Abstract
Background:
The aim was to compare gastroschisis (GS) epidemiology, management and outcome in low-income countries (LIC) in Sub-Saharan Africa (SSA) with middle- (MIC) and high-income countries (HIC).
Materials and Methods:
A 10-question survey was administered at the 2012 Pan-African Paediatric Surgery Association Congress. Results are presented as median (range); differences were analysed using contingency tests.
Results:
A total of 82 delegates (28 countries [66 institutions]) were divided into LIC (n = 11), MIC (n = 6) and HIC (n = 11). In LIC, there were fewer surgeons and more patients. LIC reported 22 cases (1-184) GS/institution/year, compared to 12 cases (3-23)/institution/year in MICs and 15 cases (1-100)/institution/year in HICs. Antenatal screening was less readily available in LIC. Access to parenteral nutrition and neonatal intensive care in LIC was 36% and 19%, compared to 100% in HIC. Primary closure rates were similar in LIC and HIC at 58% and 54%, respectively; however, the majority of staged closure utilised custom silos in LIC and preformed silos in HIC. In LIC, mortality was reported as >75% by 61% delegates and 50-75% by 33%, compared to <25% by 100% of HIC delegates (P < 0.0001).
Conclusions:
Gastroschisis is a problem encountered by surgeons in SSA. Mortality is high and resources in many centres inadequate. We propose the implementation of a combined epidemiological research, service delivery training and resource provision programme to help improve our understanding of GS in SSA whilst attempting to improve outcome.
Keywords: Africa, epidemiology, gastroschisis, management, outcome
INTRODUCTION
Gastroschisis (GS) is a congenital defect of the anterior abdominal wall through which abdominal contents protrude without a covering amniotic sac [Figure 1]. In the Western world, the incidence is rising; one in 2000-4000 live births.[1,2] Management involves resuscitation, cardiorespiratory support, initial bowel coverage then reduction of viscera. The latter can be achieved by primary or staged closure. Clinical practice varies widely in Europe and North America, but mortality is low.[3,4,5] Many infants with GS have a degree of intestinal dysmotility that delays enteral feeding, hence the requirement for parental nutrition (PN) support.[5]
Figure 1.
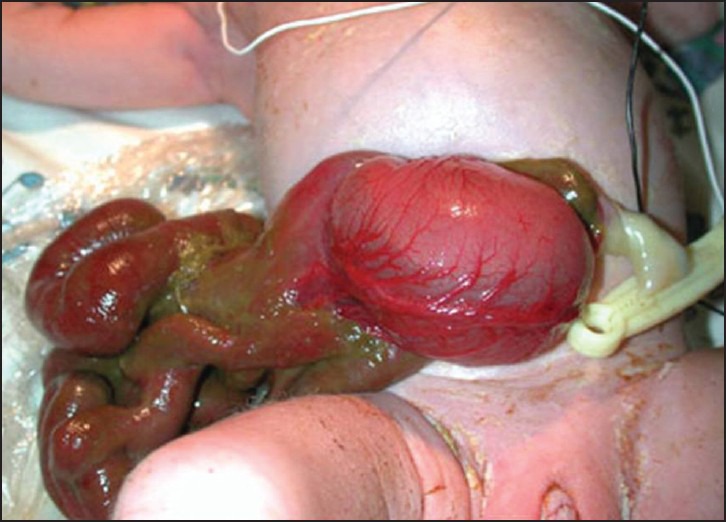
Infant with gastroschisis demonstrating abdominal wall defect to the right of the umbilical cord without a covering sac
Compared to many other parts of the world, there is a paucity of epidemiological and outcome studies regarding GS originating from Sub-Saharan Africa (SSA). Such data would help estimate burden of disease and aid health service prioritisation. Where data are available, the mortality is high; 33-100%.[6,7,8,9,10,11,12] We aimed to investigate the epidemiology, management practice patterns and outcome of GS in low-income countries (LIC) in SSA and compare them with middle- (MIC) and high-income countries (HIC) across the world.
MATERIALS AND METHODS
A 10-question survey was administered to delegates at the 9th biennial Pan-African Paediatric Surgery Association conference in Cape Town in March 2012. Respondents provided details of their grade, hospital, country of practice, catchment area and number of paediatric surgeons at their institution. Questions focused on GS frequency, antenatal care, referral patterns, management, access to PN and intensive care and estimated mortality [Table 1]. Finally, the questionnaire included a box for comments.
Table 1.
Survey questions
| Approximately, what proportion of women undergo antenatal screening in your catchment area? |
| None □ 0-25% □ 25-50% □ 50-75% □ 75-100% □ All □ |
| Are babies diagnosed with GS routinely delivered early? |
| No □ Yes, 37-40 weeks gestation □ Yes, <37 weeks gestation □ |
| Approximate number of patients with GS referred/admitted to your centre each year? |
| Approximately, what proportion are referred from outside your centre? |
| <25% □ 25-75% □ >75% □ |
| What is your primary management for patients with GS? |
| PC (custom silo if necessary) in the theatre □ |
| PC (custom silo if necessary) at the cot side □ |
| Staged reduction with custom silo and closure □ |
| Staged reduction with preformed silo and closure in theatre □ |
| Staged reduction with preformed silo and closure at the cot side □ |
| Anaesthesia used: None □ None Local □ General □ |
| Do you use PFS? Yes □ No □ |
| If yes, how often do you use? All patients primarily □ Some patients □ Rarely use □ |
| If no, what is the reason? |
| Not available □ |
| Too expensive □ |
| No experience/training in the use of preformed silo □ |
| Bad experience in the past □ |
| Not convinced the preformed silo is better □ |
| Objection from another team member (e.g. neonatologists) □ |
| Choose not to use □ |
| Other, please specify |
| Would you consider using PFS in the future? Yes □ No □ |
| Do you have access to parenteral nutrition? Yes □ No □ |
| Average time to start enteral feeding (days) |
| Average time to full enteral feeds (days) |
| Do you have a neonatal intensive care facility? Yes □ No □ |
| How would you estimate your mortality rate for patients with GS? |
| <25% □ 25-50% □ 50-75% □ 75-100% □ |
GS: Gastroschisis; PC: Primary closure; PFS: Preformed silos
Eighty-two delegates from 28 countries (66 institutions) responded and were divided into LIC (n = 11), MIC (n = 6) and HIC (n = 11) [Table 2] according to World Bank criteria.[13] Of the 66 institutions, 25 were from LIC (33 delegates), 15 MIC (21 delegates) and 26 HIC (28 delegates).
Table 2.
Paediatric surgery service provision and incidence of GS in low and middle and high-income countries
| Country type | Countries included | Catchment area per institution (population in millions) | Number of paediatric surgeons at institution | Number of cases of GS/institution/year |
|---|---|---|---|---|
| LIC | Cameroon, Cote d’Ivoire, Ethiopia, Ghana, Kenya, Madagascar, Malawi, Nigeria, Tanzania, Zambia, Zimbabwe | 10.0 (1-21) | 3 (0-12) | 22 (1-184) |
| MIC | Albania, Egypt, Lithuania, South Africa, Turkey, Ukraine | 4.8 (0.5-1.0) | 6 (2-20) | 12 (3-23) |
| HIC | Australia, Austria, Canada, Germany, Israel, Netherlands, Saudi Arabia, Slovenia, United Kingdom, United States America | 2.6 (0.5-5) | 7 (0-20) | 15 (1-100) |
LIC: Low-income countries; MIC: Middle-income countries; HIC: High-income countries; GS: Gastroschisis
Results were analysed per institution, except answers to management and estimated mortality questions, which could vary between surgeons at the same institution and were analysed per delegate. Results are presented as median (range). Differences between countries were analysed using contingency tests. P < 0.05 was considered significant.
RESULTS
Of 82 respondents, 51 (62%) were senior surgeons, 21 (25%) were registrars/middle grade, 3 (4%) were house officers/senior house officers and 7 (9%) were not documented.
Low-income countries had fewer paediatric surgeons per institution than MIC and HIC and larger catchment areas [Table 2]. LIC reported 22 cases (1-184) GS/institution/year, compared to 12 cases (3-23)/institution/year in MICs and 15 cases (1-100)/institution/year in HICs [Table 2].
Sixty-four percent (n = 16) institutions from LICs reported that fewer than 25% women have access to antenatal care compared to 96% (n = 25) institutions from HICs with over 75% women having access to this care [P < 0.0001, Table 3].
Table 3.
Provision of antenatal care in low-, middle- and high-income countries
| Country type | Percentage of women receiving antenatal care (number of institutions) | |||
|---|---|---|---|---|
| <25% | 25-50% | 50-75% | >75% | |
| LIC | 64 (16) | 16 (4) | 12 (3) | 8 (2) |
| MIC | 60 (9) | 7 (1) | 0 | 33 (5) |
| HIC | 0 | 0 | 4 (1) | 96 (25) |
LIC: Low-income countries; MIC: Middle-income countries; HIC: High-income countries
Seventy-five percent (n = 18) institutions in LICs stated neonates antenatally diagnosed with GS are allowed to proceed to term, 17% (n = 4) said they are delivered between 37 and 40 weeks and 8% (n = 2) <37 weeks. 50% (n = 13) HIC institutions stated these cases are not delivered early, 35% (n = 9) between 37 and 40 weeks and 15% (n = 4) <37 weeks.
In LICs, 58% (n = 14) institutions stated over 75% of patients are referred from outside of their centre compared to 27% (n = 7) admitting over 75% of outborn infants in HICs [Table 4].
Table 4.
Percentage of outborn patients with GS
| Country type | Percentage of patients referred from outside the hospital (number of institutions) | ||
|---|---|---|---|
| <25% | 25-75% | >75% | |
| LIC | 21 (5) | 21 (5) | 58 (14) |
| MIC | 13 (2) | 47 (7) | 40 (6) |
| HIC | 46 (12) | 27 (7) | 27 (7) |
LIC: Low-income countries; MIC: Middle-income countries; HIC: High-income countries; GS: Gastroschisis
Primary closure (PC) in theatre as the preferred management method was of similar proportions in all three groups at 58% (n = 19), 57% (n = 12) and 54% (n = 15) for LICs, MICs and HICs, respectively. PC at the cot side was 12% (n = 4) in LICs, 10% (n = 2) MICs and 21% (n = 6) in HICs. Staged closure was typically undertaken by custom silo construction in LICs (45% [n = 15]) with limited use of the preformed silos (PFS) (12% [n = 4]). By contrast, 39% (n = 11) delegates in HICs used PFS as their primary management method and only 11% (n = 3) custom silos [Figure 2]. General anaesthesia is utilised for closure at similar rates in LIC, MIC and HIC at 63% (n = 17), 79% (n = 15) and 68% (n = 17), respectively. However, local anaesthesia is used more frequently in LIC at 26% (n = 7) compared to MIC and HIC at 5% (n = 1) and 4% (n = 1), respectively [Table 5].
Figure 2.

Primary management choice for gastroschisis in low, middle and high-income countries
Table 5.
Anaesthesia used for closure
| Country type | Anaesthesia used for closure | |||
|---|---|---|---|---|
| None (%) | Local (%) | General (%) | Other (%) | |
| LIC | 3 (11) | 7 (26) | 17 (63) | 0 |
| MIC | 2 (11) | 1 (5) | 15 (79) | 1 (5) |
| HIC | 3 (12) | 1 (4) | 17 (68) | 4 (16) |
LIC: Low-income countries; MIC: Middle-income countries; HIC: High-income countries
In HIC, 74% (n = 20) delegates stated they used PFS (10 on all neonates, 8 on just some and 2 rarely) compared to 62% (n = 13; 9 on all neonates, 3 on some, 1 rarely) in MIC and 9% (n = 3; 2 on all neonates, 1 rarely) in LIC. 85% (n = 28) LIC delegates stated PFS were not available, 30% (n = 10) too expensive and 9% (n = 3) had no experience using them. 94% (n = 31) LIC delegates would consider using PFS in the future.
Only 19% (n = 5) LIC institutions had access to PN compared to 93% (n = 14) and 100% (n = 26) in MIC and HIC, respectively. The time to start enteral feeds was similar; 10 (2-28), 14 (1-21), and 9 days (0-23) in LIC, MIC and HIC, respectively. However, LIC delegates reported a shorter duration to full enteral feeds at 14 days (4-42) compared to 21 (2-35) and 25 days (8-42) in MIC and HIC, respectively. Only 36% (n = 9) LIC institutions had access to neonatal intensive care compared to 93% (n = 14) and 100% (n = 26) in MIC and HIC, respectively.
Sixty-one percent (n = 20) LIC delegates stated that mortality from GS in their centres is over 75% and a further 33% (n = 11) stated it is 50-75%. This compares to all delegates in HIC stating a mortality rate of under 25% [P < 0.0001, Figure 3].
Figure 3.
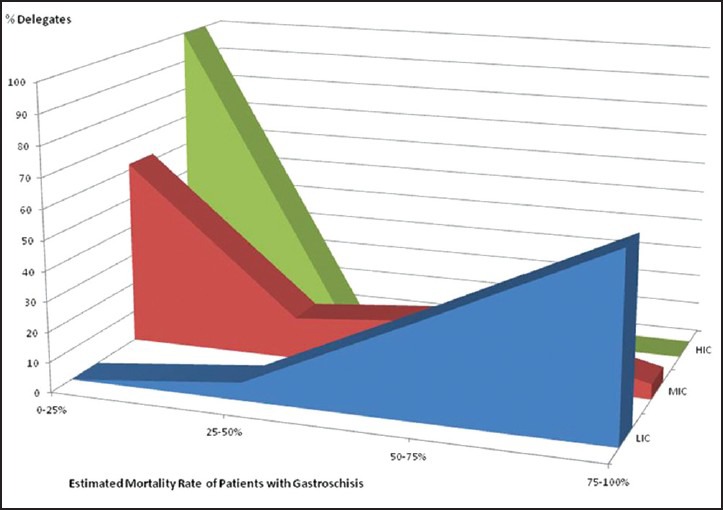
Mortality rates from gastroschisis in low, middle and high-income countries
Comments from LIC delegates included the following:
Patients come late and die of sepsis and malnutrition (Accra, Nairobi, Mahajanga)
No antenatal diagnosis, no PFS, no PN, no ventilators and sometimes no anaesthesia (Accra, Lilongwe, Dar es Salaam)
There is a need for PFS in developing countries. Availability could significantly improve outcome (Nigeria)
We are keen to try PFS (Dar es Salaam)
Because of high mortality rate, parents are discouraged from spending on care for these patients (Nigeria)
Due to high mortality, only one surgeon is prepared to attempt operative management. The others institute palliative care (Lusaka, Zambia).
Comments from MIC included:
We have begun to attempt closure with no anaesthesia in a few cases (East Cape Province, South Africa)
Although I use silo bags primarily, not all cases should be managed with a silo, “one shoe does not fit all” (Johannesburg, South Africa).
Comments from HIC included:
Preformed silos should be used selectively as they are not problem free - complications can be disastrous with bowel ischaemia and volvulus (Oxford, UK).
DISCUSSION
This study confirms that GS is encountered on a regular basis by paediatric surgeons in SSA. The data is an estimate by surgeons and does not represent a true incidence. Nevertheless, it is important as GS is considered a disease seen only in the Western world by some specialists in SSA. Others have chosen to ignore it as a result of high mortality. The high mortality should be put firmly in historical context. It is advances in foetal medicine, intensive care and PN that have resulted in the fall of mortality rates from GS in the Western World from 60% in the 1960s to 4% more recently.[5]
Respondents from LIC in SSA reported that the majority of cases are referred from outside their institution. Sekabira and Hadley previously reported that 91% of cases were born outside their tertiary centre in Durban and travelled long distances with resultant hypothermia, dehydration, sepsis and bowel necrosis.[6] This correlates with comments by LIC delegates. The lack of access to antenatal care and diagnosis in SSA contributes to this problem. Abdur-Rahman et al. noted that despite antenatal ultrasound being available in Ilorin, Nigeria, prenatal diagnosis remained poor with only 1 of the 7 GS cases in their study being detected.[12]
Eight percent of LIC delegates stated that prenatally diagnosed cases of GS are delivered prematurely. It is unclear whether this is natural or secondary to medical intervention. Elective preterm delivery has been advocated to reduce bowel exposure to potentially irritant amniotic fluid.[14,15] However, several studies, including a randomised controlled trial and a Cochrane review, have demonstrated that prematurity does not confer survival or functional advantages in infants with GS.[16,17,18,19,20] Indeed, in the context of scarce neonatal support facilities, premature delivery would almost certainly be disadvantageous.
Primary closure rates were reported as similar across the countries. Whenever PC is not feasible, delegates reported the majority of staged closure in LIC is undertaken using custom silos; PFS are unavailable or unaffordable. We have shown that staged closure using PFS reduces the risk of abdominal compartment syndrome and pulmonary barotrauma and improves early renal function.[21,22] A recent meta-analysis confirmed that in selected patients, PFS use was associated with a reduction in ventilator days, time to first feed, and infection rates, however in a wider population, the results compared to PC were mixed.[23] The use of PFS in LICs has potential advantages; allowing appropriate resuscitation of the sick infant after transfer in and the possibility of avoiding theatre altogether.
Lack of PN and neonatal intensive care facilities is a problem in SSA, with reported availability of only 19% and 36%, respectively. Agugua reported an improvement in GS survival from 35% to 65% following the introduction of PN alongside other adjunctive measures at their institution in Nigeria.[11]
It could be argued that local, national government and global health funding could be better spent elsewhere, rather than on a relatively uncommon congenital anomaly like GS. However, GS is potentially curable with disability-free long term survival achievable in the majority in the well-resourced setting.[5] If this is considered in terms of disability-adjusted life years (DALYs) (DALYs = years of life lost + years lived with disability),[24] the potentially avertable DALYs would be high, making the case for enhanced paediatric surgery capacity. The same would hold for a number of other congenital anomalies,[24,25] but GS is unique in terms of the rising worldwide incidence and the potential curability.
We propose the implementation of combined epidemiological research, service delivery training and resource provision projects to help improve our understanding of GS in SSA whilst attempting to improve outcome. These could be focused on tertiary centres highlighted as having a high number of cases initially. Partner teams managing cases of GS would receive training in the optimal management of such patients with a focus on primary resuscitation. A recent systematic review reported that resuscitation training in developing countries is commonly well received, viewed as valuable by local partners and can significantly reduce mortality.[26] López-Herce et al. noted that for such training to be sustainable, local staff must be trained as instructors to promote long-term educational independence.[27] In addition to resuscitation, training for PFS application may be valuable. We have previously described the use of an inexpensive, easy to construct model for this purpose.[28]
The use of PN in SSA has been controversial given the cost implications. Only 19% of LIC surgeons had access to PN compared with 100% of those in HIC. We consider it an essential part of the package designed to improve outcomes from GS with the added benefit of venous access and other associated skills that would grow to support the practice; this would have benefits for other infants in the units that adopt it. Now that GS is more widely recognised by professionals across SSA, our expectation is that over time, the outcome of GS in SSA will improve as has been the case in other parts of the world.
CONCLUSION
Gastroschisis is a congenital anomaly encountered by surgeons across SSA. There is limited access to key resources for the antenatal diagnosis, referral and management. This results in higher mortality rates when compared to MICs and HICs. Further research is required to define and raise awareness of the extent of the problem and calculate related DALYs. We propose this is undertaken as part of a multi-centre project aimed at improving outcome of GS through the use of training and key resource provision.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Kilby MD. The incidence of gastroschisis. BMJ. 2006;332:250–1. doi: 10.1136/bmj.332.7536.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland AJ, Walker K, Badawi N. Gastroschisis: An update. Pediatr Surg Int. 2010;26:871–8. doi: 10.1007/s00383-010-2679-1. [DOI] [PubMed] [Google Scholar]
- 3.Zani A, Ruttenstock E, Davenport M, Ade-Ajayi N. Is there unity in Europe?. First survey of EUPSA delegates on the management of gastroschisis. Eur J Pediatr Surg. 2013;23:19–24. doi: 10.1055/s-0032-1326954. [DOI] [PubMed] [Google Scholar]
- 4.Aldrink JH, Caniano DA, Nwomeh BC. Variability in gastroschisis management: A survey of North American pediatric surgery training programs. J Surg Res. 2012;176:159–63. doi: 10.1016/j.jss.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Bradnock TJ, Marven S, Owen A, Johnson P, Kurinczuk JJ, Spark P, et al. Gastroschisis: One year outcomes from national cohort study. BMJ. 2011;343:d6749. doi: 10.1136/bmj.d6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sekabira J, Hadley GP. Gastroschisis: A third world perspective. Pediatr Surg Int. 2009;25:327–9. doi: 10.1007/s00383-009-2348-4. [DOI] [PubMed] [Google Scholar]
- 7.Manson J, Ameh E, Canvassar N, Chen T, den Hoeve AV, Lever F, et al. Gastroschisis: A multi-centre comparison of management and outcome. Afr J Paediatr Surg. 2012;9:17–21. doi: 10.4103/0189-6725.93296. [DOI] [PubMed] [Google Scholar]
- 8.Ameh EA, Chirdan LB. Ruptured exomphalos and gastroschisis: A retrospective analysis of morbidity and mortality in Nigerian children. Pediatr Surg Int. 2000;16:23–5. doi: 10.1007/s003830050006. [DOI] [PubMed] [Google Scholar]
- 9.Iliff PJ. Neonatal surgery in Harare Hospital. Cent Afr J Med. 1990;36:11–5. [PubMed] [Google Scholar]
- 10.Harrison DS, Mbuwayesango BE. Factors Associated with Mortality among neonates presenting with GS in Zimbabwe. S Afr J Sci. 2006;44:157. [Google Scholar]
- 11.Agugua NE, Nwako FA. Gastroschisis a fifteen-year experience. West Afr J Med. 1990;9:147–50. [PubMed] [Google Scholar]
- 12.Abdur-Rahman LO, Abdulrasheed NA, Adeniran JO. Challenges and outcomes of management of anterior abdominal wall defects in a Nigerian tertiary hospital. Afr J Paediatr Surg. 2011;8:159–63. doi: 10.4103/0189-6725.86053. [DOI] [PubMed] [Google Scholar]
- 13.The World Bank. How we Classify Countries. [Last accessed on 2014 Jun 19]. Available from: http://www.data.worldbank.org/about/country-classifications .
- 14.Sencan A, Gümüstekin M, Gelal A, Arslan O, Ozer E, Mir E. Effects of amnio-allantoic fluid exchange on bowel contractility in chick embryos with gastroschisis. J Pediatr Surg. 2002;37:1589–93. doi: 10.1053/jpsu.2002.36190. [DOI] [PubMed] [Google Scholar]
- 15.Moore TC, Collins DL, Catanzarite V, Hatch EI., Jr Pre-term and particularly pre-labor cesarean section to avoid complications of gastroschisis. Pediatr Surg Int. 1999;15:97–104. doi: 10.1007/s003830050525. [DOI] [PubMed] [Google Scholar]
- 16.Grant NH, Dorling J, Thornton JG. Elective preterm birth for fetal gastroschisis. Cochrane Database Syst Rev. 2013;6:CD009394. doi: 10.1002/14651858.CD009394.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logghe HL, Mason GC, Thornton JG, Stringer MD. A randomized controlled trial of elective preterm delivery of fetuses with gastroschisis. J Pediatr Surg. 2005;40:1726–31. doi: 10.1016/j.jpedsurg.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Kurkchubasche AG, Carr SR, Wesselhoeft CW, Jr, Tracy TF, Jr, Luks FL. Benefits of term delivery in infants with antenatally diagnosed gastroschisis. Obstet Gynecol. 2002;100:695–9. doi: 10.1016/s0029-7844(02)02170-1. [DOI] [PubMed] [Google Scholar]
- 19.Ergün O, Barksdale E, Ergün FS, Prosen T, Qureshi FG, Reblock KR, et al. The timing of delivery of infants with gastroschisis influences outcome. J Pediatr Surg. 2005;40:424–8. doi: 10.1016/j.jpedsurg.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Charlesworth P, Njere I, Allotey J, Dimitrou G, Ade-Ajayi N, Devane S, et al. Postnatal outcome in gastroschisis: Effect of birth weight and gestational age. J Pediatr Surg. 2007;42:815–8. doi: 10.1016/j.jpedsurg.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 21.Allotey J, Davenport M, Njere I, Charlesworth P, Greenough A, Ade-Ajayi N, et al. Benefit of preformed silos in the management of gastroschisis. Pediatr Surg Int. 2007;23:1065–9. doi: 10.1007/s00383-007-2004-9. [DOI] [PubMed] [Google Scholar]
- 22.Charlesworth P, Akinnola I, Hammerton C, Praveena P, Desai A, Patel S, et al. Preformed silos versus traditional abdominal wall closure in gastroschisis: 163 infants at a single institution. Eur J Pediatr Surg. 2014;24:88–93. doi: 10.1055/s-0033-1357755. [DOI] [PubMed] [Google Scholar]
- 23.Kunz SN, Tieder JS, Whitlock K, Jackson JC, Avansino JR. Primary fascial closure versus staged closure with silo in patients with gastroschisis: A meta-analysis. J Pediatr Surg. 2013;48:845–57. doi: 10.1016/j.jpedsurg.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bickler S, Ozgediz D, Gosselin R, Weiser T, Spiegel D, Hsia R, et al. Key concepts for estimating the burden of surgical conditions and the unmet need for surgical care. World J Surg. 2010;34:374–80. doi: 10.1007/s00268-009-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bickler SW, Kyambi J, Rode H. Pediatric surgery in sub-Saharan Africa. Pediatr Surg Int. 2001;17:442–7. doi: 10.1007/s003830000516. [DOI] [PubMed] [Google Scholar]
- 26.Meaney PA, Topjian AA, Chandler HK, Botha M, Soar J, Berg RA, et al. Resuscitation training in developing countries: A systematic review. Resuscitation. 2010;81:1462–72. doi: 10.1016/j.resuscitation.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 27.López-Herce J, Urbano J, Carrillo A, Matamoros M. Red Iberoamericana de Estudio de la Parada Cardiorrespiratoria en la Infancia. Resuscitation training in developing countries: Importance of a stable program of formation of instructors. Resuscitation. 2011;82:780. doi: 10.1016/j.resuscitation.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Dabbas N, Muktar Z, Ade-Ajayi N. GABBY: An ex vivo model for learning and refining the technique of preformed silo application in the management of gastroschisis. Afr J Paediatr Surg. 2009;6:73–6. doi: 10.4103/0189-6725.54766. [DOI] [PubMed] [Google Scholar]


