Abstract
Objective
To discern the effects of continuous passive motion on inflamed temporomandibular joints (TMJ).
Methods
The effects of continuous passive motion on TMJ were simulated by exposing primary cultures of rabbit TMJ fibrochondrocyte monolayers to cyclic tensile strain (CTS) in the presence of recombinant human interleukin-1β (rHuIL-1β) in vitro. The messenger RNA (mRNA) induction of rHuIL-1β response elements was examined by semiquantitative reverse transcriptase–polymerase chain reaction. The synthesis of nitric oxide was examined by Griess reaction, and the synthesis of prostaglandin E2 (PGE2) was examined by radioimmunoassay. The synthesis of proteins was examined by Western blot analysis of the cell extracts, and synthesis of proteoglycans via incorporation of 35S-sodium sulfate in the culture medium.
Results
Exposure of TMJ fibrochondrocytes to rHuIL-1β resulted in the induction of inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2), which were paralleled by NO and PGE2 production. Additionally, IL-1β induced significant levels of collagenase (matrix metalloproteinase 1 [MMP-1]) within 4 hours, and this was sustained over a period of 48 hours. Concomitant application of CTS abrogated the catabolic effects of IL-1β on TMJ chondrocytes by inhibiting iNOS, COX-2, and MMP-1 mRNA production and NO, PGE2, and MMP-1 synthesis. CTS also counteracted cartilage degradation by augmenting expression of mRNA for tissue inhibitor of metalloproteinases 2 that is inhibited by rHuIL-1β. In parallel, CTS also counteracted rHuIL-1β–induced suppression of proteoglycan synthesis. Nevertheless, the presence of an inflammatory signal was a prerequisite for the observed CTS actions, because fibrochondrocytes, when exposed to CTS alone, did not exhibit any of the effects described above.
Conclusion
CTS acts as an effective antagonist of rHuIL-1β by potentially diminishing its catabolic actions on TMJ fibrochondrocytes. Furthermore, CTS actions appear to involve disruption/regulation of signal transduction cascade of rHuIL-1β upstream of mRNA transcription.
Temporomandibular joint (TMJ) disorders are debilitating and result in progressive degeneration of articular cartilage, the disk, and/or the subchondral bone, leading to disharmonious function of the entire masticatory apparatus (1–4). As a heterogeneous group of diseases, TMJ disorders are commonly diagnosed as arthritic conditions resulting from trauma or infections (3–5). Analysis of synovial fluid from inflamed TMJ has revealed the presence of elevated levels of cytokines and other inflammatory mediators (6–10). Proinflammatory cytokines are produced by chondrocytes, cells that line the joint cavity, and cells of the immune system that have migrated into the subsynovial space (6–10). Among the proinflammatory cytokines, local production of interleukin-1 (IL-1) appears to be directly responsible for the destruction of cartilage (6–8,10). IL-1 induces catabolic responses in chondrocytes by stimulating expression of proteases, including stromelysin, collagenase, and tissue plasminogen activator. Chondrocytes stimulated with IL-1β have been found to produce massive amounts of inducible nitric oxide synthase (iNOS) and NO, potent mediators of the destructive effects of IL-1. NO induces the synthesis of tissue-destructive enzymes and inhibits matrix synthesis (11–17). IL-1 is also a potent inducer of cyclooxygenase 2 (COX-2) and prostaglandin E2 (PGE2) synthesis (18–20). IL-1 also suppresses α1(II) procollagen messenger RNA (mRNA) expression and proteoglycan synthesis. Collectively, IL-1β–induced inhibition of matrix synthesis and the induction of proteases result in cartilage resorption and pathology of the TMJ disorder (18–24).
Interestingly, physical therapies such as continuous passive motion (CPM) exert reparative effects on diseased or inflamed TMJ (25–28), as well as in postsurgical rehabilitation of TMJ. The beneficial effects of CPM are mainly attributed to the increased circulation and the removal of inflammatory exudates from synovial fluids (29). Whether CPM also acts directly on chondrocytes is not yet discerned. Since inflammatory cytokines such as IL-1β play a major role in both the initiation and the progression of cartilage destruction, we hypothesized that CPM actions may involve suppression of proinflammatory pathways. To test this hypothesis in vitro, we examined the effects of equibiaxial cyclic tensile strain (CTS) on primary cultures of chondrocytes from rabbit TMJ in the presence of recombinant human IL-1β (rHuIL-1β), to mimic effects of CPM in vivo in arthritic TMJ disorders.
MATERIALS AND METHODS
Animals and materials
New Zealand white rabbits weighing 6–7 lb were obtained from Myrtle’s Rabbitry (Thompson Station, TN). Materials were obtained from the following suppliers: tissue culture media, sera, and antibiotics from Gibco (Grand Island, NY); crude clostridial collagenase from Worthington (Freehold, NJ); Luminol, Reflection autoradiographic film, 35sodium sulfate (1 Ci/mmole), and PGE2 radioimmunoassay (RIA) kit from NEN (Boston, MA); rHuIL-1β from Genentech (La Jolla, CA); pronectine-coated Bioflex II culture plates from Flexcell (Hillsborough, NC); primers for polymerase chain reaction (PCR) synthesized by Bio-Synthesis (Lewisville, TX); molecular biology reagents from Perkin-Elmer (Norwalk, CT); antibodies from Santa Cruz Biotechnology (Santa Cruz, CA); and all other reagents from Sigma (St. Louis, MO).
Isolation of chondrocytes from TMJ
Cartilage from the disk of TMJ was aseptically excised from the disk and condyles of TMJ, and the fibrochondrocytes were isolated by sequential enzymatic treatment with 0.2% trypsin and 0.2% clostridial collagenase (30). TMJ chondrocytes were then washed and resuspended in TCM (Ham’s F-12, 10% fetal calf serum, penicillin [100 units/ml]/streptomycin [10 μg/ml]), and plated at a rate of 2 × 105/2 ml TCM in 6-well Bioflex culture plates and cultured for 8 days at 37°C in a CO2 incubator (30). The cultures reached 90% confluence in 6–8 days. In primary cultures, these fibrochondrocytes retain their differentiated phenotype and express mRNA for aggrecan, biglycan, transforming growth factor β1 (TGFβ1), and type II collagen (Figure 1A), as well as synthesize chondroitin sulfate proteoglycans (Figure 1D), aggrecan, and type II collagen (Figure 1B) (31,32). Since fibrocartilage lacks blood, nerve, and lymphatics, these cultures are highly unlikely to be contaminated by other cell types. Also, such fibrochondrocytes respond to IL-1β in a manner similar to that of articular cartilage explants (33). Trypan blue exclusion confirmed >99% viability of cells in culture.
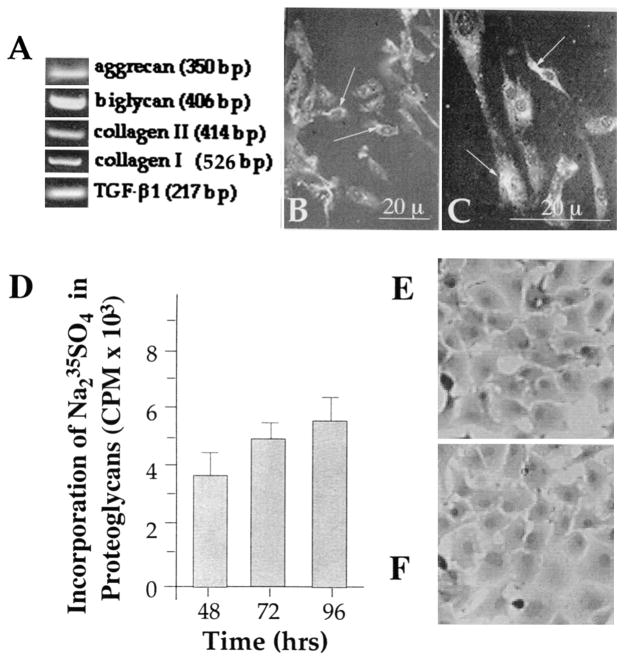
Figure 1.
Phenotypic characteristics of rabbit temporomandibular joint (TMJ) fibrochondrocytes. A, Rabbit fibrochondrocytes exhibiting the presence of aggrecan, biglycan, type I collagen, type II collagen, and transforming growth factor β1 (TGF-β1)–specific mRNA, as assessed by reverse transcriptase–polymerase chain reaction. B and C. Rabbit TMJ disk chondrocytes showing the presence of aggrecan (B) and biglycan (C) by immunofluorescence (indicated by arrows). D, Presence of proteoglycans in TMJ disk chondrocytes as assessed by incorporation of Na235SO4 into chondroitin sulfate proteoglycans during the last 8 hours of the incubation. The proteoglycans were measured in a total of 2 × 106 cells in triplicate as described in Materials and Methods. Bars represent the mean and SEM of triplicate values. E and F, Morphology of fibrochondrocytes without treatment (E) and following exposure to cyclic tensile strain for 24 hours (F).
Chondrocyte activation and exposure to equibiaxial strain
Primary fibrochondrocyte cultures that were 6–8 days old and 90% confluent were grown on Bioflex plates, washed twice with TCM, and subjected to equibiaxial strain (30) in a Flexercell unit (Flexcell). The equibiaxial strain applied was at a rate of 3 cycles/minute (0.05 Hz), i.e., 10 seconds of a maximum of 6% equibiaxial strain followed by 10 seconds of relaxation per cycle (180 cycles/hour), providing reproducible suppression of IL-1β–induced iNOS mRNA expression and NO production. The strain was calculated as follows: circumferential strain = 2π (change in radius)/2π (original radius) = (change in radius)/(original radius) = radial strain. In this system, the membrane of each well of the Bioflex plate is strained on a loading post to apply equibiaxial strain on the membrane. The cells cultured on the membrane are thus subjected to the equibiaxial strain equivalent to that applied to the membrane. The chondrocytes growing on the Bioflex plates were divided into 4 groups: untreated and unstrained control cells, cells treated with CTS alone, cells treated with rHuIL-1β (1 ng/ml) alone, and cells treated with CTS and rHuIL-1β (1 ng/ml). The cells were subjected to CTS at the time of addition of rHuIL-1β in most of the experiments.
Reverse transcriptase–PCR (RT-PCR)
The fibrochondrocytes on the Bioflex membrane growing above the loading posts were carefully scraped and subjected to RNA extraction with an RNA extraction kit (Qiagen, Santa Clara, CA). A total of 0.5 μg of RNA was mixed with 1 μg oligo-dT (12–18 oligomer) in reverse transcription buffer and incubated for 10 minutes at room temperature. Thereafter, the reaction mixture was cooled on ice and incubated with 200 units of Moloney murine leukemia virus reverse transcriptase for 60 minutes at 37°C. The complementary DNA thus obtained was amplified with 0.1 μg of specific primers in a reaction mixture containing 200 μM dNTP and 0.1 units of Taq polymerase in PCR buffer. PCR was performed in a DNA thermal cycler (Perkin-Elmer) for 30 cycles of 40 seconds at 94°C, 40 seconds at 62°C, and 60 seconds at 720°C. The sequence of sense and antisense rabbit primers used was as follows: GAPDH (293 bp) sense 5′-TCACCATCTTCCAGGAGCGA-3′, antisense 5′CAC-AATGCCGAAGTGGTCGT′; iNOS (243 bp) sense 5′-CGCCC-TTCCGCAGTTTCT-3′, antisense 5′-TCCAGGAGGACAT-GCAGCAC-3′; matrix metalloproteinase 3 (MMP-3) (322 bp) sense 5′TCAGTTCGTCCTCACTCCAG, antisense 5′-TTG-GTCCACCTGTCATCTTC; tissue inhibitor of metallopro-teinases 1 (TIMP-1) (326 bp) sense 5′-GCAACTCCGACCT-TGTCATC-3′, antisense 5′-AGCGTAGGTCTTGGTGAA GC-3′, TIMP-2 (414 bp) sense 5′-GTAGTGATCAGGGCC-AAG-3′, antisense 5′-TTCTCTGTGACCCAGTCCAT-3′; bi-glycan (406 bp) sense 5′-GATGGCCTGAAGCTCAA-3′, an-tisense 5′-GGTTTTTGAAGAGGCTG-3′; COX-2 (282 bp) sense 5′-TCAGCCACGCAGCAAATCCT-3′, antisense 5′-GTCATCTGGATGTCAGCACG-3′ (34); aggrecan (350 bp) sense 5′-CTACCTTGGAGGTCGTGGTGA-3′, antisense 5′-GTGCACGTACACGGTCCTGA-3′; type I collagen α1 (526 bp) sense 5′-TCAACGGTGCTCCTGGTGAAG-3′, antisense 5′-GGACCTTGGCTACCCTGAGAA-3′; and type II collagen (414 bp) sense 5′-TCAACAACCAGATCGAGAGCA-3′, antisense 5′-AGGTGAACCTGCTGTTGCCCT-3′ (provided by Dr. M. Heidaran, Orquest, Mountain View, CA).
Quantitative RT-PCR (RT-QCPCR)
Heterologous competitor DNA for aggrecan or iNOS was constructed by PCR using a Bam HI–Eco RI fragment of verbB gene as a template and a PCR MIMIC construction kit (Clontech, Palo Alto, CA), as described earlier (30). The equimolar concentrations of the gene products were estimated by densitometric analysis of ethidium bromide–stained DNA in each lane (Optimus software; Media Cybernetics, Silver Spring, MD), and results were expressed as the mean number of mRNA molecules synthesized per μg of RNA.
Proteoglycan synthesis
Total proteoglycan synthesis was measured by incorporation of Na2-35SO4 into chondroitin sulfate proteoglycans during the last 8 hours of the experiment. Subsequently, culture supernatants were extracted with 0.5M NaOH and the incorporated precursor was separated by size exclusion chromatography using a Pharmacia (Piscataway, NJ) PD-10 column. The 35S incorporation in proteoglycans was measured by scintillation counting (30).
Nitrite determination
NO production was determined as the nitrite concentration in culture medium, by use of a spectrophotometric assay based on the Griess reaction (30).
PGE2 measurements
PGE2 was measured in the culture supernatants of chondrocytes at various time intervals by RIA, according to the protocol recommended by the manufacturer (NEN).
Western blot analysis
Since measurable protein synthesis is apparent 8 hours after activation of cells, we selected 8 hours as the first time point at which to assess protein synthesis. Furthermore, because the effects of rHuIL-1β are sustained, we examined the effects of CTS on rHuIL-1β–induced protein synthesis 48 hours after activation of cells with rHuIL-1β. Protein synthesis in 50 μg of protein extracts was assessed by Western blot analysis (30), using goat anti–MMP-1 or anti–TIMP-2 as primary antibodies, rabbit anti-goat–horseradish peroxidase (HRP) as second antibody, and luminol as a chemiluminescent HRP substrate. The semiquantitative assessment of the luminescence in each band was performed by exposing the blots to Reflection autoradiographic film, followed by densitometric analysis of the luminescent bands using a camera equipped with a computer and Optimus software (Media Cybernetics).
Statistical analysis
Experiments were performed at least 3 times, and data are presented as the mean ± SEM. Each incubation condition was performed in triplicate, and replicates were averaged. The significance of differences between mean values was determined by Student’s t-test.
RESULTS
TMJ cells express chondrocytic phenotype
Cells from the cartilage typically synthesize type II collagen and proteins associated with proteoglycan synthesis. However, cells from the TMJ fibrocartilage are special in nature and produce type I collagen, type II collagen, and proteoglycans. Ascertainment of the fibrochondrocyte-like phenotype of cells from TMJ showed that these cells exhibited constitutive expression of aggrecan, biglycan, TGFβ1, type I collagen, and type II collagen mRNA (Figure 1A). Additionally, these cells showed the presence of aggrecan and biglycan by immunofluorescence using anti-aggrecan and biglycan immunoglobulins as primary antibodies (Santa Cruz Biotechnology) (Figures 1B and C), and proteoglycan synthesis as observed by incorporation of Na2-35SO4 into chondroitin sulfate proteoglycans (Figure 1D).
CTS suppresses IL-1β–dependent NO production
IL-1β initiates cartilage catabolism through transcriptional activation of multiple genes (7–14), including iNOS. Therefore, NO production initially was used as a marker to evaluate the effects of CTS on TMJ fibrochondrocytes. To evaluate the effects of CTS on rHuIL-1β actions, TMJ fibrochondrocytes were exposed to various magnitudes of equibiaxial CTS (3%, 6%, 9%, or 12% equibiaxial strain) in the presence or absence of rHuIL-1β (1 ng/ml). Fibrochondrocytes treated with rHuIL-1β alone exhibited high levels of iNOS mRNA, whereas cells treated simultaneously with rHuIL-1β and CTS for 4 hours or 24 hours exhibited a significant inhibition of NO production (P ≤ 0.05) at 3%, 6%, and 9% elongation (Figure 2A); 12% elongation was ineffective in this respect. Since 6% CTS inhibited NO production optimally, in the rest of the experiments 6% CTS was used to examine its antiinflammatory effects on fibrochondrocytes. In these experiments, neither untreated control cells nor cells subjected to strain alone expressed iNOS mRNA. Morphologically, fibrochondrocytes subjected to CTS exhibited minimal cell deformation, compared with unstrained control cells (Figure 1E). Similarly, neither negligible cell detachment (<0.1%), as assessed by counting of nonadherent cells in the wells, nor cell death, as assessed by DNA fragmentation, was observed (data not shown).
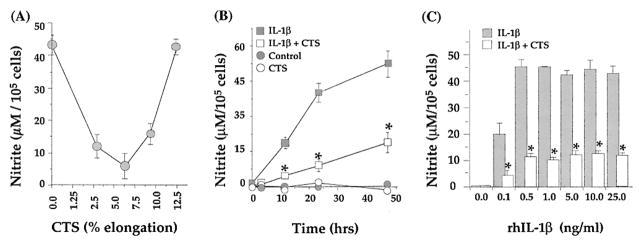
Figure 2.
A, Effect of various magnitudes of equibiaxial cyclic tensile strain (CTS) on recombinant human interleukin-1β (rhIL-1β)–induced production of nitric oxide (NO) in temporomandibular joint (TMJ) fibrochondrocytes. TMJ chondrocytes were incubated with rHuIL-1β (1 ng/ml) and simultaneously exposed to 0%, 3%, 6%, 9%, or 12% CTS for 24 hours. B, Time-dependent inhibition of rhIL-1β–induced production of NO by CTS. TMJ condylar chondrocytes were untreated, or were exposed to 6% CTS, to 1 ng/ml rhIL-1β, or to rhIL-1β and CTS for 4, 12, 24, or 48 hours. C, Effect of CTS on production of NO in response to various concentrations of rhIL-1β. Chondrocytes were treated with 0, 0,1, 0.5, 1, 5, 10, or 25 ng/ml of rHuIL-1β for 24 hours with or without simultaneous exposure to 6% CTS for 24 hours. Accumulation of NO in the culture supernatant was assessed by Griess reaction. Data represent the mean and SEM of triplicate values in all experiments. * = P < 0.05 as compared with cells treated with IL-1β alone.
Similar to articular chondrocytes, rHuIL-1β induces sustained NO production in fibrochondrocytes (30). Therefore, we determined whether the effects of CTS were paralleled in inhibiting the rHuIL-1β–dependent NO production in a sustained manner. As shown in Figure 2B, NO production increased rapidly after 12 hours of rHuIL-1β exposure, and this continued over a period of 48 hours. Interestingly, CTS consistently inhibited rHuIL-1β–induced NO production. Nevertheless, CTS alone did not induce NO production.
We next examined whether CTS inhibits rHuIL-1β–dependent NO production at higher concentrations of rHuIL-1β. TMJ chondrocytes were treated with 0, 0.5, 1, 10, or 25 ng/ml of rHuIL-1β for 24 hours, and the NO production was measured in the culture supernatants. As is apparent in Figure 2C, increasing the concentration of rHuIL-1β resulted in an increase in NO production between 0.1 and 1 ng/ml of rHuIL-1β, but higher concentrations of rHuIL-1β did not significantly elevate NO production. CTS (6% strain) suppressed rHuIL-1β–induced NO induction significantly (P ≤ 0.05) at all concentrations of rHuIL-1β tested.
CTS-mediated suppression of IL-1β–dependent NO production via inhibition of iNOS mRNA induction and iNOS synthesis
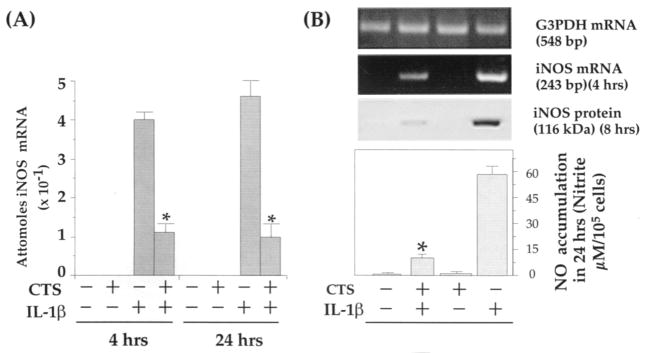
Since CTS abrogated IL-1β–induced NO production, we next examined whether this inhibition is due to the inhibition of iNOS mRNA induction. As evidenced by the data shown in Figure 3A, CTS significantly suppressed rHuIL-1β–induced iNOS mRNA expression as assessed by RT-QCPCR. Furthermore, the CTS-mediated abrogation of rHuIL-1β–induced iNOS mRNA expression was paralleled by decreased iNOS synthesis (Figure 3B). Fibrochondrocytes subjected to CTS alone, or untreated control cells, exhibited neither iNOS mRNA nor protein synthesis.
Figure 3.
CTS-mediated inhibition of recombinant human IL-1β (rHuIL-1β)–dependent inducible nitric oxide synthase (iNOS) expression and production of NO in TMJ fibrochondrocytes. A, Quantitative reverse transcriptase–polymerase chain reaction (RT-QCPCR) analysis of iNOS mRNA expression, showing inhibition of IL-1β (1 ng/ml)–induced mRNA production by CTS in TMJ fibrochondrocytes. B, Parallel experiments showing CTS-mediated inhibition of rHuIL-1β–induced iNOS mRNA synthesis, iNOS protein synthesis, and total accumulation of NO in culture supernatants of the TMJ fibrochondrocytes. Control cells and those treated with CTS alone did not exhibit iNOS gene products in RT-QCPCR, iNOS protein in Western blot analysis, or production of NO in culture supernatants. Results represent the mean and SEM in 1 of 3 separate experiments, showing similar data. * = P < 0.05 as compared with cells treated with IL-1β alone. See Figure 2 for other definitions.
CTS suppresses IL-1β–dependent induction of COX-2 mRNA and PGE2 synthesis
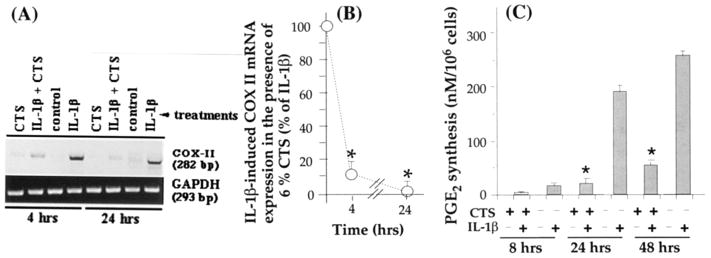
Another important proinflammatory molecule that is markedly up-regulated following IL-1β–dependent activation of chondrocytes is PGE2, synthesized by COX-2 (18,35). To examine whether the physiologic consequences of CTS action also involve inhibition of rHuIL-1β–dependent COX-2 mRNA expression and PGE2 production, we subjected TMJ fibrochondrocytes to rHuIL-1β and CTS simultaneously or individually for either 4 or 24 hours. As is apparent in Figure 4A, CTS significantly suppressed IL-1β–induced COX-2 mRNA expression. The semiquantitative measurement of the PCR products for COX-2 revealed 82% and 96% inhibition of COX-2 mRNA expression at 4 hours and 24 hours, respectively (P ≤ 0.01). The inhibition of COX-2 mRNA induction by CTS was paralleled by 88% and 81% inhibition of rHuIL-1β–dependent PGE2 production after 24 and 48 hours, respectively (Figure 4B). Unactivated control TMJ fibrochondrocytes or those exposed to CTS alone did not express COX-2 mRNA or PGE2 production.
Figure 4.
Inhibition of recombinant human IL-1β (rHuIL-1β)–dependent cyclooxygenase 2 (COX-II) mRNA expression and prostaglandin E2 (PGE2) synthesis by CTS in TMJ fibrochondrocytes. A, COX-II mRNA expression in chondrocytes either untreated or subjected to 1 ng/ml rHuIL-1β, 6% CTS, or rHuIL-1β and CTS for 4 or 24 hours, showing a marked inhibition of IL-1β–dependent mRNA expression of COX-II. B, Inhibition of COX-II mRNA expression by 6% CTS in the presence of IL-1β as compared with cells treated with IL-1β alone. C, PGE2 synthesis in chondrocytes subjected to treatment regimens described in A for 8, 24, or 48 hours. PGE2 synthesis was measured in the culture supernatants by radioimmunoassay. Data in A represent 1 of 3 separate experiments. Data in B and C represent means and SEM of triplicate values. * = P < 0.05 as compared with cells treated with IL-1β alone. See Figure 2 for other definitions.
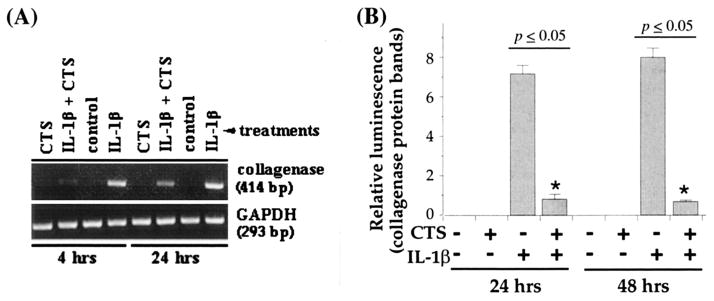
CTS suppresses IL-1β–dependent induction of collagenase (MMP-1)
Previous studies have demonstrated that besides other MMP, induction of MMP-1 in response to IL-1β plays a critical role during cartilage degradation in inflammatory joint disease (23,36–40). Therefore, to be effective in controlling catabolic effects of rHuIL-1β, CTS actions must include suppression of collagenase production. Exposure of TMJ fibrochondrocytes to rHuIL-1β induced significant amounts of MMP-1. As expected, cells subjected to rHuIL-1β and CTS exhibited a marked (98% during the first 4 hours and 83% after 24 hours) inhibition of MMP-1 mRNA expression (Figure 5A). More important, the reduction in MMP-1 mRNA expression was reflected in the inhibition of MMP-1 synthesis as assessed by Western blot analysis, showing a 92% inhibition during the first 8 hours and 87% after 24 hours (Figure 5B). As observed above, untreated control cells, or cells treated with CTS alone, did not exhibit expression of MMP-1 mRNA.
Figure 5.
Effect of CTS on recombinant human IL-1β (rHuIL-1β)–dependent collagenase mRNA expression and synthesis in TMJ fibrochondrocytes. A, Expression of collagenase mRNA in chondrocytes either untreated or subjected to rHuIL-1β, CTS, or rHuIL-1β and CTS for 4 or 24 hours. B, Collagenase synthesis by fibrochondrocytes subjected to treatment regimens described in A for 24 or 48 hours. Collagenase synthesis was measured as the relative intensity of each band in Western blot analysis. Data in A represent 1 of 3 separate experiments. Data in B represent means and SEM of triplicate values. See Figure 2 for other definitions.
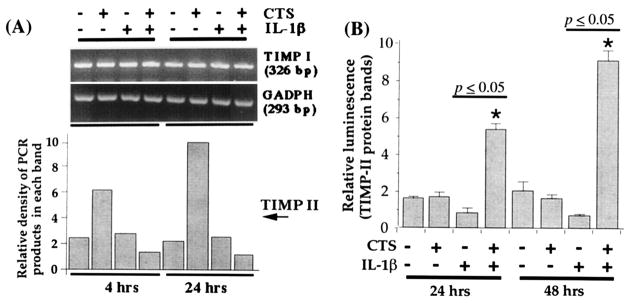
CTS abrogates IL-1β–dependent inhibition of TIMP mRNA expression
For enhanced cartilage degradation, IL-1β not only activates metalloproteinases, but also inhibits TIMP production (41–43). This results in prolonged enzymatic activity of metalloproteinases (32–35). Therefore, to examine whether CTS also abrogates rHuIL-1β–induced suppression of mRNA for TIMP (TIMP-1 and TIMP-2), TMJ chondrocytes were exposed to CTS in the presence or absence of rHuIL-1β. The densitometric analysis of the PCR products for TIMP-1 revealed that exposure to rHuIL-1β does not inhibit TIMP-1 mRNA expression significantly after either 4 or 24 hours of exposure (Figure 6). However, treatment of chondrocytes with rHuIL-1β (1 ng/ml) resulted in a consistent inhibition of the constitutive expression of TIMP-2 mRNA. More important, coexposure of chondrocytes to rHuIL-1β and CTS resulted in hyperinduction of TIMP-2 mRNA, i.e., a 3 ± 0.44–fold increase in TIMP-2 mRNA was observed during the first 4 hours and a 6.2 ± 2.1–fold increase was observed after 24 hours compared with untreated control cells (P < 0.05). CTS alone did not affect TIMP-2 mRNA expression, demonstrating that CTS acted on TMJ fibrochondrocytes solely in an rHuIL-1β–dependent manner.
Figure 6.
Effect of CTS on recombinant human IL-1β (rHuIL-1β)–dependent inhibition of tissue inhibitor of metalloproteinases (TIMP) mRNA expression. A, Expression of mRNA for TIMP I and TIMP II in TMJ chondrocytes either untreated or exposed to 1 ng/ml rHuIL-1β, 6% CTS, or rHuIL-1β and CTS. The mRNA expression was assessed by reverse transcriptase–polymerase chain reaction using 1 μg RNA from cells in each group. Amplification of GAPDH mRNA was used to ensure equal input in all lanes. B, TIMP II synthesis in cells subjected to treatment regimens described in A. Data in A represent 1 of 3 separate experiments. Data in B represent means and SEM of triplicate values. See Figure 2 for other definitions.
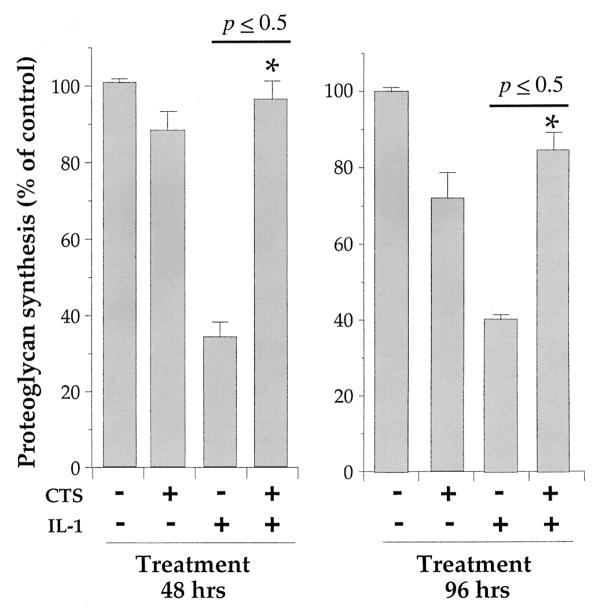
CTS abrogates the rHuIL-1β–dependent inhibition of proteoglycan synthesis in chondrocytes
A hallmark of cartilage degradation in inflamed joints is the inhibition of proteoglycan synthesis, which is attributed to the actions of IL-1β (18,44,45). Therefore, we next examined whether CTS abrogates rHuIL-1β–dependent inhibition of proteoglycan synthesis. TMJ fibrochondrocytes were exposed to rHuIL-1β, CTS, or rHuIL-1β and CTS for 24, 48, or 72 hours and pulsed with TCM containing Na2-35SO4 during the last 8 hours of incubation. The incorporation of 35SO4 into proteoglycans was used as an indicator of rHuIL-1β and/or CTS responses. Quantitative assessment of CTS-mediated abrogation of rHuIL-1β–dependent inhibition of proteoglycan synthesis showed that CTS alone down-regulated proteoglycan synthesis by a mean ± SEM of 11 ± 2%, 14 ± 3%, and 9 ± 3% at 24, 48, and 72 hours, respectively. Likewise, exposure of cells to rHuIL-1β alone resulted in a 69 ± 4.4%, 62 ± 3.6%, and 57 ± 5.6% reduction in 35SO4 incorporation in proteoglycans at 24, 48, and 72 hours, respectively. However, application of CTS almost completely abrogated rHuIL-1β–dependent inhibition of proteoglycan synthesis at all time points (Figure 7).
Figure 7.
Effect of CTS on recombinant human IL-1β (rHuIL-1β)–dependent inhibition of proteoglycan synthesis in TMJ fibrochondrocytes. Chondrocytes were either untreated or exposed to 1 ng/ml rHuIL-1β, 6% CTS, or rHuIL-1β and CTS for 48 or 96 hours. Total proteoglycans were assessed by incorporation of Na2-35SO4 during the last 8 hours of incubation followed by extraction of glycosaminoglycans with 0.5M NaOH and separated on PD-10 size exclusion chromatography columns. Data represent means and SEM of triplicate values in 1 of 3 separate experiments. See Figure 2 for other definitions.
DISCUSSION
The data presented here provide support for a critical role of CTS on TMJ fibrochondrocytes in the modulation of proinflammatory actions of rHuIL-1β, the major inflammatory agent implicated in the etiology of TMJ disorders and other arthritic diseases (12,17,19,23,36). Rehabilitation of postoperative TMJ by continuous passive motion has been shown to augment speedier and more physiologically sound recovery in TMJ disorder patients (25–30,46). To delineate the mechanisms of continuous passive motion–mediated reparative actions, we have developed an in vitro model system. In this system, the equibiaxial CTS closely mimicks the effects of continuous passive motion in vivo because cartilage exposed to continuous passive motion is subjected to tensile strain on the superficial layers (46); chondrocytes residing in the superficial layers of cartilage are exposed to inflammatory mediators in inflamed joints; and the chondrocytes in the superficial layers of cartilage are the cells most responsive to rHuIL-1β that take part in cartilage destruction (18,48,49). Thus, this in vitro system, while closely simulating the in vivo effects of continuous passive motion on inflamed or rehabilitating joints, also provided defined parameters to examine the biochemical signals generated by CTS in TMJ chondrocytes.
We have shown that fibrochondrocytes phenotypically are distinct from articular chondrocytes, because these cells synthesize type I collagen in addition to chondrocyte-associated proteins such as type II collagen and proteoglycans. The synthesis of type I collagen by these cells is not surprising since disk cartilage synthesized by fibrochondrocytes does contain type I collagen. However, fibrochondrocytes, similar to articular chondrocytes, synthesize proinflammatory proteins such as iNOS, COX-2, and MMP-1 in response to rHuIL-1β.
Similar to articular chondrocytes, rHuIL-1β also exerts catabolic effects on TMJ fibrochondrocytes via induction of multiple proteins that mediate cartilage degradation. We have observed that application of low intensities of CTS (3–9%) consistently suppresses rHuIL-1β–dependent NO production, whereas higher intensity CTS (12.5%) fails to exert antiinflammatory effects. Interestingly, continuous passive motion therapy has been shown to yield beneficial effects in some patients, but not in all. These observations, together with our present results, tempt us to speculate that the magnitude of strain exerted on the inflamed joints may be a critical determinant of the therapeutic effects of continuous passive motion. At present it is difficult to relate the magnitude of CTS exerted on chondrocytes in vitro to the magnitude of strain experienced by chondrocytes of the TMJ disk by various regimens of continuous passive motion in vivo. Nevertheless, the understanding of the effects of various magnitudes of CTS on TMJ chondrocytes in vitro may provide leads to understand the basis for the success or failure of continuous passive motion therapy in vivo. Additionally, we have used cyclic strain at 0.05 Hz to examine the antiinflammatory actions of CTS; whether CTS also exerts antiinflammatory effects at lower or higher frequencies is yet to be determined.
With regard to the antiinflammatory actions of CTS, the data suggest that 6% CTS suppresses rHuIL-1β actions over a wide range of concentrations, encompassing those frequently found in inflamed synovial fluids (6,7). This emphasizes the potential of CTS in antagonizing the effects of rHuIL-1β and is of considerable clinical relevance regarding the treatment of inflamed joints. CTS is effective in inhibiting rHuIL-1β actions at between 3% and 10% equibiaxial strain, although 6% CTS exerts maximal inhibition of the proinflammatory responses of rHuIL-1β in vitro. Therefore, we have examined the effects of 6% CTS on the proinflammatory responses of rHuIL-1β in TMJ disk fibrochondrocytes.
CTS, by reducing the mRNA abundance of 2 pivotal proinflammatory enzymes, iNOS and COX-2, suppresses their inflammatory products, NO and PGE2. Both NO and PGE2 have been implicated in mediating IL-1β–induced reduction of proteoglycan synthesis (35,45). Interestingly, the presence of an inflammatory signal was a prerequisite for the observed CTS actions, since CTS (6%) alone failed to induce a response. This supports earlier reports suggesting that the low magnitude of CTS alone is not sufficient to induce synthesis of proinflammatory mediators (50). Thus, our studies point to the importance of the magnitude of CTS in its actions, i.e., low magnitude of CTS exerts antiinflammatory actions, while it is not sufficient to induce proinflammatory responses by itself. Nevertheless, as reported by others (51–53), we have also observed that a high magnitude of CTS induces iNOS and COX-2 expression in chondrocytes. High magnitude of CTS is also associated with proteoglycan degradation, which, in turn, is regulated by intracellular levels of both NO and PGE2 (18,43,51,53,54), as well as via induction of IL-1 synthesis (51).
During cartilage degradation, cytokines such as rHuIL-1β induce extracellular matrix breakdown as well as inhibition of its synthesis. For example, chronic inflammation induces sustained collagenase production as well as inhibition of type II collagen synthesis (17,19,23,56,57). Therefore, for effective control of cartilage catabolism, prolonged suppression of collagenase production and concurrent increase in collagen synthesis is essential. In fact, our data show that CTS inhibits cartilage catabolism effectively by way of inducing prolonged suppression of rHuIL-1β–dependent collagenase synthesis via inhibition of its mRNA expression. Interestingly, application of CTS alone did not exert any effects on collagenase synthesis, which is not surprising since continuous passive motion on healthy joints has not been shown to induce cartilage degradation (51–53,58).
IL-1β has been shown to down-regulate synthesis of TIMP-2, which may facilitate increased collagen breakdown during inflammation (40–43), whereas application of TIMP-1 and TIMP-2 has been shown to inhibit IL-1β–induced collagen degradation in cartilage (41–43). Therefore, examination of the effects of CTS on the rHuIL-1β–mediated inhibition of TIMP-1 and TIMP-2 was essential to fully understand the consequences of CTS application on chondrocytes. We have observed that CTS actions indeed involve abrogation of rHuIL-1β–induced inhibition of TIMP-2 mRNA expression in parallel to inhibition of collagenase production. Furthermore, in the presence of rHuIL-1β, CTS caused hyperinduction of TIMP-2 mRNA expression, amounting to 3.2-fold and 5.6-fold increases in 4 and 24 hours, respectively. Whether all of the TIMP-2 induced by CTS is in its active form is as yet not clear. TIMP-2 blocks collagenase activity effectively at less than an equimolar ratio. This suggests that the increased production of TIMP-2 by CTS for prolonged periods may act as an effective mechanism to reduce metalloproteinase-mediated extracellular matrix degradation. Nevertheless, the presence of an inflammatory signal, such as rHuIL-1β, was essential to induce TIMP-2 mRNA expression, and exposure of chondrocytes to CTS alone was not sufficient for its induction. Interestingly, we did not observe significant down-regulation of TIMP-1 by rHuIL-1β (41,42) or its upregulation in TMJ chondrocytes by CTS alone, or following coapplication of IL-1β and CTS.
A characteristic feature of arthritic diseases is breakdown of cartilage via degradation of chondroitin sulfate proteoglycans and inhibition of their synthesis. Our data demonstrate that CTS is effective in revoking IL-1β–dependent inhibition of proteoglycan synthesis, thus providing evidence that CTS not only antagonizes rHuIL-1β–induced proinflammatory responses, but also augments proteoglycan synthesis. Since NO inhibits sulfation of proteoglycans (47,48), it is also possible that CTS negates the effects of rHuIL-1β–dependent suppression of proteoglycan synthesis via both up-regulation of proteoglycan synthesis and inhibition of NO production, which in turn results in increased chondroitin sulfate proteoglycan synthesis. Interestingly, while continuous passive motion has been widely used for postoperative rehabilitation of TMJ patients, a number of reports have shown that its application is not always beneficial (51–53,55,58). It has been shown that a high magnitude of CTS initiates collagen degradation via induction of collagenase synthesis (50), suggesting that the magnitude of mechanical strain may be a critical determinant in the success or failure of continuous passive motion therapy. For example, the failure of continuous passive motion therapy may be due to the high magnitude of mechanical load, whereas its beneficial effects may be associated with physiologic/low magnitudes of mechanical strain in vivo, which has been shown to be reparative in nature.
In summary, our results clearly demonstrate that low magnitude of CTS is an effective antagonist of IL-1β actions and may therefore play a significant role in the repair of cartilage via inhibition of proinflammatory responses. CTS actions on TMJ fibrochondrocytes involve transcriptional regulation of multiple genes activated by IL-1β. CTS appears to disrupt/down-regulate 1 or more critical step(s) in the signal transduction cascade of IL-1β upstream of mRNA synthesis that not only result in the inhibition of inflammatory responses of TMJ chondrocytes, but also aid in their repair. Nonetheless, these actions depend on the presence of IL-1β; in the absence of IL-1β, CTS of low magnitude is ineffective in inducing reparative responses. In this regard, actions of CTS on chondrocytes appear to be strikingly comparable with those of the therapeutic agents that are currently being used to minimize cartilage degradation, such as anti–IL-1 immunoglobulins, IL-1 receptor antagonist, or metalloproteinase inhibitors (17,63–65). However, while these therapies reduce the catabolic actions of IL-1, CTS appears to initiate reparative activity in chondrocytes as well. Whether CTS specifically inhibits rHuIL-1β actions or can counteract actions of other inflammatory mediators such as tumor necrosis factor α or microbial lipopolysaccharides found in inflamed synovial joints (63,67) is yet to be determined.
Acknowledgments
Supported by grants from the Oral and Maxillofacial Surgery Foundation, the NIH (grant R-15-DE-12976), and Central Medical Research Funds, University of Pittsburgh.
The authors are indebted to Dr. Mohammed Heidaran, Orquest, Mountain View, CA, for providing sequences to rabbit primers for aggrecan and type II collagen.
References
- 1.Kopp S. Degenerative and inflammatory temporomandibular joint disorders: clinical perspectives. In: Sessle BJ, Bryant PS, Dionne RA, editors. Progress in pain research and management. Vol 4. Temporomandibular disorders and related pain conditions. Seattle (WA): ISAP Press; 1995. pp. 119–32. [Google Scholar]
- 2.Albani S, Carson DA. Etiology and pathogenesis of rheumatoid arthritis. In: Koopman WJ, editor. Arthritis and allied health conditions: a textbook of rheumatology. Vol. 1. Baltimore (MD): Williams & Wilkins; 1997. pp. 979–91. [Google Scholar]
- 3.Mahan PE, Alling CC. Temporomandibular joint anatomy, function, and pathofunction. In: Mahan PE, Alling CC III, editors. Facial pain. Philadelphia: Lea & Febiger; 1991. pp. 197–216. [Google Scholar]
- 4.Zarb GA, Carlsson GE. Osteoarthrosis/osteoarthritis. In: Zarb GA, Carlsson GE, Sessle BJ, Mohl ND, editors. Temporomandibular joint and masticatory muscle disorders. Copenhagen: Munksgaard; 1994. pp. 298–327. [Google Scholar]
- 5.Israel HA, Diamond BE, Saed-Nejad F, Ratcliffe A. Correlation between arthroscopic diagnosis of osteoarthritis and synovitis of the human temporomandibular joint and keratan sulfate levels in the synovial fluid. J Oral Maxillofac Surg. 1997;55:210–7. doi: 10.1016/s0278-2391(97)90526-7. [DOI] [PubMed] [Google Scholar]
- 6.Alstergren P, Kopp S, Theodorsson E. Synovial fluid sampling from the temporomandibular joint: sample quality criteria and levels of interleukin-1 beta and serotonin. Acta Odontol Scand. 1999;57:16–22. doi: 10.1080/000163599429057. [DOI] [PubMed] [Google Scholar]
- 7.Alstergren P, Ernberg M, Kvarnstrom M, Kopp S. Interleukin-1beta in synovial fluid from the arthritic temporomandibular joint and its relation to pain, mobility, and anterior open bite. J Oral Maxillofac Surg. 1998;56:1059–65. doi: 10.1016/s0278-2391(98)90256-7. [DOI] [PubMed] [Google Scholar]
- 8.Kubota E, Kubota T, Matsumoto J, Shibata T, Murakami KI. Synovial fluid cytokines and proteinases as markers of temporomandibular joint disease. J Oral Maxillofac Surg. 1998;56:192–8. doi: 10.1016/s0278-2391(98)90868-0. [DOI] [PubMed] [Google Scholar]
- 9.Murakami KI, Shibata T, Kubota E, Maeda H. Intra-articular levels of prostaglandin E2, hyaluronic acid, and chondroitin-4 and -6 sulfates in the temporomandibular joint synovial fluid of patients with internal derangement. J Oral Maxillofac Surg. 1998;56:199–203. doi: 10.1016/s0278-2391(98)90869-2. [DOI] [PubMed] [Google Scholar]
- 10.Nordahl S, Alstergren P, Eliasson S, Kopp S. Interleukin-1beta in plasma and synovial fluid in relation to radiographic changes in arthritic temporomandibular joints. Eur J Oral Sci. 1998;106:559–63. doi: 10.1046/j.0909-8836.1998.eos106104.x. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka S, Hamanishi C, Kikuchi H, Fukuda K. Factors related to degradation of articular cartilage in osteoarthritis: a review. Semin Arthritis Rheum. 1998;27:392–9. doi: 10.1016/s0049-0172(98)80019-x. [DOI] [PubMed] [Google Scholar]
- 12.Evans CH, Watkins SC, Stefanovic-Racic M. Nitric oxide and cartilage metabolism. Methods Enzymol. 1996;269:75–88. doi: 10.1016/s0076-6879(96)69011-9. [DOI] [PubMed] [Google Scholar]
- 13.Lotz M, Blanco FJ, von Kempis J, Dudler J, Maier R, Villiger PM, et al. Cytokine regulation of chondrocyte functions. J Rheumatol Suppl. 1995;43:104–8. [PubMed] [Google Scholar]
- 14.Ratcliffe A, Israel HA, Saed-Nejad F, Diamond B. Proteoglycans in the synovial fluid of the temporomandibular joint as an indicator of changes in cartilage metabolism during primary and secondary osteoarthritis. J Oral Maxillofac Surg. 1998;56:204–8. doi: 10.1016/s0278-2391(98)90870-9. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai H, Kohsaka H, Liu MF, Higashiyama H, Hirata Y, Kanno K, et al. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J Clin Invest. 1995;96:2357–63. doi: 10.1172/JCI118292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Lent P, van de Loo FA, Holthuysen AE, van den Bersselaar LA, Vermeer LA, van den Berg WB. Major role for interleukin-1 but not TNF in early cartilage damage in immune complex arthritis in mice. J Rheumatol. 1995;22:2250–8. [PubMed] [Google Scholar]
- 17.Flugge LA, Miller-Deist LA, Petillo PA. Towards a molecular understanding of arthritis. Chem Biol. 1999;6:157–69. doi: 10.1016/S1074-5521(99)80043-X. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda K, Ohtani K, Dan H, Tanaka S. IL-1 inhibits keratan sulfate production by rabbit chondrocytes: possible role of prostaglandin E2. Inflamm Res. 1995;44:178–83. doi: 10.1007/BF01782816. [DOI] [PubMed] [Google Scholar]
- 19.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, van de Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–73. [PubMed] [Google Scholar]
- 20.Manfield L, Jang D, Murrell AC. Nitric oxide enhances cyclooxygenase activity in articular cartilage. Inflamm Res. 1996;45:254–8. doi: 10.1007/BF02259612. [DOI] [PubMed] [Google Scholar]
- 21.Van de Loo FA, Arntz OJ, Otterness IG, van den Berg WB. Protection against cartilage proteoglycan synthesis inhibition by anti-interleukin-1 antibodies in experimental arthritis. J Rheumatol. 1995;19:348–54. [PubMed] [Google Scholar]
- 22.Taskiran D, Stefanovic-Racic M, Georgescu H, Evans CH. Nitric oxide mediates suppression of cartilage proteoglycan synthesis by IL-1. Biochem Biophys Res Commun. 1994;200:142–9. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- 23.Murrell GAC, Jang D, Williams RJ. Nitric oxide activates metalloprotease in articular cartilage. Biochem Biophys Res Commun. 1995;206:15–21. doi: 10.1006/bbrc.1995.1003. [DOI] [PubMed] [Google Scholar]
- 24.Stefanovic-Racic M, Stadler J, Evans CH. Nitric oxide and arthritis. Arthritis Rheum. 1993;36:1036–44. doi: 10.1002/art.1780360803. [DOI] [PubMed] [Google Scholar]
- 25.McCarty WL, Darnell MW. Rehabilitation of the temporomandibular joint through the application of motion. Cranio. 1993;11:298–306. doi: 10.1080/08869634.1993.11677982. [DOI] [PubMed] [Google Scholar]
- 26.Kim HK, Kerr RG, Cruz TF, Salter RB. Effects of continuous passive motion and immobilization on synovitis and cartilage degradation in antigen induced arthritis. J Rheumatol. 1995;22:1714–21. [PubMed] [Google Scholar]
- 27.Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clin. 1994;10:211–8. [PubMed] [Google Scholar]
- 28.Williams JM, Moran M, Thonar E, Salter RB. Continuous passive motion stimulates repair of rabbit knee articular cartilage after matrix proteoglycan loss. Clin Orthop. 1994;304:252–9. [PubMed] [Google Scholar]
- 29.Von Schroeder HP, Coutts RD, Billings J, Mai MT, Aratow M. The changes in intramuscular pressure and femoral vein flow with continuous passive motion, pneumatic compressive stockings, and leg manipulations. Clin Orthop. 1991;266:218–26. [PubMed] [Google Scholar]
- 30.Gassner R, Buckley MJ, Georgescu H, Studer R, Stefanovich-Racic M, Piesco NP, et al. Cyclic tensile stress exerts anti-inflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol. 1999;163:2187–94. [PMC free article] [PubMed] [Google Scholar]
- 31.Fedewa MM, Oegema TR, Schwartz MH, MacLeod A, Lewis JL. Chondrocytes in cellular culture produce a mechanically functional tissue. J Orthop Res. 1998;16:227–35. doi: 10.1002/jor.1100160210. [DOI] [PubMed] [Google Scholar]
- 32.Yu H, Grynpas M, Kandel RL. Composition of cartilagenous tissue with mineralized and non-mineralized zones formed in vitro. Biomaterials. 1997;18:1425–33. doi: 10.1016/s0142-9612(97)00071-9. [DOI] [PubMed] [Google Scholar]
- 33.Hart DA, Boykiw R, Sciore P, Reno C. Complex alterations in gene expression occur in the knee ligaments of the skeletally mature multiparous rabbit during pregnancy. Biochim Biophys Acta. 1998;1397:331–41. doi: 10.1016/s0167-4781(98)00018-9. [DOI] [PubMed] [Google Scholar]
- 34.Morisset S, Patry C, Lora M, de Brum-Fernandes AJ. Regulation of cyclooxygenase-2 expression in bovine chondrocytes in culture by interleukin-1α, tumor necrosis factor-α, glucocorticoids, and 17β-estradiol. J Rheumatol. 1998;25:1146–53. [PubMed] [Google Scholar]
- 35.Evans CH. The role of proteinases in cartilage destruction. Agents Actions Suppl. 1991;32:135–52. doi: 10.1007/978-3-0348-7405-2_19. [DOI] [PubMed] [Google Scholar]
- 36.Lotz M, Blanco FJ, von Kempis J, Dudler J, Maier R, Villiger PM, et al. Cytokine regulation of chondrocyte functions. J Rheumatol Suppl. 1995;43:104–8. [PubMed] [Google Scholar]
- 37.Saito S, Katoh M, Masumoto M, Matsumoto S, Masuho Y. Involvement of MMP-1 and MMP-3 in collagen degradation induced by IL-1 in rabbit cartilage explant culture. Life Sci. 1998;62:359–65. doi: 10.1016/s0024-3205(98)00181-7. [DOI] [PubMed] [Google Scholar]
- 38.Borden P, Solymar D, Sucharczuk A, Lindman B, Cannon P, Heller RA. Cytokine control of interstitial collagenase and collagenase-3 gene expression in human chondrocytes. J Biol Chem. 1996;271:23577–81. doi: 10.1074/jbc.271.38.23577. [DOI] [PubMed] [Google Scholar]
- 39.Kozaci LD, Buttle DJ, Hollander AP. Degradation of type II collagen, but not proteoglycan, correlates with matrix metalloproteinase activity in cartilage explant cultures. Arthritis Rheum. 1997;40:164–74. doi: 10.1002/art.1780400121. [DOI] [PubMed] [Google Scholar]
- 40.Cawston T, Billington C, Cleaver C, Elliott S, Hui W, Koshy P, et al. The regulation of MMPs and TIMPs in cartilage turnover. Ann N Y Acad Sci. 1999;878:120–9. doi: 10.1111/j.1749-6632.1999.tb07678.x. [DOI] [PubMed] [Google Scholar]
- 41.Shingu M, Nagai Y, Isayama T, Naono T, Nobunaga M, Nagai Y. The effects of cytokines on metalloproteinase inhibitors (TIMP) and collagenase production by human chondrocytes and TIMP production by synovial cells and endothelial cells. Clin Exp Immunol. 1993;94:145–9. doi: 10.1111/j.1365-2249.1993.tb05992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellis AJ, Curry VA, Powell EK, Cawston TE. The prevention of collagen breakdown in bovine nasal cartilage by TIMP, TIMP-2 and a low molecular weight synthetic inhibitor. Biochem Biophys Res Commun. 1994;201:94–101. doi: 10.1006/bbrc.1994.1673. [DOI] [PubMed] [Google Scholar]
- 43.Van de Loo FA, Arntz OJ, Otterness IG, van den Berg WB. Protection against cartilage proteoglycan synthesis inhibition by antiinterleukin 1 antibodies in experimental arthritis. J Rheumatol. 1992;19:348–56. [PubMed] [Google Scholar]
- 44.Taskiran D, Stefanovic-Racic M, Georgescu H, Evans C. Nitric oxide mediates suppression of cartilage proteoglycan synthesis by interleukin-1. Biochem Biophys Res Commun. 1994;200:142–8. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- 45.Kim HK, Kerr RG, Cruz TF, Salter RB. Effects of continuous passive motion and immobilization on synovitis and cartilage degradation in antigen induced arthritis. J Rheumatol. 1995;22:1714–21. [PubMed] [Google Scholar]
- 46.Häuselmann HJ, Flechtenmacher J, Michal L, Thonar EJ-MA, Shinmei M, Kuettner KE, et al. The superficial layer of human articular cartilage is more susceptible to interleukin-1–induced damage than the deeper layers. Arthritis Rheum. 1996;39:478–88. doi: 10.1002/art.1780390316. [DOI] [PubMed] [Google Scholar]
- 47.Hickery MS, Bayliss MT. Interleukin-1 induced nitric oxide inhibits sulphation of glycosaminoglycan chains in human articular chondrocytes. Biochim Biophys Acta. 1998;1425:282–90. doi: 10.1016/s0304-4165(98)00080-4. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi T, Abe E, Yamate T, Taguchi Y, Jasin HE. Nitric oxide production by superficial and deep articular chondrocytes. Arthritis Rheum. 1997;40:261–9. doi: 10.1002/art.1780400210. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Akyuz U, Xu L, Pidaparti RM. Stress analysis of the human temporomandibular joint. Med Eng Phys. 1998;20:565–72. doi: 10.1016/s1350-4533(98)00070-8. [DOI] [PubMed] [Google Scholar]
- 50.Fujisawa T, Hattori T, Takahashi K, Kuboki T, Yamashita A, Takigawa M. Cyclic mechanical stress induces extracellular matrix degradation in cultured chondrocytes via gene expression of matrix metalloproteinases and interleukin-1. J Biochem (Tokyo) 1999;125:966–75. doi: 10.1093/oxfordjournals.jbchem.a022376. [DOI] [PubMed] [Google Scholar]
- 51.Klein-Nulend J, Burger EH, Semeins CM, Raisz LG, Pilbeam CC. Pulsating fluid flow stimulates prostaglandin release and inducible prostaglandin G/H synthase mRNA expression in primary mouse bone cells. J Bone Miner Res. 1997;12:45–51. doi: 10.1359/jbmr.1997.12.1.45. [DOI] [PubMed] [Google Scholar]
- 52.Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts—correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217:640–8. doi: 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- 53.Israel HA, Syrop SB. The important role of motion in the rehabilitation of patients with mandibular hypomobility: a review of the literature. Cranio. 1997;15:74–83. doi: 10.1080/08869634.1997.11745995. [DOI] [PubMed] [Google Scholar]
- 54.Management of temporomandibular disorders: National Institutes of Health Technology Assessment Conference Statement. J Am Dent Assoc. 1996;127:1595–606. [PubMed] [Google Scholar]
- 55.Chandrasekhar S, Harvey AK, Higginbotham JD, Horton WE. Interleukin-1-induced suppression of type II collagen gene transcription involves DNA regulatory elements. Exp Cell Res. 1990;191:105–14. doi: 10.1016/0014-4827(90)90042-9. [DOI] [PubMed] [Google Scholar]
- 56.Goldring MB, Fukuo K, Birkhead JR, Dudek E, Sandell LJ. Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. J Cell Biochem. 1994;54:85–99. doi: 10.1002/jcb.240540110. [DOI] [PubMed] [Google Scholar]
- 57.Pitsillides AA, Rawlinson SC, Suswillo RF, Bourrin S, Zaman G, Lanyon LE. Mechanical strain-induced NO production by bone cells: a possible role in adaptive bone (re)modeling? FASEB J. 1995;9:1614–22. doi: 10.1096/fasebj.9.15.8529841. [DOI] [PubMed] [Google Scholar]
- 58.Bandara G, Georgescu HI, Lin CW, Evans CH. Synovial activation of chondrocytes: evidence for complex cytokine interactions. Agents Actions. 1991;34:285–8. doi: 10.1007/BF01993304. [DOI] [PubMed] [Google Scholar]
- 59.Martel-Pelletier J, McCollum R, DiBattista J, Faure M-P, Chin JA, Fournier S, et al. The interleukin-1 receptor in normal and osteoarthritic human articular chondrocytes: identification as the type I receptor and analysis of binding kinetics and biologic function. Arthritis Rheum. 1992;35:530–40. doi: 10.1002/art.1780350507. [DOI] [PubMed] [Google Scholar]
- 60.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 61.Lane NE, Williams RJ, III, Schurman DJ, Smith RL. Inhibition of interleukin 1 induced chondrocyte protease activity by a corticosteroid and a nonsteroidal antiinflammatory drug. J Rheumatol. 1992;19:135–9. [PubMed] [Google Scholar]
- 62.Joosten LAB, Helsen MMA, van de Loo FAJ, van den Berg WB. Anticytokine treatment of established type II collagen–induced arthritis in DBA/1 mice: a comparative study using anti-TNFα, anti–IL-1α/ β, and IL-1Ra. Arthritis Rheum. 1996;39:797–809. doi: 10.1002/art.1780390513. [DOI] [PubMed] [Google Scholar]
- 63.Spirito S, Doughty J, O’Byrne E, Ganu V, Goldberg RL. Metalloprotease inhibitors halt collagen breakdown in IL-1 induced bovine nasal cartilage cultures. Inflamm Res. 1995;44(Suppl 2):S131–2. doi: 10.1007/BF01778297. [DOI] [PubMed] [Google Scholar]
- 64.Evans CH, Ghivizzani SC, Robbins PD. Blocking cytokines with genes. J Leukoc Biol. 1998;64:55–61. doi: 10.1002/jlb.64.1.55. [DOI] [PubMed] [Google Scholar]
- 65.Van den Berg WB. Lessons for joint destruction from animal models. Curr Opin Rheumatol. 1997;9:221–8. doi: 10.1097/00002281-199705000-00008. [DOI] [PubMed] [Google Scholar]