Abstract
A number of retinal degenerative diseases may be amenable to treatment with continuous intraocular delivery of therapeutic agents that cannot be delivered effectively to the retina via systemic or topical administration. Among these disorders are lysosomal storage diseases resulting from deficiencies in soluble lysosomal enzymes. Most cells, including those of the retina, are able to take up these enzymes and incorporate them in active form into their lysosomes. In theory, therefore, continuous intraocular administration of a normal form of a soluble lysosomal enzyme should be able to cure the molecular defect in the retinas of subjects lacking this enzyme. Experiments were conducted to determine whether genetically modified bone marrow-derived stem cells implanted into the vitreous could be used as vehicles for continuous delivery of such enzymes to the retina. Bone marrow-derived mesenchymal stem cells (MSCs) from normal mice were implanted into the vitreous of mice undergoing retinal degeneration as a result of a mutation in the PPT1 gene. The implanted cells appeared to survive indefinitely in the vitreous without proliferating or invading the retina. This indicates that intravitreal implantation of MSCs is likely a safe means of long-term delivery of proteins synthesized by the implanted cells. Experiments have been initiated to test the efficacy of using genetically modified autologous MSCs to inhibit retinal degeneration in a canine model of neuronal ceroid lipofuscinosis.
1 Introduction
In recent years substantial research has been conducted to assess potential therapeutic applications of stem cells. The focus of much of this work has been on utilizing stem cells to regenerate tissues, including the retina, that have been damaged as a result of injury or disease (Ramsden et al. 2013). We implanted embryonic stem cell-derived neural precursors from normal mice into the vitreous of mice undergoing progressive retinal degeneration due to a mutation in CLN8 (Meyer et al. 2006). The implanted cells migrated to and associated closely with the inner retinal surface. A fraction of the cells also migrated into the retina where they appeared to differentiate into specific types of retinal neurons appropriate to the retinal layers in which they were located. The proportion of the retinal neurons replaced by the donor cells was quite small. However, a profound preservation of host retinal photoreceptor cells occurred in areas of the retina with which the donor cells had closely associated. This suggested that the donor cells exerted a trophic effect that inhibited degeneration of the surrounding retina. The trophic factors involved in this protective effect were not identified, but the observed effect suggested that therapeutic compounds produced by donor cells may be effective in preventing retinal degeneration resulting from many causes. We are undertaking studies to further investigate this possibility.
In particular, we are studying the possibility that retinal degeneration resulting from lack of soluble lysosomal enzymes can be inhibited by secretion of these enzymes by cells implanted into the vitreous. To avoid potential problems associated with using embryonic stem cell derivatives as donor cells, we are evaluating the use of genetically modified autologous mesenchymal stem cells (MSCs) as the source of replacement enzymes. Initial experiments have been conducted to assess the behavior of such cells after implantation into the vitreous of eyes in animals undergoing progressive retinal degeneration.
2 Materials and Methods
2.1 Bone marrow-derived mesenchymal stem cells
For the mouse studies, bone marrow-derived MSCs were isolated from the femurs of 4 to 8 week old male C57BL/6-Tg(ACTB-EGFP)1Osb/J mice (Jackson Labs). These mice constitutively express eGFP in most cells, including the MSCs. Marrow was flushed from the isolated femurs with MSC culture medium (Gibco ά-MEM (Invitrogen) + 20% FBS, 2mM L-Glutamine, 1% Penicillin/Streptomycin), plated in culture flasks and grown in the MSC medium. After 24 hours, non-adherent cells were removed and the adherent cells were defined as MSCs (Williams and Hare 2011). These cells could be maintained in culture for over 60 passages, confirming that they were stem cells. They could be induced to differentiate into adipocytes and osteocytes, confirming their identity as mesenchymal progenitors.
For the dog studies, MSCs were obtained from Dachshunds homozygous for a null mutation in TPP1 that encodes the soluble lysosomal enzyme tripeptidyl peptidase-1 (Awano et al. 2006). When the dogs were 2.5 to 3 months of age, bone marrow was aspirated from the humerus using a modification of a technique described previously (Frimberger et al. 2006). The marrow was mixed with MSC culture medium and grown in culture in the same manner as the mouse MSCs. At passage 3 when the cells were near confluency, they were transduced with either AAV2-CAG-GFP or AAV2-CAG-TPP1 (SignaGen Laboratories, Gaithersburg, MD) at multiplicities of infection of 10,000 to 50,000. After transduction, the cells were maintained in culture for multiple passages.
2.2 Intravitreal implantation of mouse MSCs
Mice used as recipients for intravitreal MSC implantation had a null mutation in PPT1 that encodes the soluble lysosomal enzyme palmitoyl protein thioesterase-1 (Gupta et al. 2001). Via multiple backcrosses, the mutation was placed on a pure C57BL/6J strain background. The retina in these mice appears to develop normally and then undergoes a progressive degeneration (Lei et al. 2006).
For intravitreal implantation into the mutant mice, the eGFP-expressing normal C57BL/6J mouse MSCs were harvested after 4 to 12 passages and suspended in minimal essential medium at a concentration of 40,000 cells per μl. The recipient mice were anesthetized and approximately 2 μl of the cell suspension was injected into the vitreous. At various times up to 16 weeks after implantation, the recipient mice were euthanized. The eyes were enucleated immediately after death and prepared for and examined with either fluorescence or light microscopy (Meyer et al. 2006).
2.3 Characterization of canine MSCs
Canine MSC cultures were established and maintained as described for the mouse cells. Expression of GFP in the transduced cells was monitored with fluorescence microscopy. Expression of TPP1 by the cells transduced with AAV2-CAG-TPP1 was monitored by measuring TPP1 enzyme activity in the medium in which the cells were maintained using an established protocol (Tian et al. 2006).
All studies were performed in compliance with the ARVO Statement for the “Use of Animals in Ophthalmic and Vision Research” and were approved by the University of Missouri Animal Care and Use Committee.
3 Results
3.1 Mouse MSCs after intravitreal implantation
The mice tolerated the intravitreal injections with no apparent adverse effects, except in rare cases where the injection needle penetrated the lens capsule. In the latter cases, the mice developed cataracts within a few days of the injection. If the lens capsule was not ruptured the implanted cells formed net-like sheets within the vitreous (Fig. XX.1). The numbers of cells within these sheets remained stable over the 16 week evaluation period, with no evidence of donor cell proliferation or loss. Unlike neural precursor cells (Meyer et al. 2006), there was no evidence of donor cell migration toward or into the retina; the sheets of donor cells remained suspended in the vitreous. The presence of the donor cells in the vitreous did not appear to have a significant effect on the rate of retinal degeneration.
In cases where the lens capsule was damaged during the injection, many donor cells migrated to the lens. Some of these cells formed a layer that tightly adhered to the posterior side of the intact portions of the lens capsule. The majority of the donor cells migrated into the lens itself.
3.2 In vitro characterization of canine MSCs
Cells from the bone marrow aspirates were allowed to attach to culture plates for a period of 24 hours, after which the plates were washed repeatedly to remove non-adherent cells. Those cells remaining were identified as MSCs by their morphology and adherence to plastic (Williams and Hare 2011). The cells typically reached confluency by 96 hours after plating, at which time the cells were passaged. Subsequently the cells typically reached confluency by 48–72 hours after passaging. Cell morphology remained indicative of an MSC lineage through multiple passages (Fig. 1).
Fig. 1.
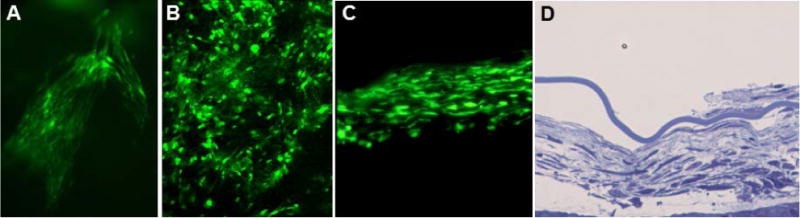
Fluorescence micrographs of a sheet of GFP donor cells in the vitreous in the intact eye (A), in a retinal flat mount (B), and in a cryostat section of the eye (C). Light micrograph of a cross-section sheet of donor cells in the vitreous after fixation and embedding the eye in epoxy resin (D). All images were from eyes collected 16 weeks after MSC implantation.
Canine MSCs were transduced with AAV2-CAG vectors at passage 4 by adding the vector to the culture media. The inoculated media was left on the plates for 96 hours, after which it was replaced with fresh media. GFP expression was detectable at 96 hours post-transduction and increased in intensity over time, reaching a stable high level of intensity approximately 5 days after transduction (Fig. 2). GFP expression remained stable for at least two passages post-transduction. P4 and P5 transduced cells kept at confluency without additional passaging maintained high levels of fluorescence for at least 70 days in vitro.
Fig. 2.
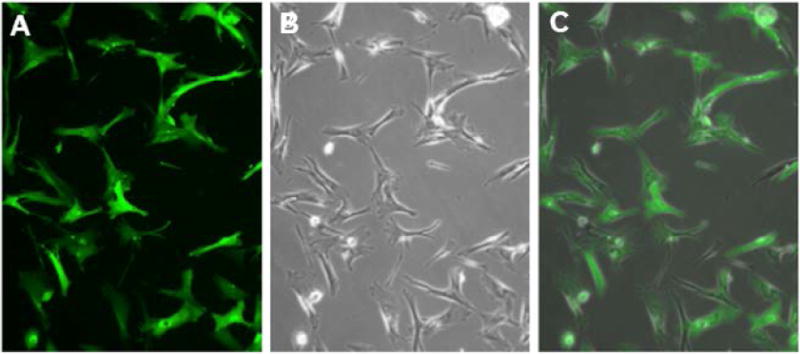
Fluorescence (A), phase contrast (B) micrographs of rAAV2-CAG-GFP transduced passage 4 canine MSCs in vitro.
To gauge TPP1 expression in vitro, the culture medium was collected once every 24 hours starting 24 hours after transduction for up to 72 hours and each sample was analyzed for TPP1 enzyme activity. Based on the TPP1 activity in the conditioned media the estimated release of enzyme by the MSCs in vitro was approximately 3 to 5 pg per cell per 24 hours.
3 Discussion
The mouse studies suggest that intravitreal implantation of MSCs may be a safe means of long-term intraocular delivery of therapeutic agents. As long as there was no damage to the lens the donor cells appeared to survive indefinitely in the vitreous without proliferating or damaging the retina or lens. As the studies with the canine MSCs demonstrated, these cells can be genetically modified to produce and release therapeutic proteins, which are then likely to reach the target eye tissues adjacent to the vitreous.
In vitro the mouse MSCs proliferate indefinitely, yet after implantation into the vitreous, no proliferation was observed. This suggests that the vitreous contains factors that inhibit proliferation. These factors are present not only in eyes in which the retina is undergoing active degeneration, as the behavior of the MSCs was essentially the same when they were implanted into the eyes of normal C57BL/6J mice. Although the vitreous is not vascularized, the donor cells apparently received enough oxygen and nutrients from the adjacent retina to support their long-term survival. In the mouse eye, the vitreous is confined to a thin layer close to the retina due to the fact that the lens occupies most of the internal volume of the eye. The consequent close proximity of the donor cells to the retina may have aided in their long-term survival. However, preliminary studies of implantation of autologous bone marrow derived MSCs into the eyes of dogs indicate that such close proximity may not be necessary for donor cell survival. MSCs implanted into the vitreous of a dog far from the retina were still present several months after implantation. This will be important in developing implantation of these cells for human therapies as the anatomy of the dog eye is more similar to that of the human eye than is the mouse eye.
The migration of the donor cells toward and into the lens when the lens capsule was damaged suggests that the lens contains trophic factors to which the donor cells respond strongly. Potential donor cell responses to endogenous trophic factors is an important consideration when considering using intravitreal implantation of such cells for treating retinal degenerative diseases. Tissues undergoing degeneration release a variety of trophic factors to which the donor cells may respond, and different donor cell types may respond differently to such trophic factors. Indeed, we found that mouse neural precursors derived from embryonic stem cells migrate toward and into the degenerating mouse retina (Meyer et al. 2006), whereas no such migration is observed with the MSCs.
These studies indicate that intravitreal implantation of genetically modified MSCs is promising as a means of long-term delivery of therapeutic agents to the retina. While the mouse and preliminary dog studies support the safety of this approach, efficacy in treating retinal degenerative disease remains to be demonstrated. Such efficacy studies are currently under way using a canine model of neuronal ceroid lipofuscinosis which exhibits a slowly progressive retinal degeneration (Katz et al. 2008).
Acknowledgments
This work was supported in part by the U.S. National Institutes of Health grants R01EY018815 and R01EY023968 and by a grant from Mizzou Advantage. We thank Lauren E. Gillespie for technical assistance and Dr. Mark Sands for the founder PPT1−/− mice.
References
- Awano T, Katz ML, Sohar I, Lobel P, Coates JR, Khan S, Johnson GC, Giger U, Johnson GS. A frame shift mutation in the canine ortholog of human CLN2 in a juvenile dachshund with neuronal ceroid lipofuscinosis. Mol Genet Matabol. 2006;89:254–260. doi: 10.1016/j.ymgme.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Frimberger AE, Moore AS, Rassnick KM, Cotter SM, O’Sullivan JL, Quesenberry PJ. A combination chemotherapy protocol with dose intensification and autologous bone marrow transplant (VELCAP-HDC) for canine lymphoma. J Vet Intern Med. 2006;20:355–364. doi: 10.1892/0891-6640(2006)20[355:accpwd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Gupta P, Soyombo AA, Atasgband A, Wysniewski KE, Shelton JM, Richardson JA, Hammer RE, Hofmann SL. Disruption of PPT1 or PPT2 causes neuronal ceroid lipofuscinosis in knockout mice. Proc Nat Acad Sci USA. 2001;98:13566–13571. doi: 10.1073/pnas.251485198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ML, Coates JR, Cooper JJ, O’Brien DP, Jeong M, Narfström K. Retinal Pathology in a Canine Model of Late Infantile Neuronal Ceroid Lipofuscinosis. Invest Ophthalmol Vis Sci. 2008;49:2686–2695. doi: 10.1167/iovs.08-1712. [DOI] [PubMed] [Google Scholar]
- Lei B, Tullis GE, Kirk MD, Zhang K, Katz ML. Ocular Phenotype in a Mouse Gene Knockout Model for Infantile Neuronal Ceroid-Lipofuscinosis. J Neurosci Res. 2006;84:1139–1149. doi: 10.1002/jnr.21008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Katz ML, Maruniak JA, Kirk MD. Embryonic stem cell derived neural precursors incorporate into the degenerating retina and enhance survival of host photoreceptors. Stem Cells. 2006;24:274–283. doi: 10.1634/stemcells.2005-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden CM, Powner MB, Carr AJ, Smart MJ, da Cruz L, Coffey PJ. Stem cells in retinal regeneration: past, present and future. Development. 2013;140:2576–2585. doi: 10.1242/dev.092270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Sohar I, Taylor JW, Lobel P. Determination of the substrate specificity of tripeptidyl-peptidase I using combinatorial peptide libraries and development of improved fluorogenic substrates. J Biol Chem. 2006;281:6559–6572. doi: 10.1074/jbc.M507336200. [DOI] [PubMed] [Google Scholar]
- Williams AR, Hare JM. Mesenchymal Stem Cells: Biology, Pathophysiology, Translational Findings, and Therapeutic Implications for Cardiac Disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]


