Abstract
Background:
We aimed to evaluate the diagnostic value of anti-smooth muscle antibodies (ASMA) and two liver markers (gamma-glutamyl transpeptidase [GGT] and alkaline phosphatase [ALP]) for differentiating between patients with extrahepatic biliary atresia (EHBA) and idiopathic neonatal hepatitis (INH).
Materials and Methods:
During April 2010–2011, all infants at 2 weeks of age who were diagnosed with cholestasis and admitted to Children's Hospital of Tabriz were enrolled. Based on the results of physical examination, laboratory, imaging and pathological studies, neonates were divided into two groups (EHBA and INH). Receiver operating characteristics analysis was used to define sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy for ASMA, GGT and ALP.
Results:
Thirty neonates with cholestasis (18 with EHBA and 12 with INH) and mean age of 54.66 ± 25.86 days were enrolled. Total and direct bilirubin, serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase and ASMA titres were highly not significant (P > 0.05) in patients with INH. GGT (P = 0.008) and ALP (P = 0.01) had statistically significant differences that were higher in patients with EHBA. The sensitivity, specificity, PPV and NPV, accuracy, LR+ and LR− of SMA in differentiating cases with BA were 66.7%, 75%, 80% 60%, 70%, 2.68 and 0.44, respectively. For GGT, the values were 88.9%, 66.7%, 80%, 80%, 79.1%, 3.08 and 0.31, respectively. Finally, for ALP, the values were 77.8%, 75%, 82.4%, 69.2%, 80%, 2.66 and 0.24, respectively.
Conclusion:
Our study showed that ASMA may be a useful biomarker for differentiation of EHBA from INH. Further studies with larger samples are recommended for confirming the results of this study.
Keywords: Anti-smooth muscles antibody, extrahepatic biliary atresia, idiopathic neonatal hepatitis, neonatal cholestasis
INTRODUCTION
Neonatal cholestasis (NC) is defined as the prolonged elevation of serum levels of conjugated bilirubin beyond 2 weeks of life. Biliary atresia (BA) and idiopathic neonatal hepatitis (INH) are the two most common causes of NC. Early and accurate differentiation between these two entities is very important and determines the prognosis and curability of cholestasis. BA requires surgical intervention as soon as possible; whereas, INH needs medical management.[1,2,3] BA is an inflammatory cholangiopathy of infancy that results in progressive fibrosis and obliteration of extrahepatic and intrahepatic bile ducts. The incidence of BA is higher in Asia and the Pacific region than in the rest of the world. The disease is diagnosed in approximately 5-6/100,000 live births in Europe and the United States, 10.6/100,000 in Japan and up to 32/100,000 in French Polynesia.[4,5] However, the precise aetiology of this disease remains largely unknown. Some factors that may contribute to its development are genetic, infective, inflammatory and even toxic insult. If untreated, affected children show progressive liver disease, with the development of portal hypertension and liver failure, invariably resulting in death within the first 2 years of life.[6] Portoenterostomy (Kasai) that was described in the 1950s by the Japanese surgeon Morio Kasai remains the only form of therapy that can be offered to these patients besides liver transplant. However, its effectiveness is variable and probably depends on early diagnosis with prompt surgical intervention. Despite all efforts, BA remains the most common indication of liver transplant in young children.[7,8] Histopathologic examination of liver biopsy specimens represents a crucial element in the diagnostic evaluation of patients with suspected BA. Biliary obstructive features must be confirmed histologically and distinguished from various nonobstructive aetiologies of NC (i.e., neonatal hepatitis syndrome).[9] Delay in diagnosis beyond 60-100 days of age may reduce longevity of the native liver and increase morbidity in infants with BA, although the ideal surgical timing remains controversial.[10,11,12] Late diagnosis also worsens the outcomes in other conditions associated with NC, such as hypopituitarism, galactosaemia and tyrosinaemia.[13,14]
Increased production of type 1 collagen, leading to hepatic fibrosis by activated hepatic stellate cells (HSCs), has been shown in pathological conditions of the adult liver, including cholestasis. HSCs were transformed to myofibroblasts (activated HSCs), which produce increased levels of fibrillar collagen and express an intracellular microfilament protein, alpha-smooth muscle actin (α-SMA), during hepatic injury. Alpha-smooth muscle actin has traditionally been used as a marker protein of the activated HSC phenotype.[15,16,17] Higher expression of anti-smooth muscle antibodies (ASMA) in BA liver was shown by Dong et al.[18]
ASMA were initially detected in serum samples of patients with liver diseases by Johnson et al. in 1965.[19] The presence of ASMA in patients with autoimmune liver disease was confirmed by Whittingham et al.[20] The association between ASMA and anti-actin antibodies in autoimmune hepatitis (AIH) was established in 1973.[21] ASMA can be detected using indirect immunofluorescence (IIF), fibroblasts, or HEp-2 cells. Immunometric methods have recently been developed, such as enzyme-linked immunosorbent assay and immunodot, as well as a new IIF method for detecting antibodies in filamentous actin.[22] ASMA with other autoimmunity markers such as antinuclear antibodies and anti-soluble liver antigen/liver pancreas are now used by clinicians to help diagnose AIH.[23]
In the previous studies, we showed relative accuracy of procalcitonin and Apo-E levels,[24] triangular cord sign[25] and ultrasound findings[26] in diagnosing extrahepatic biliary atresia (EHBA) cases in our patients. In this study, we aimed to evaluate the diagnostic value of ASMA, gamma-glutamyl transpeptidase (GGT) and alkaline phosphatase (ALP) in differentiating EHBA from INH.
MATERIALS AND METHODS
All infants with jaundice at 2 weeks of age who were diagnosed with cholestasis and admitted to Gastroenterology ward, Children's Hospital of Tabriz University of Medical Sciences between April 2010 and 2012 were enrolled. Patients with renal or liver failure or any disorder that caused reduced production and clearance of acute phase proteins, hypoxia, shock, patients receiving antibiotics, anti-inflammatory drugs, or corticosteroids and patients receiving total parenteral nutrition were the exclusion criteria of our study. This protocol was approved by the Institutional Review Board of Tabriz University of Medical Sciences and written parental consent was obtained for each study participant during the study.
Physical examination, laboratory and imaging studies were performed in all cholestatic neonates. Final diagnosis was made according to imaging studies, laboratory and pathology findings. Based on the results of final diagnosis, neonates were divided into two groups ([EHBA; n = 18] and INH [n = 12]). Along with other laboratory evaluations, ASMA levels were also measured. Fasting blood samples were obtained via venous puncture on the 1st day of admission. Samples were centrifuged within 30 min of collection and the serum was stored at −70°C before analysis for determination of ASMA. The IIF test is used for the detection of human antibody to the antigens of smooth muscle. For determination of ASMA level in patients, Euroimmun kit (FA 1710-1003) was used.
SPSS for (windows version 13) was used for all statistical analysis. Data are expressed as mean ± standard deviation or number (%) as appropriate. Student's t-test for evaluating the significance of mean for independent continuous scale data and Mann-Whitney U-test for nonparametric data, where appropriate, and Chi-square test or Fisher exact test for testing the significance of percentages were used. P ≤ 0.05 was considered as significant. Diagnostic validity of ASMA, GGT and ALP was compared with the results of final diagnosis using receiver operating characteristics (ROC) curves and the best cut-off value was calculated for ASMA. Then, sensitivity, specificity, positive and negative predictive values (PPV and NPV) were calculated for this cut-off.
RESULTS
Thirty neonates with a mean age of 54.66 ± 25.86 days suffering from jaundice were evaluated in this study. Final diagnosis was made according to imaging studies, laboratory, surgery and pathological findings; 18 of 30 patients were defined to have EHBA and other 12 patients had INH.
HIDA scan was performed in all patients and results were indicative of EHBA in 26 (86.7%), suspected atresia in 1 (3.3%) and no atresia in 3 (10%). Only 20 patients underwent cholangiography before surgery, which showed atresia in 13 (65%). Pathologic findings showed EHBA in 16 (64%) and neonatal hepatitis in 8 (40%) of 24 patients who had liver biopsy. The missing cases are due to the disagreement of parents for further imaging or invasive evaluations. Nine patients with EHBA underwent Kasai portoenterostomy, and 8 of them were candidates for liver transplantation because of liver cirrhosis.
Baseline and laboratory findings between patients with EHBA and INH are shown in Tables 1 and 2, respectively. Total and direct bilirubin, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT) and ASMA titres were higher in patients with INH, but this difference was not statistically significant [Table 2]. Only GGT and ALP had statistically significant differences and were higher in patients with EHBA [Table 2]. We did not find any correlation between the ASMA titre and the other laboratory findings.
Table 1.
Baseline findings between patients with extrahepatic biliary atresia and neonatal hepatitis
| Variable | Extra hepatic biliary atresia | Neonatal Hepatitis | P value |
|---|---|---|---|
| Gender (male) | 8 (44.4%) | 9 (75%) | 0.09 |
| Age at biopsy/surgery | 58.72 (20.71) | 48.58 (32.15) | 0.34 |
| Age at jaundice onset (day) | 5.66 (2.66) | 5.08 (4.25) | 0.84 |
| Weight at admission grams) | 4266.66 (1273.20) | 3420.00 (1140.46) | 0.69 |
| Related parents | 5 (27.8%) | 7 (58.3%) | 0.09 |
| Acholic stool | 17 (94.4%) | 10 (83.3%) | 0.32 |
| Positive cord sign | 4 (22.2%) | 1 (8.3%) | 0.31 |
Table 2.
Laboratory findings between patients with extrahepatic biliary atresia and Neonatal Hepatitis
| Variable | Extra hepatic biliary atresia | Neonatal hepatitis | P value |
|---|---|---|---|
| Total bilirubin (mg/dl) | 12.29 (4.97) | 13.24 (5.36) | 0.63 |
| Direct bilirubin (mg/dl) | 6.71 (2.70) | 8.19 (3.3.18) | 0.20 |
| SGOT (U/l) | 274.88 (207.63) | 365.75 (582.36) | 0.61 |
| SGPT (U/l) | 163.83 (124.13) | 208.00 (288.02) | 0.62 |
| ALP (U/l) | 1784.66 (683.73) | 1119.00 (717.70) | 0.01* |
| Gammaglutamyl transpeptidase (U/l) | 583.88 (445.40) | 231.74 (204.36) | 0.008* |
| PT | 21.58 (31.82) | 13.27 (1.80) | 0.28 |
| PTT | 40.33 (13.41) | 35.83 (8.1) | 0.26 |
| Albumin (g/dl) | 3.65 (0.49) | 3.96 (0.52) | 0.11 |
| SMA (ng/ml) | 0.89 (0.48) | 1.42 (0.88) | 0.07 |
Anti-smooth muscles antibody levels were between 0.016 ± 1.9 and 0.37 ± 3.60 in patients with EHBA and INH, respectively. ROC curve analysis was used to define thresholds of ASMA, GGT and ALP for differentiating between EHBA and INH [Figures 1-3]. Area under the curve was 0.78 (confidence interval [CI] 95% [0.51-0.9], P = 0.05) with cut-point <0.915 ng/ml for ASMA, 0.78 (CI 95% [0.58-0.95], P = 0.01) with cut-point >1332 U/I for ALP and 0.78 (CI 95% [0.6-0.96], P = 0.01) with cut-point >218.5 U/I [Figures 1–3].
Figure 1.
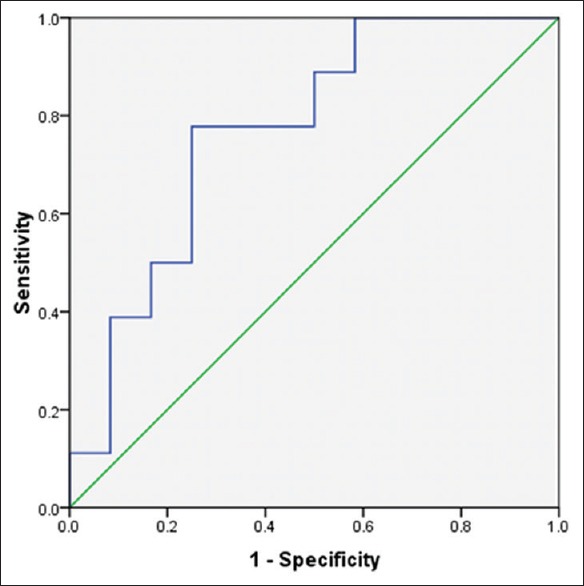
Roc curve for ALP in differentiating cases with INH and EHBA
Figure 3.
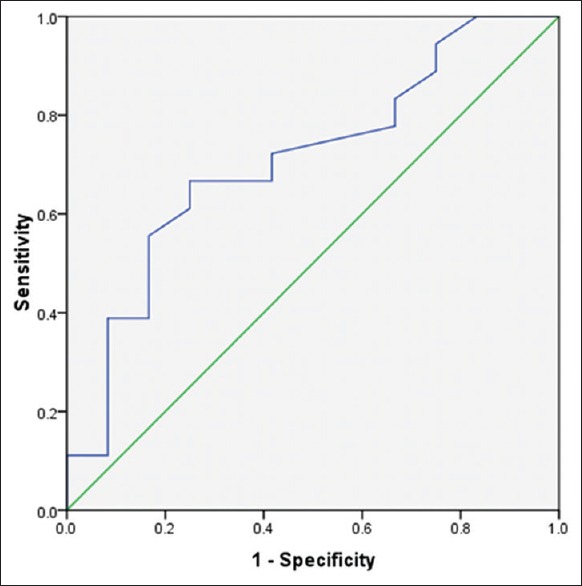
Roc carve for ASMA in differentiating cases with EHBA and INH
Figure 2.
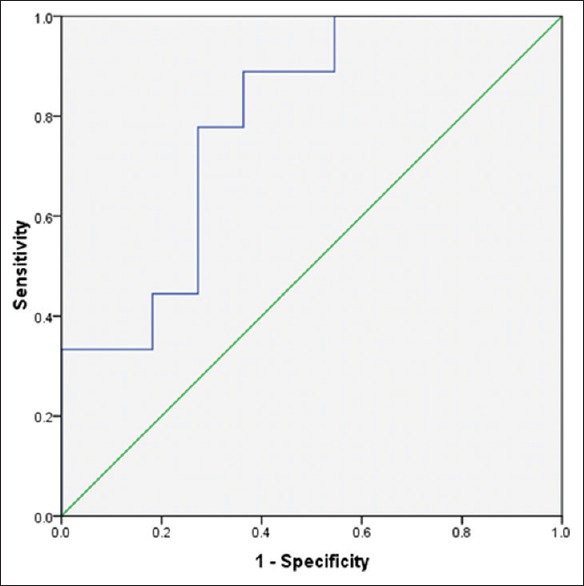
Roc curve for GGT in differentiating cases with EHBA and INH
The sensitivity, specificity, PPV, NPV, accuracy, LR+ and LR– of SMA in differentiating cases with BA were 66.7%, 75%, 80%, 60%, 70%, 2.68 and 0.44, respectively. For GGT, the values were 88.9%, 66.7%, 80%, 80%, 79.1%, 3.08 and 0.31, respectively. Finally, for ALP, the values were 77.8%, 75%, 82.4%, 69.2%, 80%, 2.66 and 0.24, respectively.
DISCUSSION
INH and EHBA are the major causes of NC. EHBA must be managed operatively as soon as possible, while the preferred treatment of INH is medical.[27,28,29] Late referral of cholestatic neonates is one of the main problems of these patients, especially in developing countries.[30] In our study, the median age of neonates that refer with cholestasis was 54.66 ± 25.86 days (9-113 days). The need for a wide range of tests to determine the cause of cholestasis in these patients leads to a loss of the golden time for Kasai portoenterostomy (60-100 days) and reduces longevity of the native liver and increases morbidity.[10,11,12] Our results showed that 9 of 18 neonates with EHBA underwent Kasai portoenterostomy, and among them, 8 were candidates for liver transplantation because of liver cirrhosis due to late referral and late diagnosis. Differentiating EHBA from other causes of cholestatic jaundice for on-time portoenterostomy and prevention of fatal consequences of EHBA is very critical.[31,32]
Dehghani et al., showed that SGOT, SGPT, ALP and GGT were significantly increased in BA group,[33] but in the study by Chu et al. the difference in the mean ALT, AST and ALP values between INH and BA patients was not significant.[34] In our study, patients with INH had higher titres of total and direct bilirubin, SGOT, SGPT and albumin, and in the other group, (EHBA), GGT, ALP, prothrombin time and partial thromboplastin time were higher, but significant differences were only observed in GGT and ALP. The sensitivity and specificity of GGT for diagnosis of BA in our study were 88.9% and 66.7%, respectively, but the results of Lai et al.[35] were different (sensitivity 51.6% and specificity 82.2%); however, the results of Chu et al.[33] were similar to those from our study (sensitivity 82.1% and specificity 79.3%). In another study that was performed by Dehghani, the values for liver enzymes tests were 68.4% and 43.5%, respectively.[34]
Many studies have evaluated the diagnostic validity of laboratory, imaging, surgical and pathological investigations in the differentiation of EHBA from other cases of cholestasis. In the study by Dehghani et al., liver biopsy with 96.6% accuracy and clinical investigation by an experienced paediatric hepatologist with 70.8% accuracy were the most reliable methods.[33] In another study that was performed in India, the accuracy rate for percutaneous liver biopsy was 88.2%.[36] PPV for positive triangular cord sign in combination with abnormal gallbladder findings was 98% in the study by Takamizawa et al.[37] and 92% by Nemati et al.[25] The diagnostic accuracy of hepatobiliary scintigraphy (HBS) single photon emission computer tomography, magnetic resonance cholangiography (MRCP) and HBS were 91.30%, 71.01% and 66.7%, respectively.[38] Motamed et al.[39] showed 60% diagnostic accuracy rate of HIDA scan for BA in their study. Conjugated bilirubin >2.5 mg/dl, GGT >150 U/L, excretion on HIDA, or a normal percutaneous cholangiogram were 100% sensitive for the exclusion of BA as demonstrated by Jancelewicz et al. In this study, clinical variables and ultrasound had low specificity and sensitivity. For liver biopsy, reported sensitivity and specificity were 98% and 84%, respectively.[40] Apolipoprotein E and procalcitonin were 67% sensitive (specificity: 61% and 67%, respectively) for diagnosis of BA as shown by Rafeey et al.[24] Evaluation of the blood levels of nitric oxide (NO) for the differentiation of BA and INH was performed in a recent study, which showed that the NO levels were significantly higher in patients with EHBA.[41]
Smooth muscle auto-antibody (SMA) is one of the serological markers of autoimmune form of hepatitis. It is present also in the sera of most cases of acute infection hepatitis.[20] Some studies have shown diagnostic accuracy and elevated level of SMA in patients with AIH and chronic abstractive pulmonary disease.[23,42,43]
In this study, we evaluated the diagnostic accuracy of ASMA in the diagnosis of EHBA and INH, and according to our results, the sensitivity, specificity, PPV, NPV and accuracy of SMA in differentiating cases with BA were 66.7%, 75%, 80%, 60% and 70%, respectively. Compared with the accuracy rates of invasive procedures (MRCP [71.1%], HBS [66.7%], HIDA scan [60%] and ultrasonography [69.2%]),[33,38,39] ASMA may be a useful marker for the differentiation of EHBA from INH.
CONCLUSION
EHBA and INH are the two common cases of NC. Because of the importance of early diagnosis of these two entities for timely intervention, we evaluated the diagnostic value of ASMA titres in the patients. Medium sensitivity and NPV, and good specificity, PPV and accuracy of ASMA in differentiating cases with EHBA and INH showed that this marker may be a useful biomarker for differentiation of EHBA from INH. Further studies with larger samples are recommended for confirming the results of this study.
Financial support and sponsorship
Pediatrics Health Research Center, Tabriz University of Medical Sciences, Iran.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Poddar U, Thapa BR, Das A, Bhattacharya A, Rao KL, Singh K. Neonatal cholestasis: Differentiation of biliary atresia from neonatal hepatitis in a developing country. Acta Paediatr. 2009;98:1260–4. doi: 10.1111/j.1651-2227.2009.01338.x. [DOI] [PubMed] [Google Scholar]
- 2.Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374:1704–13. doi: 10.1016/S0140-6736(09)60946-6. [DOI] [PubMed] [Google Scholar]
- 3.Bellomo-Brandao MA, Arnaut LT, Tommaso AM, Hessel G. Differential diagnosis of neonatal cholestasis: Clinical and laboratory parameters. J Pediatr (Rio J) 2010;86:40–4. doi: 10.2223/JPED.1970. [DOI] [PubMed] [Google Scholar]
- 4.Strickland AD, Shannon K. Studies in the etiology of extrahepatic biliary atresia: Time-space clustering. J Pediatr. 1982;100:749–53. doi: 10.1016/s0022-3476(82)80576-3. [DOI] [PubMed] [Google Scholar]
- 5.Yoon PW, Bresee JS, Olney RS, James LM, Khoury MJ. Epidemiology of biliary atresia: A population-based study. Pediatrics. 1997;99:376–82. doi: 10.1542/peds.99.3.376. [DOI] [PubMed] [Google Scholar]
- 6.Hays DM, Snyder WH., Jr Life-span in untreated biliary atresia. Surgery. 1963;54:373–5. [PubMed] [Google Scholar]
- 7.Khalil BA, Perera MT, Mirza DF. Clinical practice: Management of biliary atresia. Eur J Pediatr. 2010;169:395–402. doi: 10.1007/s00431-009-1125-7. [DOI] [PubMed] [Google Scholar]
- 8.Chitsaz E, Schreiber RA, Collet JP, Kaczorowski J. Biliary atresia: The timing needs a changin’. Can J Public Health. 2009;100:475–7. doi: 10.1007/BF03404348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreira RK, Cabral R, Cowles RA, Lobritto SJ. Biliary atresia: A multidisciplinary approach to diagnosis and management. Arch Pathol Lab Med. 2012;136:746–60. doi: 10.5858/arpa.2011-0623-RA. [DOI] [PubMed] [Google Scholar]
- 10.Moyer V, Freese DK, Whitington PF, Olson AD, Brewer F, Colletti RB, et al. Guideline for the evaluation of cholestatic jaundice in infants: Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39:115–28. doi: 10.1097/00005176-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Chardot C, Carton M, Spire-Bendelac N, Le Pommelet C, Golmard J, Reding R, et al. Is the Kasai operation still indicated in children older than 3 months diagnosed with biliary atresia? J Pediatr. 2001;138:224–8. doi: 10.1067/mpd.2001.111276. [DOI] [PubMed] [Google Scholar]
- 12.Wong KK, Chung PH, Chan IH, Lan LC, Tam PK. Performing Kasai portoenterostomy beyond 60 days of life is not necessarily associated with a worse outcome. J Pediatr Gastroenterol Nutr. 2010;51:631–4. doi: 10.1097/MPG.0b013e3181e8e194. [DOI] [PubMed] [Google Scholar]
- 13.Benchimol EI, Walsh CM, Ling SC. Early diagnosis of neonatal cholestatic jaundice: Test at 2 weeks. Can Fam Physician. 2009;55:1184–92. [PMC free article] [PubMed] [Google Scholar]
- 14.Brumbaugh D, Mack C. Conjugated hyperbilirubinemia in children. Pediatr Rev. 2012;33:291–302. doi: 10.1542/pir.33-7-291. [DOI] [PubMed] [Google Scholar]
- 15.Ramm GA, Nair VG, Bridle KR, Shepherd RW, Crawford DH. Contribution of hepatic parenchymal and nonparenchymal cells to hepatic fibrogenesis in biliary atresia. Am J Pathol. 1998;153:527–35. doi: 10.1016/S0002-9440(10)65595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang CC, Chuang JH, Huang LL, Chou MH, Wu CL, Chen CM, et al. The human delta-like 1 homologue is implicated in the progression of liver fibrosis in biliary atresia. J Pathol. 2004;202:172–9. doi: 10.1002/path.1505. [DOI] [PubMed] [Google Scholar]
- 17.Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–35. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 18.Dong R, Luo Y, Zheng S. a-SMA overexpression associated with increased liver fibrosis in infants with biliary atresia. J Pediatr Gastroenterol Nutr. 2012;55:653–6. doi: 10.1097/MPG.0b013e3182680be3. [DOI] [PubMed] [Google Scholar]
- 19.Johnson GD, Holborow EJ, Glynn LE. Antibody to smooth muscle in patients with liver disease. Lancet. 1965;2:878–9. doi: 10.1016/s0140-6736(65)92505-5. [DOI] [PubMed] [Google Scholar]
- 20.Whittingham S, Irwin J, Mackay IR, Smalley M. Smooth muscle autoantibody in “autoimmune” hepatitis. Gastroenterology. 1966;51:499–505. [PubMed] [Google Scholar]
- 21.Gabbiani G, Ryan GB, Lamelin JP, Vassalli P, Majno G, Bouvier CA, et al. Human smooth muscle autoantibody. Its identification as antiactin antibody and a study of its binding to “nonmuscular” cells. Am J Pathol. 1973;72:473–88. [PMC free article] [PubMed] [Google Scholar]
- 22.Vergani D, Alvarez F, Bianchi FB, Cançado EL, Mackay IR, Manns MP, et al. Liver autoimmune serology: A consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. J Hepatol. 2004;41:677–83. doi: 10.1016/j.jhep.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Zhang WC, Zhao FR, Chen J, Chen WX. Meta-analysis: Diagnostic accuracy of antinuclear antibodies, smooth muscle antibodies and antibodies to a soluble liver antigen/liver pancreas in autoimmune hepatitis. PLoS One. 2014;9:e92267. doi: 10.1371/journal.pone.0092267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafeey M, Saboktakin L, Shoa Hassani J, Farahmand F, Aslanabadi S, Ghorbani-Haghjou A, et al. Diagnostic value of procalcitonin and apo-e in extrahepatic biliary atresia. Iran J Pediatr. 2014;24:623–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Nemati M, Rafeey M, Shakeri AB. Ultrasound findings in biliary atresia: The role of triangular cord sign. Pak J Biol Sci. 2009;12:95–7. doi: 10.3923/pjbs.2009.95.97. [DOI] [PubMed] [Google Scholar]
- 26.Rafeey M, Nemati M, Nazarpour S. Diagnostic value of ultrasonography in biliary ducts atresia. Med J Tabriz Univ Med Sci. 2009;31:31–5. [Google Scholar]
- 27.Cauduro SM. Extrahepatic biliary atresia: Diagnostic methods. J Pediatr (Rio J) 2003;79:107–14. [PubMed] [Google Scholar]
- 28.Rafeey M, Golzar A, Javadzadeh A. Cholestatic syndromes of infancy. Pak J Biol Sci. 2008;11:1764–7. doi: 10.3923/pjbs.2008.1764.1767. [DOI] [PubMed] [Google Scholar]
- 29.Najafi M. Prevalence of different etiology in neonatal cholestasis. Iran J Pediatr. 2006;16:289–94. [Google Scholar]
- 30.Mowat AP, Davidson LL, Dick MC. Earlier identification of biliary atresia and hepatobiliary disease: Selective screening in the third week of life. Arch Dis Child. 1995;72:90–2. doi: 10.1136/adc.72.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davenport M, Ure BM, Petersen C, Kobayashi H. Surgery for biliary atresia - Is there a European consensus? Eur J Pediatr Surg. 2007;17:180–3. doi: 10.1055/s-2007-965147. [DOI] [PubMed] [Google Scholar]
- 32.Otte JB, de Ville de Goyet J, Reding R, Hausleithner V, Sokal E, Chardot C, et al. Sequential treatment of biliary atresia with Kasai portoenterostomy and liver transplantation: A review. Hepatology. 1994;20(1 Pt 2):41S–8S. doi: 10.1016/0270-9139(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 33.Dehghani SM, Haghighat M, Imanieh MH, Geramizadeh B. Comparison of different diagnostic methods in infants with cholestasis. World J Gastroenterol. 2006;12:5893–6. doi: 10.3748/wjg.v12.i36.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu B, Jiang LR, Zhou S, Xu YZ, Zhang B, Deng ZH. Value of the liver function test in differential diagnosis of infantile hepatitis syndrome and biliary atresia. Zhongguo Dang Dai Er Ke Za Zhi. 2009;11:953–6. [PubMed] [Google Scholar]
- 35.Lai MW, Chang MH, Hsu SC, Hsu HC, Su CT, Kao CL, et al. Differential diagnosis of extrahepatic biliary atresia from neonatal hepatitis: A prospective study. J Pediatr Gastroenterol Nutr. 1994;18:121–7. doi: 10.1097/00005176-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Rastogi A, Krishnani N, Yachha SK, Khanna V, Poddar U, Lal R. Histopathological features and accuracy for diagnosing biliary atresia by prelaparotomy liver biopsy in developing countries. J Gastroenterol Hepatol. 2009;24:97–102. doi: 10.1111/j.1440-1746.2008.05737.x. [DOI] [PubMed] [Google Scholar]
- 37.Takamizawa S, Zaima A, Muraji T, Kanegawa K, Akasaka Y, Satoh S, et al. Can biliary atresia be diagnosed by ultrasonography alone? J Pediatr Surg. 2007;42:2093–6. doi: 10.1016/j.jpedsurg.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 38.Yang JG, Ma DQ, Peng Y, Song L, Li CL. Comparison of different diagnostic methods for differentiating biliary atresia from idiopathic neonatal hepatitis. Clin Imaging. 2009;33:439–46. doi: 10.1016/j.clinimag.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Motamed F, Khalili A, Salamati P, Moradi G, Najafi Sani M, Khodadad A, et al. Diagnostic evaluation of neonatal cholestasis: HIDA scan and alagille criteria. Iran J Radiol. 2014;11:e6382. doi: 10.5812/iranjradiol.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jancelewicz T, Barmherzig R, Chung CT, Ling SC, Kamath BM, Ng VL, et al. A screening algorithm for the efficient exclusion of biliary atresia in infants with cholestatic jaundice. J Pediatr Surg. 2015;50:363–70. doi: 10.1016/j.jpedsurg.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 41.Goel P, Bhatnagar V, Das N, Kalaivani M. Evaluation of blood levels of nitric oxide as a means of differentiation between neonatal hepatitis and extrahepatic biliary atresia: A pilot study. J Indian Assoc Pediatr Surg. 2015;20:139–42. doi: 10.4103/0971-9261.159029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebbeson RL, Schreiber RA. Diagnosing autoimmune hepatitis in children: Is the International Autoimmune Hepatitis Group scoring system useful? Clin Gastroenterol Hepatol. 2004;2:935–40. doi: 10.1016/s1542-3565(04)00396-9. [DOI] [PubMed] [Google Scholar]
- 43.Guda TM, Yousof AA, Al Salahy MM, Al Mehy GF, Essawy TS, El Shaor OS. Study of anti nuclear and anti smooth muscle antibodies in patients with chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc. 2014;63:49–56. [Google Scholar]


