Abstract
Ovarian cancer is the leading cause of death from gynecologic malignancies. Reasons for the high mortality rate associated with ovarian cancer include a late diagnosis at which time the cancer has metastasized throughout the peritoneal cavity. Cancer metastasis is facilitated by the remodeling of the extracellular tumor matrix by a family of proteolytic enzymes known as the matrix metalloproteinases (MMPs). There are 23 members in the MMP family, many of which have been reported to be associated with ovarian cancer. In the current paradigm, ovarian tumor cells and the surrounding stromal cells stimulate the synthesis and/or activation of various MMPs to aid in tumor growth, invasion, and eventual metastasis. This review sheds light on the different MMPs in the various types of ovarian cancer and their impact on the progression of this gynecologic malignancy.
Keywords: ovary, cancer, ovulation, follicle, metalloproteinases, review
OVARIAN CANCER
Ovarian cancer is the fifth leading cause of cancer death in women today according to the National Cancer Institute (NCI) 2014 statistics. It is diagnosed in approximately 22,000 women in the United States yearly and accounts for at least 14,000 deaths. Approximately two thirds of women are diagnosed with stage III or IV disease where the five-year survival is 25–30% or less, compared to 80–95% five-year survival for those with stage I or II disease (Tingulstad et al. 2003).
There are 3 main types of ovarian cancer including epithelial ovarian cancer, sex cord stromal tumors, and germ cell tumors. Of these, epithelial tumors account for about 90% of ovarian cancers (Table 1), and are the leading cause of death from gynecological malignancies (Zhang et al. 2005, Choi et al. 2007). Sex cord stromal and germ cell tumors account for the remaining ~10% (Choi et al. 2007). Generally, germ cell tumors present at an earlier age than epithelial ovarian cancer, affecting women in their late teens and early 20’s. The average age of women with epithelial ovarian cancer is around 60 affecting mostly peri- or postmenopausal women. The different epithelial tumors are classified according to the cell types found in the reproductive tract and include serous, mucinous, endometrioid, clear cell, and transitional cell types (Table 2).
Table 1.
Classification and incidence of the major types of ovarian cancer.
| Types of Ovarian cancer | Incidence |
|---|---|
| Epithelial | 90% |
| Germ Cells | 5% |
| Sex cord | 5% |
| Primary peritoneal | Rare |
Table 2.
Major cellular subtypes of ovarian epithelial cancer.
| Type of Epithelial cancer | Subtypes | |
|---|---|---|
| Serous | Cystomas, benign cystadenomas, cystadenomas, cystadenocarcinomas | 7/10 |
| Mucinous | Cystomas, benign cystadenomas, cystadenomas, cystadenocarcinomas | 1/10 |
| Endometrioid | Benign cysts, adenocarcinomas, endometrioid tumors, adenocarcinomas | 1/20 |
| Clear Cell | Cystomas, benign cystadenomas, cystadenomas, cystadenocarcinomas | 3/100 |
| Undifferentiated/Unclassified | Tumors that do not fall in any of the other groups | 1/10 |
The risk factors associated with the development of ovarian cancer are based on an increased number of ovulatory cycles and include nulliparity, early menarche with late menopause, increasing age, and the use of fertility drugs, although the relationship of the later remains controversial (Rossing et al. 1994, Venn et al. 1999, Dor et al. 2002). Consequently, the incidence of the ovarian cancer decreases with multiparity, the use of oral contraceptives, and breastfeeding (Collaborative Group on Epidemiological Studies of Ovarian et al. 2008, Koshiyama et al. 2014). The observation that ovarian cancer increases with ovulation rate led to the “incessant ovulation” hypothesis first proposed by Fathalla in 1971 (Fathalla 1971). According to this hypothesis, follicular rupture results in an inflammatory reaction, which damages the ovarian surface epithelial cells in the vicinity of the ovulatory stigma through DNA altering reactive oxygen species. Such alterations result in potentially mutagenic lesions, such as P53 or BRCA (Fathalla 2013, Koshiyama et al. 2014). Hence, a family history of ovarian cancer is a risk factor particularly due to the genetic mutations of BRCA1 and BRCA2 as well as the presence of Lynch syndrome which is hereditary (National Cancer Institute). These mutagenic insults to the ovarian surface epithelial cells then direct the cells towards a malignant fate. Other risk factors may include the use of talc, and obesity (National Cancer Institute).
Histological similarities between serous cancers arising in the ovary and fallopian tube have led to the proposal that some ovarian cancers are of fallopian tube origin (Crum et al. 2007b). Ovulation results in bathing the distal fallopian epithelial cells with follicular fluid containing high levels of steroids, inflammatory cytokines, and reactive oxygen species. All of these molecules could lead to mutagenic changes in the tubal epithelial cells giving rise to metastasis to the ovary resulting in ovarian carcinoma (Crum et al. 2007a, Fathalla 2013).
These cellular changes set in motion the events which alter the phenotype in ovarian or fallopian tube cells from benign to malignant and allow the tumor to grow, acquire vascularization, and gain the characteristics which lead to metastasis. Chief among these changes in the tumor cell is the ability to modify the surrounding extracellular matrix (ECM). The ECM is a key regulatory component in cellular physiology providing an environment for cell migration, differentiation, and, in some cases, the ultimate fate between cell survival or cell death (Birkedal-Hansen et al. 1993). In order for tumor cells to grow, invade and metastasize, it is crucial for the cells to be able to disrupt the surrounding ECM. This matrix degradation allows tumor cells to proliferate, easily detach from their primary site, extravasate and invade other tissues (Schropfer et al. 2010). MMPs are known to be important players in the physiological process of cancer progression (John & Tuszynski 2001, Kessenbrock et al. 2010). The current review will focus on the recent literature on the involvement of the MMPs in ovarian cancer.
THE MATRIX METALLOPROTEINASE SYSTEM
The matrix metalloproteinase family in the human encompasses 23 related proteolytic enzymes which share common structural and functional similarities (Kleiner & Stetler-Stevenson 1993, Murphy et al. 1999). These functional similarities include: 1) the presence of zinc in the active site of the catalytic domain, 2) synthesis of the enzyme in an inactive or latent form, 3) activation of the latent zymogen, and 4) inhibition of enzyme action by both serum borne and tissue derived metalloproteinase inhibitors in the extracellular environment. Based on their structural similarities, the MMPs are classified into 4 broad classes: the collagenases, gelatinases, stromelysins, and membrane type enzymes (MT-MMPs) as illustrated in Figure 1. However, a few MMPs exhibit different characteristics and are classified outside of these 4 broad classes as discussed below.
Figure 1.
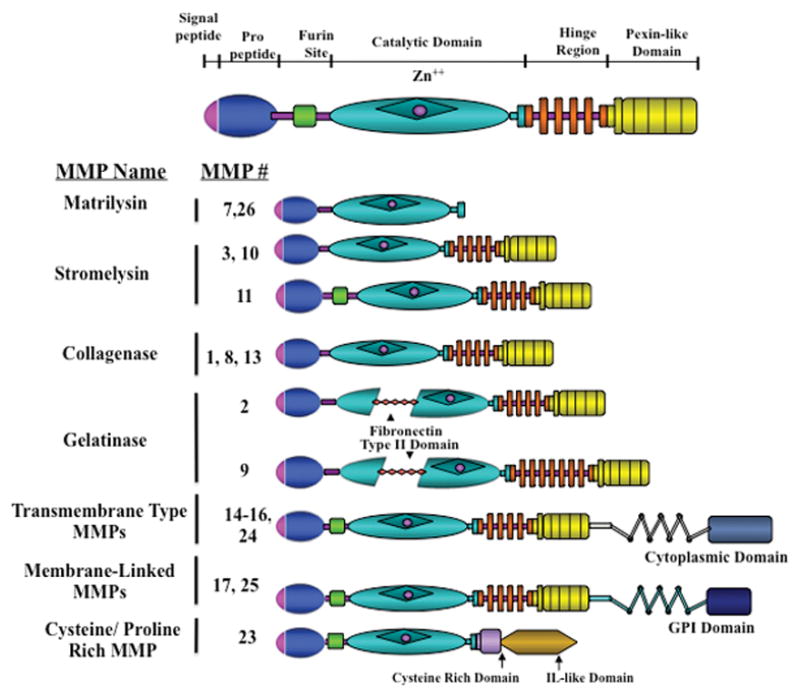
Schematic of the different classes of MMPs. The overall structure of the MMPs contains a signal peptide, pro-peptide, a furin site, catalytic domain, hinge region and a pexin-like domain. The gelatinases differ by also containing a fibronectin type II domain. Matrilysins lack the pexin-like domain and the membrane types contain a cytoplasmic or GPI domain. Figure modified from (Nagase & Woessner 1999, Curry & Osteen 2003).
Studies have shown that the MMPs act on a diverse group of ECM components including the collagens, gelatins, fibronectins and laminins (Murphy et al. 1999, Nagase & Woessner 1999, Curry & Osteen 2003, Berchuck et al. 2009). Yet the MMPs also exhibit activity towards other MMPs, growth factors, and cytokines such as IGF binding proteins, EGF, TNFα, and substance P (Sternlicht & Werb 2001). The ability of these enzymes to cleave binding proteins as well as growth factors has expanded the repertoire of MMP actions to include modulation of cell growth. In the tumor microenvironment, MMPs may be the key regulatory point in disrupting the balance between growth and antigrowth signals thereby influencing the bioavailability of growth factors to stimulate tumor cell growth as reviewed in Kessenbrock et al 2010. For example, one of the main pathways that is typically altered in cancer cells is the TGF-β receptor system leading to increased invasion and metastatic potential of cancer cells (Massague 2008). TGF-β is activated via proteolytic conversion by MMPs such as MMP2, MMP9, and MMP14 (Mu et al. 2002). The ability of the MMPs to turn on TGF-β activity suggests that MMPs indirectly have tumor-promoting effects (Kessenbrock et al. 2010).
The regulation of ECM turnover and cell growth by MMPs is rigorously controlled by MMP inhibitors. There are two major classes of inhibitors, the serum borne and tissue derived inhibitors (Curry & Osteen 2003). The serum borne inhibitors, include the macroglobulins, which have a potent ability to inhibit a broad range of proteinases. The tissue inhibitors of metalloproteinases, or TIMPs, are a family of 4 inhibitors that are locally produced and specifically inhibit MMPs. The TIMPs differ in their selectivity for different MMPs. For example, TIMP-2 has a high affinity for MMP-2 whereas TIMP-1 preferentially binds to MMP-9 (Gomez et al. 1997). TIMP-3 is able to inhibit the membrane type 1 MMP (MT1-MMP or MMP14) unlike TIMP-1 which cannot act on MT1-MMP. However, unlike TIMP-1 or TIMP-2, TIMP-3 is secreted and then bound to the ECM which has been suggested to allow TIMP3 to act as an additional regulatory stop point by working at the site of MMP action (Gomez et al. 1997).
MMPS AND OVARIAN CANCER
The Collagenases: MMP1, MMP8, MMP13
The three collagenases share structural similarities and have the ability to act on a broad variety of substrates. Although all of the collagenases cleave fibrillar collagen, these proteases have different affinities towards type I, type II, or type III collagen. MMP14, which is a membrane type MMP, also acts on collagens and will be discussed in the membrane type MMP section (below). The collagenases also have different mechanisms of reaching the extracellular environment (Nagase & Woessner 1999, Borkakoti 2000). For example, MMP8 is predominately found in neutrophils where it is synthesized and stored in granules until needed. Unlike MMP8, MMP1 and MMP13 are produced and secreted in a wide range of cell types in response to specific stimuli without being stored. The collagenases have been implicated in ovarian cancer based on their expression patterns which are dependent on the stage and tumor type (Behrens et al. 2001, Hantke et al. 2003, Stadlmann et al. 2003). For example, MMP1 and the gelatinase MMP9 were strongly expressed in both stromal and epithelial tumor cells of serous invasive carcinomas, were upregulated in the fibroblastic stroma of borderline tumors, but were expressed at very low levels in serous benign cystadenomas (Behrens et al. 2001).
In a herculean effort, Stadlmann and colleagues examined MMP expression in 302 patients using immunohistochemistry on tissue cylinder specimens (Stadlmann et al. 2003). In their study, they collected 119 serous, 40 mucinous, 68 endonetrioid, 16 undifferentiated, 16 mullerian, 24 clear cell, 5 malignant Brenner, 10 sex-cord, and 4 yolk sac tumors. The tumors were graded and correlated with prognosis as well as processed for microarrays and immunohistochemistry. Numerous MMPs were expressed in all of the ovarian cancers as discussed in detail within each MMP class below. Interestingly, only MMP8 expression levels correlated with tumor grade, tumor stage, and a poor prognosis. MMP8 was upregulated by interleukin-1 beta suggesting that that pro-inflammatory cytokines may promote the invasive potential of ovarian cancer by stimulating MMP8 expression.
Due to the association of MMP13 with ovarian cancer, it has been studied as a potential prognostic indicator. Hantke and coworkers investigated protein levels of MMP13 in ascitic fluids of 30 patients with advanced ovarian cancer (Hantke et al. 2003). Using an ELISA, MMP13 values were stratified into two subpopulations, one population with short survival (median 16 months) and one with long (median 36 months) overall survival. MMP13 was shown to be associated with shorter survival. Thus, levels of MMP13 in ascitic fluid may identify the patient’s risk and potential survival outcome.
Overexpression of the MMPs may transduce the signals for tumor cell migration and invasion through a cell surface receptor coupled to G proteins, PAR1 (protease-activated receptor-1). PAR1 is cleaved by MMP1 which promotes breast cancer migration and invasion (Boire et al. 2005). PAR1 has also been identified in ovarian cancer. Agarawal and colleagues identified a metalloprotease cascade where pro-MMP1 was activated to MMP1 which in turn directly activated PAR1 (Agarwal et al. 2008). This activation of MMP1-PAR1 induces the secretion of several angiogenic factors from ovarian carcinoma cells which cause endothelial cell proliferation, endothelial tube formation, and migration (Agarwal et al. 2010) as well as epithelial ovarian cancer cell invasion (Wang et al. 2011a). Further investigation of PAR1 has demonstrated that serum levels of PAR1 are elevated in patients with epithelial ovarian cancer but serum levels were not predictive nor of prognostic value in this group of patients (Karabulut et al. 2014).
Polymorphisms in the MMP promoter may lead to overexpression of MMPs in ovarian cancer. For example, Kanamori and colleagues (Kanamori et al. 1999) reported a guanine (G) insertion/deletion polymorphism within the promoter region of MMP1 possesses greater transcriptional activity, that the proportion of patients who contained the polymorphism was elevated in patients with ovarian cancer, and MMP1 expression was elevated in ovarian cancer tissue. Subsequent investigation into the association of promoter polymorphisms and cancer risk has questioned the role of promoter mutations in the expression of MMP1 (Wenham et al. 2003, Li et al. 2006) but has indicated a possible association between polymorphisms in the MMP7, MMP8, MMP12, or MMP13 promoters and the susceptibility to epithelium ovarian cancer in various populations (Li et al. 2006, Li et al. 2009, Arechavaleta-Velasco et al. 2014).
The Gelatinases: MMP2 and MMP9
MMP 2 and 9 have been extensively studied in cancer and there is a plethora of literature documenting their expression in ovarian cancer and cancer progression. Hence, the overview presented herein highlights some of these findings emphasizing the similarities and controversies. Brun and coworkers have extensively characterized the localization and expression levels of MMP 2, 7, 9 and MT1 in different types and stages of ovarian cancer (Brun et al. 2008, Brun et al. 2012). They report that serous tumors express higher levels of MMP2, 7, and 9 compared to mucinous tumors. However, in the surrounding stromal tissue the expression of MMP2 and 9 did not differ between tumor types. When classified into benign, borderline, and malignant, the expression levels of these MMPs were different across the tumor subtypes. For example, MMP2 expression was higher in benign tumors than borderline and malignant while MMP9 was high in malignant tumors compared to borderline tumors. In the stroma of serous tumors, the expression of MMP2 was the highest in the benign and borderline compared to malignant tumors. As for MMP9, it was highest in malignant tumors. In mucinous tumors, both MMP2 and MMP9 expression was highest in malignant tumors (Brun et al. 2008).
Although MMP2 staining was present in 76% of malignant tumors and 54% of benign tumors on immunohistochemical analysis, other studies indicate that high levels of epithelial MMPs are not necessarily specific for malignant tumors. In fact, MMP2 is more frequently expressed in benign tumors than in carcinomas (Brun et al. 2012). These discrepancies may be due to the different methods that the investigators have used to analyze the samples as well as the arbitrary thresholds that were put in place by the different groups to determine staining intensity upon immunohistochemistry analysis (Brun et al. 2012).
Both MMP2 and 9 have been extensively studied in relation to their role in migration and invasion of ovarian cancer. MMP 2 and 9 have been shown to be secreted and activated in ovarian cancer and are closely correlated with invasion and metastasis of cancer cells and correlate with poor survival (Davidson et al. 1999).
When MMP9 was silenced using siRNA, the invasive ability of cancer cells decreased suggesting a role of MMP9 in invasiveness (Hu et al. 2012). MMP9 has also has been shown to be involved in the release of VEGF from tumor cells and causes ascites in ovarian cancer (Belotti et al. 2003). MMP9 was also suggested to have two potential roles in tumor development where it acts as a tumor promoter when it is in ovarian tumor stroma but prevents tumor advancement when it is expressed in the epithelium (Sillanpaa et al. 2007).
MMP2 has been previously shown to control the attachment and adhesion of metastatic ovarian cancer cells to peritoneal surfaces via cleaving ECM proteins and enhancing their binding to integrins (Kenny et al. 2008). Similarly, Kenny and coworkers showed that the presence of MMP2 in ovarian cancer regulates the ability of the ovarian cancer to metastasize (Kenny & Lengyel 2009). MMP2, like MMP7, was measured in the serum of ovarian cancer patients and serum levels of MMP2 in those patients were lower than that of healthy controls (Acar et al. 2008).
Both MMP2 and MMP9 levels were investigated in the urine of patients in combination with CA125. Coticchia and colleagues showed that MMP2 and MMP9 levels in the urine may be clinically helpful to diagnose ovarian cancer and their results were independent of CA125 levels (Coticchia et al. 2011). Platelet derived growth factor D (PDGF-D) has been also shown to promote ovarian cancer invasion and this increase in invasion is caused by PDGF-D increasing the expression of MMP2 and MMP9 (Wang et al. 2011b). Finally, in a meta-analysis of 30 published studies on MMP9 and its prognostic use in ovarian cancer, the expression of MMP9 was generally positively correlated with poor prognosis (Li et al. 2013).
The Stromelysins: MMP3, MMP10, MMP11
MMP3 plays a significant role in regulating extracellular matrix remodeling as well as activating other MMPs. MMP3 is known to be overexpressed in cancerous hen ovaries as well as other human cancers (Choi et al. 2011). The activation of MMP3 in ovarian cancer has been linked to the down regulation of miRNA200, where induction of MMP3 overexpression caused a decrease in the ability of miR200 to inhibit ovarian cancer invasiveness. Similarly, an increase in the expression of miR200 inhibited the expression of MMP3 (Sun et al. 2014). In humans, MMP3 expression is present in cystic fluids of ovarian tumors and appears to be correlated with the activation of MMP7 and MMP9 (Furuya 1999).
MMP10 is known to have a role in, vascular remodeling (Rodriguez et al. 2008) and other functions including cancer progression (Nabeshima et al. 2002) yet very few studies exist about its role in ovarian cancer. Davidson and colleagues observed that when TP53 was mutated in ovarian cancer cell lines and exposed to hypoxic conditions, 40% (5 genes) of the genes that were upregulated were involved in ECM degradation, one of which was MMP10 (Davidson et al. 2014). Our lab has shown that the activation of the PKC pathway in human ovarian cancer cell lines caused an increase in MMP10 expression that potentially plays a role in ovarian cancer migration (Al-Alem et al. 2013). In chemotherapeutic treatment, MMP10 was highly induced in ovarian cancer cells that became resistant to platinum based chemotherapy compared to cells that were non-resistant (Solar & Sytkowski 2011). Furthermore, MMP3 and MMP10 expression increased in rat ovarian surface epithelia following Ras activation (Ulku et al. 2003).
MMP11 is known to be involved in tumor remodeling, however, a study that explored the protein expression of MMP2 and MMP11 in 100 tissue samples from patients with stage III ovarian cancer, showed that MMP2, but not MMP11, was correlated with aggressive cancer cells (Perigny et al. 2008). In contrast, Muller and coworkers showed there was a higher percentage of low malignant tumors that express MMP11 in the stroma that is adjacent to the tumor. This expression correlated positively with tumor stage (Mueller et al. 2000).
The Membrane Type MMPs: MMP14-17, MMP24, MMP25
The membrane type MMPs are unique among the MMP family because they are not secreted into the extracellular space but rather contain a domain which anchors them into or on the plasma membrane (Figure 2). An extracellular domain directs the proteolytic component of the enzyme to the exterior surface of the cell. There are 6 members of this family which are divided into type I and type II MT-MMPs (Sounni & Noel 2005). The type I MT-MMPs include MT1 (MMP14), MT2 (MMP15), MT3 (MMP16), and MT5 (MMP24) and have a transmembrane domain and an intracytoplasmic domain. The type II MT-MMPs, MT4 (MMP17) and MT6 (MMP25), have a glycosylphosphatidylinositol (GPI) link domain which anchors them onto the cell membrane (Curry & Osteen 2003, Sounni & Noel 2005). By virtue of their presence on the surface of the cell, all of the MT-MMPs are thought to participate in pericellular proteolysis to promote cell growth and migration which are a hallmarks for cancer metastasis (Murphy et al. 1999). For example, high local concentrations of active MT1 on the cell membrane of metastatic cancer cells has been proposed to play an important role in cell migration (Sabeh et al. 2004, Wolf et al. 2007, Kessenbrock et al. 2010). MMPs are mostly activated via serine proteinases that cleave prodomain peptide bonds. In addition, MT-MMPs can cleave pro-forms of other enzymes, including secreted proMMPs such as MMP2 and MMP9 as discussed above, which contributes to their involvement in ovarian cancer. MMP14 in particular activates MMP2 whereas MMP15 and MMP24 fail to activate MMP2 (Zucker et al. 1998, Miyamori et al. 2000). MT-MMPs are inhibited via TIMP2, whose C-terminal acts like a receptor for the proMMP2. A nearby uninhibited MT-MMP cleaves the adjacent proMMP2 which is further cleaved to the active form of MMP2 (Strongin et al. 1995, Deryugina et al. 2001) (Figure 2).
Figure 2.
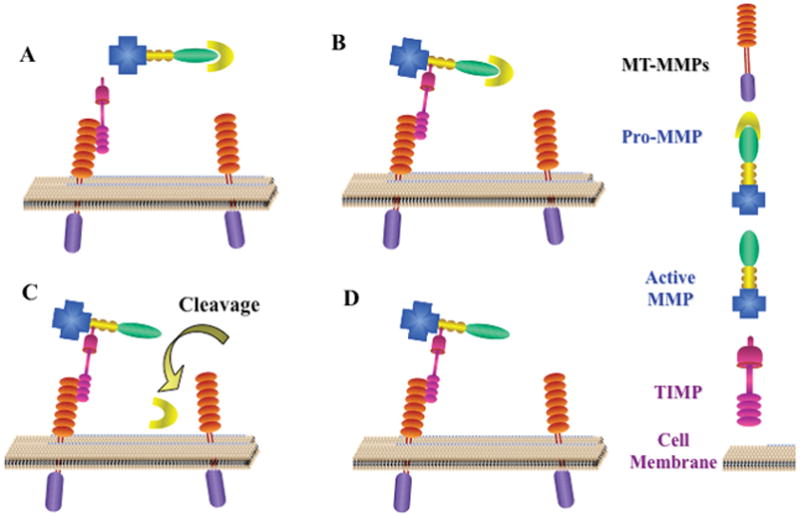
MMP activation. Certain MMPs are activated by the membrane type MMPs. (A) TIMPs attach to the active site of the MT-MMPs. This allows MMPs surrounding the cell in proximity to the MT-MMPs to be bound by TIMPs forming a MT-MMP:TIMP:ProMMP complex. (B) The complex is in close proximity to other MT-MMPs on the cell surface which are capable of cleaving the pro-peptide (C) causing the MMP to now be active (D).
An association has been described between ovarian cancer and members of the membrane type MMPs. For example, MT1 and MT2 have been reported to be associated with ovarian carcinoma (Fishman et al. 1996, Stack et al. 1998). MT1 was associated with aggressive tumor behavior (Drew et al. 2004) and a shorter disease specific survival in epithelial ovarian cancer (Kamat et al. 2006). In contrast to MT1 and MT2, MT3 mRNA was not detected in malignant pleural and peritoneal effusions (Davidson et al. 2001).
There are extremely limited reports on MT4, 5, and 6 and ovarian cancer. In the normal ovary, we have observed an increase in MT6 around the time of ovulation (Puttabyatappa et al. 2014). In ovarian cancer, the data that do exist have been performed in cell lines. For example, Delassus and coworkers reported that MMP25, along with other MMPs, was differentially regulated in SKOv3 ovarian cells (Delassus et al. 2010). However, in a provocative study these investigators reported striking variability in MMP expression in cancer cell lines. Comparison of the MMP signaling pathways in the ovarian cancer SKOV3 cells with those from cells of lung, brain, prostate or breast cancer revealed that induction of MMP expression differed so widely that almost 90% of the pathways were different in cells from one cancer to another (Delassus et al. 2010). In 18 out of 51 signaling pathways, a known suppressor of cancer progression stimulated, rather than inhibited, MMP expression. Likewise, ten signaling pathways that upregulated MMP expression in cells of some cancers resulted in downregulation in other cancer cells. These results highlight that there are pronounced differences in any signaling pathway between cells from different cancers (Delassus et al. 2010).
Support for the role of the MT-MMPs in ovarian cancer invasion is forthcoming from cell culture studies. OVCA 433 cells expressing a mutated form of MT1, which resulted in sustained cell surface activity of MT1, caused a cellular phenotypic epithelial-mesenchymal transition characterized by enhanced migration and collagen invasion (Moss et al. 2009). Likewise, MT1 increased invasion of ovarian carcinoma cells through the activation of proMMP2 (Fishman et al. 1996).
In addition to their role in invasion, the membrane type MMPs may play a key role in the vascular changes or vasculogenic mimicry associated with ovarian tumor formation and growth. MT1 is known to activate the proform of MMP2 to the active enzyme (Sood et al. 2004). Together MMP2 and MMP14 appeared to regulate the development of vasculogenic-like networks and matrix remodeling by aggressive ovarian cancer cells (Sood et al. 2004) which would allow further cell growth and proliferation.
The Matrilysins: MMP7 and MMP26
MMP7, also known as matrilysin-1, is the smallest member of the MMP family and acts on a variety of substrates (Wang et al. 2005). It is also one of the few MMPs that is secreted by tumor cells rather than stromal cells and has been shown to be expressed in almost all organ tumors in the body (Wielockx et al. 2004, Ii et al. 2006). MMP7 over expression has been implicated in numerous cancers and is linked to advanced cancer stages and poor prognosis (Ii et al. 2006). In particular, MMP7 has been shown to be elevated in 80% of malignant human ovarian cancers compared to 40% in normal or benign samples. MMP7 has also been shown to be expressed in stromal ovarian cancer tissues particularly those of serous cancers (Brun et al. 2008). Polymorphisms in the MMP7 promoter region have shown that single nucleotide polymorphisms in MMP7 are significantly higher in ovarian cancer patients than controls (Li et al. 2006). In addition, serum levels of MMP7 were higher in patients with ovarian serous (Meinhold-Heerlein et al. 2007) and mucinous (Shigemasa et al. 2000) cancers compared to controls. MMP7 serum levels were also higher in pre-operative compared to post-operative patients (Tanimoto et al. 1999) as well as being higher prior to chemotherapy (Gershtein et al. 2010) indicating that it may be useful as a biomarker. In contrast, Sillanpaa and colleagues showed that the 10-year disease-related survival was better when the tumor expression of MMP7 cells was elevated (Sillanpaa et al. 2006).
MMP7 secretion has been shown to correlate with metastasis (Shiomi & Okada 2003, Wang et al. 2005). One potential mechanism of increased invasiveness seen with MMP7 is due to its activation of MMP2 and 9 (Ii et al. 2006). MMP2 has been previously shown to control the attachment and adhesion of metastatic ovarian cancer cells to peritoneal surfaces via cleaving ECM proteins and enhancing their binding to integrins (Kenny et al. 2008). Another mechanism of MMP7 action is the degradation of IGFBP thus, increasing the bioavailability of IGF and increasing the growth of cancer cells (Ii et al. 2006).
Very few reports examine MMP26 (matrilysin-2) expression in ovarian cancer. MMP26 was not detected in ovarian cancer cell lines such as BG-1, OAW-42 (Schropfer et al. 2010) nor significantly elevated in tissues from ovarian cancer patients (Zhao et al. 2009). In contrast, Ripley and coworkers reported that immunostaining of MMP26 was increased with ovarian carcinoma tumor stage III/IV, indicating that the invading tumor cells possess the strongest staining for MMP26 (Ripley et al. 2006).
The Metalloelastase: MMP12
MMP12 shares homology with the other MMPs in that it has a similar domain structure with both the collagenases and stromelysins 1 and 2. However, MMP12 is distinct from the other MMPs as it only shares a 33 to 48% amino acid homology with the other members of this family. (Shapiro et al. 1993). MMP12 is produced by macrophages, degrades elastin, and has been shown to be associated with inflammatory skin diseases, atherosclerosis, angiogenesis and cancer (Nenan et al. 2005, Chen et al. 2013). Very few studies have explored the role of MMP12 in ovarian cancer. Polymorphism studies indicate that a 82A/G polymorphism of MMP12 may be a risk factor for the development of epithelial ovarian cancer progression (Li et al. 2009).
CONCLUSIONS
There is an extensive body of literature supporting that MMP overexpression is associated with an increased metastatic potential of ovarian tumors, which leads to poor prognosis and decreased survival. However, as the current review highlights, the expression pattern of each individual MMP varies depending upon the type of tumor, tumor stage, patient diagnosis, means of MMP identification such as PCR, enzyme activity or immunohistochemistry, and even potentially the patient population. This variability is highlighted by studies examining overexpression of MMPs related to polymorphisms in the respective MMP promoter or differences in MMP expression in different cancer cell lines as discussed above. Variability in the ability to detect MMP expression and activity may obfuscate any conclusions regarding any one MMP in the initiation, progression, metastasis and invasion of a specific MMP.
Additionally, emerging evidence suggests that MMPs may have non-proteolytic actions working through the hemopexin domain (Correia et al. 2013). With sensitive advances in technology, such as RNAseq, proteomics and 3D modeling, a more concise picture of the involvement of the MMP family in the development and progression of ovarian cancer should emerge. This will allow for the development of small molecule MMP inhibitors that block both proteolytic and non-proteolytic actions of the MMPs that could be used as an adjuvant therapy in conjunction with existing therapies to combat ovarian cancer.
Figure 3.
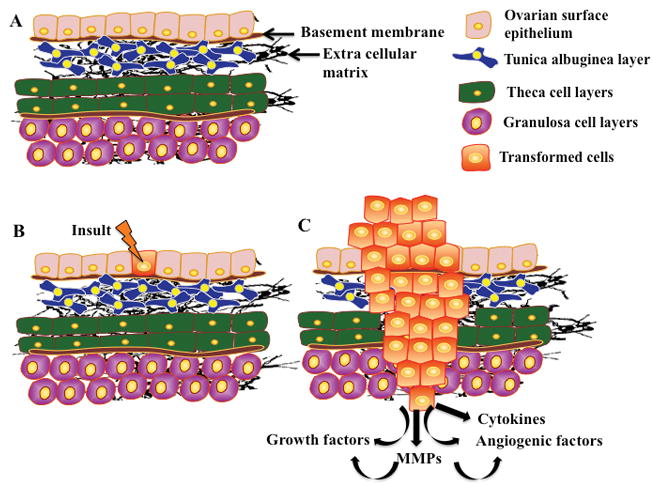
Schematic of ovarian cancer formation: The ovary is comprised of several cell layers including the surface epithelium and the underlying tunica aluginea which are separated from each other by a basement membrane. The theca layer lies under the tunica albuginea and is separated from the granulosa cell layer by a basement membrane (A). The presence of an insult or a spontaneous mutation to the cells of the surface epithelium causes cells to transform (B). This transformation leads to the uncontrolled growth of cells and the increased expression of growth factors, immune factors, MMPs and others. More importantly, for continuous cell growth, cells need to degrade the surrounding matrices, hence, ovarian cancer cells utilize an increased expression of specific MMPs to destroy the type of matrix adjacent to the tumor cells (C).
Acknowledgments
Support: This work was supported by National Institutes of Health Grants HD057446, R03HD071291, P01HD071875, UL1TR000117
Footnotes
Declaration of Interests: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Acar A, Onan A, Coskun U, Uner A, Bagriacik U, Atalay F, Unsal DK, Guner H. Clinical significance of serum MMP-2 and MMP-7 in patients with ovarian cancer. Med Oncol. 2008;25:279–283. doi: 10.1007/s12032-007-9031-1. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Covic L, Sevigny LM, Kaneider NC, Lazarides K, Azabdaftari G, Sharifi S, Kuliopulos A. Targeting a metalloprotease-PAR1 signaling system with cell-penetrating pepducins inhibits angiogenesis, ascites, and progression of ovarian cancer. Mol Cancer Ther. 2008;7:2746–2757. doi: 10.1158/1535-7163.MCT-08-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Tressel SL, Kaimal R, Balla M, Lam FH, Covic L, Kuliopulos A. Identification of a metalloprotease-chemokine signaling system in the ovarian cancer microenvironment: implications for antiangiogenic therapy. Cancer Res. 2010;70:5880–5890. doi: 10.1158/0008-5472.CAN-09-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Alem LF, McCord LA, Southard RC, Kilgore MW, Curry TE., Jr Activation of the PKC pathway stimulates ovarian cancer cell proliferation, migration, and expression of MMP7 and MMP10. Biol Reprod. 2013;89:73. doi: 10.1095/biolreprod.112.102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arechavaleta-Velasco F, Cuevas-Antonio R, Dominguez-Lopez P, Estrada-Moscoso I, Imani-Razavi FS, Zeferino-Toquero M, Diaz-Cueto L. Matrix metalloproteinase-8 promoter gene polymorphisms in Mexican women with ovarian cancer. Med Oncol. 2014;31:132. doi: 10.1007/s12032-014-0132-3. [DOI] [PubMed] [Google Scholar]
- Behrens P, Rothe M, Florin A, Wellmann A, Wernert N. Invasive properties of serous human epithelial ovarian tumors are related to Ets-1, MMP-1 and MMP-9 expression. Int J Mol Med. 2001;8:149–154. doi: 10.3892/ijmm.8.2.149. [DOI] [PubMed] [Google Scholar]
- Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224–5229. [PubMed] [Google Scholar]
- Berchuck A, Iversen ES, Luo J, Clarke JP, Horne H, Levine DA, Boyd J, Alonso MA, Secord AA, Bernardini MQ, Barnett JC, Boren T, Murphy SK, Dressman HK, Marks JR, Lancaster JM. Microarray analysis of early stage serous ovarian cancers shows profiles predictive of favorable outcome. Clin Cancer Res. 2009;15:2448–2455. doi: 10.1158/1078-0432.CCR-08-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Borkakoti N. Structural studies of matrix metalloproteinases. J Mol Med (Berl) 2000;78:261–268. doi: 10.1007/s001090000113. [DOI] [PubMed] [Google Scholar]
- Brun JL, Cortez A, Commo F, Uzan S, Rouzier R, Darai E. Serous and mucinous ovarian tumors express different profiles of MMP-2, -7, -9, MT1-MMP, and TIMP-1 and -2. Int J Oncol. 2008;33:1239–1246. [PubMed] [Google Scholar]
- Brun JL, Cortez A, Lesieur B, Uzan S, Rouzier R, Darai E. Expression of MMP-2, -7, -9, MT1-MMP and TIMP-1 and -2 has no prognostic relevance in patients with advanced epithelial ovarian cancer. Oncol Rep. 2012;27:1049–1057. doi: 10.3892/or.2011.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. 2013;2013:928315. doi: 10.1155/2013/928315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Wong AS, Huang HF, Leung PC. Gonadotropins and ovarian cancer. Endocr Rev. 2007;28:440–461. doi: 10.1210/er.2006-0036. [DOI] [PubMed] [Google Scholar]
- Choi JW, Ahn SE, Rengaraj D, Seo HW, Lim W, Song G, Han JY. Matrix metalloproteinase 3 is a stromal marker for chicken ovarian cancer. Oncol Lett. 2011;2:1047–1051. doi: 10.3892/ol.2011.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Group on Epidemiological Studies of Ovarian C. Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303–314. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- Correia AL, Mori H, Chen EI, Schmitt FC, Bissell MJ. The hemopexin domain of MMP3 is responsible for mammary epithelial invasion and morphogenesis through extracellular interaction with HSP90beta. Genes Dev. 2013;27:805–817. doi: 10.1101/gad.211383.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coticchia CM, Curatolo AS, Zurakowski D, Yang J, Daniels KE, Matulonis UA, Moses MA. Urinary MMP-2 and MMP-9 predict the presence of ovarian cancer in women with normal CA125 levels. Gynecol Oncol. 2011;123:295–300. doi: 10.1016/j.ygyno.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007a;5:35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, Lee Y. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007b;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24:428–465. doi: 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- Davidson B, Goldberg I, Berner A, Nesland JM, Givant-Horwitz V, Bryne M, Risberg B, Kristensen GB, Trope CG, Kopolovic J, Reich R. Expression of membrane-type 1, 2, and 3 matrix metalloproteinases messenger RNA in ovarian carcinoma cells in serous effusions. Am J Clin Pathol. 2001;115:517–524. doi: 10.1309/B1YX-L8DB-TGY1-7905. [DOI] [PubMed] [Google Scholar]
- Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Ben-Baruch G, Nesland JM, Berner A, Bryne M, Reich R. High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate with poor survival in ovarian carcinoma. Clin Exp Metastasis. 1999;17:799–808. doi: 10.1023/a:1006723011835. [DOI] [PubMed] [Google Scholar]
- Davidson BA, Rubatt JM, Corcoran DL, Teoh DK, Bernardini MQ, Grace LA, Soper WJ, Berchuck A, Siamakpour-Reihani S, Chen W, Owzar K, Murphy SK, Secord AA. Differential Angiogenic Gene Expression in TP53 Wild-Type and Mutant Ovarian Cancer Cell Lines. Front Oncol. 2014;4:163. doi: 10.3389/fonc.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delassus GS, Cho H, Hoang S, Eliceiri GL. Many new down- and up-regulatory signaling pathways, from known cancer progression suppressors to matrix metalloproteinases, differ widely in cells of various cancers. J Cell Physiol. 2010;224:549–558. doi: 10.1002/jcp.22157. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- Dor J, Lerner-Geva L, Rabinovici J, Chetrit A, Levran D, Lunenfeld B, Mashiach S, Modan B. Cancer incidence in a cohort of infertile women who underwent in vitro fertilization. Fertil Steril. 2002;77:324–327. doi: 10.1016/s0015-0282(01)02986-7. [DOI] [PubMed] [Google Scholar]
- Drew AF, Blick TJ, Lafleur MA, Tim EL, Robbie MJ, Rice GE, Quinn MA, Thompson EW. Correlation of tumor- and stromal-derived MT1-MMP expression with progression of human ovarian tumors in SCID mice. Gynecol Oncol. 2004;95:437–448. doi: 10.1016/j.ygyno.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- Fathalla MF. Incessant ovulation and ovarian cancer - a hypothesis re-visited. Facts Views Vis Obgyn. 2013;5:292–297. [PMC free article] [PubMed] [Google Scholar]
- Fishman DA, Bafetti LM, Stack MS. Membrane-type matrix metalloproteinase expression and matrix metalloproteinase-2 activation in primary human ovarian epithelial carcinoma cells. Invasion Metastasis. 1996;16:150–159. [PubMed] [Google Scholar]
- Furuya M. Analysis of matrix metalloproteinases and related tissue inhibitors in cystic fluids of ovarian tumors. Hokkaido Igaku Zasshi. 1999;74:145–155. [PubMed] [Google Scholar]
- Gershtein ES, Levkina NV, Digayeva MA, Laktionov KP, Tereshkina IV, Kushlinsky NE. Matrix metalloproteinases 2, 7, and 9 and tissue inhibitor of metalloproteinases-1 in tumors and serum of patients with ovarian neoplasms. Bull Exp Biol Med. 2010;149:628–631. doi: 10.1007/s10517-010-1010-4. [DOI] [PubMed] [Google Scholar]
- Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- Hantke B, Harbeck N, Schmalfeldt B, Claes I, Hiller O, Luther MO, Welk A, Kuhn W, Schmitt M, Tschesche H, Muehlenweg B. Clinical relevance of matrix metalloproteinase-13 determined with a new highly specific and sensitive ELISA in ascitic fluid of advanced ovarian carcinoma patients. Biol Chem. 2003;384:1247–1251. doi: 10.1515/BC.2003.137. [DOI] [PubMed] [Google Scholar]
- Hu X, Li D, Zhang W, Zhou J, Tang B, Li L. Matrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cell invasion. Arch Gynecol Obstet. 2012;286:1537–1543. doi: 10.1007/s00404-012-2456-6. [DOI] [PubMed] [Google Scholar]
- Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) 2006;231:20–27. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- Kamat AA, Fletcher M, Gruman LM, Mueller P, Lopez A, Landen CN, Jr, Han L, Gershenson DM, Sood AK. The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin Cancer Res. 2006;12:1707–1714. doi: 10.1158/1078-0432.CCR-05-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori Y, Matsushima M, Minaguchi T, Kobayashi K, Sagae S, Kudo R, Terakawa N, Nakamura Y. Correlation between expression of the matrix metalloproteinase-1 gene in ovarian cancers and an insertion/deletion polymorphism in its promoter region. Cancer Res. 1999;59:4225–4227. [PubMed] [Google Scholar]
- Karabulut S, Aksit E, Tas F, Ciftci R, Aydiner A, Yildiz I, Keskin S, Eralp Y, Yasasever CT, Vatansever S, Disci R, Saip P. Is there any diagnostic value of serum protease-activated receptor-1 (PAR1) levels on determination of epithelial ovarian carcinoma? Tumour Biol. 2014;35:4323–4329. doi: 10.1007/s13277-013-1567-4. [DOI] [PubMed] [Google Scholar]
- Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118:1367–1379. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny HA, Lengyel E. MMP-2 functions as an early response protein in ovarian cancer metastasis. Cell Cycle. 2009;8:683–688. doi: 10.4161/cc.8.5.7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner DE, Jr, Stetler-Stevenson WG. Structural biochemistry and activation of matrix metalloproteases. Curr Opin Cell Biol. 1993;5:891–897. doi: 10.1016/0955-0674(93)90040-w. [DOI] [PubMed] [Google Scholar]
- Koshiyama M, Matsumura N, Konishi I. Recent concepts of ovarian carcinogenesis: type I and type II. Biomed Res Int. 2014;2014:934261. doi: 10.1155/2014/934261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LN, Zhou X, Gu Y, Yan J. Prognostic value of MMP-9 in ovarian cancer: a meta-analysis. Asian Pac J Cancer Prev. 2013;14:4107–4113. doi: 10.7314/apjcp.2013.14.7.4107. [DOI] [PubMed] [Google Scholar]
- Li Y, Jia JH, Kang S, Zhang XJ, Zhao J, Wang N, Zhou RM, Sun DL, Duan YN, Wang DJ. The functional polymorphisms on promoter region of matrix metalloproteinase-12, -13 genes may alter the risk of epithelial ovarian carcinoma in Chinese. Int J Gynecol Cancer. 2009;19:129–133. doi: 10.1111/IGC.0b013e31819a1d8e. [DOI] [PubMed] [Google Scholar]
- Li Y, Jin X, Kang S, Wang Y, Du H, Zhang J, Guo W, Wang N, Fang S. Polymorphisms in the promoter regions of the matrix metalloproteinases-1, -3, -7, and -9 and the risk of epithelial ovarian cancer in China. Gynecol Oncol. 2006;101:92–96. doi: 10.1016/j.ygyno.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhold-Heerlein I, Bauerschlag D, Zhou Y, Sapinoso LM, Ching K, Frierson H, Jr, Brautigam K, Sehouli J, Stickeler E, Konsgen D, Hilpert F, von Kaisenberg CS, Pfisterer J, Bauknecht T, Jonat W, Arnold N, Hampton GM. An integrated clinical-genomics approach identifies a candidate multi-analyte blood test for serous ovarian carcinoma. Clin Cancer Res. 2007;13:458–466. doi: 10.1158/1078-0432.CCR-06-0691. [DOI] [PubMed] [Google Scholar]
- Miyamori H, Hasegawa K, Kim KR, Sato H. Expression of metastasis-associated mts1 gene is co-induced with membrane type-1 matrix metalloproteinase (MT1-MMP) during oncogenic transformation and tubular formation of Madin Darby canine kidney (MDCK) epithelial cells. Clin Exp Metastasis. 2000;18:51–56. doi: 10.1023/a:1026523418456. [DOI] [PubMed] [Google Scholar]
- Moss NM, Wu YI, Liu Y, Munshi HG, Stack MS. Modulation of the membrane type 1 matrix metalloproteinase cytoplasmic tail enhances tumor cell invasion and proliferation in three-dimensional collagen matrices. J Biol Chem. 2009;284:19791–19799. doi: 10.1074/jbc.M109.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J, Brebeck B, Schmalfeldt B, Kuhn W, Graeff H, Hofler H. Stromelysin-3 expression in invasive ovarian carcinomas and tumours of low malignant potential. Virchows Arch. 2000;437:618–624. doi: 10.1007/s004280000261. [DOI] [PubMed] [Google Scholar]
- Murphy G, Knauper V, Cowell S, Hembry R, Stanton H, Butler G, Freije J, Pendas AM, Lopez-Otin C. Evaluation of some newer matrix metalloproteinases. Ann N Y Acad Sci. 1999;878:25–39. doi: 10.1111/j.1749-6632.1999.tb07672.x. [DOI] [PubMed] [Google Scholar]
- Nabeshima K, Inoue T, Shimao Y, Sameshima T. Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol Int. 2002;52:255–264. doi: 10.1046/j.1440-1827.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Nenan S, Boichot E, Lagente V, Bertrand CP. Macrophage elastase (MMP-12): a pro-inflammatory mediator? Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):167–172. doi: 10.1590/s0074-02762005000900028. [DOI] [PubMed] [Google Scholar]
- Perigny M, Bairati I, Harvey I, Beauchemin M, Harel F, Plante M, Tetu B. Role of immunohistochemical overexpression of matrix metalloproteinases MMP-2 and MMP-11 in the prognosis of death by ovarian cancer. Am J Clin Pathol. 2008;129:226–231. doi: 10.1309/49LA9XCBGWJ8F2KM. [DOI] [PubMed] [Google Scholar]
- Puttabyatappa M, Jacot TA, Al-Alem LF, Rosewell KL, Duffy DM, Brannstrom M, Curry TE., Jr Ovarian membrane-type matrix metalloproteinases: induction of MMP14 and MMP16 during the periovulatory period in the rat, macaque, and human. Biol Reprod. 2014;91:34. doi: 10.1095/biolreprod.113.115717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley D, Tunuguntla R, Susi L, Chegini N. Expression of matrix metalloproteinase-26 and tissue inhibitors of metalloproteinase-3 and -4 in normal ovary and ovarian carcinoma. Int J Gynecol Cancer. 2006;16:1794–1800. doi: 10.1111/j.1525-1438.2006.00714.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez JA, Orbe J, Martinez de Lizarrondo S, Calvayrac O, Rodriguez C, Martinez-Gonzalez J, Paramo JA. Metalloproteinases and atherothrombosis: MMP-10 mediates vascular remodeling promoted by inflammatory stimuli. Front Biosci. 2008;13:2916–2921. doi: 10.2741/2896. [DOI] [PubMed] [Google Scholar]
- Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian tumors in a cohort of infertile women. N Engl J Med. 1994;331:771–776. doi: 10.1056/NEJM199409223311204. [DOI] [PubMed] [Google Scholar]
- Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schropfer A, Kammerer U, Kapp M, Dietl J, Feix S, Anacker J. Expression pattern of matrix metalloproteinases in human gynecological cancer cell lines. BMC Cancer. 2010;10:553. doi: 10.1186/1471-2407-10-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SD, Kobayashi DK, Ley TJ. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem. 1993;268:23824–23829. [PubMed] [Google Scholar]
- Shigemasa K, Tanimoto H, Sakata K, Nagai N, Parmley TH, Ohama K, O’Brien TJ. Induction of matrix metalloprotease-7 is common in mucinous ovarian tumors including early stage disease. Med Oncol. 2000;17:52–58. doi: 10.1007/BF02826217. [DOI] [PubMed] [Google Scholar]
- Shiomi T, Okada Y. MT1-MMP and MMP-7 in invasion and metastasis of human cancers. Cancer Metastasis Rev. 2003;22:145–152. doi: 10.1023/a:1023039230052. [DOI] [PubMed] [Google Scholar]
- Sillanpaa S, Anttila M, Voutilainen K, Ropponen K, Turpeenniemi-Hujanen T, Puistola U, Tammi R, Tammi M, Sironen R, Saarikoski S, Kosma VM. Prognostic significance of matrix metalloproteinase-9 (MMP-9) in epithelial ovarian cancer. Gynecol Oncol. 2007;104:296–303. doi: 10.1016/j.ygyno.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Sillanpaa SM, Anttila MA, Voutilainen KA, Ropponen KM, Sironen RK, Saarikoski SV, Kosma VM. Prognostic significance of matrix metalloproteinase-7 in epithelial ovarian cancer and its relation to beta-catenin expression. Int J Cancer. 2006;119:1792–1799. doi: 10.1002/ijc.22067. [DOI] [PubMed] [Google Scholar]
- Solar P, Sytkowski AJ. Differentially expressed genes associated with cisplatin resistance in human ovarian adenocarcinoma cell line A2780. Cancer Lett. 2011;309:11–18. doi: 10.1016/j.canlet.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Sood AK, Fletcher MS, Coffin JE, Yang M, Seftor EA, Gruman LM, Gershenson DM, Hendrix MJ. Functional role of matrix metalloproteinases in ovarian tumor cell plasticity. Am J Obstet Gynecol. 2004;190:899–909. doi: 10.1016/j.ajog.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Sounni NE, Noel A. Membrane type-matrix metalloproteinases and tumor progression. Biochimie. 2005;87:329–342. doi: 10.1016/j.biochi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Stack MS, Ellerbroek SM, Fishman DA. The role of proteolytic enzymes in the pathology of epithelial ovarian carcinoma. Int J Oncol. 1998;12:569–576. doi: 10.3892/ijo.12.3.569. [DOI] [PubMed] [Google Scholar]
- Stadlmann S, Pollheimer J, Moser PL, Raggi A, Amberger A, Margreiter R, Offner FA, Mikuz G, Dirnhofer S, Moch H. Cytokine-regulated expression of collagenase-2 (MMP-8) is involved in the progression of ovarian cancer. Eur J Cancer. 2003;39:2499–2505. doi: 10.1016/j.ejca.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- Sun N, Zhang Q, Xu C, Zhao Q, Ma Y, Lu X, Wang L, Li W. Molecular regulation of ovarian cancer cell invasion. Tumour Biol. 2014 doi: 10.1007/s13277-014-2434-7. [DOI] [PubMed] [Google Scholar]
- Tanimoto H, Underwood LJ, Shigemasa K, Parmley TH, Wang Y, Yan Y, Clarke J, O’Brien TJ. The matrix metalloprotease pump-1 (MMP-7, Matrilysin): A candidate marker/target for ovarian cancer detection and treatment. Tumour Biol. 1999;20:88–98. doi: 10.1159/000030051. [DOI] [PubMed] [Google Scholar]
- Tingulstad S, Skjeldestad FE, Halvorsen TB, Hagen B. Survival and prognostic factors in patients with ovarian cancer. Obstet Gynecol. 2003;101:885–891. doi: 10.1016/s0029-7844(03)00123-6. [DOI] [PubMed] [Google Scholar]
- Ulku AS, Schafer R, Der CJ. Essential role of Raf in Ras transformation and deregulation of matrix metalloproteinase expression in ovarian epithelial cells. Mol Cancer Res. 2003;1:1077–1088. [PubMed] [Google Scholar]
- Venn A, Watson L, Bruinsma F, Giles G, Healy D. Risk of cancer after use of fertility drugs with in-vitro fertilisation. Lancet. 1999;354:1586–1590. doi: 10.1016/S0140-6736(99)05203-4. [DOI] [PubMed] [Google Scholar]
- Wang FQ, Fisher J, Fishman DA. MMP-1-PAR1 axis mediates LPA-induced epithelial ovarian cancer (EOC) invasion. Gynecol Oncol. 2011a;120:247–255. doi: 10.1016/j.ygyno.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Wang FQ, So J, Reierstad S, Fishman DA. Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by activation of progelatinase. Int J Cancer. 2005;114:19–31. doi: 10.1002/ijc.20697. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hu C, Dong R, Huang X, Qiu H. Platelet-derived growth factor-D promotes ovarian cancer invasion by regulating matrix metalloproteinases 2 and 9. Asian Pac J Cancer Prev. 2011b;12:3367–3370. [PubMed] [Google Scholar]
- Wenham RM, Calingaert B, Ali S, McClean K, Whitaker R, Bentley R, Lancaster JM, Schildkraut J, Marks J, Berchuck A. Matrix metalloproteinase-1 gene promoter polymorphism and risk of ovarian cancer. J Soc Gynecol Investig. 2003;10:381–387. doi: 10.1016/s1071-5576(03)00141-2. [DOI] [PubMed] [Google Scholar]
- Wielockx B, Libert C, Wilson C. Matrilysin (matrix metalloproteinase-7): a new promising drug target in cancer and inflammation? Cytokine Growth Factor Rev. 2004;15:111–115. doi: 10.1016/j.cytogfr.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- Zhang GY, Ahmed N, Riley C, Oliva K, Barker G, Quinn MA, Rice GE. Enhanced expression of peroxisome proliferator-activated receptor gamma in epithelial ovarian carcinoma. Br J Cancer. 2005;92:113–119. doi: 10.1038/sj.bjc.6602244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YG, Xiao AZ, Ni J, Man YG, Sang QX. Expression of matrix metalloproteinase-26 in multiple human cancer tissues and smooth muscle cells. Ai Zheng. 2009;28:1168–1175. doi: 10.5732/cjc.008.10768. [DOI] [PubMed] [Google Scholar]
- Zucker S, Drews M, Conner C, Foda HD, DeClerck YA, Langley KE, Bahou WF, Docherty AJ, Cao J. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP) J Biol Chem. 1998;273:1216–1222. doi: 10.1074/jbc.273.2.1216. [DOI] [PubMed] [Google Scholar]


