CHCl3/H2O=2:2:3; (c) BOP-Cl, Et3N, MeNH2 (2 M/THF), CH2Cl2/DMF=1:1; (d) CF3CO2H, wet MeOH, 60 °C; (e) (i) 1H-tetrazole, di-t-butyl N,N-diethylphosphoramidite, THF; (ii) mCPBA, CH2Cl2; (f) DOWEX 50×8–200, MeOH/H2O=2:1; (g) (i) 1,1′-carbonyldiimidazole, DMF; (ii) 5% Et3N/MeOH; (iii) tetrabutylammonium pyrophosphate.
Scheme 3.
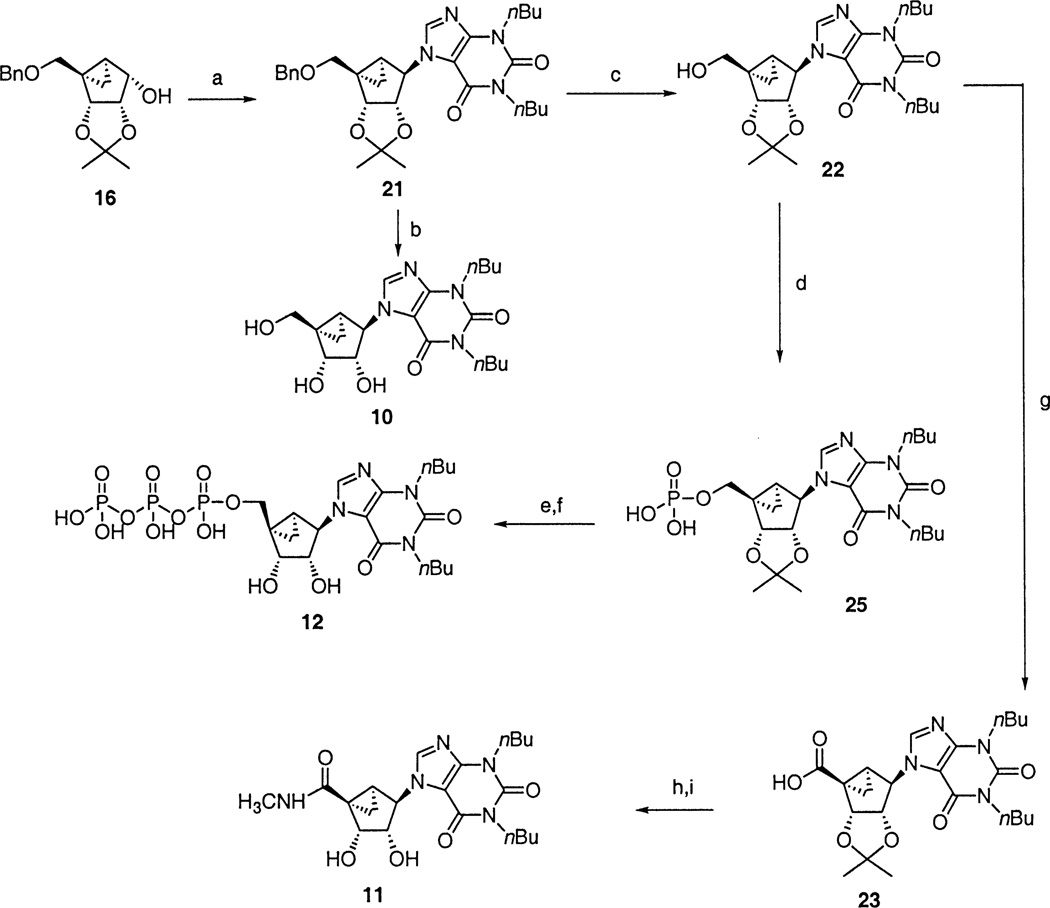
(a) 1,3-Dibutylxanthine, DEAD, Ph3P, THF; (b) BCl3, CH2Cl2; (c) Pd black, 10% HCO2H in MeOH; (d) (i) tetrazole, di-t-butyl-N,N-diethylphosphoramidite, THF; (ii) mCPBA, CH2Cl2; (e) DOWEX 50×8–200, MeOH/H2O=1:1; (f) (i) 1,1′-carbonyldiimidazole, DMF; (ii) 5% Et3N/MeOH; (iii) tetrabutylammonium pyrophosphate; (g) RuO2, NaIO4, K2CO3, H2O/CH3CN/CHCl3; (h) CH3NH2• HCl, Et3N, BOP-Cl; (i) BCl3/CH2Cl2
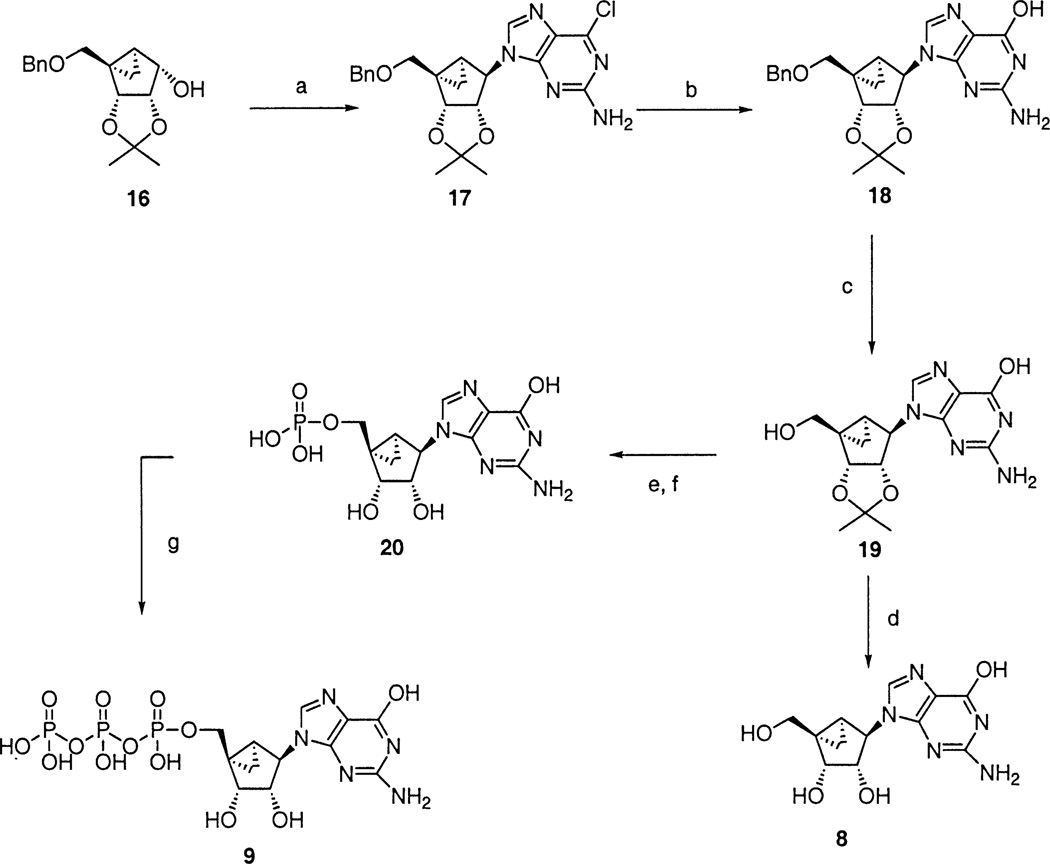
Guanosine derivative 8 was prepared by a Mitsunobu reaction of 16 and 2-amino-6-chloropurine to furnish 17, which was heated with 2N NaOH in dioxane at 100 °C to yield the guanosine derivative, 18. Debenzylation at the 5′ position using Pd to give compound 19, followed by TFA deprotection provided the guanosine derivative, 8. Compound 19 was converted into monophosphate 20 by the phosphoramidite method. This monophosphate was then converted to phosphorimidazolidate by treatment with 1,1′-carbonyldiimidazole followed by 5% Et3N/MeOH, and the phosphorimidazolidate reacted with tetrabutylammonium pyrophosphate to provide the triphosphate, 9b.
Xanthine derivatives were prepared using a Mitsunobu coupling of 16 and 1,3-dibutylxanthine to furnish 21, which was deprotected with BCl3 to yield the xanthine methanocarba nucleoside, 10. Compound 21 was debenzylated to furnish 22, which was converted to the monophosphate 25 and to the triphosphate 12 by the above phosphorylation procedures. Compound 22 was oxidized using RuO2, NaIO4, K2CO3 to furnish the acid 23, which was condensed with MeNH2 using BOP-Cl to yield intermediate 24 and finally the deprotected uronamide, 11.
The nucleoside analogues were evaluated in binding at three subtypes of ARs. While inosine itself displayed a measurable affinity at the A3 AR and was even suggested to be an endogenous ligand for this AR,6 the corresponding (N)-methanocarba analogue, 5, bound weakly. An effort to enhance the affinity in the inosine series through the introduction of 5′-methyluronamide group slightly increased the affinity of 6 (Ki 29 µM). The inosine-5′-monophosphate analogue, 7a, was inactive at the adenosine A3 AR. Curiously, the dimer 7b displaced binding at the A3 AR with a Ki value of 22 µM. Thus, this was an example of dimerization of an inactive ligand to increase the affinity, perhaps through crosslinking two sites on a receptor or by bridging two proximal receptor molecules. It is unusual for such a highly anionic molecule to bind to an AR, which may be the result of an enhancing effect of the (N)-methanocarba ring on A3 affinity.2 It will also be interesting to evaluate 7b at P2X receptors, at which diinosine polyphosphates are potent antagonists.16
A receptor for guanosine was proposed,17 based on neuroprotective effects observed for this nucleoside. However, the guanosine analogues here were examined only for interaction with ARs. The (N)-methanocarba analogue, 8, bound weakly, but selectively to the A3 AR with a Ki value of 21 µM. The corresponding triphosphate, 9, of interest for its potential interaction with G proteins, was inactive in binding to the A3 AR.
The (N)-methanocarba analogue of 1,3-dibutylxanthine-7-riboside, 10, was equipotent at A1 and A3 ARs and inactive at the A2A AR. If proven to be an agonist at both AR subtypes, this profile of selectivity would be desirable for a cardioprotective agent.18 Introduction of the 5′-N-methyluronamide group, in 11, decreased A1 affinity by 8-fold and increased A1 affinity by 2-fold. A comparison of compounds 3 and 47 with the corresponding (N)-methanocarba analogues, 10 and 11, respectively, showed that rat A1 affinities were the same, and rat A3 affinities of the ribosides were roughly similar to Ki values at the human A3 AR found in this study. The xanthine-riboside-5′-triphosphate analogue, 12, was inactive at the A3 AR.
The phosphate-containing analogues were tested for effects on intracellular calcium concentration in HL-60 leukemia cells, known to express several P2 receptor subtypes.19 Thus, a P2 receptor agonist would be expected to cause a rapid rise in intracellular calcium. Compounds 7a and 7b were essentially inactive at a concentration of 100 µM, either at stimulating calcium accumulation or inhibiting the rise in calcium produced by 1 µMUT P (acting at P2Y2 receptors). Compound 9 (100 µM) stimulated a rise in intracellular calcium equivalent to 53% of the rise produced by 1 µMUT P, suggesting that it may activate a P2Y receptor.
In conclusion, we have identified non-adenosine analogues containing the (N)-methanocarba modification which bound weakly, and in some cases selectively, at the human A3 AR. Since selectivity has been achieved in these simple derivatives, this could provide the basis for further development of more potent ligands. The phosphate analogues must also be evaluated at a range of enzymatic and receptor activities. The full evaluation of the biological activity of the new analogues is in progress.
References and Notes
- 1.Nandanan E, Jang SY, Moro S, Kim H, Siddiqi MA, Russ P, Marquez VE, Busson R, Herdewijn P, Harden TK, Boyer JL, Jacobson KA. J. Med. Chem. 2000;43:829. doi: 10.1021/jm990249v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson KA, Ji X-d, Li AH, Melman N, Siddiqi MA, Shin KJ, Marquez VE, Ravi RG. J. Med. Chem. 2000;43:2196. doi: 10.1021/jm9905965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee K, Ravi RG, Ji X-d, Marquez VE, Jacobson KA. Bioorg. Med. Chem. Lett. 2001;11:1333. doi: 10.1016/s0960-894x(01)00213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marquez VE, Siddiqui MA, Ezzitouni A, Russ P, Wang J, Wagner RW, Matteucci MD. J. Med. Chem. 1996;39:3739. doi: 10.1021/jm960306+. [DOI] [PubMed] [Google Scholar]
- 5.van Galen PJM, van Bergen AH, Gallo-Rodriguez C, Melman N, Olah ME, IJzerman AP, Stiles GL, Jacobson KA. Mol. Pharmacol. 1994;45:1101. [PMC free article] [PubMed] [Google Scholar]
- 6.Jin X, Shepherd RK, Duling BR, Linden J. J. Clin. Invest. 1997;100:2849. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HO, Ji X-d, Melman N, Olah ME, Stiles GL, Jacobson KA. J. Med. Chem. 1994;37:4020. doi: 10.1021/jm00049a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park KS, Hoffmann C, Kim HO, Padgett WL, Daly JW, Brambilla R, Motta C, Abbracchio MP, Jacobson KA. Drug Dev. Res. 1998;44:97. doi: 10.1002/(SICI)1098-2299(199806/07)44:2/3<97::AID-DDR7>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams M, Jarvis MF. Biochem. Pharmacol. 2000;59:1173. doi: 10.1016/s0006-2952(99)00341-x. [DOI] [PubMed] [Google Scholar]
- 10.Zor T, Andorn R, Sofer I, Chorev M, Selinger Z. FEBS Lett. 1998;433:326. doi: 10.1016/s0014-5793(98)00930-2. [DOI] [PubMed] [Google Scholar]
- 11.Yalowitz JA, Jayaram HN. Anticancer Res. 2000;20:2329. [PubMed] [Google Scholar]
- 12.Simons C. Nucleoside Mimetics. Amsterdam: Gordon and Breach; 2001. [Google Scholar]
- 13.Procedures in Scheme 1. A solution of 13 (40 mg, 0.119 mmol), 2-mercaptoethanol (0.03 mL, 0.475 mmol), 0.5 N NaOMe/MeOH (0.95 mL, 0.475 mmol) in MeOH (1 mL) was heated at 90 °C for 3 h and the reaction mixture was neutralized by glacial AcOH. The resulting mixture was concentrated and purified on preparative TLC (CHCl3/MeOH = 6:1) to provide the hypoxanthine derivative 14 (37 mg, 98%). 1H NMR (CD3OD) δ 8.06 (s, 1H), 7.96 (s, 1H), 5.34 (d, J = 7.1 Hz, 1H), 5.04 (s, 1H), 4.69 (d, J = 6.9 Hz, 1H), 3.97 (d, J = 11.5 Hz, 1H), 3.52 (d, J = 11.5 Hz, 1H), 1.72 (m, 1H), 1.51 (s, 3H), 1.24 (s, 3H), 1.16 (m, 1H), 0.97 (m, 1H). FAB m/z 319 (MH+). 15: A mixture of 14 (37 mg, 0.116 mmol), RuCl3 (10 mg, 0.048 mmol), NaIO4 (181 mg, 0.847 mmol) in CH3CN/CHCl3/H2O = 2:2:3 was vigorously stirred for 6 h at rt. Solvents were removed and the residue was subjected to reverse phase C18 column chromatography using H2O/MeOH (30:1–7:1) as eluents to afford the acid 15 (20.6 mg, 53.4%). 1H NM R (D2O) δ 8.15 (s, 1H), 8.11 (s, 1H), 5.74 (d, J = 7.4 Hz, 1H), 5.03 (s, 1H), 4.87 (d, J = 7.1 Hz, 1H), 1.22 (m, 1H), 1.61 (m), 1.55 (s, 3H), 1.34 (m, 1H), 1.28 (s, 3H). FAB m/z 333 (MH+). 5 (1R,2R,3S,4R,5S)-4-[6-oxo-9H-purin-9 - yl] - 1 - (hydroxymethyl)bicyclo - [3.1.0]hexane - 2,3 - diol): 1H NMR (CD3OD) δ 8.51 (s, 1H), 8.06 (s, 1H), 7.91 (s, 1H), 4.82 (s, 1H), 4.76 (d, J = 6.6 Hz, 1H), 4.24 (d, J = 11.8 Hz, 1H), 4.88 (d, J = 6.8 Hz, 1H), 1.60 (m, 1H), 1.53 (m, 1H), 0.74 (m, 1H). FAB m/z 279 (MH+). HR-MS (FAB) calcd 279.1093, found 279.1084. Acetonide intermediate of 6: BOP-Cl (26.5 mg, 0.104 mmol) was added to a solution of 15 (11.5 mg, 0.035 mmol) and Et3N (0.015 mL, 0.104 mmol) in CH2Cl2/DMF (2 mL). To the suspension 2.0 M MeNH2/THF (0.03 mL, 0.06 mmol) was added and the resulting mixture was stirred for 24 h at rt. Water was added and the solvent was removed. The residue was purified on silica gel (CHCl3/MeOH = 6:1) to give the protected amide (8.1 mg, 68.6%). 1H NMR (CD3OD) δ 8.11 (s, 1H), 8.01 (s, 1H), 5.74 (d, J = 6.8 Hz, 1H), 5.03 (s, 1H), 2.79 (s, 3H), 2.17 (m, 1H), 1.55 (m, 1H), 1.52 (s, 3H), 1.42 (m, 1H), 1.27 (s, 3H). FAB m/z 346 (MH+). 6: 1H NM R(D2O) δ 8.18 (s, 1H), 8.10 (s, 1H), 5.08 (d, J = 6.3 Hz, 1H), 4.96 (s, 1H), 4.17 (d, J = 6.6 Hz, 1H), 2.85 (s, 3H), 2.26 (m, 1H), 1.80 (t, J = 5.2 Hz, 1H), 1.51 (m, 1H), 1.51 (m, 1H). FAB m/z 306 (MH+). HR-MS (FAB) calcd 306.1202, found 306.1211. 7a t-butyl intermediate. 1H NM R (CD3OD) δ 8.45 (s, 1H), 8.20 (s, 1H), 4.92 (s, 1H), 4.44 (dd, J = 5.2, 12 Hz, 1H), 4.04 (d, J = 6.3, 1H, 2H), 4.70 (dd, J = 5.7, 11.1 Hz, 1H), 1.86 (m, 1H), 1.49 (m, 1H), 0.97 (t, J = 6.7 Hz, 1H), 1.34–1.25 (m) 1.09 (m, 1H). FAB m/z 510 (MH+). 7a: 1H NMR (D2O) δ 8.18 (s, 1H), 8.10 (s, 1H), 5.08 (d, J = 6.3 Hz, 1H), 4.96 (s, 1H), 4.17 (d, J = 6.6 Hz, 1H), 2.85 (s, 3H), 2.26 (m, 1H), 1.80 (t, J = 5.2 Hz, 1H), 1.51 (m, 1H), 1.51 (m, 1H). 31P NM R(D2O) δ 0.51. FAB m/z 357 ((M−1)−). HR-MS (FAB−) calcd 357.0600, found 357.0607. 7b: 1H NM R (D2O) δ 8.17 (s, 1H), 8.05 (s, 1H), 4.60 (d, J = 10.7 Hz, 1H), 3.93 (d, J = 6.6 Hz, 1H), 3.80 (d, J = 11.0, 1H), 1.88 (s, 1H), 1.53 (t, J = 4.7 Hz, 1H), 0.97 (t, J = 7.1 Hz, 1H). 31P NM R(D2O) δ −10.96. FAB m/z 697 ((M−1)−). HR-MS (FAB−) calcd 697.1173, found 697.1166.
- 14.Procedures in Scheme 2. To a mixture of 16 (0.2 g, 0.69 mmol), 2-amino-6-chloropurine (0.234 g, 1.38 mmol) and triphenylphosphine (0.361 g, 1.38 mmol) was added DIAD (diisopropyl azodicarboxylate, 0.27 mL, 1.38 mmol) dropwise at 0 °C. The mixture was warmed up to rt and stirred for 6 h. The solvent was evaporated under vacuum and the residue was purified by flash chromatography using 6:4 petroleum ether/ethyl acetate to furnish 17 as a solid (0.2 g, 67%). 1H NMR (CDCl3) δ 8.30 (s, 1H), 7.36 (s, 5H), 5.29 (d, J = 7.15 Hz, 1H), 5.18 (bs, 2H), 4.96 (s, 1H), 4.69–4.53 (ABq, 2H), 4.48 (d, J = 7.15 Hz, 1H), 3.92 (d, J = 10.16 Hz, 1H), 3.28 (d, J = 10.16 Hz, 1H), 1.65–1.60 (m, 1H), 1.55 (s, 3H), 1.28–1.20 (m, 1H), 1.24 (s, 3H), 0.96–0.88 (m, 1H). 18: To a solution of 17 (0.1 g, 0.23 mmol) in dioxane (2 mL) was added 2 N NaOH (8 mL) and heated at 90 °C for 3 h. The mixture was concentrated to dryness under vacuum and purified by flash chromatography using 10% MeOH in CHCl3 to furnish 18 as a solid (0.08 g, 82%). 1H NMR (CDCl3) δ 7.96 (s, 1H), 7.37 (s, 5H), 6.29 (bs, 2H), 5.27 (d, J = 6.87 Hz, 1H), 4.89 (s, 1H), 4.67–4.50 (m, 3H), 3.92 (d, J = 10.16 Hz, 1H), 3.32 (d, J = 10.16 Hz, 1H), 1.70–1.60 (m, 1H), 1.55 (s, 3H), 1.25 (s, 4H), 0.96–0.86 (m, 1H). 19: To a solution of 18 (0.06 g, 0.14 mmol) in 5% HCOOH/MeOH (5 mL) was added Pd black (0.06 g) and stirred at rt for 8 h. The reaction mixture was filtered through Celite and concentrated and purified by flash chromatography using 10% MeOH/CHCl3 to furnish 19 as a solid (0.04 g, 86%). 1H NM R (D2O) δ 789 (s, 1H), 7.83 (s, 1H), 5.28 (d, J = 6.87 Hz, 1H), 4.77 (s, 1H), 4.59 (d, J = 6.87 Hz, 1H), 3.90 (d, J = 10.54 Hz, 1H), 3.44 (d, J = 10.54 Hz, 1H), 1.64–1.54 (m, 1H), 1.43 (s, 3H), 1.18 (s, 3H), 1.10–1.00 (m, 1H), 0.94–0.82 (m, 1H). 8: Substrate 19 (0.02 g, 0.06 mmol) was dissolved in 5% TFA/MeOH (3 mL) and H2O (1 mL) and stirred at rt for 6 h. The reaction mixture was concentrated to dryness under vacuum and purified by preparative TLC using 15% MeOH/CHCl3 to furnish 8 (0.012 g, 68%). 1H NM R (D2O) δ 9.20 (s, 1H), 4.69 (d, J = 6.32 Hz, 1H), 4.25 (d, J = 11.54 Hz, 1H), 3.92 (d, J = 6.32 Hz, 1H), 3.23 (d, J = 11.54 Hz, 1H), 1.66–1.48 (m, 2H), 0.82–0.70 (m, 1H). 20: To a solution of 19 (0.02 g, 0.06 mmol) in anhydrous THF (2 mL) was added tetrazole (0.013 g, 0.18 mmol) and di-t-butyl-N-N-diethylphosphoramidite (0.025 mL, 0.09 mmol) and stirred at rt for 12 h. The reaction mixture was cooled to −78 °C and was added a solution of mCPBA (0.018 g) and warmed to rt. Methanol (2 mL) was added to the reaction mixture and concentrated under vacuum and purified by preparative TLC using 10% MeOH/CHCl3 to furnish 0.022 g product. This compound (0.02 g, 0.038 mmol) was dissolved in 5% TFA/MeOH (3 mL) and H2O (1 mL) and stirred at rt for 6 h. The reaction mixture was concentrated to dryness under vacuum to furnish 0.013 g of 20, which was used directly for the further reaction. 1H NM R (D2O) δ 8.15 (s, 1H), 4.71 (s, 1H), 4.43 (dd, J = 4.94, 11.26 Hz, 1H), 4.02 (d, J = 6.59 Hz, 1H), 3.70 (dd, J = 4.94, 11.26 Hz, 1H), 1.88–1.78 (m, 1H), 1.54–1.42 (m, 1H), 1.12–0.92 (m, 1H). 31P NM R (D2O) δ 0.87. 9: To a suspension of monophosphate (0.008 g, 0.02 mmol) in DMF (1 mL) was added 1,1′-carbonyldiimidazole (0.016 g, 0.1 mmol) and stirred for 12 h. A reaction mixture was treated with a solution of 5% Et3N/MeOH and stirred for 4 h. This mixture was concentrated to dryness under vacuum and were added tributylammonium pyrophosphate (0.055 g, 0.012 mmol) and DMF (1 mL) and stirred for 3 days. Triethylammonium bicarbonate buffer (3 mL) was added to the reaction mixture followed by water (2 mL) and lyophilized. The crude material obtained was purified on a Sephadex column using 0.5 M ammonium bicarbonate and water to furnish 3.5 mg of 9. 1H NM R (D2O) δ 8.12 (s, 1H), 4.69 (s, 1H), 4.54–4.44 (m, 1H), 4.07 (d, J = 6.32 Hz, 1H), 3.92–3.82 (m, 1H), 1.84–1.78 (m, 1H), 1.46–1.38 (m, 1H), 1.02–0.94 (m, 1H). 31P NMR (D2O) δ −8.29, −10.69, −22.26.
- 15.Procedures in Scheme 3: To a mixture of 16 (0.1 g, 0.34 mmol), 1,3-dibutylxanthine (0.18 g, 0.68 mmol) and triphenylphosphine (0.178 g, 0.68 mmol) was added DEAD (diethyl azodicarboxylate, 0.1 mL, 0.68 mmol) dropwise at 0 °C. The mixture was warmed up to rt and stirred for 6 h. The solvent was evaporated under vacuum and the residue was purified by flash chromatography using 4:1 petroleum ether/ethyl acetate to furnish 21 (0.16 g, 88%). 1H NMR (CDCl3) δ 8.25 (s, 1H), 7.40–7.28 (m, 5H), 5.55 (s, 1H), 5.21 (d, J = 7.14 Hz, 1H), 4.64 (d, J = 12.4 Hz, 1H), 4.58 (d, J = 12.4 Hz, 1H), 4.49 (d, J = 7.14 Hz, 1H), 4.16–3.84 (m, 5H), 3.16 (d, J = 9.89 Hz, 1H), 1.82–1.54 (m, 4H), 1.52 (s, 3H), 1.48–1.28 (m, 6H), 1.23 (s, 3H), 1.02–0.84 (m, 7H). 10: To a solution of 21 (0.025 g, 0.047 mmol) in anhydrous CH2Cl2 (1 mL) was added 1 M BCl3/CH2Cl2 (0.23 mL, 0.23 mmol) at 0 °C and the mixture warmed to rt and stirred for 15 min. MeOH (1 mL) was added to the reaction mixture and concentrated under vacuum. 10 was purified by preparative TLC using 10% MeOH/CHCl3 (12 mg, 63%). 1H NMR (CD3OD) δ 8.40 (s, 1H), 5.09 (s, 1H), 4.55 (d, J = 6.32 Hz, 1H), 4.17 (d, J = 11.54 Hz, 1H), 4.06–3.82 (m, 5H), 3.18 (d, J = 11.54 Hz, 1H), 1.70–1.40 (m, 5H), 1.40–1.18 (m, 5H), 0.88 (t, J = 7.14 Hz, 6H), 0.72–0.62 (m, 1H). 22: To a solution of 21 (0.07 g, 0.13 mmol) in 5% HCOOH/MeOH (10 mL) was added Pd black (0.07 g) and stirred at rt for 8 h. The reaction mixture was filtered through Celite and concentrated and purified by flash chromatography using 5% MeOH/CHCl3 to furnish 22 as a solid (0.05 g, 86%). 1H NMR (CDCl3) δ 7.88 (s, 1H), 5.46 (d, J = 7.14 Hz, 1H), 4.96 (s, 1H), 4.78 (d, J = 7.14 Hz 1H), 4.25–3.99 (m, 5H), 3.26 (d, J = 11.54 Hz, 1H), 1.77–1.58 m, 5H), 1.53 (s, 3H), 1.44–1.30 (m, 5H), 1.27 (s, 3H), 1.10–0.89 (m, 7H). 25: To a solution of 22 (0.026 g, 0.058 mmol) in anhydrous THF (2 mL) was added tetrazole (0.012 g, 0.18 mmol) and di-t-butyl-N,N-diethylphosphoramidite (0.024 mL, 0.09 mmol) and stirred at rt for 12 h. The reaction mixture was cooled to −78 °C and was added a solution of mCPBA (0.017 g) and warmed to rt. Methanol (2 mL) was added to the reaction mixture and concentrated under vacuum and purified by preparative TLC using 10% MeOH/CHCl3 to furnish 0.022 g product 25. 1H NMR (CDCl3) δ 8.02 (s, 1H), 5.48 (s, 1H), 5.20 (d, J = 6.87 Hz, 1H), 4.54 (d, J = 6.87 Hz, 1H), 4.31–3.96 (m, 6H), 1.82–1.23 (m, 34H), 1.10–0.89 (m, 7H). Compound 25 was treated with DOWEX in MeOH/H2O at 90°C for 2 h and filtered and concentrated to furnish 0.011 g of monophosphate. To a suspension of this monophosphate (0.011 g, 0.023 mmol) in DMF (1 mL) was added 1,1′-carbonyldiimidazole (0.019 g, 0.12 mmol) and stirred for 12 h. The reaction mixture was treated with a solution of 5% Et3N/MeOH and stirred for 4 h. This mixture was concentrated to dryness under vacuum, treated with tributylammonium pyrophosphate (0.063 g, 0.014 mmol) and DMF (1 mL) and stirred for 3 days. Triethylammonium bicarbonate buffer (3 mL) was added to the reaction mixture followed by water (2 mL). After lyophilization, the crude material obtained was purified on a Sephadex colum using 0.5 M ammonium bicarbonate and water to furnish 3.5 mg of 12. 1H NM R (D2O) δ 8.20 (s, 1H), 5.18 (s, 1H), 4.80–4.73 (m, 2H), 4.09–3.77 (m, 6H), 1.80–1.22 (m, 10H), 1.04–0.84 (m, 7H). 23: A mixture of 22 (0.03 g, 0.067 mmol), RuO2 (0.18 g, 1.34 mmol), NaIO4 (0.286 g, 1.34 mmol), K2CO3 (0.184 g, 1.34 mmol) in CH3CN/CHCl3/H2O = 2:2:3 (10 mL) was stirred at rt vigorously for 20 h. Reaction mixture was filtered through Celite. Organic layer was separated and the aqueous layer was acidified with HCl and extracted with EtOAc, dried and concentrated under vacuum to furnish 0.012 g product. 1H NMR (CDCl3) δ 7.64 (s, 1H), 5.69 (d, J = 7.14 Hz, 1H), 5.30 (s, 1H), 4.74 (d, J = 7.14 Hz, 1H), 4.09 (t, J = 7.42 Hz, 2H), 4.00 (t, J = 7.42 Hz, 2H), 1.85–1.59 (m, 6H), 1.55 (s, 3H), 1.44–1.33 (m, 4H), 1.28 (s, 3H), 1.02–0.86 (m, 7H). 11: A mixture of 23 (0.01 g, 0.022 mmol), BOP-Cl (0.011 g, 0.044 mmol), CH3NH2•HCl (0.006 g, 0.088 mmol), Et3N (0.012 mL, 0.088 mmol) in THF (2 mL) was stirred at rt for 8 h. Solvent was removed under vacuum and the crude material was purified by preparative TLC using 10% MeOH/CHCl3. The pure material was treated with 1 M BCl3/CH2Cl2 (0.1 mL, 0.1 mmol) at 0 °C for 10 min. Methanol was added to the reaction mixture and the solvent was evaporated and the crude material was purified by preparative TLC using 15% MeOH/CHCl3 to furnish 0.002 g product 11. 1H NMR (CDCl3) δ 8.29 (s, 1H), 5.14 (s, 1H), 4.87 (s, 1H), 4.72 (s, 1H), 4.18–3.94 (m, 4H), 3.67 (s, 3H), 1.84–1.22 (m, 10H), 1.00–0.82 (m, 7H).
- 16.Dunn PM, Liu M, Zhong Y, King BF, Burnstock G. Br. J. Pharmacol. 2000;130:1378. doi: 10.1038/sj.bjp.0703404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rathbone MP, Middlemiss PJ, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F. Prog. Neurobiol. 1999;59:663. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson KA, Xie R, Young L, Chang L, Liang BT. J. Biol. Chem. 2000;275:30272. doi: 10.1074/jbc.M001520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Communi D, Janssens R, Robaye B, Zeelis N, Boeynaems JM. FEBS Lett. 2000;475:39. doi: 10.1016/s0014-5793(00)01618-5. [DOI] [PubMed] [Google Scholar]
Scheme 2.
(a) 2-Amino-6-chloropurine, DIAD, Ph3P, THF; (b) 2N NaOH, dioxane; (c) Pd black, 10% HCO2H in MeOH; (d) TFA/MeOH/H2O; (e) (i) tetrazole, di-t-butyl-N,N-diethylphosphoramidite, THF; (ii) mCPBA, CH2Cl2; (f) TFA/MeOH/H2O (g) (i) 1,1′-carbonyldiimidazole, DMF; (ii) 5% Et3N/MeOH; (iii) tetrabutylammonium pyrophosphate.