Conspectus
The cell surface (or cell wall) of bacteria is coated with carbohydrate (or glycan) structures that play a number of important roles. These include providing structural integrity, serving as a permeability barrier to extracellular compounds (e.g., drugs) and modulating the immune system of the host. Of interest to this Account is the cell wall structure of mycobacteria. There are a host of different mycobacterial species, some of which cause human disease. The most well-known is Mycobacterium tuberculosis, the causative agent of tuberculosis. The mycobacterial cell wall is characterized by the presence of unusual carbohydrate structures that fulfill the roles described above. However, in many cases, a molecular-level understanding of how mycobacterial cell wall glycans mediate these processes is lacking.
Inspired by a seminar he heard as a postdoctoral fellow, the author began his independent research program with a focus on the chemical synthesis of mycobacterial glycans. The goals were not only to develop synthetic approaches to these unique structures but also to provide molecules that could be used to probe their biological function. Initial work addressed the preparation of fragments of two key polysaccharides, arabinogalactan and lipoarabinomannan, which contain large numbers of sugar residues in the furanose (five-membered) ring form. At the time these investigations began, there were few methods reported for the synthesis of oligosaccharides containing furanose rings. Thus, early in the program, a major area of interest was methodology development, particularly for the preparation of 1,2-cis-furanosides. To solve this challenge, a range of conformationally restricted donors have been developed, both in the author’s group and others, which provide 1,2-cis-furanosidic linkages with high stereoselectivity.
These investigations were followed by application of the developed methods to the synthesis of a range of target molecules containing arabinofuranose and galactofuranose residues. These molecules have now found application in biochemical, immunological, and structural biology investigations, which have shed light on their biosynthesis and how these motifs are recognized by both the innate and adaptive immune systems.
More recently, attention has been directed toward the synthesis of another class of immunologically active mycobacterial cell wall glycans, the extractable glycolipids. In this case, efforts have been primarily on phenolic glycolipids, and the compounds synthesized have been used to evaluate their ability to modulate cytokine release. Over the past 20 years, the use of chemical synthesis to provide increasingly complex glycan structures has provided significant benefit to the burgeoning field of mycobacterial glycobiology. Through the efforts of groups from around the globe, access to these compounds is now possible via relatively straightforward methods. As the pool of mycobacterial glycans continues to grow, so too will our understanding of their role in disease, which will undoubtedly lead to new strategies to prevent or treat mycobacterial infections.
Introduction
A challenge I, and presumably others who train graduate students, face is getting them to attend departmental seminars. At the start of a new academic year, I often hear complaints from my students about seminars being a distraction from research and that their attending a talk that is not directly aligned with their interests is a poor use of time. In response, I point out that this is an excellent opportunity to learn new things and that seminar attendance is, after all, something I require. I also describe a situation where my attending a seminar, the title of which was not directly aligned with what I thought were my “research interests”, had a profound impact on my career.
The lecture1 was given at the 17th International Carbohydrate Symposium in 1994, the time I was beginning to develop proposals for my applications for independent faculty positions. The speaker was Patrick Brennan of Colorado State University, a pioneer and international leader in determining the structures and biosynthesis of the highly complex carbohydrates (glycans) found in the cell wall of mycobacteria, including the organism that causes tuberculosis, Mycobacterium tuberculosis. Brennan is an engaging speaker and the molecules he presented had structures unlike anything I had ever seen. I knew immediately that mycobacterial glycans were something I had to study in my independent career.
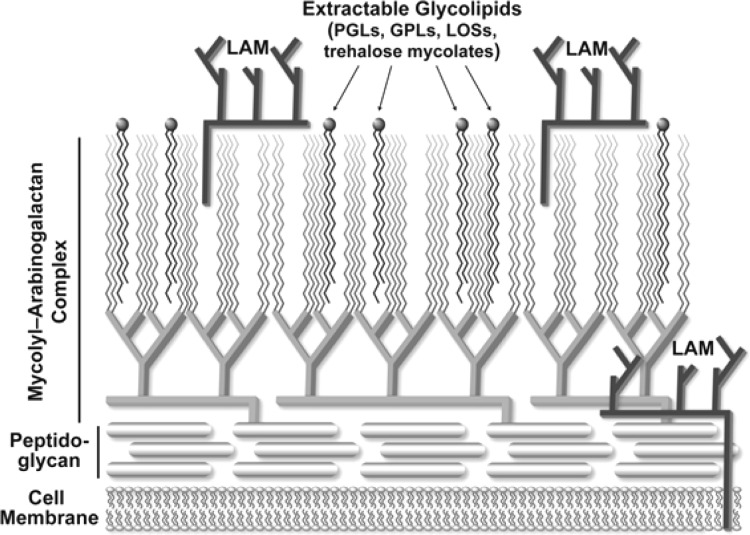
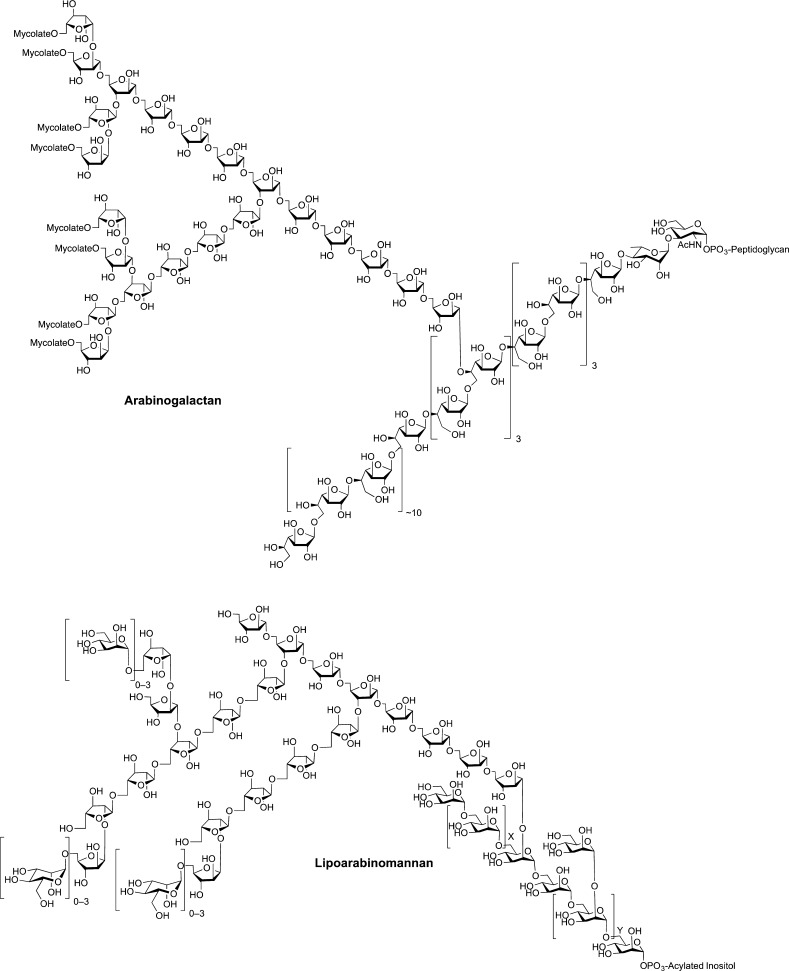
The mycobacterial cell wall is a highly complex structure composed largely of carbohydrates and lipids (Figure 1).2−4 The major components are two lipidated polysaccharides, the mycolyl–arabinogalactan (AG) complex and lipoarabinomannan (LAM). The structures (Figure 2) of both AG and LAM are characterized by the presence of a large number of sugar residues in the furanose (five-membered) ring form. It was these molecules that so intrigued me in Brennan’s talk. AG is composed nearly exclusively of such monosaccharides, galactofuranose (Galf) and arabinofuranose (Araf), while LAM contains both Araf and mannopyranose residues. In 1994, there had been relatively few investigations on the synthesis of furanose-containing glycans. For a basic science perspective, this seemed to be an excellent area in which to build a research program. Moreover, from a practical standpoint I was excited by the possibility of the target molecules being applied to study the biology of an organism that causes a disease of great importance and one for which drug resistance had become (and remains) a problem: tuberculosis.
Figure 1.
Representation of the mycobacterial cell wall with all major classes of glycans shown. LAM, lipoarabinomannan; PGLs, phenolic glycolipids; GPLs, glycopeptidolipids; LOSs, lipooligosaccharides. Reproduced with permission from ref (5). Copyright 2015 John Wiley & Sons.
Figure 2.
Composite structures of mycobacterial arabinogalactan (AG) and lipoarabinomannan (LAM).
Synthesis of Arabinofuranosides
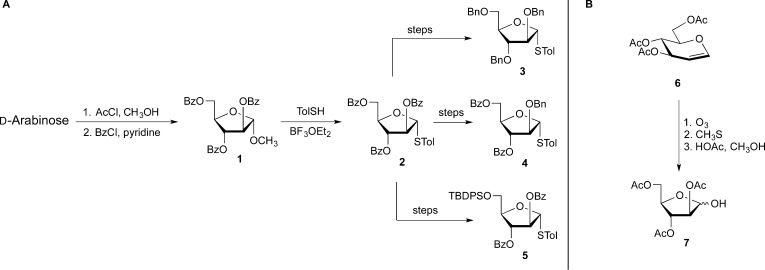
In charting a course of action, the initial goal was to develop chemistry that could be used for the synthesis of Araf-containing oligosaccharides, which could then serve as substrates for the glycosyltransferases involved in AG and LAM biosynthesis or as ligands for Araf-binding proteins. An initial challenge in the synthesis of furanosides is to obtain derivatives in the less thermodynamically stable five-membered ring form.6 There are a number of methods for doing this, each with their pros and cons. For arabinose, we found the most straightforward access was through a classic method: Fischer glycosylation under kinetic control. We adopted and refined a reported method7 to prepare methyl glycoside 1 in two steps from d-arabinose (Scheme 1A). This method is robust enough that it can be reliably done in undergraduate teaching laboratories.8 We subsequently could convert 1, in one step, into the corresponding thioglycoside 2(9) and then into other glycosyl donors (e.g., 3–5).10,11 Early in our investigations, we also developed an alternate approach to the Araf system though ozonolysis of protected glucal derivatives (6 → 7, Scheme 1B).12 This latter method was useful for the preparation of 13C labeled materials (from commercially available 13C-labeled d-glucose), which were of interest for NMR spectroscopic studies. However, it is less convenient than Fischer glycosylation for the preparation of materials on large scale.
Scheme 1. Access to the Arabinofuranose Ring System and Donors (3–5) for the Synthesis of Arabinofuranose-Containing Glycans.
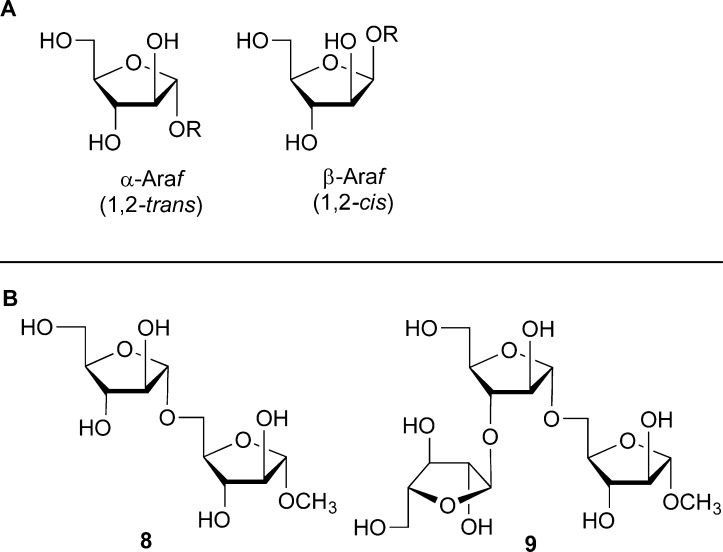
The Araf residues in AG and LAM are found as part of α-(1,2-trans) and β-(1,2-cis) linkages (Figure 3A). We assumed that the α-linkages could be straightforwardly assembled from glycosyl donors possessing O-2 acylation, for example, 2. The acyl groups, through neighboring group participation, would ensure the 1,2-trans stereochemistry. Indeed, the use of donors analogous to 2 were used by graduate student Joe Ayers to synthesize α-Araf-containing glycans (e.g., 8 and 9, Figure 3B), which could then be used as acceptor substrates for mycobacterial arabinosyltransferases.13
Figure 3.
(A) Structures of the α-Araf and β-Araf ring systems and (B) structures of α-Araf-containing oligosaccharides 8 and 9.
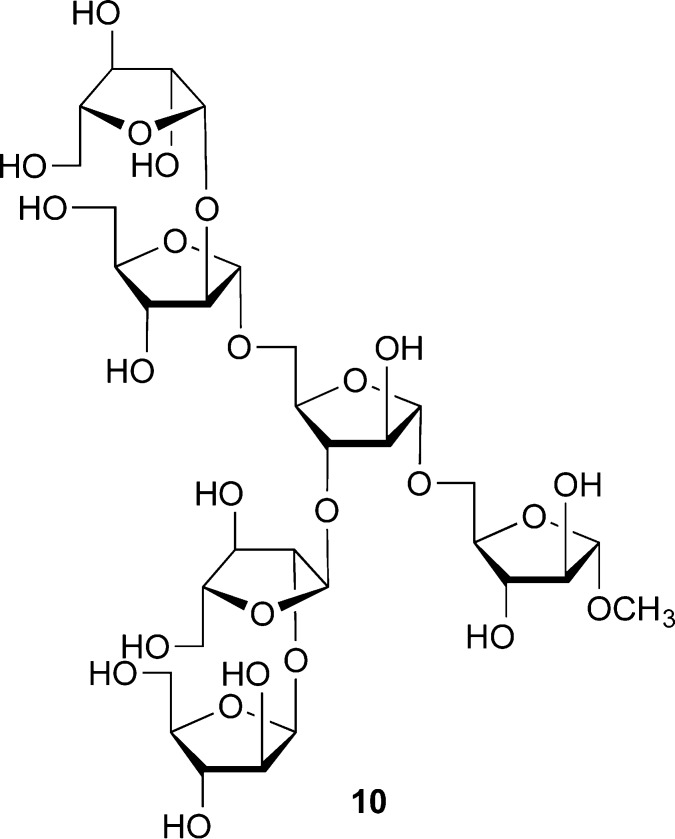
On the other hand, we anticipated that stereoselective access to the β-Araf-linkages would be more difficult, given their stereochemical similarity to β-mannopyranosides. The stereochemistry of these 1,2-cis-β systems is such that one can rely on neither neighboring group participation nor thermodynamics (through the anomeric effect) to give the desired product.14 That said, we did explore the possibility that 2-O-benzylated donors (e.g., 3, 4) could be used to access β-Araf linkages. We discovered that by carefully controlling the reaction conditions, some donor and acceptor pairs could be coupled with good to excellent β-selectivity.10 Using this approach a postdoctoral fellow, Haifeng Yin, could synthesize a panel of mycobacterial arabinofuranoside fragments, including the common Ara6 motif (10, Figure 4), which is found at the nonreducing terminus of both AG and LAM.11 Subsequent work from the Kim group showed similar results with another class of glycosyl donors.15
Figure 4.
Structure of the Ara6 motif (as the methyl glycoside), which is present in both AG and LAM.
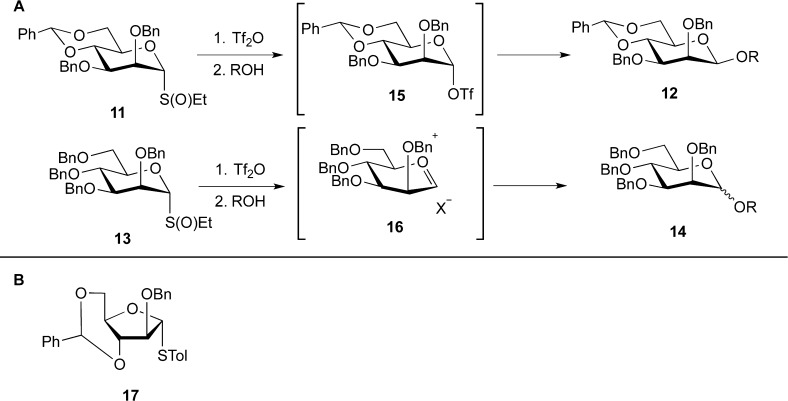
Although we could access many targets through donors such as 3, the lack of generality of the method for synthesizing the β-Araf glycosides was a limitation. Around the same time, the Crich group had demonstrated the influence of conformational restriction of the pyranose ring in the synthesis of β-mannopyranosides. In particular, it was shown that donors possessing a 4,6-O-benzylidene acetal (e.g., 11, Figure 5A) could, under the proper conditions, produce β-mannopyranosides (12) with high stereoselectivity, whereas donors lacking this cyclic acetal (13) are unselective or even α-selective.16,17 It was later demonstrated that the torsional restraints induced by the benzylidene acetal led to the in situ generation of an α-mannopyranosyl triflate (15, Figure 5B), which could then react with the alcohol in an SN2-like manner to produce the β-mannopyranoside. In the absence of this cyclic protecting group an ion-pair structure (e.g., 16) is produced in place of 15, which leads to increases in the amount of α-glycoside product.18,19
Figure 5.
(A) Studies by Crich on the synthesis of β-mannopyranosides, showing the β-selective glycosyl triflate intermediate (15) and an α-selective/unselective intermediate (16).19 (B) A conformationally restricted Araf-thioglycoside (17).
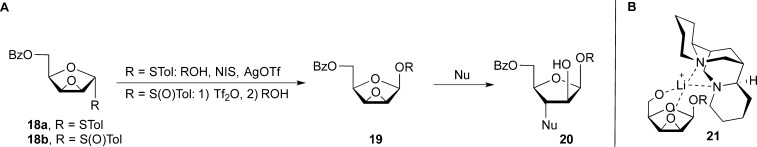
These studies prompted us to consider an analogous approach for synthesis of β-Araf linkages. The obvious extension would be to prepare a benzylidene acetal such as 17 (Figure 5B). However, we were aware that the yield of compounds of this type was generally low given the strain present in the trans-fused 5/6 ring system. These considerations were underway when, for other reasons, we prepared a series of 2,3-anhydro-furanoside thioglycosides (e.g., 18, Figure 6). Out of simple curiosity, the postdoctoral fellow carrying out this work, Raj Gadikota, tested how 18 behaved in glycosylation reactions. We were surprised to discover that it was indeed a highly stereoselective glycosyl donor, in most cases producing a single glycoside product. Even more surprising was that the product obtained was the one in which the glycosidic linkage was cis to the epoxide moiety, 19.20 We subsequently showed that the sulfoxide derivative 20 gave the same results when glycosylations were done under “Crich” conditions.18,19 These results provided an alternative, albeit indirect, route to β-arabinofuranosides: glycosylation with donors such as 18a/18b, followed by regioselective opening of the epoxide ring (19) with a nucleophile resulting in 20.21
Figure 6.
(A) 2,3-anhydrosugar donors 18a/18b and their use in the synthesis of β-arabinofuranosides (20). (B) Postulated complex (21) formed in (−)-sparteine-mediated nucleophilic opening of O-5 deprotonated 2,3-anhydro-β-d-lyxofuranosides (e.g., 19) by lithium alkoxides.
The opening of the epoxide in a regioselective manner proved to be more challenging than expected. Previous work22 suggested that these substrates should be predisposed to nucleophilic attack at the desired C-3 position; however, with alkoxides as the nucleophile, the selectivities were modest.21 Moreover, the substrates were quite robust. Heating at over 100 °C was necessary for reasonable rates to be achieved. A serendipitous discovery that lithium alkoxides provided better regioselectivity than their sodium or potassium counterparts led ultimately to a solution. We investigated the possibility that lithium binding additives might enhance the rate and regioselectivity of the reaction. After screening a number of such additives, graduate student Chris Callam found that both a rate enhancement and a substantial increase in regiocontrol could be obtained with (−)-sparteine. The temperature could be lowered to 70 °C, the reaction was complete in less than 30 min, and only the desired arabinofuranoside product was produced. While our initial thoughts were that the chirality of the additive was essential, that was shown not to be the case.21 The reasons underlying this effect remain unclear, but the presence of a free OH group at C-5 in 19 (either present initially or liberated under the basic conditions of the reaction) is essential for good regiocontrol. Presumably, a ternary complex (e.g., 21) between the lithium ion, the (−)-sparteine, and the substrate forms, which then directs the nucleophile to C-3. However, attempts to determine the structure of this intermediate have been unsuccessful.
Although we did not elucidate reasons for the high regioselectivity in the epoxide ring opening in glycosides of the type 19, we did succeed in understanding the origins of the selectivity in the glycosylation reactions with 2,3-anhydro-furanoside sulfoxides.23 Using experimental techniques pioneered by Crich,18 coupled with ab initio calculations, we demonstrated that the origin of the high stereoselectivity was due to the formation of an intermediate α-glycosyl triflate that underwent an SN2-like nucleophilic displacement by the nucleophile. Thus, these reactions proceed in a way analogous to the Crich β-mannoside method,19 with the epoxide serving as the conformational restraint.
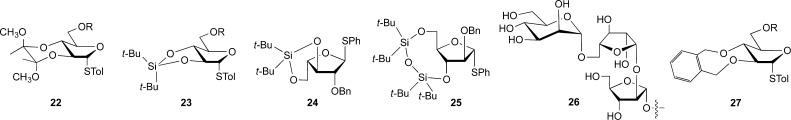
The 2,3-anhydrosugar methodology could be applied to the preparation of complex arabinans, including the Ara6 motif (10, Figure 4), and it was also extended to hexofuranosides through the preparation of α-galactofuranosides by graduate student Yu Bai.24,25 Nevertheless, the two step approach and the rather complex conditions required for the epoxide ring opening prompted us to explore other conformationally restricting groups that could achieve the same effect. Our initial focus was on groups that spanned O-2 and O-3 of the arabinofuranose ring, in particular 22 and 23 (Figure 7). Unfortunately, we were never able to produce these compounds in yields sufficient for further studies. While engaged in these investigations, we were “scooped” by Boons26 and soon after Ito.27 Both groups reported that conformational restriction of the arabinofuranose ring through O-3/O-5, using silyl-based protecting groups, provided reagents (e.g., 24 and 25) that were highly β-selective. The rationale for the selectivity builds from the “inside-attack” model proposed by Woerpel and co-workers to predict the stereoselectivity of nucleophilic attack onto five-membered ring oxacarbenium ions.28 The silyl protecting group effectively locks the five-membered ring into an envelope conformation that is predisposed to attack leading to the β-glycoside.
Figure 7.
Conformationally restricted arabinofuranose donors (22–25, 27) and an O-5 substituted β-arabinofuranoside motif present in mycobacterial LAM (26).
While regents such as 24 and 25 are very useful, and generally superior to 2,3-anhydrosugar donors, they have limitations with regard to the preparation of β-arabinofuranosides that are substituted on O-5. Such motifs (e.g., 26) are present in mycobacterial LAM.2 The use of O-3/O-5-protected reagents to prepare compounds of this type requires a number of transformations after introduction of the β-Araf residue. To address this limitation, a postdoctoral fellow, Aki Imamura, explored the use of another class of donors possessing conformational restriction across O-2 and O-3: 2,3-O-xylylene-protected thioglycosides (e.g., 27). Whereas our attempts to prepare 22 and 23 had failed, compounds of the general class 27 could be prepared straightforwardly. When explored in glycosylation reactions,29 we were pleased to discover that these compounds, like 24 and 25, are indeed generally β-selective, thus allowing straightforward access to compounds such as 26. Following glycosylation, the O-5 protecting group can be cleaved, allowing further modification at that position.
The studies described above, as well as work carried out by others, have led to an arsenal of methods for the synthesis of essentially any mycobacterial arabinan fragment. Such syntheses can now be done with confidence and an increasingly diverse range of compounds have now been synthesized.30−37 Notable among these are large fragments of these molecules including species with more than 20 Araf residues.38−40 Access to these compounds is now facilitating downstream biological/immunological investigations, including mapping of the epitopes of anti-LAM monoclonal antibodies,41,42 the development of novel anti-TB vaccines,37 and characterization of the specificity of the immune response that arises from LAM.
Synthesis of Mannopyranosides
A desire to carry-out structure–function studies of the biological role of LAM has led to interest in synthesizing fragments containing both Araf and mannopyranose (Manp) residues.31,37,43−46 The Manp residues in LAM are α-(1→2)-, α-(1→6)-, and, in one strain, α-(1→3)-linked.3 α-Manp residues in these linkages are found in a broad range glycoconjugates and are generally straightforward to prepare. In most cases, established approaches have led to the successful preparation of these molecules, although streamlined methods specific to these fragments have been reported.47
Synthesis of Galactofuranosides
Several years after initiating the work on arabinofuranosides, we turned our attention to the other major furanose component in the mycobacterial cell wall: the galactan domain of the AG. Our interest was motivated by the identification of a galactofuranosyltransferase enzyme,48 which, at the time, appeared to be the primary glycosyltransferase involved in galactan biosynthesis. Compared with the arabinan, the galactan is relatively simple. It is a linear chain of alternating β-(1→5) and β-(1→6)-linked Galf residues, attached to a disaccharide containing rhamnose and N-acetylglucosamine.2 Moreover, the linkages between the Galf residues are 1,2-trans, and hence we envisioned that the assembly of galactan fragments would be straightforward through the use of donors with acyl protecting groups on O-2. We were aware of the pioneering work on galactofuranoside synthesis done by de Lederkremer, particularly the development and application of suitable donor and acceptor species, and our work was informed by those previous investigations.49−51
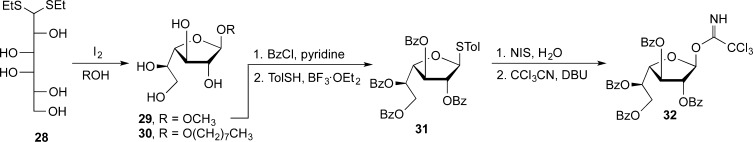
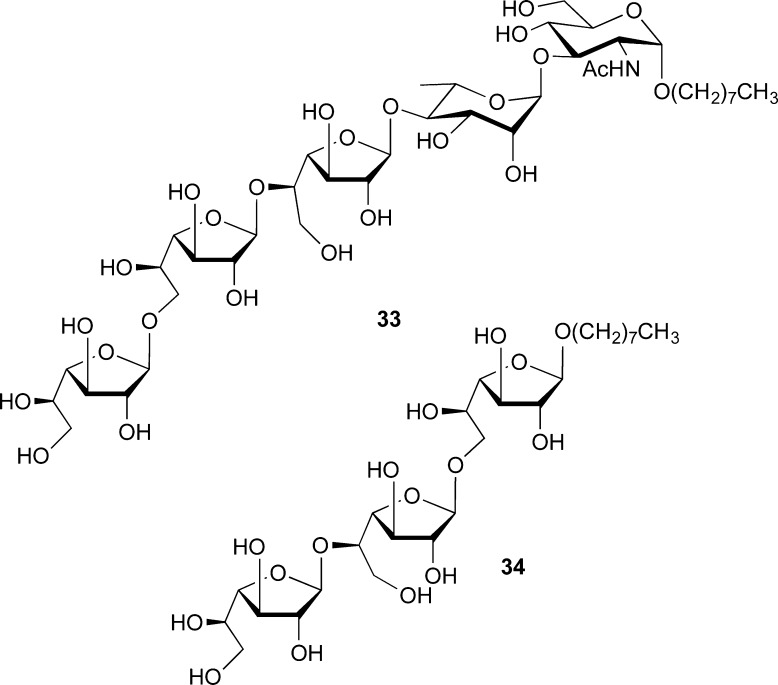
Although access to Galf derivatives via a kinetically controlled Fischer glycosylation is possible,52 significant amounts of pyranosides are often formed, which greatly complicates purification. For this reason, we generally access Galf derivatives through the cyclization of the readily prepared galactose diethyl dithioacetal (28, Scheme 2). This method, which dates to the 1930s,53 is robust, and through the use of iodine as a promoter,54 we have used it to prepare both methyl and octyl galactofuranosides, 29 and 30, respectively.55 The former can be acylated and then converted into the corresponding thioglycoside (31) and in turn the trichloroacetimidate 32. In continuation of this work, graduate student Gladys Completo then showed that 31 and 32 are useful donors for the preparation of β-Galf-containing oligosaccharides (e.g., 33 and 34, Figure 8), by both traditional and one-pot approaches.55
Scheme 2. Access to the Galactofuranose Ring System from Dithioacetal 28 and Donors (31 and 32) Used in the Synthesis of β-Galactofuranosides.
Figure 8.
Representative structures of synthetic β-galactofuranoside fragments of mycobacterial AG.
Using the general approaches outlined above, both we55 and others56,57 have synthesized a range of galactofuranosides. Such compounds have found significant application in studying mycobacterial galactan biosynthesis, in particular the two transferase enzymes involved in that process, GlfT1 and GlfT2.58 Such studies have included kinetics studies of the enzyme,59 the evaluation of active site probes,60−66 and X-ray crystallographic investigations.67
Other Mycobacterial Cell Wall Glycans
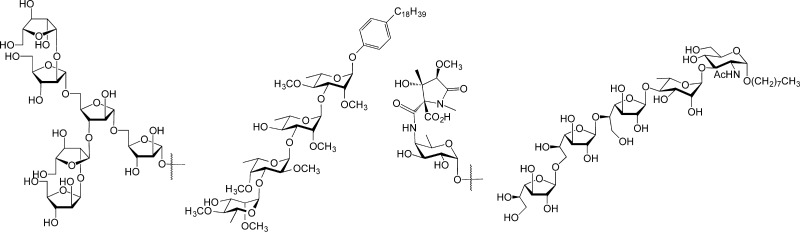
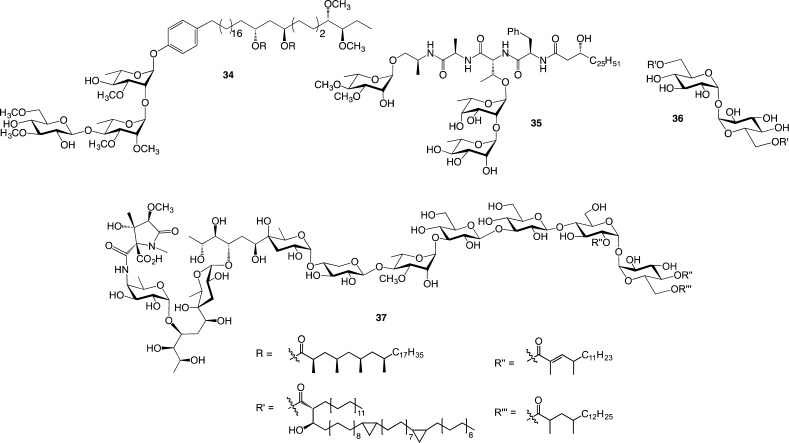
After developing approaches for the synthesis of the furanoside-containing glycans of the mycobacterial cell wall, we looked to another fascinating group of structures present in this complex assembly: the “extractable” glycolipids.68 These species are found intercalated into the mycolic acid layer of the organism (Figure 1) and often serve as modulators of the host immune system.69 The extractable lipids can be grouped into four general classes: phenolic glycolipids (PGLs, e.g, 34, Figure 9),69 glycopeptidolipids (GPLs, e.g., 35),70 trehalose mycolates (e.g., 36), and lipooligosaccharides (LOSs, e.g., 37).5 These molecules are produced in a species specific manner. That is, not all mycobacterial strains synthesize all of the structures, and moreover, the molecules that are produced generally differ across species. Although the immunomodulatory properties of the extractable lipids are well established, detailed structure–function studies were not available when we began to consider synthesizing these compounds. We therefore set as a goal the synthesis of all known representatives of these glycolipids, both as the free glycan and as their lipidated (natural) forms. It was anticipated that access to such a library would greatly facilitate the development of a molecular-level appreciation of how these molecules interact with the immune system of the host.
Figure 9.
Representative structures of phenolic glycolipids (34), glycopeptidolipids (35), trehalose mycolates (36), and lipooligosaccharides (37).
On the surface, these molecules seem to be more straightforwardly accessible than the LAM and AG fragments described above. Indeed, while there is considerable prior art that could be used to develop synthetic routes to the extractable glycolipids, there are nevertheless challenges. One is the preparation of the unusual monosaccharides present in many of the targets (e.g., 37). Another is the development of strategies that allow the introduction of fatty acyl acids, which effectively precludes the use of most ester protecting groups.
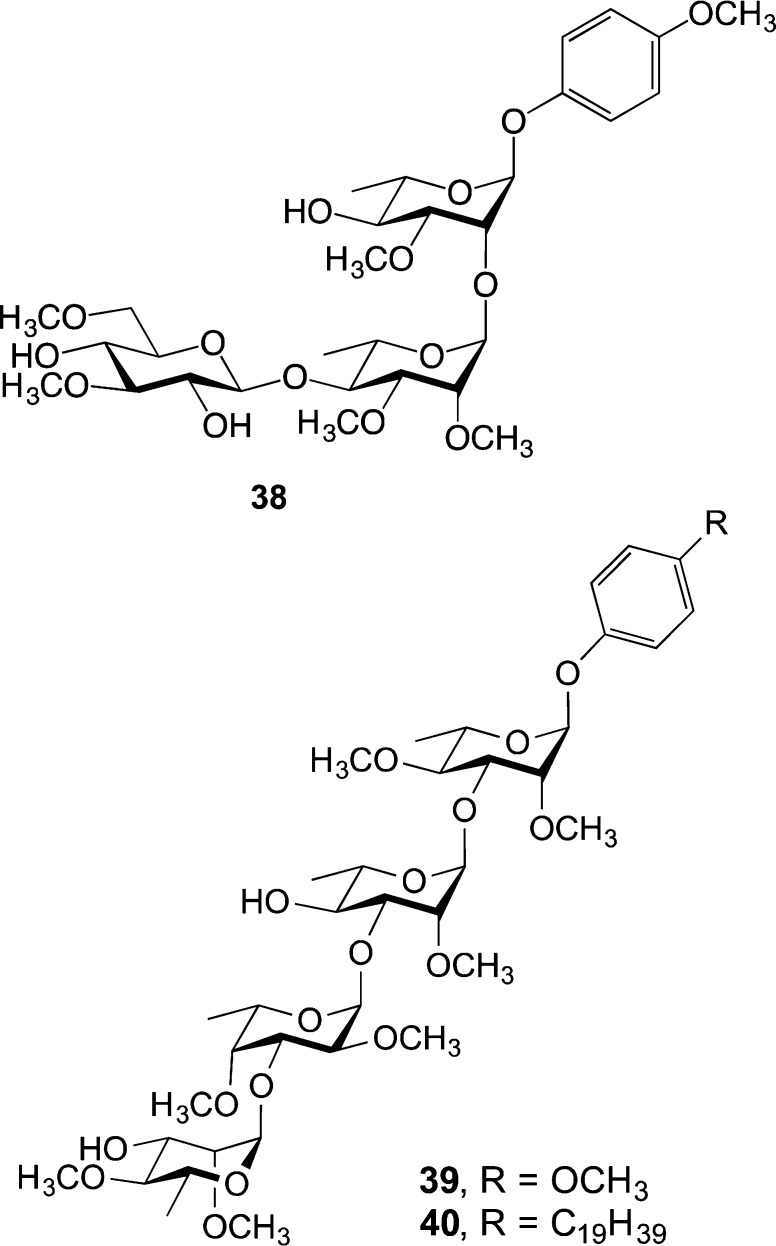
Mindful of these challenges, we first targeted what we considered to be the most tractable of the extractable glycolipids: PGLs. Although there is now a total synthesis of a complete PGL,71 that paper had not appeared when we began our investigations. At the outset, to probe the effect of the glycan on cytokine function in the absence of the lipid domain, we targeted a family of all of the reported PGL structures, prepared as their p-methoxyphenyl glycosides (e.g., 38, 39, Figure 10).72−74 This work, done by a single graduate student, Hassan Elsaidi, was a significant undertaking. It was envisioned that the p-methoxyphenyl aglycone would serve as a suitable surrogate for the phenyl group of the native PGLs. Moreover, the presence of this group could facilitate the synthesis of more complex analogs, through its selective cleavage75 and then coupling of the resulting oligosaccharide to other aglycones. In addition, strategies for the introduction of the methyl groups that characterize these species were investigated. In most cases it turned out to be more efficient to carry out methylation on the monosaccharide building blocks rather than on the more complex oligosaccharide products.
Figure 10.
Synthetic PGL analogs used to probe cytokine modulation.
After synthesizing the panel of more than 25 compounds, Hassan then evaluated their ability modulate the activity of proinflammatory cytokines (e.g., TNF-α) and nitric oxide. Although none of the compounds were inducers of these cytokines, many inhibited their production and these effects were structure dependent. Hassan went on to demonstrate that replacement of the aryl methoxy group with a longer, and yet still simple, lipid (40) enhanced this effect. Moreover, the potency of this compound approached that for phenolic glycolipid 1 (PGL-1) from Mycobacterium leprae, the only PGL available in usable quantities for purposes of comparison. Through these studies, we could conclude that the carbohydrate domain is essential for the ability of these molecules to inhibit the production of proinflammatory cytokines and that addition of a lipid domain enhances the effect, although the complex lipid present in the natural product appears not to be required for activity.
Conclusions and Future Directions
Through our studies of the last 20 years, we have systematically developed approaches for the chemical synthesis of mycobacterial cell wall glycans and then applied them to the preparation of a range of target molecules. The compounds we and others have made are increasingly being used in studies to probe the biosynthesis and biological function of the fascinating glycans produced by mycobacteria. Given the importance of tuberculosis as a global health threat, such studies will impact the development of novel drugs for treating mycobacterial disease, novel vaccines for preventing them, and new diagnostics. Current efforts in the group are focused on broadening our collection of extractable lipids structures, with a particular emphasis on the glycopeptidolipids and the lipooligosaccharides.76
Acknowledgments
I am grateful for the contributions of the talented graduate students and postdoctoral fellows who, over the past two decades, have carried out the work described in this Account. Some of their names are mentioned in the text above, but many many others made significant contributions as well. This research was supported in the United States by the National Science Foundation and the National Institutes of Health and in Canada by the Natural Sciences Engineering Research Council of Canada and the Alberta Glycomics Centre. I also thank the Canada Research Chair Program for a Tier I Chair. Finally, I thank Professor Patrick J. Brennan for an inspirational seminar 22 years ago that got me started on this research path.
Biography
Todd L. Lowary was born in Canton, Illinois, in 1966. He received a B.A. in chemistry from the University of Montana (1988) and a Ph.D. in organic chemistry from the University of Alberta (1993). Following postdoctoral appointments at the University of Alberta and the Carlsberg Research Laboratory, he started his academic career at The Ohio State University. Following tenure, he returned to the University of Alberta where he is currently the Raymond U. Lemieux Professor of Carbohydrate Chemistry. He also serves as the Scientific Director for the Canadian Glycomics Network (GlycoNet), a pan-Canadian research network focused on providing solutions to unmet medical needs through exploiting the biological role of carbohydrates. His research interests are in the area of synthetic carbohydrate chemistry and carbohydrate biochemistry, in particular as these fields relate to microbial glycans.
The authors declare no competing financial interest.
References
- Brennan P. J.ILC2.1 The Surface Structures of Mycobacteria: Their Roles in the Host Response, Their Biogenesis and Potential As Drug Targets, Presented at the 17th International Carbohydrate Symposium, Ottawa, ON Canada July17–22, 1994.
- Angala S. K.; Belardinelli J. M.; Huc-Claustre E.; Wheat W. H.; Jackson M. The Cell Envelope Glycoconjugates of Mycobacterium tuberculosis. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 361–399. 10.3109/10409238.2014.925420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B.; Williams S. J. Chemical Approaches for the Study of the Mycobacterial Glycolipids Phosphatidylinositol Mannosides, Lipomannan and Lipoarabinomannan. Nat. Prod. Rep. 2010, 27, 919–947. 10.1039/c000604a. [DOI] [PubMed] [Google Scholar]
- Umesiri F. E.; Sanki A. K.; Boucau J.; Ronning D. R.; Sucheck S. J. Recent Advances Toward the Inhibition of mAG and LAM Synthesis in Mycobacterium tuberculosis. Med. Res. Rev. 2010, 30, 290–326. 10.1002/med.20190. [DOI] [PubMed] [Google Scholar]
- Bai B.; Chu C.; Lowary T. Lipooligosaccharides from Mycobacteria: Structure, Function, and Synthesis. Isr. J. Chem. 2015, 55, 360–372. 10.1002/ijch.201400194. [DOI] [Google Scholar]
- Imamura A.; Lowary T. L. Chemical Synthesis of Furanose Glycosides. Trends Glycosci. Glycotechnol. 2011, 23, 134–152. 10.4052/tigg.23.134. [DOI] [Google Scholar]
- Fletcher H. G. Jr. The Anomeric Tri-O-Benzozyl-Arabinofuranosyl Bromides. Methods Carbohydr. Chem. 1963, 2, 228–230. [Google Scholar]
- Callam C. S.; Lowary T. L. Synthesis of Methyl 2,3,5-tri-O-Benzoyl-α-D-Arabinofuranoside in the Organic Laboratory. J. Chem. Educ. 2001, 78, 73–74. 10.1021/ed078p73. [DOI] [Google Scholar]
- Callam C. S.; Gadikota R. R.; Lowary T. L. Sensitivity of 1J(C1-H1) Magnitudes to Anomeric Stereochemistry in 2,3-anhydro-O-Furanosides. J. Org. Chem. 2001, 66, 4549–4558. 10.1021/jo001747a. [DOI] [PubMed] [Google Scholar]
- Yin H.; Lowary T. L. Synthesis of Arabinofuranosides via Low-Temperature Activation of Thioglycosides. Tetrahedron Lett. 2001, 42, 5829–5832. 10.1016/S0040-4039(01)01133-9. [DOI] [Google Scholar]
- Yin H.; D’Souz F. W.; Lowary T. L. Arabinofuranosides from Mycobacteria: Synthesis of a Highly Branched Hexasaccharide and Related Fragments Containing β-Arabinofuranosyl Residues. J. Org. Chem. 2002, 67, 892–903. 10.1021/jo010910e. [DOI] [PubMed] [Google Scholar]
- D’Souza F. W.; Cheshev P.; Ayers J. A.; Lowary T. L. Conversion of Pyranose Glycals to Furanose Derivatives: A New Route to Oligofuranosides. J. Org. Chem. 1998, 63, 9037–9044. 10.1021/jo9815406. [DOI] [Google Scholar]
- Ayers J. D.; Lowary T. L.; Morehouse C. B.; Besra G. S. Synthetic Arabinofuranosyl Oligosaccharides as Mycobacterial Arabinosyltransferase Substrates. Bioorg. Med. Chem. Lett. 1998, 8, 437–442. 10.1016/S0960-894X(98)00049-3. [DOI] [PubMed] [Google Scholar]
- Crich D. Methodology Development and Physical Organic Chemistry: A Powerful Combination for the Advancement of Glycochemistry. J. Org. Chem. 2011, 76, 9193–9209. 10.1021/jo2017026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. J.; Lee K.; Jung E. H.; Jeon H. B.; Kim K. S. Acceptor-Dependent Stereoselective Glycosylation: 2′-CB Glycoside-Mediated Direct β-D-Arabinofuranosylation and Efficient Synthesis of the Octaarabinofuranoside in Mycobacterial Cell Wall. Org. Lett. 2005, 7, 3263–3266. 10.1021/ol0510668. [DOI] [PubMed] [Google Scholar]
- Crich D.; Sun S. Formation of β-Mannopyranosides of Primary Alcohols Using the Sulfoxide Method. J. Org. Chem. 1996, 61, 4506–4507. 10.1021/jo9606517. [DOI] [PubMed] [Google Scholar]
- Crich D.; Sun S. Direct Synthesis of β-Mannopyranosides by the Sulfoxide Method. J. Org. Chem. 1997, 62, 1198–1199. 10.1021/jo962345z. [DOI] [Google Scholar]
- Crich D.; Sun S. Are Glycosyl Triflates Intermediates in the Sulfoxide Glycosylation Method? A Chemical and 1H, 13C, and 19F NMR Spectroscopic Investigation. J. Am. Chem. Soc. 1997, 119, 11217–11223. 10.1021/ja971239r. [DOI] [Google Scholar]
- Crich D. Mechanism of a Chemical Glycosylation Reaction. Acc. Chem. Res. 2010, 43, 1144–1153. 10.1021/ar100035r. [DOI] [PubMed] [Google Scholar]
- Gadikota R.; Callam C.; Lowary T. Stereocontrolled Synthesis of 2,3-Anhydro-β-D-Lyxofuranosyl Glycosides. Org. Lett. 2001, 3, 607–610. 10.1021/ol007008y. [DOI] [PubMed] [Google Scholar]
- Gadikota R.; Callam C.; Wagner T.; Del Fraino B.; Lowary T. 2,3-Anhydrosugars in Glycoside Bond Synthesis. Highly Stereoselective Syntheses of Oligosaccharides Containing α- and β-Arabinofuranosyl Linkages. J. Am. Chem. Soc. 2003, 125, 4155–4165. 10.1021/ja029302m. [DOI] [PubMed] [Google Scholar]
- Williams N. Oxirane Derivatives of Aldoses. Adv. Carbohydr. Chem. Biochem. 1970, 25, 109–179. 10.1016/S0065-2318(08)60427-8. [DOI] [Google Scholar]
- Callam C. S.; Gadikota R. R.; Krein D. M.; Lowary T. L. 2,3-Anhydrosugars in Glycoside Bond Synthesis. NMR and Computational Investigations into the Mechanism of Glycosylations with 2,3-Anhydrofuranosyl Glycosyl Sulfoxides. J. Am. Chem. Soc. 2003, 125, 13112–13119. 10.1021/ja0349610. [DOI] [PubMed] [Google Scholar]
- Bai Y.; Lowary T. L. 2,3-Anhydrosugars in Glycoside Bond Synthesis. Application to α-D-Galactofuranosides. J. Org. Chem. 2006, 71, 9658–9671. 10.1021/jo061713o. [DOI] [PubMed] [Google Scholar]
- Bai Y.; Lowary T. L. Synthesis of a Pentasaccharide Fragment of Varianose, a Cell Wall Polysaccharide from Penicillium varians. J. Org. Chem. 2006, 71, 9672–9680. 10.1021/jo061821a. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Kawatkar S.; Rao Y.; Boons G. Practical Approach for the Stereoselective Introduction of β-Arabinofuranosides. J. Am. Chem. Soc. 2006, 128, 11948–11957. 10.1021/ja0629817. [DOI] [PubMed] [Google Scholar]
- Ishiwata A.; Akao H.; Ito Y. Stereoselective Synthesis of a Fragment of Mycobacterial Arabinan. Org. Lett. 2006, 8, 5525–5528. 10.1021/ol062198j. [DOI] [PubMed] [Google Scholar]
- Larsen C. H.; Ridgway B. H.; Shaw J. T.; Woerpel K. A. A Stereoelectronic Model To Explain the Highly Stereoselective Reactions of Nucleophiles with Five-Membered-Ring Oxocarbenium Ions. J. Am. Chem. Soc. 1999, 121, 12208–12209. 10.1021/ja993349z. [DOI] [Google Scholar]
- Imamura A.; Lowary T. L. β-Selective Arabinofuranosylation Using a 2,3-O-Xylylene-Protected Donor. Org. Lett. 2010, 12, 3686–3689. 10.1021/ol101520q. [DOI] [PubMed] [Google Scholar]
- Sahloul K.; Lowary T. L. Development of an Orthogonal Protection Strategy for the Synthesis of Mycobacterial Arabinomannan Fragments. J. Org. Chem. 2015, 80, 11417–11434. 10.1021/acs.joc.5b02083. [DOI] [PubMed] [Google Scholar]
- Holemann A.; Stocker B. L.; Seeberger P. H. Synthesis of a Core Arabinomannan Oligosaccharide of Mycobacterium tuberculosis. J. Org. Chem. 2006, 71, 8071–8088. 10.1021/jo061233x. [DOI] [PubMed] [Google Scholar]
- Deng L. M.; Liu X.; Liang X. Y.; Yang J. S. Regioselective Glycosylation Method Using Partially Protected Arabino- and Galactofuranosyl Thioglycosides as Key Glycosylating Substrates and its Application to One-Pot Synthesis of Oligofuranoses. J. Org. Chem. 2012, 77, 3025–3037. 10.1021/jo300084g. [DOI] [PubMed] [Google Scholar]
- Kandasamy J.; Hurevich M.; Seeberger P. H. Automated Solid Phase Synthesis of Oligoarabinofuranosides. Chem. Commun. 2013, 49, 4453–4455. 10.1039/c3cc00042g. [DOI] [PubMed] [Google Scholar]
- Liu Q. W.; Bin H. C.; Yang J. S. β-arabinofuranosylation Using 5-O-(2-quinolinecarbonyl) Substituted Ethyl Thioglycoside Donors. Org. Lett. 2013, 15, 3974–3977. 10.1021/ol401755e. [DOI] [PubMed] [Google Scholar]
- Islam M.; Gayatri G.; Hotha S. Influence of Steric Crowding on Diastereoselective Arabinofuranosylations. J. Org. Chem. 2015, 80, 7937–7945. 10.1021/acs.joc.5b00964. [DOI] [PubMed] [Google Scholar]
- Podvalnyy N. M.; Abronina P. I.; Fedina K. G.; Kondakov N. N.; Zinin A. I.; Chizhov A. O.; Torgov V. I.; Kachala V. V.; Kononov L. O. Synthesis of Hexasaccharide Fragment of Lipoarabonomannan from Mycobacteria: Advantages of the Benzyl-Free Approach. Russ. Chem. Bull. 2015, 64, 1149–1162. 10.1007/s11172-015-0992-5. [DOI] [Google Scholar]
- Wang L.; Feng S.; An L.; Gu G.; Guo Z. Synthetic and Immunological Studies of Mycobacterial Lipoarabinomannan Oligosaccharides and Their Protein Conjugates. J. Org. Chem. 2015, 80, 10060–10075. 10.1021/acs.joc.5b01686. [DOI] [PubMed] [Google Scholar]
- Fraser-Reid B.; Lu J.; Jayaprakash K. N.; Lopez J. C. Synthesis of a 28-mer Oligosaccharide Core of Mycobacterial Lipoarabinomannan (LAM) Requires only Two n-Pentenyl Orthoester Progenitors. Tetrahedron: Asymmetry 2006, 17, 2449–2463. 10.1016/j.tetasy.2006.09.008. [DOI] [Google Scholar]
- Joe M.; Bai Y.; Nacario R. C.; Lowary T. L. Synthesis of the Docosanasaccharide Arabinan Domain of Mycobacterial Arabinogalactan and a Proposed Octadecasaccharide Biosynthetic Precursor. J. Am. Chem. Soc. 2007, 129, 9885–9901. 10.1021/ja072892+. [DOI] [PubMed] [Google Scholar]
- Ishiwata A.; Ito Y. Synthesis of Docosasaccharide Arabinan Motif of Mycobacterial Cell Wall. J. Am. Chem. Soc. 2011, 133, 2275–2291. 10.1021/ja109932t. [DOI] [PubMed] [Google Scholar]
- Rademacher C.; Shoemaker G.; Kim H.; Zheng R.; Taha H.; Liu C.; Nacario R.; Schriemer D.; Klassen J.; Peters T.; Lowary T. Ligand Specificity of CS-35, a Monoclonal Antibody that Recognizes Mycobacterial Lipoarabinomannan: A Model System for Oligofuranoside-Protein Recognition. J. Am. Chem. Soc. 2007, 129, 10489–10502. 10.1021/ja0723380. [DOI] [PubMed] [Google Scholar]
- Murase T.; Zheng R. B.; Joe M.; Bai Y.; Marcus S. L.; Lowary T. L.; Ng K. K. S. Structural Insights into Antibody Recognition of Mycobacterial Polysaccharides. J. Mol. Biol. 2009, 392, 381–392. 10.1016/j.jmb.2009.06.074. [DOI] [PubMed] [Google Scholar]
- Subramaniam V.; Lowary T. L. Synthesis of Oligosaccharide Fragments of Mannosylated Lipoarabinomannan from Mycobacterium tuberculosis. Tetrahedron 1999, 55, 5965–5976. 10.1016/S0040-4020(99)00260-4. [DOI] [Google Scholar]
- Joe M.; Sun D.; Taha H.; Completo G.; Croudace J.; Lammas D.; Besra G.; Lowary T. The 5-Deoxy-5-Methylthio-Xylofuranose Residue in Mycobacterial Lipoarabinomannan. Absolute Stereochemistry, Linkage Position, Conformation, and Immunomodulatory Activity. J. Am. Chem. Soc. 2006, 128, 5059–5072. 10.1021/ja057373q. [DOI] [PubMed] [Google Scholar]
- Patil P. S.; Cheng T.-J. R.; Zulueta M. M. L.; Yang S.-T.; Lico L. S.; Hung S.-C. Total Synthesis of Tetraacylated Phosphatidylinositol Hexamannoside and Evaluation of its Immunomodulatory Activity. Nat. Commun. 2015, 6, 7239. 10.1038/ncomms8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Stocker B. L.; Seeberger P. H. Total Synthesis of Phosphatidylinositol Mannosides of Mycobacterium tuberculosis. J. Am. Chem. Soc. 2006, 128, 3638–3648. 10.1021/ja0565368. [DOI] [PubMed] [Google Scholar]
- Watts A.; Williams S. J. Rapid, Iterative Assembly of Octyl α-1,6-Oligomannosides and their 6-Deoxy Equivalents. Org. Biomol. Chem. 2005, 3, 1982–1992. 10.1039/b503919c. [DOI] [PubMed] [Google Scholar]
- Kremer L.; Dover L. G.; Morehouse C.; Hitchin P.; Everett M.; Morris H. R.; Dell A.; Brennan P. J.; McNeil M. R.; Flaherty C.; Duncan K.; Besra G. S. Galactan Biosynthesis in Mycobacterium tuberculosis: Identification of a Bifunctional UDP-Galactofuranosyltransferase. J. Biol. Chem. 2001, 276, 26430–26440. 10.1074/jbc.M102022200. [DOI] [PubMed] [Google Scholar]
- Marino C.; Varela O.; de Lederkremer R. M. Synthesis of Galactofuranose Disaccharides of Biological Significance. Carbohydr. Res. 1989, 190, 65–76. 10.1016/0008-6215(89)84147-3. [DOI] [PubMed] [Google Scholar]
- du Mortier C.; Varela O.; de Lederkremer R. M. A New Approach to the Synthesis of Disaccharide Derivatives Having a Furanose as the Reducing Unit. Carbohydr. Res. 1989, 189, 79–86. 10.1016/0008-6215(89)84087-X. [DOI] [Google Scholar]
- Marino C.; Gallo-Rodriguez C.; de Lederkremer R. M.. Galactofuranosyl-containing Glycans: Occurrence, Synthesis and Biochemistry. In Glycans: Biochemistry, Characterization and Applications; 1st ed.; Mora-Montes H. M., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, 2012; Chapter 10, pp 207–268. [Google Scholar]
- Ferrieres V.; Bertho J.-N.; Plusquellec D. A Convenient Synthesis of Alkyl D-glycofuranosiduronic Acids and Alkyl D-Glycofuranosides from Unprotected Carbohydrates. Carbohydr. Res. 1998, 311, 25–35. 10.1016/S0008-6215(98)00197-9. [DOI] [Google Scholar]
- Green J. W.; Pacsu E. Glycofuranosides and Thioglycofuranosides. I. A Method of Preparation and its Application to Galactose and Glucose. J. Am. Chem. Soc. 1937, 59, 1205–1210. 10.1021/ja01286a015. [DOI] [Google Scholar]
- Szarek W. A.; Zamojski A.; Tiwari K. N.; Ison E. R. A New, Facile Method for Cleavage of Acetals and Dithioacetals in Carbohydrate Derivatives. Tetrahedron Lett. 1986, 27, 3827–3830. 10.1016/S0040-4039(00)83890-3. [DOI] [Google Scholar]
- Completo G.; Lowary T. Synthesis of Galactofuranose-Containing Acceptor Substrates for Mycobacterial Galactofuranosyltransferases. J. Org. Chem. 2008, 73, 4513–4525. 10.1021/jo800457j. [DOI] [PubMed] [Google Scholar]
- Gandolfi-Donadío L.; Gallo-Rodriguez C.; De Lederkremer R. Synthesis of a Tetrasaccharide Fragment of Mycobacterial Arabinogalactan. Carbohydr. Res. 2008, 343, 1870–1875. 10.1016/j.carres.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Peltier P.; Euzen R.; Daniellou R.; Nugier-Chauvin C.; Ferrieres V. Recent Knowledge and Innovations Related to Hexofuranosides: Structure, Synthesis and Applications. Carbohydr. Res. 2008, 343, 1897–1923. 10.1016/j.carres.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Belánová M.; Dianiskova P.; Brennan P.; Completo G.; Rose N.; Lowary T.; Mikušová K. Galactosyl Transferases in Mycobacterial Cell Wall Synthesis. J. Bacteriol. 2008, 190, 1141–1145. 10.1128/JB.01326-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N. L.; Completo G. C.; Lin S. J.; McNeil M.; Palcic M. M.; Lowary T. L. Expression, Purification, and Characterization of a Galactofuranosyltransferase Involved in Mycobacterium tuberculosis Arabinogalactan Biosynthesis. J. Am. Chem. Soc. 2006, 128, 6721–6729. 10.1021/ja058254d. [DOI] [PubMed] [Google Scholar]
- Szczepina M. G.; Zheng R. B.; Completo G. C.; Lowary T. L.; Pinto B. M. STD-NMR Studies Suggest that Two Acceptor Substrates for GlfT2, a Bifunctional Galactofuranosyltransferase Required for the Biosynthesis of Mycobacterium tuberculosis Arabinogalactan, Compete for the Same Binding Site. ChemBioChem 2009, 10, 2052–2059. 10.1002/cbic.200900202. [DOI] [PubMed] [Google Scholar]
- Szczepina M.; Zheng R. B.; Completo G.; Lowary T. L.; Pinto B. M. STD-NMR Studies of Two Acceptor Substrates of GlfT2, a Galactofuranosyltransferase from Mycobacterium tuberculosis: Epitope Mapping Studies. Bioorg. Med. Chem. 2010, 18, 5123–5128. 10.1016/j.bmc.2010.05.069. [DOI] [PubMed] [Google Scholar]
- Peltier P.; Beláňová M.; Dianišková P.; Zhou R.; Zheng R. B.; Pearcey J. A.; Joe M.; Brennan P. J.; Nugier-Chauvin C.; Ferrières V.; Lowary T. L.; Daniellou R.; Mikušová K. Synthetic UDP-Furanoses as Potent Inhibitors of Mycobacterial Galactan Biogenesis. Chem. Biol. 2010, 17, 1356–1366. 10.1016/j.chembiol.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin M. B.; Zhou R.; Lowary T. L. Synthetic UDP-Galactofuranose Analogs Reveal Critical Enzyme–Substrate Interactions in GlfT2-Catalyzed Mycobacterial Galactan Assembly. Org. Biomol. Chem. 2012, 10, 4074–4087. 10.1039/c2ob25159k. [DOI] [PubMed] [Google Scholar]
- May J. F.; Splain R. A.; Brotschi C.; Kiessling L. L. A Tethering Mechanism for Length Control in a Processive Carbohydrate Polymerization. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 11851–11856. 10.1073/pnas.0901407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levengood M. R.; Splain R. A.; Kiessling L. L. Monitoring Processivity and Length Control of a Carbohydrate Polymerase. J. Am. Chem. Soc. 2011, 133, 12758–12766. 10.1021/ja204448t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J. F.; Levengood M. R.; Splain R. A.; Brown C. D.; Kiessling L. L. A Processive Carbohydrate Polymerase That Mediates Bifunctional Catalysis Using a Single Active Site. Biochemistry 2012, 51, 1148–1159. 10.1021/bi201820p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley R. W.; Zheng R. B.; Richards M. R.; Lowary T. L.; Ng K. K. S. Tetrameric Structure of the GlfT2 Galactofuranosyltransferase Reveals a Scaffold for the Assembly of Mycobacterial Arabinogalactan. J. Biol. Chem. 2012, 287, 28132–28143. 10.1074/jbc.M112.347484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall G. O.; Chatterjee D.; Brennan P. J. The Variable Surface Glycolipids of Mycobacteria: Structures, Synthesis of Epitopes, and Biological Properties. Adv. Carbohydr. Chem. Biochem. 1995, 51, 169–242. 10.1016/S0065-2318(08)60194-8. [DOI] [PubMed] [Google Scholar]
- Blaauw G. J.; Appelmelk B. J.: Mycobacterial Glycolipids and the Host: Role of Phenolic Glycolipids and Lipoarabinomannan. In Protein–Carbohdrate Interactions in Infectious Diseases; Bewley C., Ed.; RSC Publishing: Cambridge, 2006; pp 6–29. [Google Scholar]
- Schorey J.; Sweet L. The Mycobacterial Glycopeptidolipids: Structure, Function, and their Role in Pathogenesis. Glycobiology 2008, 18, 832–841. 10.1093/glycob/cwn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso S.; Castelli R.; Baggelaar M. P.; Geerdink D.; Ter Horst B.; Casas-Arce E.; Overkleeft H. S.; van der Marel G. A.; Codee J. D. C.; Minnaard A. J. Total Synthesis of the Triglycosyl Phenolic Glycolipid PGL-tb1 from Mycobacterium tuberculosis. Angew. Chem., Int. Ed. 2012, 51, 11774–11777. 10.1002/anie.201206221. [DOI] [PubMed] [Google Scholar]
- Elsaidi H. R. H.; Barreda D. R.; Cairo C. W.; Lowary T. L. Mycobacterial Phenolic Glycolipids with a Simplified Lipid Aglycone Modulate Cytokine Levels Through Toll-Like Receptor 2. ChemBioChem 2013, 14, 2153–2159. 10.1002/cbic.201300505. [DOI] [PubMed] [Google Scholar]
- Elsaidi H. R. H.; Lowary T. L. Inhibition of Cytokine Release by Mycobacterium tuberculosis Phenolic Glycolipid Analogues. ChemBioChem 2014, 15, 1176–1182. 10.1002/cbic.201402001. [DOI] [PubMed] [Google Scholar]
- Elsaidi H. R. H.; Lowary T. L. Effect of Phenolic Glycolipids from Mycobacterium kansasii on Proinflammatory Cytokine Release. A Structure–Activity Relationship Study. Chemical Science 2015, 6, 3161–3172. 10.1039/C4SC04004J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki Y.; Ito Y.; Nakahara Y.; Ogawa T. Synthesis of Branched Poly-N-acetyl-Lactosamine Type Pentaantennary Pentacosasaccharide: Glycan part of a Glycosyl Ceramide from Rabbit Erythrocyte Membrane. Tetrahedron Lett. 1993, 34, 1061–1064. 10.1016/S0040-4039(00)77492-2. [DOI] [Google Scholar]
- Wang L.; Dong M.; Lowary T. L. Synthesis of Unusual N-Acylated Aminosugar Fragments of Mycobacterium marinum Lipooligosaccharide IV. J. Org. Chem. 2015, 80, 2767–2780. 10.1021/acs.joc.5b00064. [DOI] [PubMed] [Google Scholar]