Abstract
This review will define the role of collagen and within-bone heterogeneity and elaborate the importance of trabecular and cortical architecture with regard to their effect on the mechanical strength of bone. For each of these factors, the changes seen with osteoporosis and ageing will be described and how they can compromise strength and eventually lead to bone fragility.
Keywords: Bone resorption, Bone loss, Bone fragility, Collagen Biomechanics
Introduction
Osteoporotic fractures occur spontaneously or as a result of minimal trauma from day-to-day activities [1]. In 90% of all hip fractures, the leading mechanism of trauma is a simple fall, [2–5] indicating bone fragility in these patients. Early detection of an impaired quality of bone is crucial in the prevention of osteoporotic fractures. Previous studies suggest broad under-diagnosis of osteoporosis [6], and the opportunity to start bone modulating therapies before the occurrence of an osteoporotic fracture is missed in up to 84% of osteoporotic fracture cases [7].
The assessment of bone mineral density (BMD) as a surrogate marker of bone strength using non-invasive methods like dual-energy X-ray absorptiometry is widely regarded as the gold-standard for diagnostic screening and as a guide prior to therapeutic decisions [8]. However, BMD accounts for only 60% of the variation in bone fragility [9], because it is unable to depict differences in bone material composition and structural design. Both characteristics influence bone strength to a large extent [10].
The unique mechanical properties of bone reflect the need to provide at the same time strength and lightweight design, stiffness and elasticity, the ability to resist deformation and to absorb energy [11]. This is possible because of the complex arrangements in compositional and micro-architectural characteristics of bone as well as continuous adjustments over time in response to dynamic extrinsic and intrinsic factors. Ageing and other factors like estrogen deficiency can affect these components and eventually result in decreased bone strength and fracture toughness [12]. Osteoporotic fractures, therefore, are the macroscopic result of a sequence of multiple nano- and microstructural events.
This review will define the roles of (1) trabecular and cortical bone architecture, (2) structural and compositional heterogeneity in trabecular bone, and (3) alterations in collagen in determining mechanical integrity of bone. For each of these factors, the changes seen with osteoporosis and ageing will be described and how they can compromise strength and toughness, eventually lead to bone fragility.
Differences between trabecular and cortical bone
Macroscopically, the two most apparent structural features of bone are those of trabecular and cortical bone. Cortical bone forms a solid osseous shell around the bone and consists of dense and parallel, concentric, lamellar units – the osteons. Each is surrounded by a layer of cement-like substance, forming the so called cement line. The osteons are nurtured and interconnected by a system of Haversian and Volkmann’s canals as well as canaliculi [11]. On its outer surface, cortical bone is covered by an envelope of connective tissue, the periosteum; and on its inner surface it is covered by the endosteum.
In contrast, trabecular bone shows a characteristic network of lamellar bone plates and rods that presents with less density, less homogeneity, and a lesser degree of parallel orientation. The trabecular bone is supplied by diffusion from the surrounding bone marrow; there are no vessels within trabeculae. Trabecular bone is always surrounded by a cortical bone but the thickness and strength of the cortical shell depends on location. Long bones, for example, show a higher cortex-to-trabecular bone volume ratio than vertebrae and the diaphyseal areas of long bones show a higher cortex-to-trabecular bone ratio than the metaphyseal areas [10].
Cortical bone is stiffer and able to resist higher ultimate stresses than trabecular bone, but it is also more brittle [10,13,14]. Trabecular bone in vitro can withstand strains up to 30%, cortical bone fails with strains of only 2%. While the biomechanical behaviourof cortical bone is rather uniform, trabecular bone shows a wide variability in strength and stiffness. This variability to the largest part depends on the trabecular bone’s apparent density. Due to its heterogeneity, the apparent density and thus the trabecular bone modulus can vary 100-fold from one location to another within the same metaphysis [14].
Besides apparent density, stiffness and strength of cortical and trabecular bone depend on the loading direction, indicating its anisotropic microstructure [10,15,16]. In general, bone can resist to higher compression loads than tension loads and to higher tension loads than shear loads [15,16]. In line with this, the trabecular connectivity inside a bone – as a measure of anisotropy – contributes more to the bone’s biomechanical strength than the trabecular thickness or the bone mineral density [17].
The mechanical response to loading, differs widely between cortical and trabecular bone. Cortical bone, for instance, shows small load carrying capacity when loaded beyond its range of elastic deformation (post-yield) both with tensile and compression loads [10,14]. In contrast, the load carrying capacity of trabecular bone is insignificant after tensile fracture, but even larger than for cortical bone after compressive fracture [14,18].
Each bone’s location in the body and the forces acting on it determine its characteristic microstructure and composition. For example, vertebral bodies must resist high and repetitive axial compression loads but experience much less shear or tension loads. If the trabecular bone is removed from a vertebral body, this leads to increased cortical shell stresses and a disproportionate decrease in the vertebral bone’s ability to withstand compression forces [19].
The femoral neck or the proximal humerus, on the other hand, is mainly subjected to shear forces and bending moments, the latter of which create a combination of compression, tension, and shear. Both show a distinct cortical structure. There is only little change in the biomechanical strength if the trabecular components are removed from a proximal femur [20], but any reduction in cortical thickness or change in cortical shape can increase the risk for sustaining a hip fracture [21] or a proximal humerus fracture [22].
In vivo, bone experiences different loads from different directions and in different intensity and frequency over time. Bone has two main structural responses to changing loading patterns: altering structural density and increasing the degree of structural orientation along the acting force vectors, i.e. anisotropy [10,14].
These adaptive responses would not be possible without the existence of continuous bone remodelling. In bone remodelling, bone tissue is removed by osteoclastic resorption and new bone is formed by osteoblasts. In the early life span after skeletal maturity the amounts of bone removed and replaced with each cycle of bone remodelling are usually equal to each other, leaving the total volume of bone unchanged. With ageing and in the setting of osteoporosis, the balance of bone resorption and formation becomes negative. The bone loss in aged and osteoporotic bone is a consequence of imbalanced and excessive bone remodelling [11].
As bone remodelling occurs on osseous surfaces, osteoporotic bone loss is a function of surface available for bone remodelling [23]. In individuals less than 65 years of age, the largest surface available for bone remodelling is the trabecular bone. In this population, trabecular bone – due to its lesser density when compared to cortical bone – provides only about 20% of the skeletal bone mass but it is responsible for most of the turnover [10,13]. Thus, the bone loss in early osteoporosis is mainly a trabecular bone loss. With increasing age, the cortical bone becomes more and more porous and, therefore, its endocortical surface increases (Figure 1). As a consequence, the largest loss of absolute bone mass due to osteoporosis occurs in cortical bone by intracortical rather than endocortical or trabecular remodelling [23].
Fig. 1. Cortical bone trabecularization.
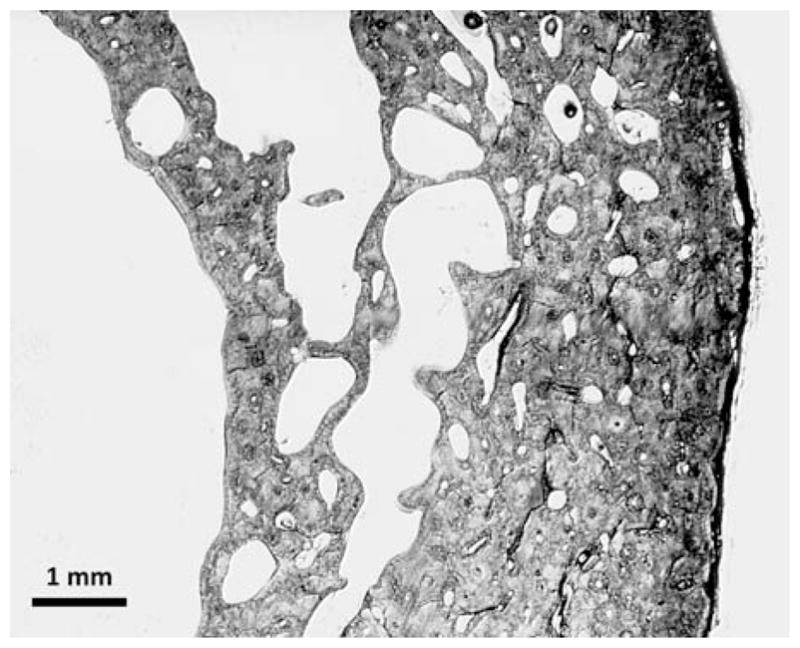
Trabecularization of cortical bone at the endocortical aspect of the cortex. Light microscopy of a quadrant of a female (age 91 years) femoral cortex at midshaft level.
The transition from early trabecular to later cortical bone loss is consistent with the epidemiological data on osteoporotic fractures. Vertebral compression fractures, being “trabecular fractures”, are more common in individuals aged less than 65 years [24]. With increasing cortical bone loss after the age of 65 years, hip fractures, being rather “cortical fractures”, become more frequent (Figure 2).
Fig. 2. Association between bone loss and fracture incidence.
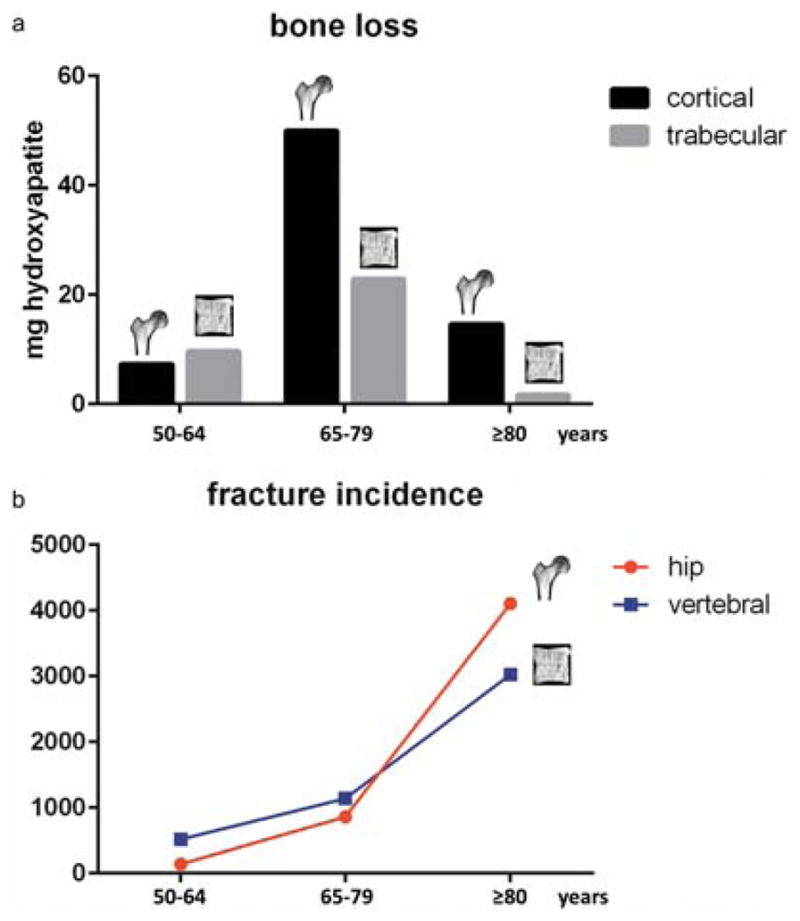
(a) Cortical and trabecular bone loss in different age groups as shown by Zebaze et al. [20]. Early bone loss occurs in the trabecular bone, but with increasing age the bone loss becomes mainly cortical. (b) Incidence of osteoporotic hip and vertebral compression fractures in different age groups in Switzerland as shown by Svedbom et al. [21]. Vertebral compression fractures are more common in individuals aged less than 65 years. With increasing cortical bone loss after the age of 65 years, hip fractures become the most frequent entity.
The knowledge about these differences between trabecular and cortical bone and the changes of their relation due to ageing has multiple potential implications for the understanding and treatment of osteoporotic fractures. It might be advantageous to apply anti-resorptive or anabolic medication regimens that aim for modification of trabecular bone remodelling in younger patients and for modification of cortical bone remodelling in the elderly. When a fracture has occurred, different surgical approaches might be favourable that either address the “trabecular” or “cortical” character of the bone that is fractured. Bone cement, for instance, which is strong in compression and weak in shear and tension forces, is an excellent adjunct tool in the treatment of osteoporotic vertebral or even metaphyseal “trabecular fractures” [25,26]. In proximal humeral or femoral “cortical fractures,” in contrast, a focus on cortical alignment is of more importance and the use of additional support by cortical grafts might be beneficial [27,28].
Changes in trabecular bone with osteoporosis and aging
Structural heterogeneity
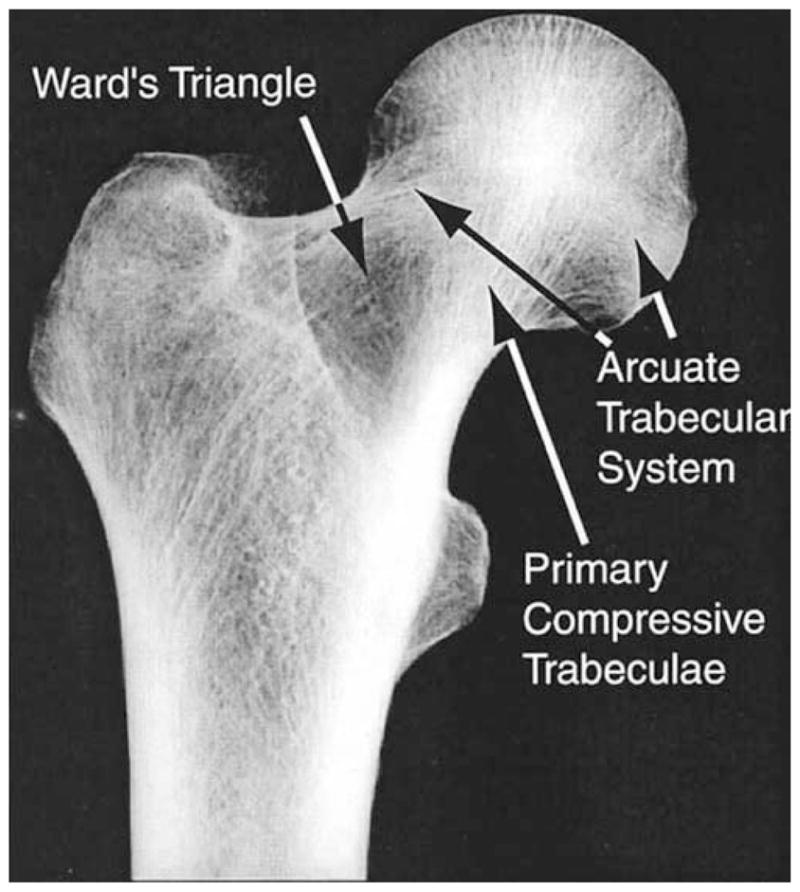
Even a cursory examination of anatomic sites with high risk of osteoporotic fracture reveals that bone density and microstructure are not uniform throughout the trabecular compartment. This regional heterogeneity in density and microstructure is common knowledge for the proximal femur: Ward’s triangle is the region of low density between the femoral neck and greater trochanter, and the primary compressive group is the region of high density and strong microstructural alignment in the femoral head and neck (Figure 3).
Fig. 3. Radiographic frontal view of the proximal femur. Courtesy of Dennis Carter.
Density and microstructure are also not uniform throughout the vertebral centrum. Volume fraction and bone mineral density are highest in the regions of the centrum closest to the endplates and in the posterio-lateral regions [29–34]. Trabecular separation (Tb.Sp.*) and degree of anisotropy are highest in the middle and anterior regions of the centrum [33–36]. The relatively low density and high degree of anisotropy in the anterior region has been suggested as a primary cause of the high proportion of anterior wedge fractures among vertebral fractures [37,38]. In addition, the spatial variations in density and architecture throughout the vertebra change with age [30,35] and with degeneration of the intervertebral disc [38,39]. Within the population, bone loss occurs with age at a higher rate on average in the regions near the endplates than in the central regions — resulting in a more uniform density distribution — but the data also show that in many elderly individuals, the density distribution remains highly nonuniform [35,37].
The heterogeneity in density and architecture throughout bones such as the femur and vertebra have been proposed [40–43] as a major reason why the average BMD of the bone explains only ~60% of the variation in whole-bone strength. Biomechanical studies support the hypothesis that heterogeneity is important for mechanical strength. An early study using finite element modeling of the femur found that increases in bone density in a fairly small region (~5 cm3) at the femoral neck could produce a relatively greater increase in bone strength as compared to a uniform increase throughout the entire bone [44]. Studies in the vertebra have found that the compressive failure properties of the vertebra in both static and fatigue loading conditions were predicted better by measures of density from one or several sub-regions of the centrum as compared to average density of the entire centrum [40,41].
However, the literature on the mechanisms by which regional variations in density and microstructure affect bone strength is mixed. Studies of excised specimens of trabecular bone have found that failure in compression initiates in regions of low local volume fraction [45] and that larger intra-specimen variations in trabecular thickness and tissue properties are associated with lower apparent elastic moduli [46,47]. Supporting these findings, Snyder and colleagues have reported that estimating the weakest cross-section of the vertebral body provides good predictions of vertebral strength [48,49] and fracture risk [50]. A study on a small sample of human vertebrae also reported that increased heterogeneity in volume fraction in the centrum was associated with decreased compressive strength [51]. In contrast, more recent studies have found that, increased intravertebral heterogeneity in density is associated with increased vertebral strength [52].
Ideally, the measures of heterogeneity that will emerge are those that have biomechanical underpinnings. For example, increased intravertebral heterogeneity may confer higher vertebral strength if this heterogeneity arises from the existence of regions of high density that are strategically placed in a centrum that is otherwise of low average density. In other words, larger structural heterogeneity could be advantageous if the particular spatial distribution of bone density matches the way that load is distributed throughout the vertebral body. Prior measurements have shown that in erect spinal postures, less than half of the total load applied to the vertebral body is distributed over the anterior half, and that this fraction decreases with age [53]. Vertebral bodies with higher density posteriorly than anteriorly would be expected to exhibit higher strength under this type of load distribution, as has been shown [52]. In addition, a prevailing hypothesis has emerged that degeneration of the intervertebral disc results in transfer of more of the applied load to the outer regions of the vertebral body, thus causing resorption in the central and mid-transverse regions [54]. Vertebrae that have undergone this adaptation may thus be less likely to fracture [53].
Even considering regional variations in density and microstructure within small but critical areas of the vertebral body may provide further insight into the mechanisms of fracture. For example, collapse of the superior endplate has long been associated with vertebral fracture, and this collapse initiates in and propagates to regions overlying trabecular bone of low density and mechanically inferior microstructure [55] (Figure 4).
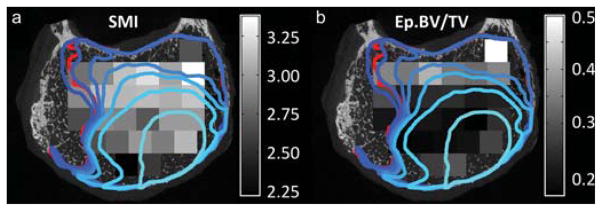
Fig. 4. Bone heterogeneity and vertebral endplate collapse.
Regions of endplate collapse (outlined in blue and red) and distribution of structure model index (SMI) in the trabecular bone directly underlying the endplate (grayscale): The lightest blue outline corresponds to the loading increment at which endplate collapse clearly initiated. The boundaries at subsequent loading increments are represented with progressively darker shades of blue. The red outline corresponds to the region of endplate collapse that remained after loading was complete and all load was removed. Modified from Jackman et al. [52].
In summary, large amounts of heterogeneity in density and microstructure exist throughout the trabecular compartment of the bones with high prevalence of osteoporotic fracture. Substantial evidence exists that this heterogeneity has important biomechanical consequences, but further work is required to establish mechanisms and clinical implementation of these insights.
Tissue heterogeneity
Changes in tissue composition and mechanical properties at the material/tissue level (lamellae, individual trabeculae) likely contribute to fracture risk, but up until recently these changes have been less well understood. A number of studies have sought to address this, using a combination of mechanical testing (nano-indentation, micro-mechanical testing) [56–60] and compositional analyses at the tissue level [57–59,61–64], and their findings regarding changes in tissue properties and composition during osteoporosis are conflicting. It has been reported for example that trabecular bone tissue from the proximal femur of ovariectomized sheep (12 months post-surgery) had a lower tissue modulus, as measured by nano-indentation, compared to age matched controls [56,57]. These changes were associated with a decrease in mineral content in the osteoporotic trabecular bone tissue [57,62]. Interestingly, the differences were not maintained 31 months post-surgery [57]. In contrast, micro-tensile testing showed that the stiffness and strength of ovariectomized rat trabeculae was increased by 40–90% by 54 weeks post-ovariectomy [58,59]. These increases were associated with a significant increase (11%) in the mineral content of these trabeculae, although overall bone mineral density and mass were reduced [58,59]. It has also been reported that increased calcium content and stiffness occur within individual trabeculae from human osteoporotic bone [64,65].
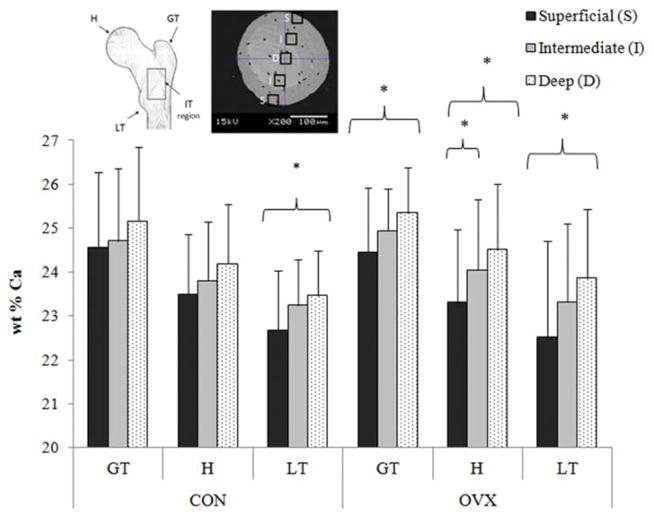
Variations in experimental methods, animal model or the anatomical location from which bone was chosen for analysis might explain the discrepancies between previous studies. For example decreased trabecular stiffness was reported based on nanoindentation of trabeculae from the anteromedial region of the proximal femur of the ovariectomized sheep [56,57], whereas increased trabecular stiffness was based on micro-tensile testing of trabeculae from a region below the growth plate of the tibia of ovariectomized rat bones [58,59]. Nanoindentation characterises the mechanical properties (elastic modulus, hardness) of nanometer areas of bone tissue (typically within individual lamellae), whereas micro-tensile testing assesses the mechanical behaviour of entire trabeculae. Therefore, to understand these discrepancies further a recent study sought to distinguish (1) the spatial distribution of mineral within different lamellae across individual trabeculae and (2) the variation in trabecular mineralisation in different anatomical regions of the proximal femur following the onset of estrogen deficiency [66]. Mineral content (wt% Ca) was determined using a quantitative backscattered scanning electron microscopy approach, for individual trabeculae harvested from the proximal femur of ovariectomized sheep (12 months post-OVX) and age-matched controls. It was found that the difference in mineralization between the superficial and deep lamellae of trabeculae was more pronounced in ovariectomized sheep (Figure 5), representing an increase in mineral heterogeneity of approximately 13%, compared to trabeculae from aged matched controls [66]. Moreover the distribution of bone mineral was shown to be dependent on anatomical location within the proximal femur, with a higher variability of mineralization between the greater and lesser trochanter regions of ovariectomized sheep (Figure 5), which coincides with the intertrochanteric fracture line [66]. These findings were undetectable by focusing solely on bone mineral density and are corroborated by studies of human osteoporotic trabeculae [64,67].
Fig. 5. Trabecular mineralization in estrogen deficiency.
Spatial distribution of calcium (wt% Ca) between superficial, intermediate, and deep lamellae in the greater trochanter (GT), head (H) and lesser trochanter (LT) regions of the proximal femur from 12 month ovariectomized sheep (OVX) and aged matched controls (CON). * indicates statistical significance between trabecular regions indicated by brackets ( p ≤ 0.02). Figure adapted and data from [64].
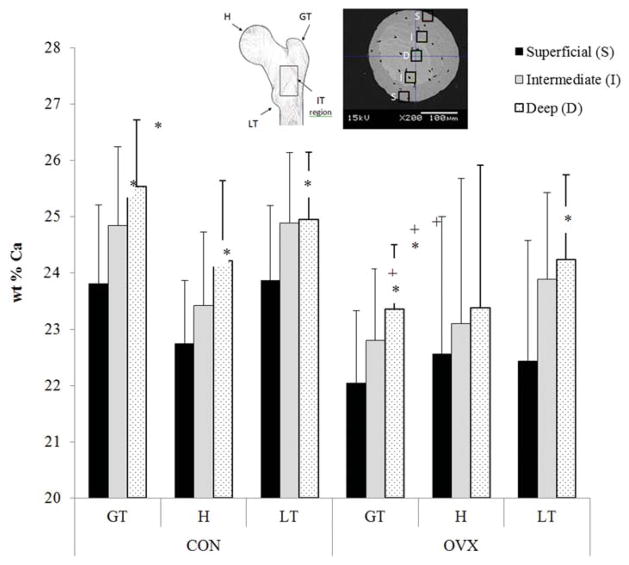
Rapid increases in bone resorption by osteoclasts occur at the onset of osteoporosis but abate over time. As such the disparity between different studies might also relate to the extent of disease progression, the timing of which likely varies between animal models and human bone. A recent study sought to understand how trabecular tissue mineralization is altered over prolonged estrogen depletion and compared this to normal age-related changes in trabecular bone tissue composition [68]. Bone mineral density distribution parameters were compared in trabeculae from the proximal femora of ovariectomized sheep that underwent estrogen deficiency for 12 or 31 months and age-matched controls. It was reported that normal ageing increases mean mineralization and mineral heterogeneity at a trabecular level and that these differences arise due to an increase in the mineralisation of the deep lamellae of the trabeculae with ageing (Figure 6). However, prolonged estrogen deficiency (31 months) leads to significantly decreased mean mineralization compared to trabeculae from both aged matched controls and a shorter duration of estrogen deficiency (12 months) (compare with Figure 5). Increased rates of bone turnover during estrogen deficiency could explain this lower mean mineralization. However, reductions in mineralization were nonuniform within the proximal femur [68]. The underlying mechanisms by which trabecular mineral heterogeneity is altered during osteoporosis might be due to hypermineralized osteocyte lacunae in osteoporotic trabecular bone and an increased bone turnover [69]. Additionally, this variability might be related to local variations in the mechanical environment, which might lead to alterations in tissue mineral content at those regions regulated by mechanosensitive bone cells [69]. Together these recent studies [66,68] reveal the importance of duration and anatomical location in assessing the effects of estrogen deficiency on trabecular bone mineralization and may explain discrepancies regarding the effect of estrogen deficiency between previous studies.
Fig. 6. Trabecular mineralization in prolonged estrogen deficiency.
Spatial distribution of calcium (wt% Ca) between superficial, intermediate, and deep lamellae in the greater trochanter (GT), head (H) and lesser trochanter (LT) regions of the proximal femur from 31 month ovariectomized sheep (OVX) and aged matched controls (CON). * indicates significantly different to deep lamellae within the same femoral region of the indicated group. + indicates significant difference to the same ROI of the CON group. Data from [65].
In summary, it is becoming increasingly clear that, even though overall trabecular bone mass and strength are reduced during osteoporosis, the scarce trabecular tissue that remains is more heterogeneous, with regions of trabecular tissue that are more mineralized, stiffer and stronger. It would also appear that these changes are a transient and site-specific characteristic of osteoporosis, whereby the trabecular tissue properties are altered varyingly as the disease progresses.
Changes in cortical bone with aging and osteoporosis
The biomechanical competence of a bone is determined by the amount and quality of bone material and even more importantly by the arrangement of the material in space. Geometrical measures including bone size, cross-sectional area or area moment of inertia explain up to 80% of the biomechanical competence of whole bones. For the distal radius, the best predictors of fracture load were measures of cortical bone mass, cortical area and cortical width [70]. For the proximal femur cortical area, size of the femoral neck and area moment of inertia were the strongest predictors of fracture load [70]. The combination of individual parameters in multiple regression models has provided further evidence that geometrical measurements considerably improve the prediction of bone strength beyond measurement of bone mineral density [71]. Consequently it has been found that fracture risk in patients is associated with certain geometrical features such as local thinning of cortical bone [72].
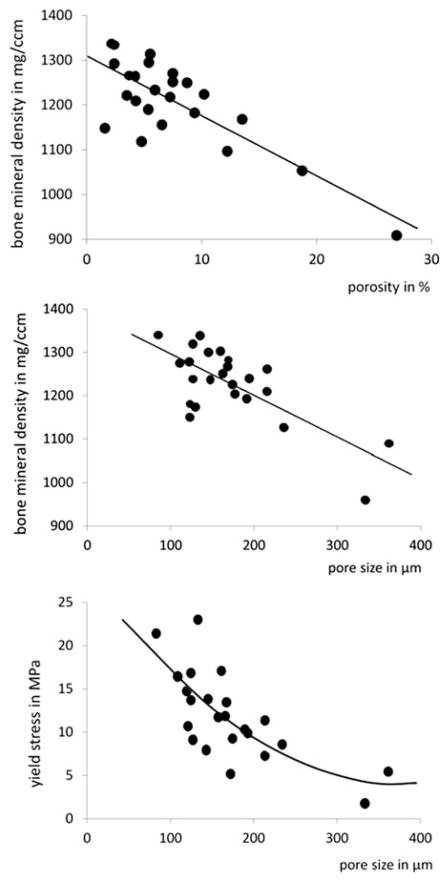
Furthermore, the mechanical competence of cortical bone strongly depends on its porosity. Cortical bone tissue is composed of osteons and interstitial bone. The longitudinally oriented Haversian canals and the perpendicular Volkmann canals perforate the cortical bone matrix. Towards the endocortical bone surface Haversian canals can unite and also connect with the intramedullary cavity. The Haversian canals and the resorption cavities produce a porous bone tissue with pore diameters ranging from a few up to several hundred micrometers. The number and size of the pores determine intracortical porosity and bone mineral density (Figure 7). With increasing pore size the mechanical properties of cortical bone considerably degrade. Thus porosity accounts for about 70% of elastic modulus and 55% of yield stress of cortical bone [73]. Accordingly, fracture toughness also decreases significantly with increasing porosity possibly by reducing the available area for the propagation of microcracks [74].
Fig. 7. Cortical bone porosity and mechanical strength.
Relationships among bone mineral density, and pore size in cortical bone and mechanical strength assessed by yield stress. Data from [4,6]
Age-related degradation of mechanical competence of bone appears to be more pronounced for mechanical properties associated with failure than for those associated with stiffness. Energy absorption, fracture toughness and ultimate tensile strain show age-related decrease of about 5–10% per decade, while elastic moduli in tension or compression degrade by only about 2% per decade [12]. It appears, therefore, that the relationship between failure properties and stiffness properties changes with increasing tissue maturity. This makes the accurate prediction of fracture risk even more difficult. Fracture risk prediction largely relies on non-invasive image assessment and the measurement of mineral density. However, while bone mineral density is closely related to stiffness properties of bones its association with failure strength or toughness is less pronounced.
Changes in bone’s mechanical competence are explained by functional adaptation of bone structure and age-related deterioration of intrinsic mechanical properties both being directly related to bone remodeling. When bone remodeling is suppressed, the ratio of highly mineralized to new, less mineralized bone tissue is increased resulting in an increase in the homogeneity of cortical bone tissue. A more homogenous tissue allows cracks to grow more easily and thus reduces the toughness of the composite material. Furthermore, remodeling reduces the regional variability of collagen fiber orientation, leading to changes in mechanical properties. It has been shown that the collagen network itself experiences up to 50% loss in its capability to absorb energy during ageing probably because of an increase in the percentage of denatured collagen [75]. With increasing age, the degree of mineralization increases, which is reflected in an increase in mineral content of cortical bone tissue. As micro-damage in cortical bone accumulates with increasing age, there is a concomitant progressive increase in micro-crack density [76]. After the age of 50, micro-cracks accumulate in cortical bone and this occurs much more quickly in women than in men.
But not only cortical bone material changes with age, bone geometry also adapts to a modified mechanical environment. In essence, both the outer and inner diameter of the cortex increases while the thickness of the cortex is reduced [77]. In addition, the porosity of the cortex increases with age and results in a dramatic increase of the intracortical bone surface. The increase in porosity results from coalescence of Haversian channels within the cortex and from fragmentation of the endocortical bone surface. The remaining cortical remnants have similarity to trabecular bone and can be described by trabecularization of the endocortical bone (Figure 1). The porosity in cortical bone increases from about 4% in young healthy bone to around 12% at age 60 years [14] and up to almost 50% in very elderly individuals [23]. The increasing surface area of the cortical bone provides more surface to receive signals for remodeling to be initiated and thus further accelerates cortical bone loss with age. In fact, most of the trabecular appearing bone is likely to be trabecularized cortical bone fragments [78]. While at early ages bone loss dominates at trabecular sites, with increasing age bone is primarily lost in the cortex of peripheral bones. Fifty percent of the bone loss occurs at the endocortical aspect of cortical bone, thinning the cortex and leaving trabecular like cortical fragments [23].
The adaptive changes of cortical bone tissue with age are largely site-dependent. In the femoral neck bone loss is lowest in inferior regions that bear the largest loads during normal gait, whereas regions at the superior aspect which are less loaded undergo thinning of the cortex by endocortical absorption. These regions with reduced thickness however, experience highest stresses during falling and are more likely to fracture at advanced age. In the femoral shaft, a similar mechanism has been reported long ago [79]. In the distal forearm, the age-related adaptation is reflected in endosteal absorption together with periosteal apposition, increasing the area moment of inertia and thus preserving bone rigidity and strength [80] to some extent. Although this adaptive response has been observed in both women and men, it appears to be more effective in men.
Although the crucial role of cortical bone for the mechanical competence of bone and the risk of fracture has been recognized it has not really been transferred to clinical practice for fracture risk assessment or for monitoring of osteoporosis treatment. Future clinical imaging techniques will have to consider measures cortical bone geometrical features and also its local porosity.
The role of collagen
The matrix of bone is composed of both inorganic (i.e. mineral) and organic (i.e. water, collagen, and non-collagenous proteins) components. The role of mineral composition in skeletal fragility has been studied in depth, and it is generally understood that in normal bone, the mineral content provides strength and stiffness [81]. There is less known about the effect of collagen and non-collagenous proteins, but there is increasing evidence suggesting that changes in protein content and structure play important roles in age- and disease-related changes in bone. In particular, the organic matrix is considered to be responsible for bone’s ductility and its ability to absorb energy prior to fracturing [82].
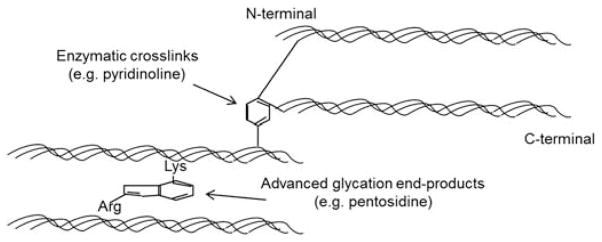
Ninety percent of bone’s organic matrix is composed of type I collagen, a structural protein comprised of three polypeptide chains with a defined amino acid sequence, glycine-X-hydroxyproline or glycine-proline-X (X is an amino acid such as lysine). This particular sequence of amino acids allows the polypeptide chains to twist into a triple helical structure with the small glycine in the middle, and amino acids that remain exposed on the surface of the triple helix are involved in the formation of collagen crosslinks [83]. Collagen undergoes numerous post-translational modifications with aging and disease, including both enzymatic and non-enzymatic crosslinking. In general, enzymatic crosslinking is considered to be a normal process for healthy collagen and has a beneficial effect on its mechanical properties, while non-enzymatic crosslinking results in a brittle collagen network that leads to deteriorated bone mechanical properties if its accumulation exceeds normal repair [84].
Enzymatic crosslinking requires the enzyme lysyl oxidase to aid the formation of intra- or inter-fibrillar crosslinks such as pyridinoline and deoxypyridinoline [85]. The lysine-based crosslinks form in the overlap regions of fibrils in a head-to-tail fashion (Figure 8) [86]. In the maturation process, bivalent crosslinks slowly transform into a more stable, trivalent, non-reducible conformation. Mature crosslinks accumulate, inhibit collagen fibril remodeling, increase the stiffness of the fibril, and provide increased strength to the tissue [86,87]. Pyridinoline and deoxypyridinoline serve as markers of bone resorption and are indicators of collagen maturity [88]. Enzymatic crosslinks are most reliably quantified and characterized with mass spectrometry [89] or HPLC (high performance liquid chromatography) [90], but some studies indicate that FTIR (Fourier transform infrared) spectroscopy can illustrate collagen crosslink characteristics [91]. Using these methods, enzymatic crosslinks have been shown to be reduced in osteoporotic patients with hip fractures compared to healthy controls [92,93].
Fig. 8. Collagen cross-links.
A schematic illustration of enzymatic crosslinks (e.g. pyridinoline [PYD], deoxypyridinoline [DPD]) and non-enzymatic crosslinks (e.g. pentosidine [PEN]) at the molecular level.
The second pathway for collagen crosslinking does not involve any enzymes, and is termed non-enzymatic glycation. Unlike the enzymatic crosslinks, which link the ends of the collagen molecules, non-enzymatic crosslinks are found at any position along the collagen. Non-enzymatic glycation involves a reaction between an aldehyde group of a sugar (e.g. glucose) and the ε-amino group of hydroxylysine or lysine. This reaction results in the formation of glucosyl-lysine, which undergoes further reactions to form an Amadori product or Schiff base adduct. Both of these intermediate products undergo additional reactions to create crosslinks that form within and across collagen fibers and are known as advanced glycation end-products (AGEs) [86], which have been shown to accumulate in numerous tissues including skin, cartilage, tendons, and bone [94]. AGEs accumulate with age and disease [85]. Specifically, osteoporotic bone has significantly more AGEs than normal healthy bone [92,93]. The increased AGE levels can result in brittleness of tissues undergoing non-enzymatic glycation [95].
There are two methods used for quantifying AGEs in bone, and these techniques incorporate measurement of the autofluorescence emitted by most AGEs. One technique quantifies pentosidine, a single AGE crosslink and the only non-enzymatic crosslink that has been successfully isolated and quantified in bone, using HPLC [96]. As pentosidine composes less than 1% of total fluorescent AGEs in bone and is weakly correlated to the amount of total fluorescent AGEs in human bone [83,97], it is valuable to measure total fluorescent AGEs in addition to pentosidine content. The second technique quantifies the bulk fluorescence of AGEs from enzyme-digested or acid-hydrolyzed bone samples relative to a quinine sulfate standard [98], and the amount of fluorescence is normalized to collagen content. Wavelengths used in this fluorometric assay capture the excitation and emission wavelengths of several major AGE crosslinks including pentosidine, carboxymethyllysine, vesperlysines, crossline, and carboxyethyllysine [83], and thus, the relative contributions of each of these crosslinks to the total fluorescence cannot be determined from this assay.
Increased non-enzymatic glycation has been shown to reduce mechanical strength and/or toughness of bone [99,100]. Glycation levels have also been shown to be greater in cadaver specimens from hip fracture patients compared to controls, and the glycation content was correlated with several biomechanical properties in cancellous bone, but not in cortical bone [92,93]. Although it is generally understood that AGEs accumulate in bone, stiffen the collagen matrix, and in turn, deteriorate bone’s mechanical properties, the contradictions in current literature arise for a number of reasons: (1) few in vitro glycation studies have been conducted, and most in vitro studies have been primarily conducted in cancellous bone, (2) studies conducted on in vivo glycation levels report pentosidine content only while a few studies report total AGEs, making the studies difficult to compare, (3) range of values for glycation levels reported vary greatly depending on the bone, location, and age range of specimens used, and (4) various mechanical testing techniques, animal models, or disease states have been used in these studies. Thus, the exact contribution of AGEs to age-related skeletal fragility remains undefined.
There is increasing evidence that AGEs directly affect cellular function through the receptor for AGE (RAGE), a surface receptor on many cell types [101]. RAGE activation is associated with inflammation, cellular dysfunction, and localized tissue destruction. In bone, activation of the RAGE receptor inhibits osteoblast proliferation and differentiation [102], reduces matrix production [103], reduces bone formation [104] and increases osteoblast apoptosis [105]. This indicates that crosslinking properties of the matrix not only alter the tissue properties, but directly control cellular function and may play an important role in the decreased bone formation found in osteoporosis [106].
In addition to enzymatic and non-enzymatic modifications of collagen, non-collagenous proteins (e.g. osteopontin, osteocalcin), which compose 10% of bone’s organic matrix, also may affect bone mechanical properties. Osteocalcin stimulates mineral maturation, inhibits bone formation, recruits osteoclast precursors to bone resorption sites, and helps with their differentiation into mature osteoclasts [107]. Osteopontin plays a role in mineralization and assists the bone resorption process by anchoring osteoclasts to the mineral matrix of the bone surface [88]. More importantly, these proteins have been recently considered to act as the glue that holds mineralized collagen fibers together. When a force is applied, these components stretch, help dissipate energy by breaking sacrificial bonds between adjacent collagen fibrils, and prevent harmful crack formation and propagation [108]. Thus, alterations to the matrix composition of both collagenous and non-collagenous proteins may alter bone biomechanical properties. Increased serum osteocalcin and osteopontin has been reported in postmenopausal women with osteoporosis compared to healthy controls [109,110].
In summary, there is increasing evidence of the role of bone’s organic matrix on age- and disease-related changes in bone’s mechanical properties. Enzymatic crosslinking of collagen is generally considered to have a positive effect on bone’s mechanical properties, while non-enzymatic crosslinking can lead to deteriorated bone mechanical properties with aging and disease. Non-collagenous proteins play a role in the prevention of harmful microdamage formation. Though osteoporosis is generally defined as a loss of bone mass, there are considerable matrix changes, particularly in collagen crosslinks, which cause a loss of bone quality.
Conclusions
The bone’s inorganic and organic composition, its trabecular and cortical nano-, micro-, and macroscopic architecture, and the heterogeneity of these structural features all have impact on age- and disease-related changes in bone’s mechanical properties. Though osteoporosis is generally defined as a loss of bone mass, there are considerable changes of the structure and matrix itself, which can cause a loss of bone quality.
It is known, that cortical bone plays a major role in determining the mechanical competence of bone and the risk of fracture; the age-related alterations of its geometrical features and its local porosity, though, have long been poorly understood and underestimated. The number of trabeculae in trabecular bone, trabecular thickness and the degree of connectivity all influence the mechanical strength of a bone. In osteoporosis a decrease of all these characteristics is seen. Especially in bones with increased risk for osteoporotic fractures, however, the remaining trabecular tissue is largely heterogeneous, with regions of different mineralization, stiffness and strength.
Both, the trabecular and the cortical component undergo different changes at different times. Bone remodelling occurs on osseous surfaces and, thus, osteoporotic bone loss is a function of surface available for bone remodelling. The bone loss in early osteoporosis is mainly trabecular and with increasing age the bone loss becomes primarily endo- and intracortical.
The knowledge about this evolution in matrix and structure in osteoporotic bone and about the differences between trabecular and cortical bone could help with predicting, avoiding and treating osteoporotic fractures. Future clinical imaging techniques will have to consider structural measures of cortical and trabecular bone rather than focusing on bone mineral density alone. In prophylactic treatment regimens, the aimed for therapeutic region (i.e. trabecular versus cortical) and mechanisms of action within the cascade of bone remodelling might have to be chosen according to the patient’s age and the individual advancement of bone changes. Eventually, when a fracture has occurred, the non-operative or surgical treatment has to be guided by both: the personality of a patient and the personality of their bone.
Footnotes
Conflict of interest
The authors report no conflict of interest related to the content of the manuscript.
References
- 1.Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ., 3rd Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7:221–7. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz AV, Kelsey JL, Maggi S, Tuttleman M, Ho SC, Jonsson PV, et al. International variation in the incidence of hip fractures: cross-national project on osteoporosis for the World Health Organization Program for Research on Aging. Osteoporos Int. 1999;9:242–53. doi: 10.1007/s001980050144. [DOI] [PubMed] [Google Scholar]
- 3.Tosounidis TH, Castillo R, Kanakaris NK, Giannoudis PV. Common complications in hip fracture surgery: Tips/tricks and solutions to avoid them. Injury. 2015;46(Suppl 5):S3–11. doi: 10.1016/j.injury.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Makridis KG, Karachalios T, Kontogeorgakos VA, Badras LS, Malizos KN. The effect of osteoporotic treatment on the functional outcome, re-fracture rate, quality of life and mortality in patients with hip fractures: a prospective functional and clinical outcome study on 520 patients. Injury. 2015;46:378–83. doi: 10.1016/j.injury.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Guerado E, Cruz E, Cano JR, Crespo PV, Alaminos M, Del Carmen Sánchez-Quevedo M, Campos A. Bone mineral density aspects in the femoral neck of hip fracture patients. Injury. 2016;47(Suppl 1):S21–4. doi: 10.1016/S0020-1383(16)30005-5. [DOI] [PubMed] [Google Scholar]
- 6.Greenspan SL, Perera S, Nace D, Zukowski KS, Ferchak MA, Lee CJ, et al. FRAX or fiction: determining optimal screening strategies for treatment of osteoporosis in residents in long-term care facilities. J Am Geriatr Soc. 2012;60:684–90. doi: 10.1111/j.1532-5415.2011.03884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith MG, Dunkow P, Lang DM. Treatment of osteoporosis: missed opportunities in the hospital fracture clinic. Ann R Coll Surg of Engl. 2004;86:344–6. doi: 10.1308/147870804371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleerekoper M, Nelson DA. Which bone density measurement? J Bone Miner Res. 1997;12:712–4. doi: 10.1359/jbmr.1997.12.5.712. [DOI] [PubMed] [Google Scholar]
- 9.Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14(Suppl 3):S13–8. doi: 10.1007/s00198-002-1345-4. [DOI] [PubMed] [Google Scholar]
- 10.Nordin M, Frankel VH. Biomechnics of bone. In: Nordin M, Frankel VH, editors. Basic Biomechanics of the musculoskeletal system. 4. North American: LWW; 2012. p. 472. [Google Scholar]
- 11.Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–61. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 12.Burstein AH, Reilly DT, Martens M. Aging of bone tissue: mechanical properties. J Bone Joint Surg Am. 1976;58:82–6. [PubMed] [Google Scholar]
- 13.Carter DR, Hayes WC. Compact bone fatigue damage: a microscopic examination. Clin Orthop Relat Res. 1977:265–74. [PubMed] [Google Scholar]
- 14.Keaveny TM, Hayes WC. A 20-year perspective on the mechanical properties of trabecular bone. J Biomech Eng. 1993;115:534–42. doi: 10.1115/1.2895536. [DOI] [PubMed] [Google Scholar]
- 15.Galante J, Rostoker W, Ray RD. Physical properties of trabecular bone. Calcif Tissue Res. 1970;5:236–46. doi: 10.1007/BF02017552. [DOI] [PubMed] [Google Scholar]
- 16.Dempster WT, Liddicoat RT. Compact bone as a non-isotropic material. Am J Anat. 1952;91:331–62. doi: 10.1002/aja.1000910302. [DOI] [PubMed] [Google Scholar]
- 17.Mittra E, Rubin C, Gruber B, Qin YX. Evaluation of trabecular mechanical and microstructural properties in human calcaneal bone of advanced age using mechanical testing, microCT, and DXA. J Biomech. 2008;41:368–75. doi: 10.1016/j.jbiomech.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Carter DR, Schwab GH, Spengler DM. Tensile fracture of cancellous bone. Acta Orthop Scand. 1980;51:733–41. doi: 10.3109/17453678008990868. [DOI] [PubMed] [Google Scholar]
- 19.Mizrahi J, Silva MJ, Keaveny TM, Edwards WT, Hayes WC. Finite-element stress analysis of the normal and osteoporotic lumbar vertebral body. Spine (Phila Pa 1976) 1993;18:2088–96. doi: 10.1097/00007632-199310001-00028. [DOI] [PubMed] [Google Scholar]
- 20.Holzer G, von Skrbensky G, Holzer LA, Pichl W. Hip fractures and the contribution of cortical versus trabecular bone to femoral neck strength. J Bone Miner Res. 2009;24:468–74. doi: 10.1359/jbmr.081108. [DOI] [PubMed] [Google Scholar]
- 21.Gluer CC, Cummings SR, Pressman A, Li J, Gluer K, Faulkner KG, et al. Prediction of hip fractures from pelvic radiographs: the study of osteoporotic fractures. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1994;9:671–7. doi: 10.1002/jbmr.5650090512. [DOI] [PubMed] [Google Scholar]
- 22.Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C. Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg. 2009;129:1251–9. doi: 10.1007/s00402-009-0889-6. [DOI] [PubMed] [Google Scholar]
- 23.Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–36. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 24.Svedbom A, Ivergard M, Hernlund E, Rizzoli R, Kanis JA. Epidemiology and economic burden of osteoporosis in Switzerland. Arch Osteoporos. 2014;9:187. doi: 10.1007/s11657-014-0187-y. [DOI] [PubMed] [Google Scholar]
- 25.Edidin AA, Ong KL, Lau E, Kurtz SM. Mortality risk for operated and nonoperated vertebral fracture patients in the medicare population. J Bone Miner Res. 2011;26:1617–26. doi: 10.1002/jbmr.353. [DOI] [PubMed] [Google Scholar]
- 26.Jacquot F, Letellier T, Atchabahian A, Doursounian L, Feron JM. Balloon reduction and cement fixation in calcaneal articular fractures: a five-year experience. Int Orthop. 2013;37:905–10. doi: 10.1007/s00264-013-1865-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osterhoff G, Baumgartner D, Favre P, Wanner GA, Gerber H, Simmen HP, et al. Medial support by fibula bone graft in angular stable plate fixation of proximal humeral fractures: an in vitro study with synthetic bone. J Shoulder Elbow Surg. 2011;20:740–6. doi: 10.1016/j.jse.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 28.Paul O, Barker JU, Lane JM, Helfet DL, Lorich DG. Functional and radiographic outcomes of intertrochanteric hip fractures treated with calcar reduction, compression, and trochanteric entry nailing. J Orthop Trauma. 2012;26:148–54. doi: 10.1097/BOT.0b013e31821e3f8c. [DOI] [PubMed] [Google Scholar]
- 29.Nepper-Rasmussen J, Mosekilde L. Local differences in mineral content in vertebral trabecular bone measured by dual-energy computed tomography. Acta Radiol. 1989;30:369–71. [PubMed] [Google Scholar]
- 30.Sandor T, Felsenberg D, Kalender WA, Brown E. Global and regional variations in the spinal trabecular bone: single and dual energy examinations. J Clin Endocrinol Metab. 1991;72:1157–68. doi: 10.1210/jcem-72-5-1157. [DOI] [PubMed] [Google Scholar]
- 31.Keller TS, Moeljanto E, Main JA, Spengler DM. Distribution and orientation of bone in the human lumbar vertebral centrum. J Spinal Disord. 1992;5:60–74. doi: 10.1097/00002517-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Antonacci MD, Hanson DS, Leblanc A, Heggeness MH. Regional variation in vertebral bone density and trabecular architecture are influenced by osteoarthritic change and osteoporosis. Spine. 1997;22:2393–401. doi: 10.1097/00007632-199710150-00014. discussion 401–2. [DOI] [PubMed] [Google Scholar]
- 33.Banse X, Devogelaer JP, Munting E, Delloye C, Cornu O, Grynpas M. Inhomogeneity of human vertebral cancellous bone: systematic density and structure patterns inside the vertebral body. Bone. 2001;28:563–71. doi: 10.1016/s8756-3282(01)00425-2. [DOI] [PubMed] [Google Scholar]
- 34.Hulme PA, Boyd SK, Ferguson SJ. Regional variation in vertebral bone morphology and its contribution to vertebral fracture strength. Bone. 2007;41:946–57. doi: 10.1016/j.bone.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Thomsen JS, Ebbesen EN, Mosekilde L. Zone-dependent changes in human vertebral trabecular bone: clinical implications. Bone. 2002;30:664–9. doi: 10.1016/s8756-3282(02)00686-5. [DOI] [PubMed] [Google Scholar]
- 36.Gong H, Zhang M, Yeung HY, Qin L. Regional variations in microstructural properties of vertebral trabeculae with aging. J Bone Miner Metab. 2005;23:174–80. doi: 10.1007/s00774-004-0557-4. [DOI] [PubMed] [Google Scholar]
- 37.Oda K, Shibayama Y, Abe M, Onomura T. Morphogenesis of vertebral deformities in involutional osteoporosis. Age-related, three-dimensional trabecular structure. Spine. 1998;23:1050–5. doi: 10.1097/00007632-199805010-00016. discussion 6. [DOI] [PubMed] [Google Scholar]
- 38.Simpson EK, Parkinson IH, Manthey B, Fazzalari NL. Intervertebral disc disorganization is related to trabecular bone architecture in the lumbar spine. J Bone Miner Res. 2001;16:681–7. doi: 10.1359/jbmr.2001.16.4.681. [DOI] [PubMed] [Google Scholar]
- 39.Keller TS, Ziv I, Moeljanto E, Spengler DM. Interdependence of lumbar disc and subdiscal bone properties: a report of the normal and degenerated spine. J Spinal Disord. 1993;6:106–13. [PubMed] [Google Scholar]
- 40.Cody DD, Goldstein SA, Flynn MJ, Brown EB. Correlations between vertebral regional bone mineral density (rBMD) and whole bone fracture load. Spine. 1991;16:146–54. [PubMed] [Google Scholar]
- 41.McCubbrey DA, Cody DD, Peterson EL, Kuhn JL, Flynn MJ, Goldstein SA. Static and fatigue failure properties of thoracic and lumbar vertebral bodies and their relation to regional density. J Biomech. 1995;28:891–9. doi: 10.1016/0021-9290(94)00155-w. [DOI] [PubMed] [Google Scholar]
- 42.Liebschner MA, Kopperdahl DL, Rosenberg WS, Keaveny TM. Finite element modeling of the human thoracolumbar spine. Spine. 2003;28:559–65. doi: 10.1097/01.BRS.0000049923.27694.47. [DOI] [PubMed] [Google Scholar]
- 43.Crawford RP, Cann CE, Keaveny TM. Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography. Bone. 2003;33:744–50. doi: 10.1016/s8756-3282(03)00210-2. [DOI] [PubMed] [Google Scholar]
- 44.Oden ZM, Selvitelli DM, Bouxsein ML. Effect of local density changes on the failure load of the proximal femur. J Orthop Res. 1999;17:661–7. doi: 10.1002/jor.1100170507. [DOI] [PubMed] [Google Scholar]
- 45.Nazarian A, Stauber M, Zurakowski D, Snyder BD, Muller R. The interaction of microstructure and volume fraction in predicting failure in cancellous bone. Bone. 2006;39:1196–202. doi: 10.1016/j.bone.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Yeh OC, Keaveny TM. Biomechanical effects of intra-specimen variations in trabecular architecture: a three-dimensional finite element study. Bone. 1999;25:223–8. doi: 10.1016/s8756-3282(99)00092-7. [DOI] [PubMed] [Google Scholar]
- 47.Jaasma MJ, Bayraktar HH, Niebur GL, Keaveny TM. Biomechanical effects of intraspecimen variations in tissue modulus for trabecular bone. J Biomech. 2002;35:237–46. doi: 10.1016/s0021-9290(01)00193-2. [DOI] [PubMed] [Google Scholar]
- 48.Hong J, Cabe GD, Tedrow JR, Hipp JA, Snyder BD. Failure of trabecular bone with simulated lytic defects can be predicted non-invasively by structural analysis. J Orthop Res. 2004;22:479–86. doi: 10.1016/j.orthres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Whealan KM, Kwak SD, Tedrow JR, Inoue K, Snyder BD. Noninvasive imaging predicts failure load of the spine with simulated osteolytic defects. J Bone Joint Surg Am. 2000;82:1240–51. doi: 10.2106/00004623-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Snyder BD, Hauser-Kara DA, Hipp JA, Zurakowski D, Hecht AC, Gebhardt MC. Predicting fracture through benign skeletal lesions with quantitative computed tomography. J Bone Joint Surg Am. 2006;88:55–70. doi: 10.2106/JBJS.D.02600. [DOI] [PubMed] [Google Scholar]
- 51.Kim DG, Hunt CA, Zauel R, Fyhrie DP, Yeni YN. The effect of regional variations of the trabecular bone properties on the compressive strength of human vertebral bodies. Ann Biomed Eng. 2007;35:1907–13. doi: 10.1007/s10439-007-9363-1. [DOI] [PubMed] [Google Scholar]
- 52.Hussein AI, Morgan EF. The effect of intravertebral heterogeneity in microstructure on vertebral strength and failure patterns. Osteoporos Int. 2013;24:979–89. doi: 10.1007/s00198-012-2039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polikeit A, Nolte LP, Ferguson SJ. Simulated influence of osteoporosis and disc degeneration on the load transfer in a lumbar functional spinal unit. J Biomech. 2004;37:1061–9. doi: 10.1016/j.jbiomech.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 54.Hussein AI, Mason ZD, Morgan EF. Presence of intervertebral discs alters observed stiffness and failure mechanisms in the vertebra. J Biomech. 2013;46:1683–8. doi: 10.1016/j.jbiomech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackman TM, Hussein AI, Adams AM, Makhnejia KK, Morgan EF. Endplate deflection is a defining feature of vertebral fracture and is associated with properties of the underlying trabecular bone. J Orthop Res. 2014;32:880–6. doi: 10.1002/jor.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brennan O, Kennedy OD, Lee TC, Rackard SM, O’Brien FJ. Biomechanical properties across trabeculae from the proximal femur of normal and ovariectomised sheep. J Biomech. 2009;42:498–503. doi: 10.1016/j.jbiomech.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 57.Brennan O, Kennedy OD, Lee TC, Rackard SM, O’Brien FJ, McNamara LM. The effects of estrogen deficiency and bisphosphonate treatment on tissue mineralisation and stiffness in an ovine model of osteoporosis. J Biomech. 2011;44:386–90. doi: 10.1016/j.jbiomech.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 58.McNamara LM, Prendergast PJ, Schaffler MB. Bone tissue material properties are altered during osteoporosis. J Musculoskelet Neuronal Interact. 2005;5:342–3. [PubMed] [Google Scholar]
- 59.McNamara LM, Ederveen AG, Lyons CG, Price C, Schaffler MB, Weinans H, et al. Strength of cancellous bone trabecular tissue from normal, ovariectomized and drug-treated rats over the course of ageing. Bone. 2006;39:392–400. doi: 10.1016/j.bone.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 60.Guo X, Goldstein S. Vertebral trabecular bone microscopic tissue elastic modulus and hardness do not change in ovariectomized rats. J Orthop Res. 2000;18:333–6. doi: 10.1002/jor.1100180224. [DOI] [PubMed] [Google Scholar]
- 61.Bohic S, Rey C, Legrand A, Sfihi H, Rohanizadeh R, Martel C, et al. Characterization of the trabecular rat bone mineral: effect of ovariectomy and bisphosphonate treatment. Bone. 2000;26:341–8. doi: 10.1016/S8756-3282(99)00276-8. [DOI] [PubMed] [Google Scholar]
- 62.Gadeleta SJ, Boskey AL, Paschalis E, Carlson C, Menschik F, Baldini T, et al. A physical, chemical, and mechanical study of lumbar vertebrae from normal, ovariectomized, and nandrolone decanoate-treated cynomolgus monkeys (macaca fascicularis) Bone. 2000;27:541–50. doi: 10.1016/s8756-3282(00)00362-8. [DOI] [PubMed] [Google Scholar]
- 63.Loveridge N, Power J, Reeve J, Boyde A. Bone mineralization density and femoral neck fragility. Bone. 2004;35:929–41. doi: 10.1016/j.bone.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 64.Busse B, Hahn M, Soltau M, Zustin J, Püschel K, Duda GN, et al. Increased calcium content and inhomogeneity of mineralization render bone toughness in osteoporosis: Mineralization, morphology and biomechanics of human single trabeculae. Bone. 2009;45:1034–43. doi: 10.1016/j.bone.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 65.Nicholson P, Cheng X, Lowet G, Boonen S, Davie M, Dequeker J, et al. Structural and material mechanical properties of human vertebral cancellous bone. Med Eng Phys. 1997;19:729–37. doi: 10.1016/s1350-4533(97)00030-1. [DOI] [PubMed] [Google Scholar]
- 66.Brennan MA, Gleeson JP, Browne M, O’Brien FJ, Thurner PJ, McNamara LM. Site specific increase in heterogeneity of trabecular bone tissue mineral during oestrogen deficiency. Eur Cell Mater. 2011;21:396–406. doi: 10.22203/ecm.v021a30. [DOI] [PubMed] [Google Scholar]
- 67.Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42:456–66. doi: 10.1016/j.bone.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 68.Brennan MA, Gleeson JP, O’Brien FJ, McNamara LM. Effects of ageing, prolonged estrogen deficiency and zoledronate on bone tissue mineral distribution. J Mech Behav Biomed Mater. 2014;29:161–70. doi: 10.1016/j.jmbbm.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 69.McNamara LM. Perspective on post-menopausal osteoporosis: establishing an interdisciplinary understanding of the sequence of events from the molecular level to whole bone fractures. J R Soc Interface. 2010;7:353–72. doi: 10.1098/rsif.2009.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Augat P, Reeb H, Claes LE. Prediction of fracture load at different skeletal sites by geometric properties of the cortical shell. J Bone Miner Res. 1996;11:1356–63. doi: 10.1002/jbmr.5650110921. [DOI] [PubMed] [Google Scholar]
- 71.Lang TF, Keyak JH, Heitz MW, Augat P, Lu Y, Mathur A, et al. Volumetric quantitative computed tomography of the proximal femur: precision and relation to bone strength. Bone. 1997;21:101–8. doi: 10.1016/s8756-3282(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 72.Crabtree N, Loveridge N, Parker M, Rushton N, Power J, Bell KL, et al. Intracapsular hip fracture and the region-specific loss of cortical bone: analysis by peripheral quantitative computed tomography. J Bone Miner Res. 2001;16:1318–28. doi: 10.1359/jbmr.2001.16.7.1318. [DOI] [PubMed] [Google Scholar]
- 73.Wachter NJ, Krischak GD, Mentzel M, Sarkar MR, Ebinger T, Kinzl L, et al. Correlation of bone mineral density with strength and microstructural parameters of cortical bone in vitro. Bone. 2002;31:90–5. doi: 10.1016/s8756-3282(02)00779-2. [DOI] [PubMed] [Google Scholar]
- 74.Yeni YN, Brown CU, Wang Z, Norman TL. The influence of bone morphology on fracture toughness of the human femur and tibia. Bone. 1997;21:453–9. doi: 10.1016/s8756-3282(97)00173-7. [DOI] [PubMed] [Google Scholar]
- 75.Wang X, Bank RA, TeKoppele JM, Agrawal CM. The role of collagen in determining bone mechanical properties. J Orthop Res. 2001;19:1021–6. doi: 10.1016/S0736-0266(01)00047-X. [DOI] [PubMed] [Google Scholar]
- 76.Schaffler MB, Choi K, Milgrom C. Aging and matrix microdamage accumulation in human compact bone. Bone. 1995;17:521–25. doi: 10.1016/8756-3282(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 77.Tong X, Burton IS, Isaksson H, Jurvelin JS, Kroger H. Cortical bone histomorphometry in male femoral neck: the investigation of age-association and regional differences. Calcif Tissue Int. 2015 Apr;94(4):295–306. doi: 10.1007/s00223-015-9957-9. [DOI] [PubMed] [Google Scholar]
- 78.Seeman E. Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J Gerontol A Biol Sci Med Sci. 2013;68:1218–25. doi: 10.1093/gerona/glt071. [DOI] [PubMed] [Google Scholar]
- 79.Martin RB, Atkinson PJ. Age and sex-related changes in the structure and strength of the human femoral shaft. J Biomech. 1977;10:223–31. doi: 10.1016/0021-9290(77)90045-8. [DOI] [PubMed] [Google Scholar]
- 80.Bouxsein ML, Myburgh KH, van der Meulen MC, Lindenberger E, Marcus R. Age-related differences in cross-sectional geometry of the forearm bones in healthy women. Calcif Tissue Int. 1994;54:113–8. doi: 10.1007/BF00296061. [DOI] [PubMed] [Google Scholar]
- 81.Turner CH. Bone strength: current concepts. Ann N Y Acad Sci. 2006;1068:429–46. doi: 10.1196/annals.1346.039. [DOI] [PubMed] [Google Scholar]
- 82.Currey J. Role of collagen and other organics in the mechanical properties of bone. Osteop Int. 2003;14 doi: 10.1007/s00198-003-1470-8. tbd. [DOI] [PubMed] [Google Scholar]
- 83.Vashishth D. Advanced glycation end-products and bone fractures. IBMS BoneKEy. 2009;6:268–78. doi: 10.1138/20090390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saito M, Marumo K, Kida Y, Ushiku C, Kato S, Takao-Kawabata R, et al. Changes in the contents of enzymatic immature, mature, and non-enzymatic senescent cross-links of collagen after once-weekly treatment with human parathyroid hormone (1–34) for 18 months contribute to improvement of bone strength in ovariectomized monkeys. Osteoporos Int. 2011;22:2373–83. doi: 10.1007/s00198-010-1454-4. [DOI] [PubMed] [Google Scholar]
- 85.Garnero P. The contribution of collagen crosslinks to bone strength. BoneKEy Rep. 2012;1:182. doi: 10.1038/bonekey.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998;106:1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 87.Depalle B, Qin Z, Shefelbine SJ, Buehler MJ. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J Mech Behav Biomed Mater. 2015;52:1–13. doi: 10.1016/j.jmbbm.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sroga GE, Vashishth D. Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr Osteoporos Rep. 2012;10:141–50. doi: 10.1007/s11914-012-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eyre DR, Weis MA, Wu JJ. Advances in collagen cross-link analysis. Methods. 2008;45:65–74. doi: 10.1016/j.ymeth.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sroga GE, Vashishth D. UPLC methodology for identification and quantitation of naturally fluorescent crosslinks in proteins: a study of bone collagen. J Chromatog B Analyt Technol Biomed Life Sci. 2011;879:379–85. doi: 10.1016/j.jchromb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paschalis EP, Mendelsohn R, Boskey AL. Infrared assessment of bone quality: a review. Clin Orthop Relat Res. 2011;469:2170–8. doi: 10.1007/s11999-010-1751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saito M, Fujii K, Marumo K. Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcif Tissue Int. 2006;79:160–8. doi: 10.1007/s00223-006-0035-1. [DOI] [PubMed] [Google Scholar]
- 93.Saito M, Fujii K, Soshi S, Tanaka T. Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int. 2006;17:986–95. doi: 10.1007/s00198-006-0087-0. [DOI] [PubMed] [Google Scholar]
- 94.Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006;17:319–36. doi: 10.1007/s00198-005-2035-9. [DOI] [PubMed] [Google Scholar]
- 95.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J hypertens. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 96.Saito M, Marumo K, Fujii K, Ishioka N. Single-column high-performance liquid chromatographic-fluorescence detection of immature, mature, and senescent cross-links of collagen. Anal Biochem. 1997;253:26–32. doi: 10.1006/abio.1997.2350. [DOI] [PubMed] [Google Scholar]
- 97.Karim L, Tang SY, Sroga GE, Vashishth D. Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone. Osteoporos Int. 2013;24:2441–7. doi: 10.1007/s00198-013-2319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karim L, Vashishth D. Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS ONE. 2012;7:e35047. doi: 10.1371/journal.pone.0035047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saito M, Fujii K, Mori Y, Marumo K. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int. 2006;17:1514–23. doi: 10.1007/s00198-006-0155-5. [DOI] [PubMed] [Google Scholar]
- 100.Knott L, Whitehead CC, Fleming RH, Bailey AJ. Biochemical changes in the collagenous matrix of osteoporotic avian bone. Biochem J. 1995;310:1045–51. doi: 10.1042/bj3101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tobon-Velasco JC, Cuevas E, Torres-Ramos MA. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol Disord Drug Targets. 2014;13:1615–26. doi: 10.2174/1871527313666140806144831. [DOI] [PubMed] [Google Scholar]
- 102.Dong XN, Qin A, Xu J, Wang X. In situ accumulation of advanced glycation endproducts (AGEs) in bone matrix and its correlation with osteoclastic bone resorption. Bone. 2011;49:174–83. doi: 10.1016/j.bone.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yamamoto Y, Kato I, Doi T, Yonekura H, Ohashi S, Takeuchi M, et al. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest. 2001;108:261–8. doi: 10.1172/JCI11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanguineti R, Storace D, Monacelli F, Federici A, Odetti P. Pentosidine effects on human osteoblasts in vitro. Ann NY Acad Sci. 2008;1126:166–72. doi: 10.1196/annals.1433.044. [DOI] [PubMed] [Google Scholar]
- 105.Alikhani M, Alikhani Z, Boyd C, MacLellan CM, Raptis M, Liu R, et al. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40:345–53. doi: 10.1016/j.bone.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Willett TL, Pasquale J, Grynpas MD. Collagen modifications in postmenopausal osteoporosis: advanced glycation endproducts may affect bone volume, structure and quality. Curr Osteoporos Rep. 2014;12:329–37. doi: 10.1007/s11914-014-0214-3. [DOI] [PubMed] [Google Scholar]
- 107.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 108.Fantner GE, Oroudjev E, Schitter G, Golde LS, Thurner P, Finch MM, et al. Sacrificial bonds and hidden length: unraveling molecular mesostructures in tough materials. Biophys J. 2006;90:1411–8. doi: 10.1529/biophysj.105.069344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lumachi F, Ermani M, Camozzi V, Tombolan V, Luisetto G. Changes of bone formation markers osteocalcin and bone-specific alkaline phosphatase in postmenopausal women with osteoporosis. Ann N Y Acad Sci. 2009;1173(Suppl 1):E60–3. doi: 10.1111/j.1749-6632.2009.04953.x. [DOI] [PubMed] [Google Scholar]
- 110.Fodor D, Bondor C, Albu A, Simon SP, Craciun A, Muntean L. The value of osteopontin in the assessment of bone mineral density status in postmenopausal women. J Invest Med. 2013;61:15–21. doi: 10.2310/JIM.0b013e3182761264. [DOI] [PubMed] [Google Scholar]