Abstract
Helminth infections are ubiquitous worldwide and can trigger potent immune responses that differ from and potentially antagonize host protective responses to microbial pathogens. In this Review we focus on the three main killers in infectious disease—AIDS, tuberculosis and malaria—and critically assesses whether helminths adversely influence host control of these diseases. We also discuss emerging concepts for how M2 macrophages and helminth-modulated dendritic cells can potentially influence the protective immune response to concurrent infections. Finally, we present evidence advocating for more efforts to determine how and to what extent helminths interfere with the successful control of specific concurrent coinfections.
Parasitic worms, or helminths, have probably coevolved with their vertebrate hosts for hundreds of millions of years. There is also abundant archaeological evidence that helminths have chronically infected humans, including the 5,000-year-old Neolithic ‘Tyrolean Iceman’, who was infected with the whipworm Trichuris trichiura1. Ample evidence now indicates that helminths compromise fitness in wild vertebrate populations2,3. In humans, although helminth infections are rarely lethal, they can contribute to morbidity in adults and impair physical and cognitive development in children4,5. Generally, although the number of people infected by helminths in certain populations may be high, the frequency of people with high worm counts is usually low6. That binomial distribution may be an important factor in considering the effects of helminths on the immune response to coinfecting microbes.
Helminths are handled very differently by the host immune system than are microorganisms, such as fungi, protozoa, bacteria and viruses. Helminths include many very different multicellular worms that can reside mainly in tissues, such as filaria or schistosomes, or (alternatively) in the intestinal lumen, such as hookworms or Ascaris species. Generally, in both humans and mice, the characteristic helminth-induced type 2 immune response includes marked elevations in interleukin 4 (IL-4), IL-5 and IL-13 (ref. 7). That response includes activation of cells of the innate and adaptive immune systems that together can mediate a potent host protective response to helminth parasites. Those cells contribute to parasite resistance and also promote host tolerance to (i.e., coexistence with) helminths; this can include both anti-inflammatory and wound-healing properties that are probably important when these large multicellular eukaryotes migrate through vital host tissues8,9. In this context, helminths also stimulate potent regulatory cell populations of the innate and adaptive immune systems, which can function through mechanisms distinct from the effects mediated by cytokines of the TH2 subset of helper T cells (type 2 helper T cells). In contrast, pathogenic microorganisms typically trigger a type 1 immune response, which instead results in elevations in IL-12, IL-23, interferon-γ (IFN-γ) and IL-17 (ref. 10). This potent response develops rapidly, which is critical for the control of potentially lethal pathogens that can rapidly divide and disseminate throughout the host; however, a cost of this rapid response can be tissue-damaging inflammation.
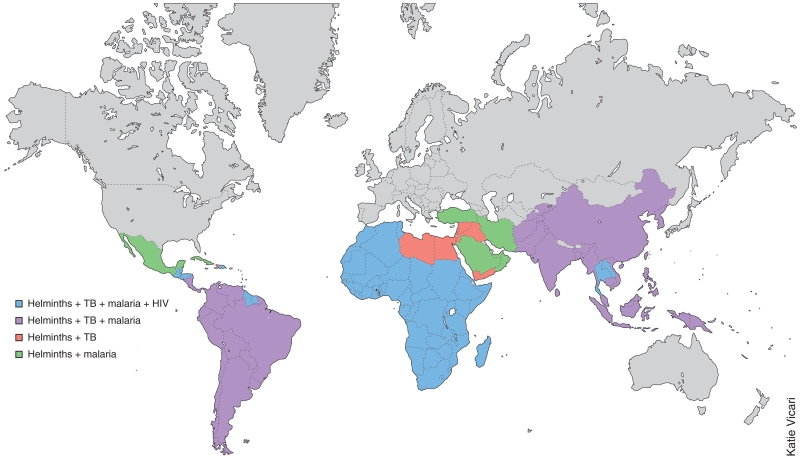
Both the TH2 cytokines and the immunoregulatory pathways activated by helminth infection have the potential to downregulate the effector functions that mediate resistance during type 1 immune responses. Major global pathogens, including Mycobacterium tuberculosis, human immunodeficiency virus (HIV) and the Plasmodium species that cause malaria, can overlap in geographic distribution with helminth infection (Fig. 1). This map (Fig. 1) emphasizes the point that in sub-Saharan Africa there is extensive overlap of the presence of helminths, infection with HIV, Plasmodium species that cause malaria, and M. tuberculosis. In fact, each of those pathogens is responsible for substantial disease burdens in sub-Saharan Africa3. Widespread concurrent infections with helminths and other pathogens may pose a clinical and immunological quandary, as coinfected hosts must manage two classes of pathogens that generate disparate and potentially conflicting effector-cell responses for their control and resolution. It is thus plausible that in many areas of the world, host immune responses to these prominent disease-causing microbial pathogens is modulated by coinfection with helminths, and in this context, helminth infection may also potentially impair vaccine efficacy11,12.
Figure 1.
World map showing the geographic distribution of coinfection with helminths together with tuberculosis, malaria and/or HIV infection of adults. This simplified map (constructed for illustrative purposes) includes only data obtained from the following sources: for helminths, lymphatic filariasis, onchocerciasis, schistosomiasis or soil-transmitted helminthiasis123; for tuberculosis (TB), incidence rates124; for malaria, the countries and territories affected125; and for HIV, the reported prevalence in adults126. Countries with fewer than 25–49 new cases of tuberculosis per 100,000 people and with a prevalence of HIV infection below 0.5% have not been included here. Because of that cutoff for HIV, many parts of the world (such as India and South America) are not presented here as regions with coinfection with helminths and HIV, although there is a substantial HIV burden in those countries.
The chronicity of helminth infections from childhood through adult life may affect overall immunological function. Studies have suggested that helminth infection may influence homeostasis of the immune system, activating immunoregulatory cell populations and TH2 effector cells that together control the development of harmful autoimmune and inflammatory disorders. Consistent with that model, populations in regions without endemic helminth infection show an increased prevalence of inflammatory diseases and in particular autoimmunity13. Studies of experimental models have further demonstrated the efficacy of helminth infection or the administration of helminth products in mitigating disease severity14–16, which has provided the basis for many ongoing clinical trials. Thus, although eradication of helminths may enhance resistance to certain microbial pathogens, one cost may be altered homeostasis of the immune system and increased susceptibility to inflammatory diseases. Also, antihelminth treatments can potentially influence the course of microbial infection by affecting host tolerance as well as resistance.
In this Review, we examine evidence of a role for helminth infection in modulating the immune response to major microbial pathogens that affect global health, including M. tuberculosis, HIV and Plasmodium species that cause malaria. We then discuss how, in the context of coinfection, individual parameters of the type 2 immune response may potentially regulate type 1 immunity, affecting both host resistance and tolerance. Such an analysis may provide a functional framework for more in-depth studies of the effects of the helminth-induced type 2 immune response to coinfection by microrganisms.
Tuberculosis and concurrent helminth infection
Tuberculosis and helminth infections are coendemic in many parts of the world, which raises the possibility of modulating tuberculosis through host responses to helminths. Furthermore, the larvae of many soil-transmitted helminths migrate through the lungs. Hence, the localized helminth-induced pulmonary response may directly influence the outcome of a concurrent infection with M. tuberculosis. Volunteers experimentally infected with the hookworm Necator americanus have been shown to generate not only a strong local TH2 response and regulatory T cell (Treg cell) response (characterized by IL-10 and transforming growth factor-β (TGF-β)) but also a similar systemic response17, which indicates that helminth-induced intestinal responses may also affect the outcome of concurrent infections at distant sites, including M. tuberculosis–specific lung responses. Indeed, findings from many clinical studies have provided evidence that helminths modulate host susceptibility to tuberculosis. In a case-control study in Ethiopia, a significant association was found between tuberculosis and intestinal helminth infection. Notably, the association increased with the number of concurrently infecting helminth species18. Another study also found a higher frequency of helminth infection in patients with pulmonary tuberculosis than in a control group matched by age, sex and neighborhood19. A lower IFN-γ response and greater IL-10 response has also been observed in mycobacteria-stimulated whole-blood cultures from helminth-coinfected patients with tuberculosis than in those from patients with tuberculosis20. A similar reduction in the production of antigen-specific IFN-γ has also been seen in peripheral blood mononuclear cells from helminth-infected children with responsiveness to bacillus Calmette-Guerin (BCG). However, depletion of CD4+CD25+ T cells from those in vitro cultures resulted in a significantly enhanced IFN-γ response, which suggests that Treg cells might be responsible for antagonizing the production of IFN-γ21. Patients with tuberculosis who are coinfected with helminths also present with more advanced disease20. Whether helminth-induced increases in bacterial burden or direct damage to the lungs by helminths cause(s) the exacerbated immunopathology remains to be determined. In asymptomatic pediatric household contacts of patients with tuberculosis who had sputum smear positive for acid-fast bacilli, a positive tuberculin skin test was significantly associated with concurrent helminth infection22. This indicates that a concurrent helminth infection in people exposed to M. tuberculosis can increase their risk of becoming latently infected with M. tuberculosis. Consistent with that finding, people with latent infection with M. tuberculosis from areas hyperendemic for onchocerciasis, the helminth responsible for ‘river blindness’, had a delayed tuberculin skin test response23,24 and lower T cell proliferation and IFN-γ responses to the M. tuberculosis antigen PPD25. The expression and function of Toll-like receptors 2 and 9 (ref. 26) and TH1 and TH17 responses27 of people with latent tuberculosis and coincident filarial infection were also significantly lower than those of people with latent tuberculosis without filarial infection. The marked improvement in the Mycobacteria-specific immune responses of peripheral blood mononuclear cells from latently infected people after deworming26,28 suggests that eliminating helminth infection in coendemic areas may reduce the risk of progression to active tuberculosis.
Experimental models of coinfection have also been used to delineate the mechanisms of immunomodulation. Mice with an ongoing type 2 response induced by a filarial parasite29 or Schistosoma mansoni30 also develop a type 2 response to mycobacterial infection. Mice coinfected with S. mansoni and M. tuberculosis31 or with S. mansoni and BCG32 had a higher bacterial burden in their lungs than did mice without S. mansoni coinfection. Similarly, mice coinfected with Nippostrongylus brasiliensis, an intestinal helminth with a lung stage, had impaired resistance to M. tuberculosis infection33. The mechanistic basis for the enhanced-susceptibility to M. tuberculosis of those coinfected mice was mainly IL-4 receptor–mediated alternative activation of macrophages. In contrast, another study has reported that acute infection with N. brasiliensis actually boosts early macrophage-mediated control of infection with BCG34. However, it must be noted that BCG does not express certain genes35 encoding molecules that control the virulence and intracellular survival of mycobacteria36; therefore, it is not clear that such a boosting effect would occur during infection with M. tuberculosis. Several other contrasting studies have also been published showing a lack of modulation of mycobacteria-specific responses during concurrent helminth infection37–39. In this context, a study of the cotton-rat model of coinfection has also demonstrated a lack of exacerbated of M. tuberculosis infection during chronic filarial infection40. Also, a longitudinal study of a bovine model of onchocerciasis has demonstrated that although the response in the cattle during TH2 polarization was dominated by IL-4 expression, there were periods of strong and sustained IFN-γ expression41, which indicates that some of the divergent findings could be due to differences in the timing of the coinfection in the host.
The effect of helminths on efficacy of a vaccine against BCG in humans has also been investigated. Deworming of people before vaccination against BCG enhanced the IFN-γ response, whereas the TGF-β response of that group was lower than that of the control group that did not receive deworming treatment42; this suggests that the poor immunogenicity of BCG in the helminth-infected population could have been due to increased TGF-β production. The finding that downmodulation of the immune response affects vaccine efficacy was confirmed in a mouse model in which it was shown that the partial protection against M. tuberculosis infection mediated by BCG was significantly abrogated in helminth-coinfected mice43. Maternal helminth infection during pregnancy can sensitize neonates in utero to parasitic antigens44. Indeed, infants born to mothers who had filarial or schistosomal infections during pregnancy generate a significantly lower IFN-γ response to the vaccine against BCG than do infants born to uninfected mothers45. The mechanism behind such altered BCG immunity is probably in utero sensitization by parasitic antigens that have crossed the placenta46. Those parasite antigens induce a helminth-specific TH2 response that lasts several weeks after birth and has the potential to subsequently skew the immune response to the vaccine against BCG46. How the parasite antigens cross the placental barrier remains unclear, but such findings suggest that treating mothers during pregnancy may improve the efficacy of the vaccine against BCG in their newborns44. However, in a large randomized, double-blind, placebo-controlled study in Uganda47, the response to the vaccine against BCG in infants was not affected by treatment of the mothers with antihelminthic drugs47. Such opposing results may have been due to differences in the types of helminths and their numbers present in the pregnant mothers, which could have led to differences in the transmission of parasite antigens and subsequent modulation of the infant’s immune response. Thus, it is still an open question whether helminth infections in utero or after birth alter the immune response to the vaccine against BCG and other subunit vaccines against tuberculosis.
Effect of helminth infection on malaria
Malaria is highly endemic in sub-Saharan Africa, in certain parts of Asia and in South America, where there is also a high prevalence of schistosomiasis, filariasis and gastrointestinal infection with nematodes. Thus, during the past decade, the effect of helminth coinfection on infection with Plasmodium species that cause malaria and the pathogenesis of malaria has been investigated in both human populations and experimental animal models. In studies in Senegal and Ethiopia, a high prevalence of coinfection with helminths (including Ascaris lumbricoides and T. trichiura) was associated with the severity of malaria attacks, higher densities of malaria parasites and lower hemoglobin concentrations48,49. However, in separate studies in Malian and Thai subjects, helminth infections were associated with protection from acute malaria caused by infection with Plasmodium falciparum50, decreased severity of renal pathology and jaundice51 and diminished severity of cerebral malaria52. A prospective study in Mali of the effects of filarial coinfection on the clinical course of malaria found that pre-existent filarial infection conferred protection against anemia without necessarily affecting the severity of the malarial infection itself53. Overall, these epidemiological studies suggest that helminth infection in humans may alter the development of malaria not only by increasing the replication of Plasmodium parasites but also by modulating the severity of the pathological sequelae associated with malaria. Helminth coinfection has been shown to alter the cytokine response to malarial antigens and extracts: helminth-coinfected children exhibited an overall higher IL-10 response to an extract of infected erythrocytes or to recombinant malarial antigens, without a concomitant decrease in IFN-γ production54,55. That response might correspond to enhanced activation of CD4+ T cells that produce both IFN-γ and IL-10. A separate study of Malian subjects coinfected with filarial parasites and P. falciparum demonstrated that malarial antigen–induced IL-12, IFN-γ and chemokine CXCL10 responses were diminished in patients with patent filarial infection56. That diminished TH1 response was associated with lower expression of the transcription factor IRF1 and was dependent on concomitant IL-10 production56,57. In Senegal, higher circulating concentrations of IL-10 were also found in the plasma of Schistosoma haematobium–coinfected patients with malaria, and this correlated with decreased concentrations of protective cytophilic immunoglobulin G1 (IgG1) and IgG3 antibodies to the malarial antigens MSP1, MSP2 and GLURP58. To explore the possibility that helminth coinfection may be an important factor in delaying the acquisition of clinical immunity to malaria, an intervention study was done in Madagascar involving the treatment of children with an antihelminth agent (levamisole)59. A 2.5-fold decrease in malaria attacks was observed after treatment with levamisole, with the effect manifesting only after an 18- to 24-month lag period. Such a delay in the effect suggests that the anti-helminthic might have promoted class switching in B lymphocytes toward the production of cytophilic IgG1 and IgG3. That hypothesis has gained support from a prospective study of over 200 Senegalese children over a 51-month period. Carriage of helminths (hookworms in particular) was strongly associated with increased malarial attacks and significantly decreased IgG1 and IgG3 responses to the protective MSP3 antigen with an increase in noncytophilic IgG4 antibody responses60. Thus, by altering the TH2-TH1 balance, helminth infection may be a substantial contributing factor to the delayed acquisition of clinical immunity to malaria.
Studies of the mouse model of infection with the intestinal helminth Heligmosomoides polygyrus bakeri and Plasmodium chabaudi has enabled careful analysis of how chronic helminthiasis affects the course of infection with the Plasmodium species that cause malaria and the pathogenesis of malaria. In this model, prior infection with the intestinal nematode results in greater malaria parasitemia and a resultant higher incidence of mortality due to hemolysis-induced anemia61. That impaired resistance is associated with decreased IFN-γ, whereas the production of TGF-β and IL-10 in mice with prior infection is greater than that of mice infected only with P. chabaudi. Nevertheless, in mice (which do not die of malarial hyperparasitemia), coinfection with H. polygyrus protects them from cachexia, hypothermia and hypoglycemia, which are some of the pathogenic sequelae associated with acute malaria62. Despite the importance of IL-4 and IL-13 and the downstream transcription factor STAT6 in the development of a type 2 response, most of the effects of helminth coinfection on both parasitemia and pathogenesis is preserved in mice that lack STAT6. This indicates a minor role for helminth-induced type 2 polarization itself and instead suggests the prominence of independent regulatory mechanisms mediated by IL-10 and TGF-β, which do not signal through STAT6.
Helminth-induced modulation of HIV infection
Whereas helminths have infected humans throughout their evolution, HIV has only recently become an important infectious agent in humans, with little time for the evolution of specific host resistance mechanisms. In contrast, the long-term helminth-vertebrate coevolutionary dynamic may have resulted in relatively benign coexistence in much of the infected population. HIV has a widespread geographic distribution throughout sub-Saharan Africa, overlapping areas in which there is also a high frequency of both soil-transmitted helminths and water-borne schistosomes3,63. The partially protective host immune response to HIV includes several cell populations of the immune system associated with type 1 immunity, including CD8+ T cells, CD4+ TH1 cells, natural killer cells and antibody-producing B cells, which together can contribute to a stable viral ‘set point’ that can endure for years. When HIV neutralizes those and other protective functions of effector cells of the immune system, an increased viral load and immunodeficiency can result, followed by progression to AIDS and susceptibility to other pathogens.
The helminth-induced immune response may have several components that compromise the immune responses needed to keep the HIV infection in check, including the production of TH2 cytokines, activation of Treg cells and impaired function of antigen-presenting cells. However, those same mechanisms, through dampening the activation of cells of the innate and adaptive immune systems, may potentially impair the early stages of HIV infection63. For example, macrophages treated with IL-4 and IL-13 inhibit the entry of macrophage-tropic virus by downregulating expression of the CCR5, CXCR4 and CD4 receptors for viral entry64. Furthermore, TH2 cytokines also inhibit the replication of HIV in primary blood-derived monocytes65,66 and bronchoalveolar macrophages67 and could block the completion of reverse transcription68. Other studies have reported that IL-13 negatively regulates the replication of HIV69, whereas IL-4 enhances infection with HIV69,70. Thus, although the viral load of HIV may in some cases ultimately be increased after helminth infection, those potential mechanisms that decrease infectivity may offset the advantages of antihelminth treatments. The actual physical site of HIV infection may affect the effects of infection with metazoan parasites. Although HIV infects the host systemically, transmission occurs in mucosal tissues, mainly through the genital tract. Lesions in that region may increase the accessibility of local mucosal cells of the immune system to HIV. For example, the trematode parasite Schistosoma hematobium causes urogenital schistosomiasis. Those parasites trigger the formation of granulomas, which includes activated cells of the immune system. The granulomas then migrate through the tissue, causing lesions as they leave the body. Both the lesions and the associated activated cells of the immune system may create favorable entry points for HIV71,72. A study in Tanzania has shown a threefold greater prevalence of HIV in women with urogenital schistosomiasis than in women without urogenital schistosomiasis73, which provides some of the strongest evidence yet linking helminths to enhanced susceptibility to infection with HIV. It should also be noted that modulation of the immune response by intestinal helminths may also ‘preferentially’ affect HIV, as gut-associated lymphoid tissue is an important site of HIV replication and death of CD4+ T cells71,74. Helminth coinfection also increases the risk of mother-to-child transmission of HIV. HIV+ pregnant mothers with chronic intravascular helminth coinfection were found to have sevenfold greater odds of transmitting HIV to their offspring. That increased risk was attributed to the in utero priming of T cells by soluble parasite antigens75. The finding that there is enhanced activation of proviral gene-transcription pathways in cord-blood T cells from infants primed in utero is consistent with enhancement of the susceptibility of T cells to HIV infection by in utero activation of the immune system76.
Several other clinical studies have suggested that immune responses to and/or control of viral load after infection with HIV are (is) compromised by helminth infection77–80, whereas others have indicated little effect80,81, and still others have raised the possibility of adverse effects after antihelminth treatment82,83. As noted in reviews63,84, although overall there are indications of beneficial effects of antihelminth therapy on viral load and immunological parameters, a major caveat with many studies so far has been the small sample size, lack of sufficient power and overall paucity of extensive longitudinal studies. One potential confounding factor in such coinfection studies is the actual diagnosis of active helminth infection, as the most common approach of simply detecting eggs in stool can be misleading85,86. That was addressed in a coinfection study in South Africa, in which the helminth-infection phenotype was classified into four distinct subtypes on the basis of high and low counts of eggs in stool and high and low concentrations of helminth antigen–specific IgE. It was hypothesized that this more granular approach may help in distinguishing helminth-infected people with high-intensity type 2 responses from those with low-intensity type 2 responses. Intriguingly, impaired immune responses to HIV were ‘preferentially’ associated with high fecal egg counts and high IgE concentrations87. Such findings suggest that the actual magnitude and quality of the host immune response to helminth infection are important parameters that should necessarily be included in future coinfection studies; indeed, the magnitude of the type 2 immune response may be a better indicator of the effects on coinfecting pathogens than is infection status.
A confounding factor in many randomized clinical trials is that they examine only whether a single intervention is effective. They often do not consider partial effects or the possibility that multiple different simultaneous interventions may be needed to produce highly significant results. For HIV in sub-Saharan Africa, conventional dogma has focused on sexual behavior as a cause of a greater incidence of infection with HIV. Increasing evidence now suggests that may be erroneous and that instead people in sub-Saharan Africa are more susceptible to infection with HIV for environmental reasons that impair an effective immune response88. Such factors that contribute to impaired immunity to HIV may include nutritional deficiencies88–90, such as iron-deficiency anemia or protein-energy malnutrition, either of which may result not only from diet but also from coinfection with helminths. Thus, malnutrition, as well as immunoregulatory networks elicited by helminth infection, may lead to immunosuppression and diminished ability of the host to control infection with HIV and progression to AIDS.
Together, several studies have now provided strongly suggestive evidence that helminth coinfection influences the immune response to M. tuberculosis, Plasmodium species that cause malaria and HIV and may in some cases affect the course of disease. In particular, it is increasingly clear that urogenital schistosomiasis can increase susceptibility to HIV infection. However, the results may be more complex for other helminth-microbe coinfection combinations, with immunoregulatory effects reducing infectivity in some cases, promoting immunosuppression and increased susceptibility in others, and affecting host tolerance in still others. More extensive clinical research and mechanistic studies are needed to develop robust models of how such widespread helminth infections affect the course of globally important infectious diseases.
Potential mechanisms of helminth-induced modulation
The characteristic type 2 immune response is very similar after infection with different helminth species7. Furthermore, the effects of this response on immunity to different microbial pathogens share common features, in part because microbial pathogens generally trigger a characteristic type 1 immune response. Thus, rather than discussing helminth-induced immunological mechanisms that influence responses to specific microbial pathogens, in this section we instead more broadly examine how helminth-induced immune responses influence specific components of type 1 immunity and use specific pathogens as examples.
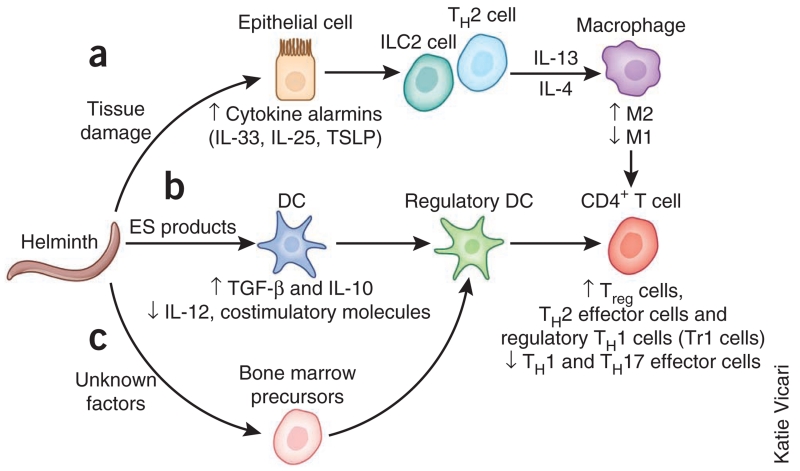
Helminth infection triggers a complex immune response that includes activation of TH2 cytokine–producing cell populations of the innate and adaptive immune systems in response to alarmin cytokines (Fig. 2). We will first discuss how in the first compartment, the TH2 cytokines modulate the antimicrobial functions of macrophages. In addition to that effect, helminth excretory-secretory products can directly influence antigen presentation, suppressing the differentiation of TH1 or TH17 cells and supporting the development of TH2 cells and Treg cells. Together those stimuli trigger a potent highly polarized type 2 response and also a regulatory cell compartment of the immune system that at least in some cases can develop independently of TH2 cytokines. That second compartment includes macrophages and Treg cells that produce IL-10 and TGF-β, which may dampen the immune response to microbial pathogens. In this section, we will consider how these two compartments of the helminth-induced immune response may potentially influence the development of an effective type 1 immune response to microorganisms, with a particular emphasis on innate regulatory cell populations.
Figure 2.
Mechanisms of helminth-induced inhibition of effector CD4+ T cells and macrophages required for protective immunity to microbial pathogens. Specific stimuli characteristic of helminth invasion trigger different aspects of the overall type 2 immune response. (a) Induction of tissue injury by helminths induces the release of cytokine alarmins (IL-33, IL-25 and TSLP), which promote the production of TH2 cytokines by ILC2 cells and TH2 cells and also by eosinophils and basophils. Exposure of macrophages to IL-4 or IL-13 suppresses classical (M1) activation of macrophages and diverts their differentiation toward the M2 phenotype. (b) Excretory-secretory (ES) molecules produced and shed by worms can shut down DC synthesis of proinflammatory cytokines, chemokines and costimulatory molecules and also promote DC production of the immunoregulatory molecules IL-10 and TGF-β. (c) Helminth infection has also been shown to induce the in vivo differentiation of a CD103−CD11Clo population of regulatory DCs, which are inefficient in priming effector T cells and instead favor the generation of Treg cells. Together all these mechanisms act in synergy to produce a helminth-modulated immunoregulatory environment that compromises TH1 and TH17 responses and favors Treg cell activities.
Helminth-induced M2 macrophages and microbial replication
The cytokines made by polarized TH1 and TH2 cells induce functionally distinct subsets of macrophages: classically activated (M1) macrophages and alternatively activated (M2) macrophages, respectively91. M1 macrophages secrete proinflammatory cytokines, have high expression of reactive oxygen and nitrogen intermediates and are key effectors against intracellular pathogens. In contrast, M2 macrophages secrete the anti-inflammatory cytokines IL-10 and TGF-β, have high expression of arginase-1 (ref. 92), directly promote wound healing9 and mediate resistance against helminths7. Studies reporting that mice with arginase-deficient macrophages are better able to control M. tuberculosis93 and that macrophages treated in vitro with IL-4 have increased M. tuberculosis replication94 were early indicators that M2 macrophages generated during helminth infection may potentially reduce macrophage effector activity against microbial pathogens. Indeed, a study has identified the IL-4 receptor–induced M2 macrophage as a potential niche for augmented growth of M. tuberculosis in helminth-coinfected hosts95. M2 macrophages96 and alternatively activated dendritic cells (DCs)97 are also present during coinfection of humans, but whether they affect the growth of M. tuberculosis has yet to be determined.
The signaling pathways, transcription factors, epigenetic modifications and microRNAs that regulate the polarization of M1 and M2 macrophages are being rapidly identified. Furthermore, accumulating evidence also indicates that macrophages have a continuum of activation profiles between the two polarized forms, which suggests extensive plasticity in the developmental program of M1 and M2 macrophages98. Thus, it remains to be determined whether in coinfected hosts macrophages newly recruited to the site of infection acquire an M2 phenotype or whether M1 macrophages harboring microbial pathogens are reprogrammed to become M2 cells under the influence of IL-4 and IL-13. Another unexplored issue is the source of IL-4 and IL-13 that drives the polarization of macrophages to the M2 phenotype, particularly during the early stages of coinfection. The discovery of type 2 innate lymphoid cells (ILC2 cells), which develop under the influence of IL-25 or IL-33 and, like TH2 cells, produce IL-5 and IL-13, raises the possibility that ILC2 cells, as well as TH2 cells, could be a potential source of the TH2 cytokines that drive M2 polarization. In a surgical-implant model with Brugia malayi, tissue injury alone was found to be sufficient to transiently induce M2 macrophages in a manner dependent on signaling through the IL-4 receptor α-chain but independent of an adaptive TH2 response99. Although the source of the innate IL-4 or IL-13 was not identified, one could now speculate that they were probably derived from ILC2 cells. Thus, during concurrent infections, it is possible that ILC2 cells activated subsequent to helminth-induced tissue damage may promote the development of M2 macrophages. Further work is needed to delineate the in vivo dynamics of the development of M1 and M2 macrophages during the progression of coinfection and to determine how their cross-regulation affects the effector functions that mediate resistance to microbial pathogens. In this context, the discovery that macrophages can proliferate in situ during a TH2 response100 further highlights the potential for M2 macrophages in downregulating the effector functions of M1 macrophages during coinfection.
Helminths inhibit DC activation
A major mechanism for the downregulation of TH1 responses and cytotoxic T cell responses to intracellular pathogens is the well-known ability of helminth infection to target the classical activation of DCs101. This seems to occur despite the reported ability of DCs to compartmentalize the handling of antigens and to simultaneously prime for both TH1 responses and TH2 responses102.
Among antigen-presenting cells, DCs are known to be the most critical for the initiation and also the maintenance of protective T cell responses to many infectious pathogens. In contrast to the exposure of DCs to microbial ligands of Toll-like receptors, exposure of DCs to helminth excretory-secretory products fails to upregulate the surface expression of costimulatory molecules such as CD40, CD80 and CD86 and does not result in the synthesis of proinflammatory cytokines and chemokines such as IL-12, CCL2 (MCP-1) and TNF103,104. Perhaps a far more insidious effect of prior exposure to helminths is the inhibition of the responses of both human DCs and mouse DCs to microbial ligands, including lipopolysaccharide and CpG oligonucleotides, which normally promotes the antigen presentation, costimulation and proinflammatory cytokine production of DCs105–108. That dominant-negative effect on the activation of classical DCs has been documented after in vitro exposure to schistosome soluble egg antigens and various nematodes (Brugia, Trichinella and Nippostrongylus) and helminth-derived preparations97,109–112. Exposure to helminths not only inhibits the proinflammatory activation of DCs but also promotes an alternative regulatory program, which could further dampen TH1 and cytotoxic T lymphocyte responses111,113,114. After being exposed to helminths, DCs promote the differentiation of Treg cells from naive T cell precursors or the further expansion of pre-existing Treg cell subpopulations111,115. DC production of TGF-β and/or IL-10 in response to exposure to helminth excretory-secretory products may be the critical mechanism underlying the ability of helminth infection to promote the population expansion of Treg cells. It is not known to what extent helminth inhibition of cell-mediated immunity relies on the shutdown of the proinflammatory functions of antigen-presenting cells versus the active promotion of Treg cell activities. It is conceivable that defective costimulation of antigen-presenting cells in helminth-infected people would have a more profound effect during the initiation of a nascent T cell response, such as during vaccination or primary exposure. In contrast, a negative regulatory regime imposed by the population expansion and activation of Treg cells might have a more prominent role in restricting the function of preformed effector memory and effector T cells located at peripheral tissue sites. Another study has also suggested the deviation of TH1 cells into Foxp3+ regulatory cells mediated by helminth-induced production of IL-10, rather than Treg cell–mediated downmodulation itself, as the underlying mechanism for the diminished TH1 responses to vaccination116. A similar mechanism of localized recruitment of Foxp3+ Treg cells may be involved in the exertion of anti-inflammatory effects by IL-10-producing regulatory B cells induced by helminth infection117.
Most of the aforementioned studies examining the effects of helminths on the activation and function of DCs have used in vitro cultures of bone marrow–derived DCs, which may not be representative of CD103+ DCs (presumably relevant at mucosal sites) or CD8α+ DCs (required for priming TH1 and cytotoxic T lymphocyte responses). As a consequence, very little is known about how DC subpopulations are affected in the helminth-infected host. Moreover, how helminth coinfection alters or suppresses the in vivo response of those relevant DC subsets to microbial agonists needs to be investigated. Two studies have demonstrated the emergence of a CD103−CD11clo DC population, distinct from plasmacytoid DCs, during chronic helminth infection118,119. CD11clo DCs were ineffective stimulators of an effector response by CD4+ T cells and instead strongly drove expression of the transcription factor Foxp3 in naive CD4+ T cells, which resulted in the generation of Treg cells. Whether CD11clo DCs negatively affect the in situ activation of T cells in lymphoid tissues and how helminth infection induces a numerical expansion of this regulatory DC subset is not known. Amplification in this compartment may be a direct and specific response to parasite products or, more likely, a host regulatory response to damage and stress. Regardless of those considerations, that CD11clo DC response, together with the ILC2 cell responses discussed above, may be representative of systematic deviations from basal hematopoiesis that result in an altered immunoregulatory milieu in the helminth-infected host. An important question for future investigations is whether competitive or antagonistic interactions exist between the demands for generating regulatory and ILC2 cell–type lineages and the IFN-γ-driven recruitment and population expansion of IL-7R+c-Kithi myelolymphoid hemopoietic progenitor cells required for antimicrobial protection120. Advances in this area will increase the understanding of how chronic helminth infestation reconfigures the innate immune compartment, which could fundamentally explain not only the dampened effector response to microbes but also perhaps enhanced tissue and/or host tolerance.
CONCLUSIONS
As discussed here, increasing evidence indicates that helminths can negatively affect resistance to microbes, including M. tuberculosis, Plasmodium species and HIV. Control of helminthiasis may also have a substantial effect on vaccine efficacy. However, the ability of the host to tolerate microbial infections may also be compromised by the control of helminthiasis. This is because although resistance to those microbes may be enhanced, exacerbation of the associated harmful inflammation, in the absence of helminth-induced immunoregulatory controls, could potentially contribute to increased disease severity. Such interplay between the effects of helminth coinfection on microbial resistance and tolerance will vary with the particular pathogens involved and with the severity of the helminth or microbial infection. It must also be considered that chronic helminth infection may generally be associated with a robust immunoregulatory system more able to control harmful autoimmune diseases. The helminth-induced immune mechanisms that affect microbial infection are still poorly understood. However, as discussed here, it is increasingly clear that they include separable components induced through different mechanisms triggered by either tissue damage caused by multicellular helminths, as they transit through the host, or the release of factors by helminths that can potently influence host immunological function. This complex response includes both classical type 2 immunity and innate and adaptive immunoregulatory compartments.
More extensive randomized and longitudinal clinical studies, with the use of relevant biomarkers to assess actual immune responses, are needed to further define the effect of helminth infections on the host response to microbial pathogens. An important gap that needs to be addressed is elucidating how antigen-specific T cells and B cells reactive to microbial pathogens are intrinsically reprogrammed by helminth coinfection. Gene profiling and detailed functional analyses of distinct effector lymphocytes will probably reveal convergent pathways used by diverse helminths to inactivate these cells. Knowledge generated from such comparative studies could then further guide biomarker selection as well as provide therapeutic targets for reversing the effects of helminth coinfection. In conjunction with clinical and laboratory studies, mathematical modeling of micro- and macroparasite coinfections121 should be used as an adjunct tool to model the effect of coinfection on disease evolution. However, the need for such studies should not preclude the current use of antihelminth treatments to decrease susceptibility to deadly microbial pathogens, to enhance vaccine effectiveness or to prevent mother-to-child transmission of HIV.
Further studies are also needed to investigate the mechanisms by which helminth-induced immune responses affect both resistance and tolerance to microbial pathogens, as such knowledge may lead to future targeted therapeutic interventions or vaccines that avoid the harmful effects of helminth coinfection. Future immunotherapies may include harnessing the immunoregulatory networks characteristic of helminth infections that promote tolerance to microbial pathogens by controlling harmful associated inflammation. In this context, the application of systems-biology approaches, including proteomics, transcriptomics and high-throughput functional assays, to identify immunomodulatory components of helminth parasites promises not only advances in scientific understanding but also a new class of immunotherapeutic compounds122. Even though understanding of the effects of helminth coinfections on host susceptibility to microbial pathogens is still evolving, nevertheless an increasing number of studies now demonstrate their translational relevance to human health and justify the need for increased efforts in this important area of research.
ACKNOWLEDGMENTS
We thank T. Nutman for critical comments on this manuscript. Supported by the US National Institutes of Health (AI031678 and AIO66188 to W.C.G., AI065663 and A1069395 to P.S. and AI83405 to G.S.Y.).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Dickson JH, et al. The omnivorous Tyrolean Iceman: colon contents (meat, cereals, pollen, moss and whipworm) and stable isotope analyses. Phil. Trans. R. Soc. Lond. B. 2000;355:1843–1849. doi: 10.1098/rstb.2000.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gulland FM. The role of nematode parasites in Soay sheep (Ovis aries L.) mortality during a population crash. Parasitology. 1992;105:493–503. doi: 10.1017/s0031182000074679. [DOI] [PubMed] [Google Scholar]
- 3.Jackson JA, Friberg IM, Little S, Bradley JE. Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunology. 2009;126:18–27. doi: 10.1111/j.1365-2567.2008.03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King CH. Health metrics for helminthic infections. Adv. Parasitol. 2010;73:51–69. doi: 10.1016/S0065-308X(10)73003-7. [DOI] [PubMed] [Google Scholar]
- 6.Schad GA, Anderson RM. Predisposition to hookworm infection in humans. Science. 1985;228:1537–1540. doi: 10.1126/science.4012307. [DOI] [PubMed] [Google Scholar]
- 7.Anthony RM, Rutitzky LI, Urban JF, Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 9.Chen F, et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat. Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J. Infect. Dis. 1996;173:269–272. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- 12.Elliott AM, et al. Effects of maternal and infant co-infections, and of maternal immunisation, on the infant response to BCG and tetanus immunisation. Vaccine. 2010;29:247–255. doi: 10.1016/j.vaccine.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott DE, Weinstock JV. Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Ann. NY Acad. Sci. 2012;1247:83–96. doi: 10.1111/j.1749-6632.2011.06292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott DE, et al. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 15.Zaccone P, et al. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur. J. Immunol. 2009;39:1098–1107. doi: 10.1002/eji.200838871. [DOI] [PubMed] [Google Scholar]
- 16.Mishra PK, Patel N, Wu W, Bleich D, Gause WC. Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response. Mucosal Immunol. 2013;6:297–308. doi: 10.1038/mi.2012.71. [DOI] [PubMed] [Google Scholar]
- 17.Gaze S, et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012;8:e1002520. doi: 10.1371/journal.ppat.1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elias D, Mengistu G, Akuffo H, Britton S. Are intestinal helminths risk factors for developing active tuberculosis? Trop. Med. Int. Health. 2006;11:551–558. doi: 10.1111/j.1365-3156.2006.01578.x. [DOI] [PubMed] [Google Scholar]
- 19.Tristão-Sá R, Ribeiro-Rodrigues R, Johnson LT, Pereira FE, Dietze R. Intestinal nematodes and pulmonary tuberculosis. Rev. Soc. Bras. Med. Trop. 2002;35:533–535. doi: 10.1590/s0037-86822002000500020. [DOI] [PubMed] [Google Scholar]
- 20.Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin. Exp. Immunol. 2007;147:45–52. doi: 10.1111/j.1365-2249.2006.03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wammes LJ, et al. Regulatory T cells in human geohelminth infection suppress immune responses to BCG and Plasmodium falciparum. Eur. J. Immunol. 2010;40:437–442. doi: 10.1002/eji.200939699. [DOI] [PubMed] [Google Scholar]
- 22.Verhagen LM, et al. Helminths and skewed cytokine profiles increase tuberculin skin test positivity in Warao Amerindians. Tuberculosis (Edinb.) 2012;92:505–512. doi: 10.1016/j.tube.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Buck AA, Anderson RI, Kawata K, Hitchcock JC., Jr. Onchocerciasis: some new epidemiologic and clinical findings. Results of an epidemiologic study in the Republic of Chad. Am. J. Trop. Med. Hyg. 1969;18:217–230. doi: 10.4269/ajtmh.1969.18.217. [DOI] [PubMed] [Google Scholar]
- 24.Rougemont A, Boisson-Pontal ME, Pontal PG, Gridel F, Sangare S. Tuberculin skin tests and B.C.G. vaccination in hyperendemic area of onchocerciasis. Lancet. 1977;1:309. doi: 10.1016/s0140-6736(77)91857-8. [DOI] [PubMed] [Google Scholar]
- 25.Stewart GR, et al. Onchocerciasis modulates the immune response to mycobacterial antigens. Clin. Exp. Immunol. 1999;117:517–523. doi: 10.1046/j.1365-2249.1999.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babu S, et al. Attenuation of toll-like receptor expression and function in latent tuberculosis by coexistent filarial infection with restoration following antifilarial chemotherapy. PLoS Negl. Trop. Dis. 2009;3:e489. doi: 10.1371/journal.pntd.0000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babu S, et al. Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen-4 and programmed death-1. J. Infect. Dis. 2009;200:288–298. doi: 10.1086/599797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elias D, et al. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin. Exp. Immunol. 2001;123:219–225. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearlman E, Kazura JW, Hazlett FE, Jr., Boom WH. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. J. Immunol. 1993;151:4857–4864. [PubMed] [Google Scholar]
- 30.Sacco R, et al. H1 granulomatous responses induced by active Mycobacterium avium infection switch to TH2 following challenge with Schistosoma mansoni. Clin. Immunol. 2002;104:274–281. doi: 10.1006/clim.2002.5263. [DOI] [PubMed] [Google Scholar]
- 31.Frantz FG, et al. Helminth coinfection does not affect therapeutic effect of a DNA vaccine in mice harboring tuberculosis. PLoS Negl. Trop. Dis. 2010;4:e700. doi: 10.1371/journal.pntd.0000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elias D, Akuffo H, Thors C, Pawlowski A, Britton S. Low dose chronic Schistosoma mansoni infection increases susceptibility to Mycobacterium bovis BCG infection in mice. Clin. Exp. Immunol. 2005;139:398–404. doi: 10.1111/j.1365-2249.2004.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potian JA, et al. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J. Exp. Med. 2011;208:1863–1874. doi: 10.1084/jem.20091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.du Plessis N, et al. Acute helminth infection enhances early macrophage mediated control of mycobacterial infection. Mucosal Immunol. 2013;6:931–941. doi: 10.1038/mi.2012.131. [DOI] [PubMed] [Google Scholar]
- 35.Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 1996;178:1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DiGiuseppe Champion PA, Cox JS. Protein secretion systems in Mycobacteria. Cell. Microbiol. 2007;9:1376–1384. doi: 10.1111/j.1462-5822.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- 37.Al-Riyami L, Wilson EH, Watson CA, Harnett W. T-helper type 1 responses to the BCG vaccine component PPD in mice are unaffected by the filarial nematode immunomodulatory molecule ES-62. J. Parasitol. 2009;95:1201–1204. doi: 10.1645/GE-2017.1. [DOI] [PubMed] [Google Scholar]
- 38.Lipner EM, et al. Coincident filarial, intestinal helminth, and mycobacterial infection: helminths fail to influence tuberculin reactivity, but BCG influences hookworm prevalence. Am. J. Trop. Med. Hyg. 2006;74:841–847. [PubMed] [Google Scholar]
- 39.Cooper PJ, Guderian RH, Nutman TB, Taylor DW. Human infection with Onchocerca volvulus does not affect the T helper cell phenotype of the cellular immune response to mycobacterial antigen. Trans. R. Soc. Trop. Med. Hyg. 1997;91:350–352. doi: 10.1016/s0035-9203(97)90103-6. [DOI] [PubMed] [Google Scholar]
- 40.Hubner MP, et al. Chronic helminth infection does not exacerbate Mycobacterium tuberculosis infection. PLoS Negl. Trop. Dis. 2012;6:e1970. doi: 10.1371/journal.pntd.0001970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhodes SG, Graham SP. Is ‘timing’ important for cytokine polarization? Trends Immunol. 2002;23:246–249. doi: 10.1016/s1471-4906(02)02200-7. [DOI] [PubMed] [Google Scholar]
- 42.Elias D, Britton S, Aseffa A, Engers H, Akuffo H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-β production. Vaccine. 2008;26:3897–3902. doi: 10.1016/j.vaccine.2008.04.083. [DOI] [PubMed] [Google Scholar]
- 43.Elias D, et al. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine. 2005;23:1326–1334. doi: 10.1016/j.vaccine.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 44.Labeaud AD, Malhotra I, King MJ, King CL, King CH. Do antenatal parasite infections devalue childhood vaccination? PLoS Negl. Trop. Dis. 2009;3:e442. doi: 10.1371/journal.pntd.0000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malhotra I, et al. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J. Immunol. 1999;162:6843–6848. [PubMed] [Google Scholar]
- 46.Malhotra I, et al. In utero exposure to helminth and mycobacterial antigens generates cytokine responses similar to that observed in adults. J. Clin. Invest. 1997;99:1759–1766. doi: 10.1172/JCI119340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Webb EL, et al. Effect of single-dose anthelmintic treatment during pregnancy on an infant’s response to immunisation and on susceptibility to infectious diseases in infancy: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:52–62. doi: 10.1016/S0140-6736(10)61457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Degarege A, Legesse M, Medhin G, Animut A, Erko B. Malaria and related outcomes in patients with intestinal helminths: a cross-sectional study. BMC Infect. Dis. 2012;12:291. doi: 10.1186/1471-2334-12-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Hesran JY, et al. Severe malaria attack is associated with high prevalence of Ascaris lumbricoides infection among children in rural Senegal. Trans. R. Soc. Trop. Med. Hyg. 2004;98:397–399. doi: 10.1016/j.trstmh.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Lyke KE, et al. Association of Schistosoma haematobium infection with protection against acute Plasmodium falciparum malaria in Malian children. Am. J. Trop. Med. Hyg. 2005;73:1124–1130. [PMC free article] [PubMed] [Google Scholar]
- 51.Nacher M, et al. Helminth infections are associated with protection from malaria-related acute renal failure and jaundice in Thailand. Am. J. Trop. Med. Hyg. 2001;65:834–836. doi: 10.4269/ajtmh.2001.65.834. [DOI] [PubMed] [Google Scholar]
- 52.Nacher M, et al. Ascaris lumbricoides infection is associated with protection from cerebral malaria. Parasite Immunol. 2000;22:107–113. doi: 10.1046/j.1365-3024.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 53.Dolo H, et al. Filariasis attenuates anemia and proinflammatory responses associated with clinical malaria: a matched prospective study in children and young adults. PLoS Negl. Trop. Dis. 2012;6:e1890. doi: 10.1371/journal.pntd.0001890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartgers FC, et al. Responses to malarial antigens are altered in helminth-infected children. J. Infect. Dis. 2009;199:1528–1535. doi: 10.1086/598687. [DOI] [PubMed] [Google Scholar]
- 55.Diallo TO, et al. Schistosomiasis coinfection in children influences acquired immune response against Plasmodium falciparum malaria antigens. PLoS ONE. 2010;5:e12764. doi: 10.1371/journal.pone.0012764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Metenou S, et al. Patent filarial infection modulates malaria-specific type 1 cytokine responses in an IL-10-dependent manner in a filaria/malaria-coinfected population. J. Immunol. 2009;183:916–924. doi: 10.4049/jimmunol.0900257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Metenou S, et al. Interferon regulatory factor modulation underlies the bystander suppression of malaria antigen-driven IL-12 and IFN-γ in filaria-malaria co-infection. Eur. J. Immunol. 2012;42:641–650. doi: 10.1002/eji.201141991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Courtin D, et al. Schistosoma haematobium infection affects Plasmodium falciparum-specific IgG responses associated with protection against malaria. Parasite Immunol. 2011;33:124–131. doi: 10.1111/j.1365-3024.2010.01267.x. [DOI] [PubMed] [Google Scholar]
- 59.Druilhe P, Tall A, Sokhna C. Worms can worsen malaria: towards a new means to roll back malaria? Trends Parasitol. 2005;21:359–362. doi: 10.1016/j.pt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Roussilhon C, Brasseur P, Agnamey P, Perignon JL, Druilhe P. Understanding human-Plasmodium falciparum immune interactions uncovers the immunological role of worms. PLoS ONE. 2010;5:e9309. doi: 10.1371/journal.pone.0009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Su Z, Segura M, Morgan K, Loredo-Osti JC, Stevenson MM. Impairment of protective immunity to blood-stage malaria by concurrent nematode infection. Infect. Immun. 2005;73:3531–3539. doi: 10.1128/IAI.73.6.3531-3539.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Segura M, Matte C, Thawani N, Su Z, Stevenson MM. Modulation of malaria-induced immunopathology by concurrent gastrointestinal nematode infection in mice. Int. J. Parasitol. 2009;39:1525–1532. doi: 10.1016/j.ijpara.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 63.Webb EL, Ekii AO, Pala P. Epidemiology and immunology of helminth-HIV interactions. Curr. Opin. HIV AIDS. 2012;7:245–253. doi: 10.1097/COH.0b013e32835210cd. [DOI] [PubMed] [Google Scholar]
- 64.Bailer RT, Lee B, Montaner LJ. IL-13 and TNF-α inhibit dual-tropic HIV-1 in primary macrophages by reduction of surface expression of CD4, chemokine receptors CCR5, CXCR4 and post-entry viral gene expression. Eur. J. Immunol. 2000;30:1340–1349. doi: 10.1002/(SICI)1521-4141(200005)30:5<1340::AID-IMMU1340>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 65.Montaner LJ, et al. Interleukin 13 inhibits human immunodeficiency virus type 1 production in primary blood-derived human macrophages in vitro. J. Exp. Med. 1993;178:743–747. doi: 10.1084/jem.178.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cassol E, Cassetta L, Rizzi C, Alfano M, Poli G. M1 and M2a polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. J. Immunol. 2009;182:6237–6246. doi: 10.4049/jimmunol.0803447. [DOI] [PubMed] [Google Scholar]
- 67.Denis M, Ghadirian E. Interleukin 13 and interleukin 4 protect bronchoalveolar macrophages from productive infection with human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses. 1994;10:795–802. doi: 10.1089/aid.1994.10.795. [DOI] [PubMed] [Google Scholar]
- 68.Montaner LJ, Bailer RT, Gordon S. IL-13 acts on macrophages to block the completion of reverse transcription, inhibit virus production, and reduce virus infectivity. J. Leukoc. Biol. 1997;62:126–132. doi: 10.1002/jlb.62.1.126. [DOI] [PubMed] [Google Scholar]
- 69.Mikovits JA, et al. IL-4 and IL-13 have overlapping but distinct effects on HIV production in monocytes. J. Leukoc. Biol. 1994;56:340–346. doi: 10.1002/jlb.56.3.340. [DOI] [PubMed] [Google Scholar]
- 70.Naif H, Ho-Shon M, Chang J, Cunningham AL. Molecular mechanisms of IL-4 effect on HIV expression in promonocytic cell lines and primary human monocytes. J. Leukoc. Biol. 1994;56:335–339. doi: 10.1002/jlb.56.3.335. [DOI] [PubMed] [Google Scholar]
- 71.Mouser EE, Pollakis G, Paxton WA. Effects of helminths and Mycobacterium tuberculosis infection on HIV-1: a cellular immunological perspective. Curr. Opin. HIV AIDS. 2012;7:260–267. doi: 10.1097/COH.0b013e3283521144. [DOI] [PubMed] [Google Scholar]
- 72.Jourdan PM, Holmen SD, Gundersen SG, Roald B, Kjetland EF. HIV target cells in Schistosoma haematobium-infected female genital mucosa. Am. J. Trop. Med. Hyg. 2011;85:1060–1064. doi: 10.4269/ajtmh.2011.11-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Downs JA, et al. Urogenital schistosomiasis in women of reproductive age in Tanzania’s Lake Victoria region. Am. J. Trop. Med. Hyg. 2011;84:364–369. doi: 10.4269/ajtmh.2011.10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat. Rev. Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gallagher M, et al. The effects of maternal helminth and malaria infections on mother-to-child HIV transmission. AIDS. 2005;19:1849–1855. doi: 10.1097/01.aids.0000189846.90946.5d. [DOI] [PubMed] [Google Scholar]
- 76.Steiner KL, et al. In utero activation of fetal memory T cells alters host regulatory gene expression and affects HIV susceptibility. Virology. 2012;425:23–30. doi: 10.1016/j.virol.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wolday D, et al. Treatment of intestinal worms is associated with decreased HIV plasma viral load. J. Acquir. Immune Defic. Syndr. 2002;31:56–62. doi: 10.1097/00126334-200209010-00008. [DOI] [PubMed] [Google Scholar]
- 78.Ndhlovu PD, et al. Prevalence of urinary schistosomiasis and HIV in females living in a rural community of Zimbabwe: does age matter? Trans. R. Soc. Trop. Med. Hyg. 2007;101:433–438. doi: 10.1016/j.trstmh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 79.Kjetland EF, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20:593–600. doi: 10.1097/01.aids.0000210614.45212.0a. [DOI] [PubMed] [Google Scholar]
- 80.Hosseinipour MC, et al. HIV and parasitic infection and the effect of treatment among adult outpatients in Malawi. J. Infect. Dis. 2007;195:1278–1282. doi: 10.1086/513274. [DOI] [PubMed] [Google Scholar]
- 81.Elliott AM, et al. Associations between helminth infection and CD4+ T cell count, viral load and cytokine responses in HIV-1-infected Ugandan adults. Trans. R. Soc. Trop. Med. Hyg. 2003;97:103–108. doi: 10.1016/s0035-9203(03)90040-x. [DOI] [PubMed] [Google Scholar]
- 82.Brown M, et al. Treatment of Schistosoma mansoni infection increases helminth-specific type 2 cytokine responses and HIV-1 loads in coinfected Ugandan adults. J. Infect. Dis. 2005;191:1648–1657. doi: 10.1086/429668. [DOI] [PubMed] [Google Scholar]
- 83.Lawn SD, et al. The effect of treatment of schistosomiasis on blood plasma HIV-1 RNA concentration in coinfected individuals. AIDS. 2000;14:2437–2443. doi: 10.1097/00002030-200011100-00004. [DOI] [PubMed] [Google Scholar]
- 84.Sangaré LR, Herrin BR, John-Stewart G, Walson JL. Species-specific treatment effects of helminth/HIV-1 co-infection: a systematic review and meta-analysis. Parasitology. 2011;138:1546–1558. doi: 10.1017/S0031182011000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 86.Adams VJ, et al. Recall of intestinal helminthiasis by HIV-infected South Africans and avoidance of possible misinterpretation of egg excretion in worm/HIV co-infection analyses. BMC Infect. Dis. 2006;6:88. doi: 10.1186/1471-2334-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mkhize-Kwitshana ZL, Taylor M, Jooste P, Mabaso ML, Walzl G. The influence of different helminth infection phenotypes on immune responses against HIV in co-infected adults in South Africa. BMC Infect. Dis. 2011;11:273. doi: 10.1186/1471-2334-11-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stillwaggon E. Living with uncertainty. Trends Parasitol. 2012;28:261–266. doi: 10.1016/j.pt.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Chan DJ. Factors affecting sexual transmission of HIV-1: current evidence and implications for prevention. Curr. HIV Res. 2005;3:223–241. doi: 10.2174/1570162054368075. [DOI] [PubMed] [Google Scholar]
- 90.Dreyfuss ML, Fawzi WW. Micronutrients and vertical transmission of HIV-1. Am. J. Clin. Nutr. 2002;75:959–970. doi: 10.1093/ajcn/75.6.959. [DOI] [PubMed] [Google Scholar]
- 91.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 92.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.El Kasmi KC, et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat. Immunol. 2008;9:1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harris J, et al. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–517. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 95.Potian JA, et al. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J. Exp. Med. 2011;208:1863–1874. doi: 10.1084/jem.20091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Babu S, Kumaraswami V, Nutman TB. Alternatively activated and immunoregulatory monocytes in human filarial infections. J. Infect. Dis. 2009;199:1827–1837. doi: 10.1086/599090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Talaat KR, Bonawitz RE, Domenech P, Nutman TB. Preexposure to live Brugia malayi microfilariae alters the innate response of human dendritic cells to Mycobacterium tuberculosis. J. Infect. Dis. 2006;193:196–204. doi: 10.1086/498912. [DOI] [PubMed] [Google Scholar]
- 98.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Loke P, et al. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J. Immunol. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 100.Jenkins SJ, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.White RR, Artavanis-Tsakonas K. How helminths use excretory secretory fractions to modulate dendritic cells. Virulence. 2012;3:668–677. doi: 10.4161/viru.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cervi L, MacDonald AS, Kane C, Dzierszinski F, Pearce EJ. Cutting edge: dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J. Immunol. 2004;172:2016–2020. doi: 10.4049/jimmunol.172.4.2016. [DOI] [PubMed] [Google Scholar]
- 103.Chaussabel D, et al. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood. 2003;102:672–681. doi: 10.1182/blood-2002-10-3232. [DOI] [PubMed] [Google Scholar]
- 104.Kane CM, et al. Helminth antigens modulate TLR-initiated dendritic cell activation. J. Immunol. 2004;173:7454–7461. doi: 10.4049/jimmunol.173.12.7454. [DOI] [PubMed] [Google Scholar]
- 105.Klaver EJ, et al. Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int. J. Parasitol. 2013;43:191–200. doi: 10.1016/j.ijpara.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 106.Segura M, Su Z, Piccirillo C, Stevenson MM. Impairment of dendritic cell function by excretory-secretory products: a potential mechanism for nematode-induced immunosuppression. Eur. J. Immunol. 2007;37:1887–1904. doi: 10.1002/eji.200636553. [DOI] [PubMed] [Google Scholar]
- 107.Everts B, et al. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J. Exp. Med. 2012;209:1753–1767. doi: 10.1084/jem.20111381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Al-Riyami L, Harnett W. Immunomodulatory properties of ES-62, a phosphorylcholine-containing glycoprotein secreted by Acanthocheilonema viteae. Endocr. Metab. Immune Disord. Drug Targets. 2012;12:45–52. doi: 10.2174/187153012799278893. [DOI] [PubMed] [Google Scholar]
- 109.Jankovic D, Kullberg MC, Caspar P, Sher A. Parasite-induced Th2 polarization is associated with down-regulated dendritic cell responsiveness to Th1 stimuli and a transient delay in T lymphocyte cycling. J. Immunol. 2004;173:2419–2427. doi: 10.4049/jimmunol.173.4.2419. [DOI] [PubMed] [Google Scholar]
- 110.Semnani RT, Law M, Kubofcik J, Nutman TB. Filaria-induced immune evasion: suppression by the infective stage of Brugia malayi at the earliest host-parasite interface. J. Immunol. 2004;172:6229–6238. doi: 10.4049/jimmunol.172.10.6229. [DOI] [PubMed] [Google Scholar]
- 111.Aranzamendi C, et al. Trichinella spiralis-secreted products modulate DC functionality and expand regulatory T cells in vitro. Parasite Immunol. 2012;34:210–223. doi: 10.1111/j.1365-3024.2012.01353.x. [DOI] [PubMed] [Google Scholar]
- 112.Massacand JC, et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc. Natl. Acad. Sci. USA. 2009;106:13968–13973. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Walsh KP, Brady MT, Finlay CM, Boon L, Mills KH. Infection with a helminth parasite attenuates autoimmunity through TGF-β-mediated suppression of Th17 and Th1 responses. J. Immunol. 2009;183:1577–1586. doi: 10.4049/jimmunol.0803803. [DOI] [PubMed] [Google Scholar]
- 114.Setiawan T, et al. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect. Immun. 2007;75:4655–4663. doi: 10.1128/IAI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grainger JR, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hartmann W, Haben I, Fleischer B, Breloer M. Pathogenic nematodes suppress humoral responses to third-party antigens in vivo by IL-10-mediated interference with Th cell function. J. Immunol. 2011;187:4088–4099. doi: 10.4049/jimmunol.1004136. [DOI] [PubMed] [Google Scholar]
- 117.Amu S, et al. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J. Allergy Clin. Immunol. 2010;125:1114–1124. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 118.Smith KA, et al. Chronic helminth infection promotes immune regulation in vivo through dominance of CD11cloCD103− dendritic cells. J. Immunol. 2011;186:7098–7109. doi: 10.4049/jimmunol.1003636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Z, et al. The phenotype and function of naturally existing regulatory dendritic cells in nematode-infected mice. Int. J. Parasitol. 2011;41:1129–1137. doi: 10.1016/j.ijpara.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 120.Belyaev NN, et al. Induction of an IL7-R+c-Kithi myelolymphoid progenitor critically dependent on IFN-γ signaling during acute malaria. Nat. Immunol. 2010;11:477–485. doi: 10.1038/ni.1869. [DOI] [PubMed] [Google Scholar]
- 121.Fenton A, Lamb T, Graham AL. Optimality analysis of Th1/Th2 immune responses during microparasite-macroparasite co-infection, with epidemiological feedbacks. Parasitology. 2008;135:841–853. doi: 10.1017/S0031182008000310. [DOI] [PubMed] [Google Scholar]
- 122.Moreno Y, et al. Proteomic analysis of excretory-secretory products of Heligmosomoides polygyrus assessed with next-generation sequencing transcriptomic information. PLoS Negl. Trop. Dis. 2011;5:e1370. doi: 10.1371/journal.pntd.0001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lustigman S, et al. A research agenda for helminth diseases of humans: the problem of helminthiases. PLoS Negl Trop Dis. 2012;6:e1582. doi: 10.1371/journal.pntd.0001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.World Health Organization . Global tuberculosis report 2012. World Health Organization; 2012. http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 125.World Health Organization . Public Health Information and Geographic Information System (GIS) World Health Organization; 2012. WHO Map Production. http://gamapserver.who.int/mapLibrary/Files/Maps/malaria_003.jpg. [Google Scholar]
- 126.Joint United Nations Programme on HIV/AIDS (UNAIDS) UNAIDS Report on the Global AIDS Epidemic 2010. UNAIDS; 2010. http://www.unaids.org/globalreport/global_report.htm. [Google Scholar]