Abstract
Regulators of G protein signaling (RGS) proteins of the B/R4 family are widely expressed in the cardiovascular system where their role in fine tuning G protein signaling is critical to maintaining homeostasis. Among members of this family, RGS2 and RGS5 have been shown to play key roles in cardiac and smooth muscle function by tightly regulating signaling pathways that are activated through Gq/11 and Gi/o classes of heterotrimeric G proteins. This chapter reviews accumulating evidence supporting a key role for RGS2 in vascular function and the implication of changes in RGS2 function and/or expression in the pathogenesis of blood pressure disorders, particularly hypertension. With such understanding, RGS2 and the signaling pathways it controls may emerge as novel targets for developing next-generation anti-hypertensive drugs/agents.
Keywords: RGS2, vascular function, G protein signaling, smooth muscle, endothelium, vasoactive hormones
Introduction
G protein coupled receptor (GPCR) signaling mediates the biological effects of many physiologically important vasoactive substances such as norepinephrine, epinephrine, angiotensin II (ANG II), endothelin-1 (ET-1), vasopressin, acetylcholine (ACh) and bradykinin (BK). These vasoactive substances activate GPCRs that couple to one or more of four heterotrimeric G-protein families distinguished by the type of α subunit in the heterotrimer (Gs, Gq/11, Gi/o and G12/13)[1–4]. RGS proteins play key roles in G protein signaling by acting in part as GTPase-activating proteins (GAPs) that accelerate the rate that Gα subunits hydrolyze GTP and consequently deactivate signal transduction. By facilitating reformation of inactive GDP-bound Gαβγ heterotrimers, this mechanism also resets the system for further rounds of activation by agonist-activated GPCRs. Growing evidence indicates that RGS proteins also regulate cell signaling by mechanisms independent of GAP activity[5–7].
In vertebrates, RGS proteins are expressed in essentially all cell types, tissues and organ systems where they play critical roles in physiology and disease[8, 9]. Among >30 RGS proteins encoded by the human or mouse genome, RGS2 has proved to have diverse functions in the cardiovascular system, including blood pressure regulation. Whereas studies of RGS2 and related B/R4-class RGS proteins in cardiovascular biology and other processes were reviewed several years ago[10], significant progress has been made since then[11–13]. Accordingly, this chapter reviews current understanding of RGS2 structure, function and regulation in vascular biology, and highlights current questions that are driving research in the field.
Structure and Biochemical Functions of RGS2
In mice and humans, the Rgs2 locus is located on chromosome 1 and contains 5 exons. This locus encodes a 212-residue protein containing a RGS domain of ~120 amino acids that is flanked by an ~80-residue N-terminal domain and a short C-terminal tail[14], similar to other B/R4-class RGS proteins[15]. RGS2 possesses intrinsic GAP activity that is potent and selective in vitro for Gq-class Gα subunits[16, 17], in contrast to other B/R4-family RGS proteins that have intrinsic GAP activity for both Gq- and Gi/o-class Gα subunits. However, GAP activity of RGS2 toward Gi/o-class Gα subunits can be detected in receptor-driven systems[18], suggesting that interaction with GPCRs or other components influence which G proteins can be regulated by RGS2. RGS2 possesses an N-terminal amphipathic α-helix and a hydrophobic motif that facilitate targeting of the protein to the plasma membrane where GPCRs and G proteins reside[14]. Plasma membrane targeting of RGS2 is enhanced by cGMP-dependent protein kinase Iα-mediated phosphorylation of serine 46 and 64[19]. The N-terminal domain of RGS2 also binds other proteins, including spinophilin, muscarinic and α-adrenergic receptors, and directly inhibits certain adenylyl cyclase isoforms[6, 20–24]. Furthermore, RGS2 is capable of regulating protein synthesis independently of its GAP activity by binding elongation factor 2b[5].
Regulation of RGS2 Expression
RGS2 expression is regulated at the levels of transcription and protein degradation. RGS2 was first identified as an immediate early gene in activated blood mononuclear cells[25]. Subsequent investigations in many cell types identified various stimuli and signaling pathways that induce Rgs2 gene transcription. For example, ANG II and Gs-coupled GPCR agonists and pharmacological agents that increase cAMP levels or intracellular Ca2+ increase RGS2 mRNA expression in several cell types, including neonatal cardiomyocytes, neurons, HEK293 and vascular smooth muscle cell lines[26–28]. Moreover, CREB elements in the Rgs2 gene promoter have been shown to drive expression in response to GPCR agonists [29].
Like many regulatory proteins whose transcription is tightly controlled, the half-life of RGS2 protein is short (e.g. ~30 min in vascular smooth muscle cells[19]) due to proteasome-mediated degradation. Degradation of RGS2 is augmented by a polymorphism (RGS2-Q2L) identified initially in a cohort of Japanese hypertension patients[30, 31] and subsequently in other genetic studies of human hypertension[32, 33]. Defining how RGS2 is degraded may provide avenues to increase its expression level and function in diseases linked to RGS2 deficiency or dysfunction, including hypertension, anxiety disorders and airway hyper reactivity[33–36].
Expression of RGS2 in the Cardiovascular System
RGS2 is widely expressed in the cardiovascular system including brain, heart, vasculature, and kidney[37]. Whereas all B/R4-class RGS proteins are expressed at the mRNA level in myocardium[38], only a few (RGS1, 2, 4, and 5) have been shown thus far to be expressed detectably at the mRNA or protein level in vascular smooth muscle or endothelial cells of the arterial system[39–43]. In mouse heart, RGS2 has been found to regulate pathologic remodeling elicited by pressure overload[44, 45]. In this model, RGS2 deficiency increases the susceptibility and extent of pathologic cardiac hypertrophic responses that progress rapidly to dilated heart failure. Cardiomyocyte-specific overexpression of Gqα in RGS2 knockout mice is sufficient to trigger cardiac hypertrophy and dilated cardiomyopathy, indicating that impaired regulation of Gq signaling by RGS2 in cardiomyocytes plays a causal role[44]. Moreover, loss of Gq-dependent regulation by RGS2 enhances ANG II-induced pro-fibrotic responses, which can be reversed by adenovirus-mediated overexpression of RGS2 in ventricular myocytes[45].
RGS2 Function in the Vasculature
A. Smooth muscle function
Homozygous and heterozygous RGS2 knockout mice exhibit elevated blood pressure[40], indicating that the mouse Rgs2 gene is a quantitative trait locus (QTL) affecting blood pressure. Consistent with the function of RGS2 as a Gq GAP, agonists that activate Gq-coupled GPCRs evoke augmented Ca2+ transients in vascular smooth muscle cells from mesenteric resistance arteries of RGS2 knockout mice[19]. Furthermore, RGS2 deficiency enhances contraction of mesenteric resistance arteries or renal interlobar arteries stimulated by an α1-adrenergic receptor agonist[46, 47]. Thus, Gq-mediated smooth muscle contraction is augmented by RGS2 deficiency in several regions of the arterial tree (Figure 1).
Figure 1.
The role of RGS2 in vascular smooth muscle contraction. RGS2 tightly regulates the duration and amplitude of G protein signaling that mediates smooth muscle contraction triggered by vasoactive ligands that activate Gq-coupled GPCRs. Upon activation, Gq signaling evokes a rise in intracellular Ca2+ concentration resulting in myosin phosphorylation by myosin light chain kinase (MLCK) and actomyosin crossbridge formation. Decreased RGS2 expression or function can lead to increased signaling duration and intensity that in turn cause augmented smooth muscle contraction. Cam, calmodulin; DAG, diacylglycerol; PIP2, phosphatidylinositol 4,5-bisphosphate; PLCβ, Phospholipase C-beta; IP3, inositol 1,3,5-trisphosphate; SR, sarcoplasmic reticulum; IP3R, IP3 receptor; NE, norepinephrine; ET-1, endothelin-1; ANG II, angiotensin II.
RGS2 also promotes smooth muscle-intrinsic relaxation by functioning as an effector of the nitric oxide/cGMP-dependent protein kinase Iα (PKGIα) signaling pathway that attenuates Gq-evoked vasoconstriction. In this mechanism, nitric oxide (NO) produced by endothelial cells stimulates production of cGMP in vascular smooth muscle cells to activate PKGIα. RGS2 is phosphorylated by PKGIα on serine-46 and -64, which promotes its association with the plasma membrane and attenuation of Gq-evoked vasoconstriction[19, 48]. Indeed, the absence of RGS2 abolishes the ability of cGMP analogs to blunt vasopressin-evoked Ca2+ transients in resistance artery smooth muscle cells[49]. Moreover, RGS2 deficiency blunts the ability of a NO donor to antagonize the pressor effect of an α1-adrenergic receptor agonist that directly stimulates contraction of the resistance vasculature[49].
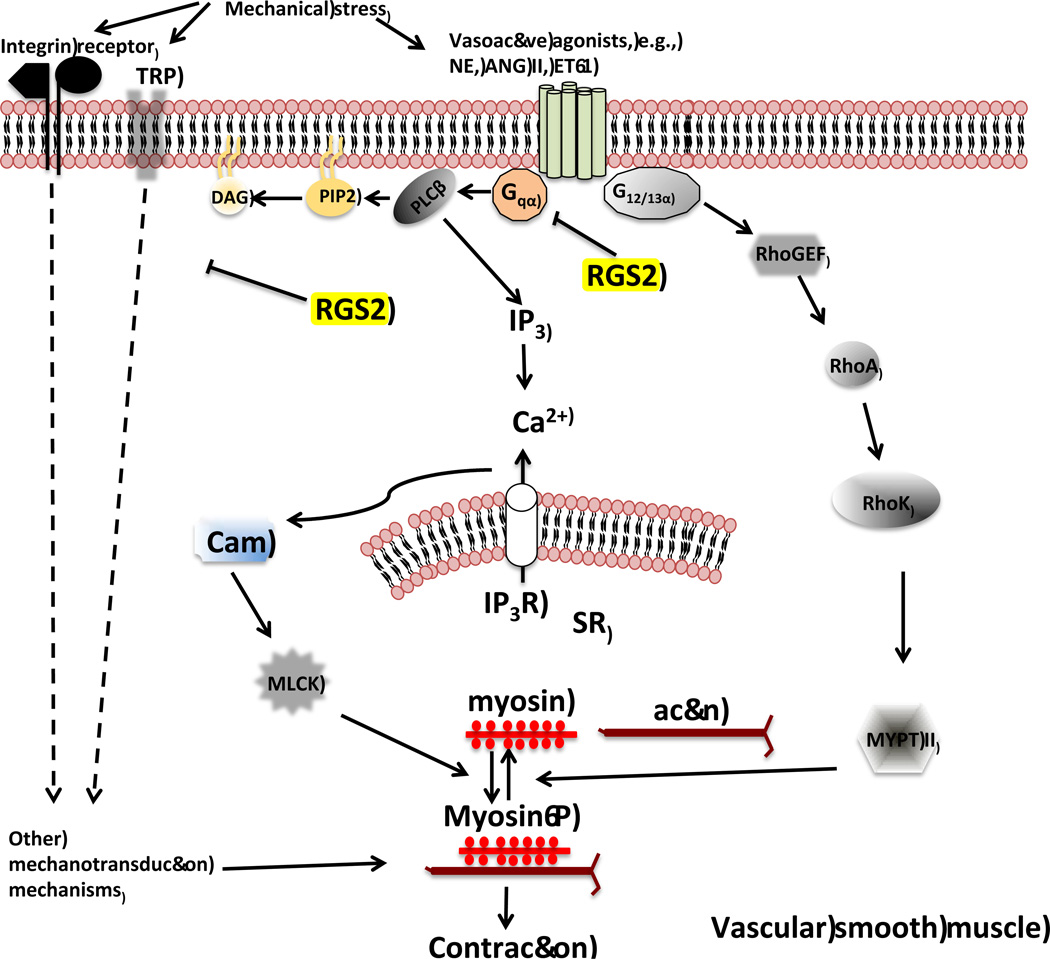
RGS2 deficiency also appears to augment vasoconstriction of resistance arteries by affecting stretch-induced contraction or myogenic tone. Renal interlobar arteries from RGS2 knockout mice exhibit enhanced constriction triggered by rises in transmural pressure ex vivo[46]. How RGS2 regulates myogenic tone is unknown. Myogenic response evoked by stretch of smooth muscle cells depends on activation of stretch receptors mediating mechanotransduction signaling[50, 51]. Although the components of this signaling mechanism in smooth muscle remain to be fully defined, receptors studied to date include members of the transient receptor potential (TRP) superfamily[52], vascular epithelial sodium channel (ENaC)[53], integrins[54], and the angiotensin type 1 receptor (AT1R)[55]. Whereas RGS2 is known to negatively regulate vascular AT1R signaling, it remains possible that RGS2 regulates other mediators of vascular mechanotransduction (Figure 2) that are not known to couple to heterotrimeric G proteins. Because mechanotransduction occurs prominently in resistance as compared to conduit arteries, elucidating how RGS2 regulates mechanotransduction may reveal new insight into mechanisms of vascular dysfunction in hypertension or other cardiovascular diseases in which augmented vascular resistance occurs.
Figure 2.
A model of contractile signaling pathways potentially regulated by RGS2 in smooth muscle cells in the resistance vasculature. In addition to regulating Gq-dependent smooth muscle contraction, RGS2 could potentially regulate stretch-induced contraction mediated by putative receptors and ion channels that can be activated by mechanical stimuli. TRP, transient receptor potential; NE, norepinephrine; ET-1, endothelin-1; ANG II, angiotensin II; myosin-P, phosphorylated myosin; RhoK, RhoA associated kinase; MYPT II, myosin phosphatase regulatory subunit II.
B. Endothelial function
Because RGS2 is expressed in vascular endothelium, smooth muscle and adventitia[56, 57], it potentially regulates vascular function in several ways. Indeed, recent studies have revealed a novel endothelium-specific function for RGS2[47]. Because Gq signaling in vascular endothelium elicits Ca2+ fluxes to stimulate production of NO and endothelium-derived hyperpolarizing factor (EDHF), RGS2 deficiency would be expected to augment Gq activity and consequent endothelium-dependent vasodilatation. Surprisingly, however, the opposite effect has been found. Global or endothelium-specific knockout of RGS2 markedly impairs endothelium-dependent relaxation of mesenteric resistance arteries stimulated by acetylcholine (ACh)[47]. Whereas global RGS2 deficiency causes relatively modest impairment of ACh-evoked relaxation mediated by NO, it nearly completely eliminates ACh-evoked relaxation mediated by EDHF. This defect in EDHF-mediated relaxation can be rescued by blocking Gi/o signaling with pertussis toxin. Thus, in contrast to its role in vascular smooth muscle as a GAP for Gq, RGS2 apparently functions in endothelium of mesenteric resistance arteries as a GAP that squelches Gi/o signaling, which otherwise would inhibit EDHF-dependent vasodilatation (Figure 3).
Figure 3.
Regulation of G protein signaling in vascular endothelium. Vasodilatory ligands such as ACh and BK activate Gq-coupled receptors in the vascular endothelium of resistance arteries causing a rise in intracellular Ca2+ required to stimulate endothelial nitric oxide synthase (eNOS) for producing nitric oxide (NO). A rise in endothelial Ca2+ is also necessary for activation of potassium channels SKCa and IKCa that mediate the production of endothelium-derived hyperpolarizing factor (EDHF). The activity of SKCa and IKCa are negatively regulated by Gα subunits of the Gi-class in this vascular compartment. By acting as GAP, RGS2 squelches the activity of Gi-class G proteins to promote EDHF production. Thus, RGS2 deficiency in the endothelium is proposed to result in impairment of endothelium-dependent vasodilatation due to increased Gi signaling, inhibiting EDHF production. IP3, inositol 1,4,5-trisphosphate; PLCβ, Phospholipase C-beta; BH4, tetrahydrobiopterin; eNOS, endothelium nitric oxide synthase.
In contrast to its effect on endothelial function in mesenteric resistance arteries, RGS2 deficiency does not significantly affect endothelium-dependent relaxation of aortic rings[48]. This finding is consistent with evidence indicating that the relative contributions of various endothelium-derived relaxing factors, including NO, EDHF, and prostacyclin, change as the diameter of the vessel lumen decreases from conduit vessels where NO predominates, to small arterioles where EDHF is a primary mediator of endothelium-dependent vasodilation[58, 59]. However, it is inconsistent with evidence indicating that RGS2 deficiency significantly impairs relaxation of aortic rings evoked by cGMP, which is a direct effector of the NO system. Despite such unresolved questions, what remains clear is that RGS2 has distinct functions in different types of arteries and in different compartments within arteries.
Although significant understanding of RGS2 function in the vasculature has been obtained, an important unanswered question is how RGS2 deficiency causes hypertension. Whereas global RGS2 deficiency causes hypertension, endothelium- or vascular smooth muscle-specific deletion of RGS2 is insufficient to increase blood pressure[47]. However, transplantation of kidneys lacking RGS2 into wild type recipient mice is sufficient to elevate blood pressure[60], as expected since the kidney plays a dominant role in regulating blood pressure. Because RGS2 is expressed widely throughout the kidney, including renal vasculature and parenchyma[46, 61], future experiments that analyze the effects of deleting RGS2 in specific renal structures and cell types will be required to assess the vascular and tubular contributions of this RGS protein in renal control of blood pressure homeostasis. Such studies may reveal whether integrated versus compartment-specific functions of RGS2 in renal vasculature and tubules are required for normal regulation of blood pressure.
RGS2 and Human Hypertension
The initial discovery of hypertension in heterozygous or homozygous RGS2 knockout mice[40] prompted investigations whether RGS2 dysfunction occurs in human hypertension. Initial studies examined RGS2 expression and function in human skin fibroblasts and peripheral blood mononuclear cells (PBMNC) in normotensive and hypertensive individuals[62]. They found that cells from hypertensive subjects exhibited decreased RGS2 mRNA expression and increased ANG II-induced ERK phosphorylation and Ca2+ release from internal stores[62]. Human genetic studies subsequently have identified Rgs2 single nucleotide polymorphisms (SNPs) in hypertension cohorts from several regions of the globe including, Japan[31], Netherlands[32], United States[33] and China[63, 64]. Some of these SNPs apparently decrease RGS2 protein expression by increasing its susceptibility to arginylation/ubiquitination and subsequent degradation by the proteasome pathway[30]. Other mutations or SNPs impair GAP activity or affect the subcellular localization of RGS2[36]. Whether or how these SNPs affect RGS2-dependent control of organ systems critical for blood pressure homeostasis remains to be determined.
In contrast to evidence linking decreased expression or function of RGS2 in hypertension, evidence of augmented RGS2 expression has been reported in Bartter/Gitelman syndrome, a human disorder characterized by hypotension and sodium and potassium wasting due to defects in renal electrolyte transport proteins[65, 66]. Interestingly, PBMNC from Bartter/Gitelman syndrome patients show elevated RGS2 expression, and refractory response to ANG II-induced Ca2+ mobilization from internal stores[57]. Thus, identifying regulatory mechanisms that up or downregulate RGS2 expression or function to increase or decrease blood pressure may provide new targets for drug discovery in cardiovascular diseases.
RGS2 in Preeclampsia
Pregnancy triggers physiological remodeling of the cardiovascular system that is necessary to maintain normal blood pressure and organ perfusion in the face of increased maternal extracellular fluid volume[67, 68]. Marked increase in the levels of vasoactive hormones, particularly ANG II, occur during pregnancy, which has the potential to augment vasoconstriction, peripheral resistance, sodium retention and blood pressure[69–71]. However, such effects ordinarily are countered by increased production of endothelium-derived relaxing factors, mainly NO[72]. Furthermore, the arterial vasculature in pregnancy becomes refractory to vasoconstriction by ANG II[73], although the mechanisms involved remain poorly understood. Defects in such compensatory mechanisms therefore may contribute to gestational hypertension and preeclampsia[74, 75].
RGS2 may function in compensatory mechanisms that normally maintain blood pressure during pregnancy and are impaired in preeclampsia. This hypothesis is attractive because ANG II triggers vasoconstriction by activating Gq-coupled AT1 receptors such that increased expression or activity of RGS2 or other Gq regulatory proteins could provide adaptive mechanisms that normally maintain blood pressure during pregnancy. Impairment of such adaptive mechanisms potentially could contribute to the pathophysiology of preeclampsia. Indeed, recent studies of G protein signaling pathways associated with blood pressure regulation in preeclamptic and normotensive pregnant women identified the Rgs2 SNP rs4606 in the 3’ untranslated region as associated with risk and progression of preeclampsia[76]. Further evidence indicated that women experiencing preeclampsia and carrying this Rgs2 SNP have increased risk of developing hypertension after delivery[77]. These findings should motivate further studies aimed at unraveling how G protein signaling is functionally remodeled during pregnancy, whether RGS2 is an important component of this regulatory network, and whether RGS2 may provide a therapeutic target in preeclampsia.
RGS2 in Other Disorders Affecting Smooth Muscle
Because contraction and relaxation of smooth muscle in a variety of organs and tissues occur by similar mechanisms, the functions of RGS2 established in the vasculature may be recapitulated in other organ systems and diseases. This hypothesis has been explored in asthma and chronic obstructive pulmonary disease (COPD), a central feature of which is increased constriction and spasms of airway smooth muscle driven by various GPCRs, principally Gq-coupled muscarinic receptors[78]. Recent studies have shown that RGS2 expression is decreased in lungs of asthmatic relative to non-asthmatic individuals[35], and that RGS2 knockout mice exhibit spontaneous hyper-responsiveness of airway smooth muscle accompanied by augmented GPCR-induced intracellular Ca2+ mobilization[79]. In humans, the Rgs2 SNPs rs2746071 and rs2746072 are in linkage disequilibrium, exhibiting higher prevalence in asthmatics compared to controls[79]. Moreover, RGS2 expression in human airway smooth muscle is increased by currently used therapeutics for asthma and COPD, including long-acting β2-adrenergic agonists alone or combined with glucocorticoids[80]. Thus, RGS2 dysfunction in some patients may play a causal role in airway smooth muscle hyper-contraction, and upregulation of RGS2 may promote therapeutic outcome in asthma or COPD patients.
Conclusions and Future Perspectives
RGS2 has emerged as a critical component of G protein signaling mechanisms in various systems that regulate cardiovascular function. In the heart, RGS2 tightly regulates Gq signaling to maintain normal cardiac structure and function[44]. Similarly, Gq regulation by RGS2 in the vasculature is key to maintaining normal vascular tone by controlling constriction triggered by ligands that activate Gq-coupled GPCRs[40]. In addition, by serving as a substrate of the NO-cGMP-PKG signaling pathway, RGS2 promotes smooth muscle intrinsic relaxation, thereby reducing vascular tone[19, 48]. Moreover, RGS2 in endothelium reduces vascular tone by acting as a GAP towards Gi/o-class Gα proteins to facilitate EDHF-dependent vasodilatation of resistance arteries[47]. These roles of RGS2 are consistent with studies showing that defective RGS2 regulation of Gq and Gi/o-class Gα proteins play a primary role in the pathogenesis of cardiovascular and other smooth muscle disorders such as heart failure, COPD, asthma, and hypertension. Thus, mechanisms that impinge on RGS2 expression could be critical to disease onset or progression.
Certain Rgs2 SNPs or mutations identified to date that are linked to human diseases are capable of altering RGS2 expression and/or function[36]. Following the discovery more than a decade ago that RGS2 has an important role in blood pressure homeostasis[40], subsequent human genetics studies have identified association of several Rgs2 SNPs with hypertension in various populations[32, 33]. Some of the hypertension-linked SNPs of Rgs2 have been shown to decrease RGS2 GAP activity towards Gq in vitro [36]. In contrast, decreased RGS2 expression is associated with increased Gq signaling and hypotension in Bartter/Gitelman syndrome[57]. These studies highlight the relevance of the function of RGS2 in human cardiovascular function.
Several outstanding questions must be answered to obtain a more complete understanding of how RGS2 regulates vascular function in normal physiology and disease. First, how does RGS2 deficiency cause hypertension? Recent transplantation studies establish that RGS2 deficiency in the kidney is sufficient to increase blood pressure[60], although defining the renal mechanisms that are dysregulated in the absence of RGS2 remains an important goal. Second, what mechanisms functionally shift the GAP activity of RGS2 toward Gi/o- as opposed to Gq-class Gα proteins in the vascular endothelium? Addressing this question may have significant impact because endothelial dysfunction is a central feature of several cardiovascular disorders, particularly hypertension and preeclampsia, which are also associated with Rgs2 SNPs[33, 76]. Third, what mechanisms regulate RGS2 expression levels, and how are they altered to up- or down-regulate RGS2 in health and disease? Unraveling these mechanisms may provide novel therapeutic targets for increasing or decreasing the endogenous level of RGS2 in diseases and disorders as diverse as hypertension, asthma, COPD, Bartter’s/Gitelman’s syndrome, and anxiety that have been linked to RGS2.
Acknowledgments
We would like to thank Dr. Harpreet Singh for critical reading of the manuscript. The authors were supported by NIH grants HL075632 and GM44592 to Kendall J. Blumer, and institutional support from Drexel University College of Medicine to Patrick Osei-Owusu.
References
- 1.Wess J. G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 1997;11(5):346–354. [PubMed] [Google Scholar]
- 2.Hur EM, Kim KT. G protein-coupled receptor signalling and cross-talk: achieving rapidity and specificity. Cell Signal. 2002;14(5):397–405. doi: 10.1016/s0898-6568(01)00258-3. [DOI] [PubMed] [Google Scholar]
- 3.Conklin BR, Bourne HR. Structural elements of G alpha subunits that interact with G beta gamma, receptors, and effectors. Cell. 1993;73(4):631–641. doi: 10.1016/0092-8674(93)90245-l. [DOI] [PubMed] [Google Scholar]
- 4.Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995;80(2):249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen CH, et al. Translational control by RGS2. J Cell Biol. 2009;186(5):755–765. doi: 10.1083/jcb.200811058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy AA, et al. RGS2 interacts with Gs and adenylyl cyclase in living cells. Cell Signal. 2006;18(3):336–348. doi: 10.1016/j.cellsig.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Sethakorn N, Yau DM, Dulin NO. Non-canonical functions of RGS proteins. Cell Signal. 2010;22(9):1274–1281. doi: 10.1016/j.cellsig.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54(3):527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, Mende U. Functional role, mechanisms of regulation, and therapeutic potential of regulator of G protein signaling 2 in the heart. Trends Cardiovasc Med. 2014;24(2):85–93. doi: 10.1016/j.tcm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansal G, Druey KM, Xie Z. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacol Ther. 2007;116(3):473–495. doi: 10.1016/j.pharmthera.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nisancioglu MH, et al. Generation and characterization of rgs5 mutant mice. Mol Cell Biol. 2008;28(7):2324–2331. doi: 10.1128/MCB.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho H, et al. Rgs5 targeting leads to chronic low blood pressure and a lean body habitus. Mol Cell Biol. 2008;28(8):2590–2597. doi: 10.1128/MCB.01889-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grillet N, et al. Generation and characterization of Rgs4 mutant mice. Mol Cell Biol. 2005;25(10):4221–4228. doi: 10.1128/MCB.25.10.4221-4228.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heximer SP, et al. Mechanisms governing subcellular localization and function of human RGS2. J Biol Chem. 2001;276(17):14195–14203. doi: 10.1074/jbc.M009942200. [DOI] [PubMed] [Google Scholar]
- 15.Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci. 2005;1(2):51–66. doi: 10.7150/ijbs.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heximer SP, et al. RGS2/G0S8 is a selective inhibitor of Gqalpha function. Proc Natl Acad Sci U S A. 1997;94(26):14389–14393. doi: 10.1073/pnas.94.26.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nance MR, et al. Structural and functional analysis of the regulator of G protein signaling 2-galphaq complex. Structure. 2013;21(3):438–448. doi: 10.1016/j.str.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingi T, et al. Dynamic regulation of RGS2 suggests a novel mechanism in G-protein signaling and neuronal plasticity. J Neurosci. 1998;18(18):7178–7188. doi: 10.1523/JNEUROSCI.18-18-07178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osei-Owusu P, et al. Regulation of RGS2 and second messenger signaling in vascular smooth muscle cells by cGMP-dependent protein kinase. J Biol Chem. 2007;282(43):31656–31665. doi: 10.1074/jbc.M706360200. [DOI] [PubMed] [Google Scholar]
- 20.Salim S, Dessauer CW. Analysis of the interaction between RGS2 and adenylyl cyclase. Methods Enzymol. 2004;390:83–99. doi: 10.1016/S0076-6879(04)90006-7. [DOI] [PubMed] [Google Scholar]
- 21.Salim S, et al. Identification of RGS2 and type V adenylyl cyclase interaction sites. J Biol Chem. 2003;278(18):15842–15849. doi: 10.1074/jbc.M210663200. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, et al. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat Cell Biol. 2005;7(4):405–411. doi: 10.1038/ncb1237. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein LS, et al. RGS2 binds directly and selectively to the M1 muscarinic acetylcholine receptor third intracellular loop to modulate Gq/11alpha signaling. J Biol Chem. 2004;279(20):21248–21256. doi: 10.1074/jbc.M312407200. [DOI] [PubMed] [Google Scholar]
- 24.Hague C, et al. Selective inhibition of alpha1A-adrenergic receptor signaling by RGS2 association with the receptor third intracellular loop. J Biol Chem. 2005;280(29):27289–27295. doi: 10.1074/jbc.M502365200. [DOI] [PubMed] [Google Scholar]
- 25.Siderovski DP, Heximer SP, Forsdyke DR. A human gene encoding a putative basic helix-loop-helix phosphoprotein whose mRNA increases rapidly in cycloheximide-treated blood mononuclear cells. DNA Cell Biol. 1994;13(2):125–147. doi: 10.1089/dna.1994.13.125. [DOI] [PubMed] [Google Scholar]
- 26.Zou MX, et al. RGS2 is upregulated by and attenuates the hypertrophic effect of alpha1-adrenergic activation in cultured ventricular myocytes. Cell Signal. 2006;18(10):1655–1663. doi: 10.1016/j.cellsig.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Grant SL, et al. Specific regulation of RGS2 messenger RNA by angiotensin II in cultured vascular smooth muscle cells. Mol Pharmacol. 2000;57(3):460–467. doi: 10.1124/mol.57.3.460. [DOI] [PubMed] [Google Scholar]
- 28.Song L, De Sarno P, Jope RS. Muscarinic receptor stimulation increases regulators of G-protein signaling 2 mRNA levels through a protein kinase C-dependent mechanism. J Biol Chem. 1999;274(42):29689–29693. doi: 10.1074/jbc.274.42.29689. [DOI] [PubMed] [Google Scholar]
- 29.Xie Z, et al. Identification of a cAMP-response element in the regulator of G-protein signaling-2 (RGS2) promoter as a key cis-regulatory element for RGS2 transcriptional regulation by angiotensin II in cultured vascular smooth muscles. J Biol Chem. 2011;286(52):44646–44658. doi: 10.1074/jbc.M111.265462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodenstein J, Sunahara RK, Neubig RR. N-terminal residues control proteasomal degradation of RGS2, RGS4, and RGS5 in human embryonic kidney 293 cells. Mol Pharmacol. 2007;71(4):1040–1050. doi: 10.1124/mol.106.029397. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, et al. Genetic variations of regulator of G-protein signaling 2 in hypertensive patients and in the general population. J Hypertens. 2005;23(8):1497–1505. doi: 10.1097/01.hjh.0000174606.41651.ae. [DOI] [PubMed] [Google Scholar]
- 32.Hahntow IN, et al. Are RGS2 gene polymorphisms associated with high blood pressure in an ethnicity- and gender-specific manner? Am J Hypertens. 2009;22(1):80–86. doi: 10.1038/ajh.2008.310. [DOI] [PubMed] [Google Scholar]
- 33.Riddle EL, et al. Polymorphisms and haplotypes of the regulator of G protein signaling-2 gene in normotensives and hypertensives. Hypertension. 2006;47(3):415–420. doi: 10.1161/01.HYP.0000200714.81990.61. [DOI] [PubMed] [Google Scholar]
- 34.Hettema JM, et al. Genetic association study between RGS2 and anxiety-related phenotypes. Psychiatr Genet. 2013;23(2):92. doi: 10.1097/YPG.0b013e32835d70b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie Y, et al. Regulator of G protein signaling 2 is a key modulator of airway hyperresponsiveness. J Allergy Clin Immunol. 2012;130(4):968–76. e3. doi: 10.1016/j.jaci.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Gu S, Tirgari S, Heximer SP. The RGS2 gene product from a candidate hypertension allele shows decreased plasma membrane association and inhibition of Gq. Mol Pharmacol. 2008;73(4):1037–1043. doi: 10.1124/mol.107.044214. [DOI] [PubMed] [Google Scholar]
- 37.Kehrl JH, Sinnarajah S. RGS2: a multifunctional regulator of G-protein signaling. Int J Biochem Cell Biol. 2002;34(5):432–438. doi: 10.1016/s1357-2725(01)00141-8. [DOI] [PubMed] [Google Scholar]
- 38.Riddle EL, et al. Multi-tasking RGS proteins in the heart: the next therapeutic target? Circ Res. 2005;96(4):401–411. doi: 10.1161/01.RES.0000158287.49872.4e. [DOI] [PubMed] [Google Scholar]
- 39.Timofeeva AV, et al. Comparative transcriptome analysis of human aorta atherosclerotic lesions and peripheral blood leukocytes from essential hypertension patients. Kardiologiia. 2009;49(9):27–38. [PubMed] [Google Scholar]
- 40.Heximer SP, et al. Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. J Clin Invest. 2003;111(4):445–452. doi: 10.1172/JCI15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bondjers C, et al. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol. 2003;162(3):721–729. doi: 10.1016/S0002-9440(10)63868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albig AR, Schiemann WP. Identification and Characterization of Regulator of G Protein Signaling 4 (RGS4) as a Novel Inhibitor of Tubulogenesis: RGS4 Inhibits Mitogen-activated Protein Kinases and Vascular Endothelial Growth Factor Signaling. Molecular Biology of the Cell. 2005;16(2):609–625. doi: 10.1091/mbc.E04-06-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, et al. RGS5, RGS4, and RGS2 expression and aortic contractibility are dynamically co-regulated during aortic banding-induced hypertrophy. J Mol Cell Cardiol. 2008;44(3):539–550. doi: 10.1016/j.yjmcc.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Takimoto E, et al. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest. 2009;119(2):408–420. doi: 10.1172/JCI35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang P, et al. Regulator of G protein signaling 2 is a functionally important negative regulator of angiotensin II-induced cardiac fibroblast responses. Am J Physiol Heart Circ Physiol. 2011;301(1):H147–H156. doi: 10.1152/ajpheart.00026.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hercule HC, et al. Regulator of G protein signalling 2 ameliorates angiotensin II-induced hypertension in mice. Exp Physiol. 2007;92(6):1014–1022. doi: 10.1113/expphysiol.2007.038240. [DOI] [PubMed] [Google Scholar]
- 47.Osei-Owusu P, et al. Regulator of G protein signaling 2 deficiency causes endothelial dysfunction and impaired endothelium-derived hyperpolarizing factor-mediated relaxation by dysregulating Gi/o signaling. J Biol Chem. 2012;287(15):12541–12549. doi: 10.1074/jbc.M111.332130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang KM, et al. Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat Med. 2003;9(12):1506–1512. doi: 10.1038/nm958. [DOI] [PubMed] [Google Scholar]
- 49.Sun X, et al. RGS2 is a mediator of nitric oxide action on blood pressure and vasoconstrictor signaling. Mol Pharmacol. 2005;67(3):631–639. doi: 10.1124/mol.104.007724. [DOI] [PubMed] [Google Scholar]
- 50.Hill MA, Meininger GA. Arteriolar vascular smooth muscle cells: mechanotransducers in a complex environment. Int J Biochem Cell Biol. 2012;44(9):1505–1510. doi: 10.1016/j.biocel.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kauffenstein G, et al. Emerging role of G protein-coupled receptors in microvascular myogenic tone. Cardiovasc Res. 2012;95(2):223–232. doi: 10.1093/cvr/cvs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beech DJ. Emerging functions of 10 types of TRP cationic channel in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2005;32(8):597–603. doi: 10.1111/j.1440-1681.2005.04251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim EC, et al. Epithelial Na+ channel proteins are mechanotransducers of myogenic constriction in rat posterior cerebral arteries. Exp Physiol. 2012;97(4):544–555. doi: 10.1113/expphysiol.2011.062232. [DOI] [PubMed] [Google Scholar]
- 54.Chao JT, Davis MJ. The roles of integrins in mediating the effects of mechanical force and growth factors on blood vessels in hypertension. Curr Hypertens Rep. 2011;13(6):421–429. doi: 10.1007/s11906-011-0227-6. [DOI] [PubMed] [Google Scholar]
- 55.Mederos y Schnitzler M, Storch U, Gudermann T. AT1 receptors as mechanosensors. Curr Opin Pharmacol. 2011;11(2):112–116. doi: 10.1016/j.coph.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Grayson TH, et al. Vascular microarray profiling in two models of hypertension identifies caveolin-1, Rgs2 and Rgs5 as antihypertensive targets. BMC Genomics. 2007;8:404. doi: 10.1186/1471-2164-8-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calo LA, et al. Increased expression of regulator of G protein signaling-2 (RGS-2) in Bartter's/Gitelman's syndrome. A role in the control of vascular tone and implication for hypertension. J Clin Endocrinol Metab. 2004;89(8):4153–4157. doi: 10.1210/jc.2004-0498. [DOI] [PubMed] [Google Scholar]
- 58.Feletou M, Vanhoutte PM. The alternative: EDHF. J Mol Cell Cardiol. 1999;31(1):15–22. doi: 10.1006/jmcc.1998.0840. [DOI] [PubMed] [Google Scholar]
- 59.Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 2009;117(4):139–155. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- 60.Gurley SB, et al. Renal actions of RGS2 control blood pressure. J Am Soc Nephrol. 2010;21(11):1847–1851. doi: 10.1681/ASN.2009121306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zuber AM, et al. Increased renal responsiveness to vasopressin and enhanced V2 receptor signaling in RGS2−/− mice. J Am Soc Nephrol. 2007;18(6):1672–1678. doi: 10.1681/ASN.2007010032. [DOI] [PubMed] [Google Scholar]
- 62.Semplicini A, et al. Reduced expression of regulator of G-protein signaling 2 (RGS2) in hypertensive patients increases calcium mobilization and ERK1/2 phosphorylation induced by angiotensin II. J Hypertens. 2006;24(6):1115–1124. doi: 10.1097/01.hjh.0000226202.80689.8f. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, et al. Association between the regulator of G-protein signaling 2 gene 1891-1892del TC polymorphism with hypertension in Xinjiang Kazakh population. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2010;27(1):29–33. doi: 10.3760/cma.j.issn.1003-9406.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 64.Zhao Q, et al. Interactions among genetic variants from contractile pathway of vascular smooth muscle cell in essential hypertension susceptibility of Chinese Han population. Pharmacogenet Genomics. 2008;18(6):459–466. doi: 10.1097/FPC.0b013e3282f97fb2. [DOI] [PubMed] [Google Scholar]
- 65.Deschenes G, Feldmann D, Doucet A. Primary molecular changes and secondary biological problems in Bartter and Gitelman syndrome. Arch Pediatr. 2002;9(4):406–416. doi: 10.1016/s0929-693x(01)00801-6. [DOI] [PubMed] [Google Scholar]
- 66.Knoers NV, Levtchenko EN. Gitelman syndrome. Orphanet J Rare Dis. 2008;3:22. doi: 10.1186/1750-1172-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katz VL. Physiologic changes during normal pregnancy. Curr Opin Obstet Gynecol. 1991;3(6):750–758. [PubMed] [Google Scholar]
- 68.Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin. 2012;30(3):317–329. doi: 10.1016/j.ccl.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 69.Wong AY, et al. Maternal cardiovascular changes during pregnancy and postpartum in mice. Am J Physiol Heart Circ Physiol. 2002;282(3):H918–H925. doi: 10.1152/ajpheart.00641.2001. [DOI] [PubMed] [Google Scholar]
- 70.Clapp JF, 3rd, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol. 1997;80(11):1469–1473. doi: 10.1016/s0002-9149(97)00738-8. [DOI] [PubMed] [Google Scholar]
- 71.Slangen BF, et al. Hemodynamic changes in early pregnancy in chronically instrumented, conscious rats. Am J Physiol. 1996;270(5 Pt 2):H1779–H1784. doi: 10.1152/ajpheart.1996.270.5.H1779. [DOI] [PubMed] [Google Scholar]
- 72.Chu ZM, Beilin LJ. Nitric oxide-mediated changes in vascular reactivity in pregnancy in spontaneously hypertensive rats. Br J Pharmacol. 1993;110(3):1184–1188. doi: 10.1111/j.1476-5381.1993.tb13939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conrad KP, et al. The renin-angiotensin system during pregnancy in chronically instrumented, conscious rats. Am J Obstet Gynecol. 1989;161(4):1065–1072. doi: 10.1016/0002-9378(89)90785-0. [DOI] [PubMed] [Google Scholar]
- 74.Shah DM. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. Am J Physiol Renal Physiol. 2005;288(4):F614–F625. doi: 10.1152/ajprenal.00410.2003. [DOI] [PubMed] [Google Scholar]
- 75.Friedman SA, et al. Preeclampsia and related disorders. Clinical aspects and relevance of endothelin and nitric oxide. Clin Perinatol. 1995;22(2):343–355. [PubMed] [Google Scholar]
- 76.Kvehaugen AS, et al. Single nucleotide polymorphisms in G protein signaling pathway genes in preeclampsia. Hypertension. 2013;61(3):655–661. doi: 10.1161/HYPERTENSIONAHA.111.00331. [DOI] [PubMed] [Google Scholar]
- 77.Kvehaugen AS, et al. Hypertension after preeclampsia and relation to the C1114G polymorphism (rs4606) in RGS2: data from the Norwegian HUNT2 study. BMC Med Genet. 2014;15:28. doi: 10.1186/1471-2350-15-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gosens R, et al. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res. 2006;7:73. doi: 10.1186/1465-9921-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang H, et al. RGS2 Repression Exacerbates Airway Hyperresponsiveness and Remodeling in Asthma. Am J Respir Cell Mol Biol. 2014 doi: 10.1165/rcmb.2014-0319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holden NS, et al. beta2-Adrenoceptor agonist-induced RGS2 expression is a genomic mechanism of bronchoprotection that is enhanced by glucocorticoids. Proc Natl Acad Sci U S A. 2011;108(49):19713–19718. doi: 10.1073/pnas.1110226108. [DOI] [PMC free article] [PubMed] [Google Scholar]